ABSTRACT
Bacterial antimicrobial resistance, especially phenotypic resistance to multiple drugs (MDR), has posed a serious threat to public health worldwide. To clarify the mechanism of transmission of multidrug resistance encoding plasmids in Enterobacterales, all seven plasmids of an Escherichia coli (E. coli) strain 1108 obtained from a chicken meat sample were extracted and sequenced by Illumina Nextseq 500 and MinION platforms. Plasmids in strain 1108 possessed 16 known antimicrobial resistance genes, with p1108-NDM (~97K) being the most variable plasmid. The multidrug resistance region of p1108-NDM was punctuated by eight IS26 insertion sequences; thus, four MDR regions were found in the backbone of this plasmid. The plasmid p1108-MCR (~65K) was found to lack the ISApl1 element and harbor the blaCTX-M-64-ISEcp1 transposition unit. Moreover, the ISEcp1-blaCMY-2 transposition unit was found in plasmid p1108-CMY2 (~98K), whereas plasmid p1108-emrB (~102K) was associated with resistance to erythromycin (emrB) and streptomycin (aadA22). p1108-IncY (96K) was a phage P1-like plasmid, while p1108-IncFIB (~194K) was found to harbor a virulence region similar to ColV plasmids, and they were found to encode a conserved conjugative transfer protein but harbor no resistance genes. Finally, no mobile element and resistant genes were found in p1108-ColV (~2K). Carriage of mcr-1-encoding elements in carbapenemase-producing Escherichia coli will potentially render all antimicrobial treatment regimens ineffective. Enhanced surveillance and effective intervention strategies are urgently needed to control the transmission of such multidrug resistance plasmids.
IMPORTANCE Antimicrobial resistance (AMR) has been increasingly prevalent in agricultural and clinical fields. Understanding the genetic environment involved in AMR genes is important for preventing transmission and developing mitigation strategies. In this study, we investigated the genetic features of an E. coli strain (1108) isolated from food product and harboring 16 AMR genes, including blaNDM-1 and mcr-1 genes encoding resistance to last line antibiotics, meropenem, and colistin. Moreover, this strain also carried virulence genes such as iroBCDEN, iucABCD, and iutA. Our findings confirmed that multiple conjugative plasmids that were formed through active recombination and translocation events were associated with transmission of AMR determinants. Our data warrant the continuous monitoring of emergence and further transmission of these important MDR pathogens.
KEYWORDS: resistance genes, foodborne E. coli, phage, genetic analysis, virulence genes
INTRODUCTION
Bacterial antimicrobial resistance, especially phenotypic resistance to multiple drugs (MDR), has posed a serious threat to human and animal health worldwide. The situation has continued to be even worse as a result of emergence of the New Delhi metallo-β-lactamase (NDM-1), which confers resistance to almost all antibiotics, including carbapenems (1). Colistin is considered one of the last-resort agents for antimicrobial treatment of serious infections caused by carbapenemase-producing Enterobacterales (CPE). Nevertheless, since the first discovery of horizontal transfer of the colistin resistance gene (mcr-1) (2), mcr variants (mcr-1 to mcr-10) have subsequently been reported on a global scale (3, 4). More worrisome, cocarriage of mcr variants and carbapenemase genes (particularly blaNDMs) among Enterobacterales, which makes clinical treatment more difficult, heralds the advent of the era of pan-drug resistance (5, 6). Phages play an important role in mediating horizontal gene transfer between bacterial cells. Moreover, production of phage, which promotes the spread of virulence-related genes, can be induced by antibiotics (7). Until now, carriage of AMR genes in virulence plasmids has been reported in strains such as Klebsiella (8) and Salmonella (9), but there are few reports about the existence of blaNDMs and mcr genes in foodborne E. coli harboring virulence and phage-like plasmids.
Here, we report the genetic characteristics of a multidrug-resistant E. coli strain recovered from chicken meat in a supermarket of Shenzhen, China in 2017. Such a strain was found to harbor as many as seven plasmids, in which 16 resistance genes were detectable, among which the plasmid carrying the blaNDM-1 gene was found to be the most variable. Findings in this work therefore provide new insights into the mechanism of transmission of MDR-encoding plasmids in Enterobacterales. Meanwhile, the carriage of multiple MDR plasmids in foodborne pathogens implied the risk of resistance genes transmission among food products.
RESULTS AND DISCUSSION
Antimicrobial susceptibility tests showed that E. coli strain 1108 was resistant to most of the antimicrobials tested; however, it exhibited intermediate susceptibility to fosfomycin. Multilocus sequence typing (MLST) was performed, with results showing that the strain belonged to ST88. PCR analysis and DNA sequencing revealed that it carried the blaNDM-1, mcr-1, and blaCTX-M-64 genes, which presumably accounted for the corresponding drug-resistance phenotypes. The results of filter mating conjugation assays, S1-PFGE, and Southern hybridization analysis of blaNDM-1- and mcr-1-bearing plasmids in strain 1108 showed that the blaNDM-1- and mcr-1-bearing plasmids were transferable and the blaNDM-1 gene was located in a plasmid of approximately 90 kb, whereas the mcr-1 gene was located in a plasmid with a size of ~60 kb (Fig. S1 in the supplemental material). The corresponding transconjugants harboring blaNDM-1 and mcr-1 genes were designated MTC1108 and CTC1108, respectively. Strain MTC1108 was found to be resistant to most of the antibiotics tested but susceptible to fosfomycin, kanamycin, chloramphenicol, and nalidixic acid; however, strain CTC1108 was only resistant to colistin, cefotaxime, ampicillin, and sulfamethoxazole/trimethoprim, but susceptible to the other antibiotics.
E. coli strain 1108 was found to possess 16 known antimicrobial resistance encoding genes, matching the resistance phenotypes observed. Seven plasmids of different incompatibility types were identified and designated p1108-NDM, p1108-MCR, p1108-emrB, p1108-CMY2, p1108-IncY, p1108-IncFIB, and p1108-Col, respectively. The basic plasmid information of the seven plasmids is provided in Table 1, and p1108-NDM was the most variable plasmid among them. The complete sequences of these plasmids were subjected to BLASTN against the NCBI database to identify previously characterized plasmids for further comparative analysis.
TABLE 1.
Genetic features of seven plasmids identified in E. coli strain 1108
| Plasmid | Size (bp) | G+C (%) | Inc type | Antimicrobial resistance genes | IS elements | Accession no. |
|---|---|---|---|---|---|---|
| p1108-NDM | 96,688 | 53.0 | IncN | blaNDM-1, bleMBL, sul1, strA, oqxB, oqxA, sul2, blaTEM-1, dfrA12, aadA2, rmtB | IS903B, IS903, ISPa40, IS26, IS6100, IS50R, IS1294, ISKpn26, ISCR1, ISAba125, ISCR2 | MG825381 |
| p1108-MCR | 64,906 | 42.3 | IncI2 | mcr-1, blaCTX-M-64 | ISEcp1 | MG825380 |
| p1108-emrB | 101,660 | 53.5 | IncI1 | emrB, aadA1 | IS26, IS1A, IS1294 | MG825377 |
| p1108-CMY2 | 98,157 | 50.0 | IncI1 | bla CMY-2 | IS1294, ISEcp1, ISEc46, IS629 | MG825376 |
| p1108-IncY | 96,082 | 48.1 | IncY | NDa | IS1294, IS186B, IS1203 | MG825379 |
| p1108-IncFIB | 193,873 | 50.0 | IncFIB | NDa | ISCro1, IS629, IS186B, IS2, IS1294, IS1A, IS640, IS3, ISEc8, IS30, IS1A, IS26 | MG825378 |
| p1108-Col | 2,096 | 55.6 | ColpVC | NDa | NDa | MH425140 |
ND, not detected.
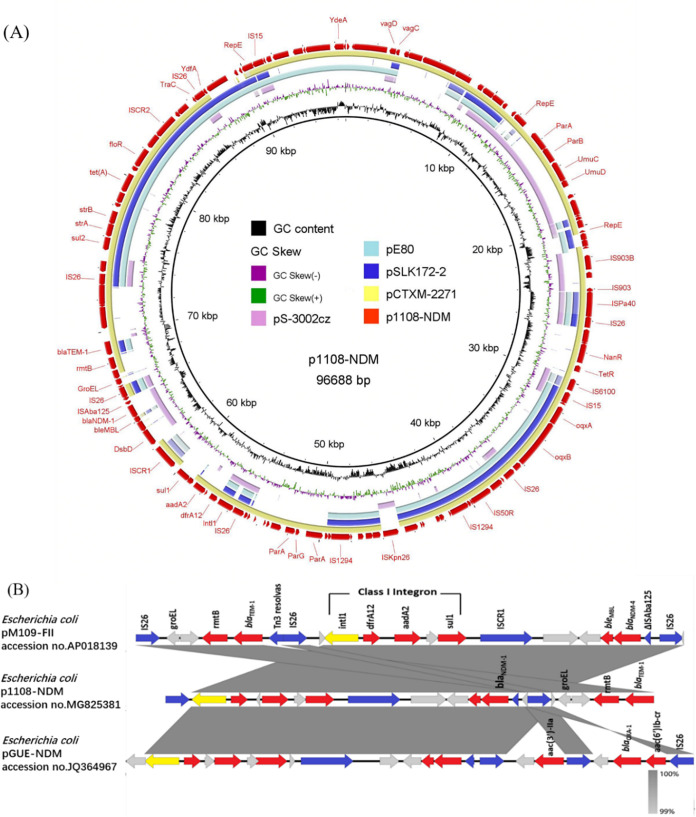
The blaNDM-1-harboring plasmid, p1108-NDM, belongs to the IncN replication type, and additional IncR and IncX replicon genes were also identified. Several of the genes located in p1108-NDM were associated with resistance to aminoglycosides (strA), sulfonamides (sul1, sul2), tetracycline (tetA, tetR), penicillin (blaTEM-1), bleomycin (bleMBL), quinolone (oqxA, oqxB), phenicol (floR), streptomycin (aadA2), trimethoprim (dfrA12), and carbapenems (blaNDM-1) (Table 1). In addition, p1108-NDM revealed 58% query coverage and 99% nucleotide identity with an IncFII type plasmid pE80, which also harbored an IncN replicon gene (10). pE80 was carried by an E. coli strain E80 isolated from chicken meat in Hong Kong and harbored blaCTX-M-55, oqxAB, fosA3, and blaTEM-1 genes; however, the MDR region encompassing the blaNDM-1 gene was absent in it (Fig. 1A). This plasmid also exhibited sequence similarity to an IncR type plasmid pS-3002cz, which was carried by a K. pneumoniae strain in the Czech Republic (11). Interestingly, the multidrug-resistance region of p1108-NDM was punctuated by eight IS26 insertion sequences, a key mobile element associated with transmission of antimicrobial-resistance determinants (12). Consequently, four MDR regions were found in the backbone of this plasmid, including IS26-blaTEM-1-rmtB-IS26, IS26-oqxB-oqxA-IS26, IS26-sul2-strA-strB-tet(A)-floR-IS26, and IS26-intI1-dfrA12-aadA2-sul1-ISCR1-bleMBL-blaNDM-1-ΔISAba125-IS26, indicating that IS26 plays an important role in transferring the blaNDM-1 gene into the backbone. As previously stated, the blaNDM gene was generally found in transposon Tn125, with two flanking ISAba125 mobile elements or various lengths of truncated ΔTn125 (13). In p1108-NDM, only a small fragment of ISAba125 (195 bp) remains located upstream of the blaNDM-1 gene, which may suggest the genetic transmission from Acinetobacter baumannii to E. coli (14). Moreover, ISCR1 was previously reported to be responsible for mobilization of antibiotic-resistant genes (15, 16). Here, an intact ISCR1 element was found to be located upstream of various genetic elements containing the trpF phosphoribosylanthranilate isomerase gene, the bleomycin resistance gene, the blaNDM-1 gene, and the truncated transposase ΔISAba125 gene. This configuration was commonly observed in plasmids harbored by NDM-1-producing Pseudomonas aeruginosa HIABP11 isolated in France (17), a strain of non-baumannii Acinetobacter spp. ABC7926, isolated in China (18), and a Providencia rettgeri strain pPrY2001 isolated in Canada (19); however, the backbone of these plasmids exhibited significant structural differences. Remarkably, the 14,381-bp mobile mosaic MDR region (IS26-intI1-dfrA12-aadA2-sul1-ISCR1-bleMBL-blaNDM-1-ΔISAba125-IS26) in p1108-NDM represented a class I integron designated In27 (intI1-dfrA12-aadA2-sul1), as previously reported in PKOX-P1 (KY913897) (20). Comparison analysis showed that the MDR region of p1108-NDM showed a high nucleotide identity with E. coli pM109-FII (AP018139) derived from patients’ blood specimens in Yangon, Myanmar. In blaNDM-4-bearing plasmid pM109_FII, the blaTEM-1 and rmtB gene cassette was bracketed by two IS26 sequences and is located downstream of this region (21); however, it was located upstream of the blaNDM-1 gene in p1108-NDM (Fig. 1B). Moreover, pGUE-NDM (JQ364967) acquired in India also displayed a similar MDR region to p1108-NDM, with a class I integron and a module consisting of the blaNDM-1 gene, ΔISAba125, and the bleMBL gene (22). Nonetheless, p1108-NDM lacked the large transfer region compared to pM109_FII and pGUE-NDM (Fig. 1B). Meanwhile, the bleMBL-blaNDM-1-ΔISAba125 transposition unit, the sul1 gene, and the ISKpn26 element were absent in pCTXM-2271, but all the other regions of p1108-NDM could be found in the backbone of pCTXM-2271, indicating that the bleMBL-blaNDM-1-ΔISAba125 transposition unit was most likely captured by this plasmid backbone and incorporated into the backbone with the aid of ISCR1 and IS26 elements.
FIG 1.
Genetic feature of blaNDM-1-bearing plasmid in E. coli strain 1108. (A) Sequence alignment of pS-3002cz (KJ958927), pE80 (KU321583), pSLK172-2 (CP017633), pCTXM-2271 (MF589339), and p1108-NDM (MG825381), with the latter being used as a reference. The outer circle with red arrows denotes annotation of the reference sequence; the gaps represent missing sequences compared to the reference plasmid. (B) Schematic representation of the structure of the blaNDM-1-surrounding sequences in plasmid p1108-NDM. Results of alignment with blaNDM-1-associated genetic structures identified in E. coli plasmid pM109-FII (AP018139) and E. coli plasmid pGUE-NDM (JQ364967) are shown. Gray shading indicates homologies between the corresponding genetic loci in each plasmid. Arrows indicate coding sequences (CDSs), with arrowheads indicating the direction of transcription: red, antibiotic resistance-encoding genes; blue, mobile elements; yellow, intl1 integrase; gray, maintenance/stability functioning genes, or hypothetical proteins.
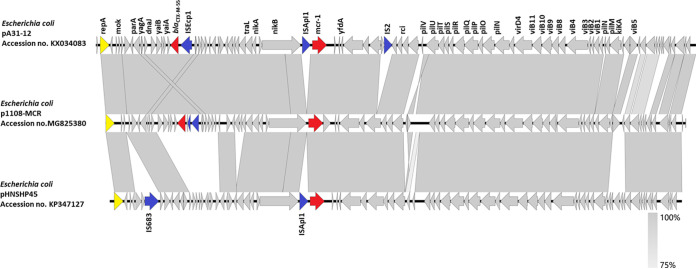
Resembling other IncI2-type plasmid backbones, p1108-MCR contains the gene encoding the RepA replicon protein, genes involved in plasmid stability (yafA, mok, hok and yafB), the chromosome (plasmid) partitioning protein (parA and parB), genes encoding proteins related to the type IV secretion complex (virB and virD4), as well as a plasmid-borne site-specific recombinase (Rci) gene and the conserved genes in all IncI2 plasmids, which have the pilus-encoding gene (pil) and the ones responsible for plasmid transfer (tra) (23). BLASTN comparison revealed that p1108-MCR had a structure highly similar to pSCS23 (KU934209), which was isolated from Salmonella enterica of chicken origin in China, and BA76-MCR-1 (KX013540), which was isolated in E. coli BA76 in a sacral wound swab in a patient in the Arabian Peninsula. In p1108-MCR, two acquired resistance genetic regions were identified. The blaCTX-M-64 gene was located in a 1,783-bp ISEcp1 transposition unit containing a truncated ISEcp1 (336 bp), blaCTX-M-64, orf477, and a 194-bp fragment. A 5-bp direct repeats (TTTTC) flanking the ΔISEcp1 was presented in the IncI2 backbone. Moreover, the left inverted repeats (5′-CCTAGATTCTACGT-3′) and right inverted repeats (5′-CCTAAATTCCACGT-3′) were observed flanking ΔISEcp1, and a perfect IRR (5′-ACGTAGAATCTAGG-3′) was located upstream of orf477, which was similar to the configuration of pA31-12 (KX034083) (23). This configuration was widely presented in other IncI2 plasmids, such as pCTXM64_C0967 harboring blaCTX-M-64 and pHN1122-1 carrying the blaCTX-M-55 gene, indicating that these IncI2 plasmids originated from a common ancestor (24). However, the IS683 and ISApl1 elements, which were commonly observed in other reported IncI2 types of plasmid harboring the mcr-1 gene, were not found in plasmid p1108-MCR compared with pHNSHP45 (KP347127) (Fig. 2).
FIG 2.
Schematic representation of the structure of the mcr-1- and blaCTX-M-64-surrounding sequences in plasmid p1108-MCR (MG825380). Results of sequence alignments with mcr-1- and blaCTX-Ms-associated genetic structures identified in Eshcerichia coli plasmid pA31-12 (KX034083) and E. coli plasmid pHNSHP45 (KP347127) are shown. CDSs without labels represent hypothetical proteins. The shaded parallelograms denote genetic regions that exhibit sequence homology among different segments. Light shading denotes regions with a lower level of sequence identity. Arrows indicate CDSs, with arrowheads indicating the direction of transcription: red, antibiotic resistance-encoding genes; blue, mobile elements; yellow, replication protein; gray, maintenance/stability functioning genes, or hypothetical proteins.
The backbone of p1108-IncY showed a high nucleotide sequence identity with the bacterial phage P1 and the phage P1-like region of pKP1226 (Fig. S2), suggesting that p1108-IncY was most likely to be lysogenized into a plasmid via phage sequences. Plasmid p1108-IncFIB harbored a transfer region, including tra and trb genes and also a virulence region from iroBCDEN of the salmochelin siderophore system, to iucABCD and iutA of the aerobactin iron transport system with a size of 62.1 kb, which was similar to that of pAPEC-O1-ColBM, p1ColV5155, and an IncF-type plasmid (pC59-153) (Fig. S3). Plasmid p1108-ermB revealed sequence similarities with pS68 and plasmid II, which were obtained from China and the United Kingdom, respectively. Moreover, two resistance genes (emrB and aadA22) and a class I integron (In155; AM261837) were found in p1108-ermB (Fig. S4). Plasmid p1108-CMY2 harbored the ISEcp1-blaCMY-2 transposition unit, and no other resistance genes were present in this plasmid other than blaCMY-2 (Fig. S5). The last plasmid in this strain, p1108-Col, was a small ColpVC plasmid with a size of 2,096 bp, and no antimicrobial resistance gene or IS element was found in it (Table 1).
Conclusion.
In conclusion, this study described the complete genetic features of seven plasmids in a foodborne CPE E. coli strain harboring 16 resistance genes, a phage P1-like region, and multiple virulence-related genes. Importantly, the concurrence of blaNDM-1 and mcr-1 genes (in different plasmids) in retail chicken meat sample provides a warning that colistin- and carbapenem-resistant genes have been disseminated into food products. Considering the fact that colistin is the last choice for treating human and animal infections caused by MDR Enterobacterales, infections due to strains that simultaneously carry the mcr-1 and CPE genes are expected to become almost untreatable. Effective surveillance and intervention approaches to control the transmission of such MDR plasmids are urgently required.
MATERIALS AND METHODS
Bacterial isolation.
E. coli isolate 1108 was obtained from chicken meat purchased from a supermarket in Shenzhen, Guangdong Province, China on 27 March 2017. This isolate was among a number of meropenem-resistant E. coli strains isolated from chicken samples using the following approach: 25 g of chicken sample were placed in a sterile homogeneous bag containing 50 mL of sterilized saline; 1 mL of homogenate was transferred to lactose broth and incubated at 42°C for 12 to 16 h; and 1 mL each of pre-enriched broth was transferred to a MacConkey agar plate supplemented with 0.5 μg/mL meropenem. Following incubation at 37°C for 16 h, two or three putative E. coli isolates were purified on MacConkey agar plates containing 0.5 μg/mL meropenem. The MALDI-TOF MS was used to identify E. coli isolate 1108 by Bruker MicroFlex LT mass spectrometer (Bruker Daltonics); the species identity of this strain was further confirmed by an API20E test strip (bioMérieux, Inc.).
Antimicrobial susceptibility testing.
Antimicrobial susceptibility of strain 1108 was tested based on previous reports (25), following the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (26). Twelve antibiotics (including colistin, meropenem, ceftazidime-avibactam, fosfomycin, kanamycin, chloramphenicol, nalidixic acid, amikacin, ciprofloxacin, cefotaxime, ampicillin, and trimethoprim-sulfamethoxazole) were tested. E. coli strain ATCC 25922 and Staphylococcus aureus ATCC 29213 were used as the quality control strain.
Genetic characterization of E. coli strain 1108.
E. coli strain 1108 was subjected to screening for the presence of mcr-1 genes and β-lactamase genes, including blaNDM-1 gene by PCR; primers were used as previously described (2, 27, 28). The genetic identity was confirmed by Sanger sequencing of purified PCR products (28106, Qiagen).
Conjugation, S1-PFGE, and Southern hybridization.
A filter-mating experiment was carried out to test the transferability of resistance phenotypes of strain 1108. Overnight cultures of donor (E. coli 1108) and recipient (sodium-azide-resistant E. coli J53) were mixed together in a ratio of 4:1 and plated on a filter membrane (0.45 μm) on LB agar medium without selection. E. coli strain 1108 was expected to undergo conjugative transfer of two types of plasmid. For plasmid carrying the blaNDM-1 gene, MacConkey Agar containing meropenem (1 μg/mL) and sodium azide (200 μg/mL) was used for selection of transconjugants that have acquired such plasmid, followed by verification of the presence of the blaNDM-1 gene by PCR. For the mcr-1 gene, Eosin Methylene Blue Agar containing colistin (2 μg/mL) and sodium azide (100 μg/mL) was used, followed by verification of the presence of mcr-1 in the plasmid by PCR. The plasmid profiles were characterized by S1-nuclease PFGE using the Chef-Mapper system (Bio-Rad, USA). The locations of blaNDM and mcr-1 in E. coli strain 1108 and the corresponding transconjugants were identified by Southern hybridization, using digoxigenin-labeled probes in accordance with the manufacturer’s instructions. The whole genome of E. coli 1108 was sequenced and was then subjected to do multilocus sequence typing (MLST) according to the protocol at an online database (http://bigsdb.pasteur.fr/) for E. coli.
Sequencing and bioinformatics analyses of plasmids.
To determine the complete nucleotide sequences of plasmids in E. coli strain 1108, the plasmids were extracted by the Qiagen Plasmid Midi kit (Qiagen, Germany) and were decoded by whole-plasmid sequencing using the Illumina Nextseq 500 and MinION platforms (Oxford Nanopore Technologies) as described previously (29). Briefly, paired-end Illumina reads (2 × 150 bp) and MinION long reads were generated with the NEBNext Ultra DNA Library Prep kit and Rapid Barcoding Sequencing kit, respectively. Hybrid assembly strategy was used to perform de novo assembly with Unicycler (30) combining short- and long-read data. Gene prediction and annotation were conducted by RAST (31) and edited manually. Alignment with complete sequences of plasmids available in the NCBI database was conducted with the BRIG (32) and Easyfig (33) tools.
Data availability.
The completed plasmid sequences for p1108-NDM, p1108-MCR, p1108-emrB, p1108-CMY2, p1108-IncY, p1108-IncFIB, and p1108-Col were deposited in NCBI with accession numbers MG825381, MG825380, MG825377, MG825376, MG825379, MG825378, and MH425140, respectively.
ACKNOWLEDGMENTS
The work described in this article was fully supported by the Basic Research Fund of Shenzhen JCYJ20200109143220716 and grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (C7003-20G, C7147-20G), the Natural Science Foundation of Changsha (kq2208418), and the Education Department of Scientific Research Project of Hunan Province (21C0147).
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Sheng Chen, Email: shechen@cityu.edu.hk.
Cezar M. Khursigara, University of Guelph College of Biological Science
REFERENCES
- 1.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L-F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J-H, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y. 2018. Transferability of MCR-1/2 polymyxin resistance: complex dissemination and genetic mechanism. ACS Infect Dis 4:291–300. doi: 10.1021/acsinfecdis.7b00201. [DOI] [PubMed] [Google Scholar]
- 4.Li R, Xie M, Zhang J, Yang Z, Liu L, Liu X, Zheng Z, Chan EW, Chen S. 2017. Genetic characterization of mcr-1-bearing plasmids to depict molecular mechanisms underlying dissemination of the colistin resistance determinant. J Antimicrob Chemother 72:393–401. doi: 10.1093/jac/dkw411. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang R, Li J, Wu Z, Yin W, Schwarz S, Tyrrell JM, Zheng Y, Wang S, Shen Z, Liu Z, Liu J, Lei L, Li M, Zhang Q, Wu C, Zhang Q, Wu Y, Walsh TR, Shen J. 2017. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol 2:16260. doi: 10.1038/nmicrobiol.2016.260. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, Zhang S, Shen J, Shen Z, Wang Y. 2018. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect 7:122. doi: 10.1038/s41426-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Barcelo C. 2018. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerging Microbes & Infections 7:1–12. doi: 10.1038/s41426-018-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Dong N, Chan EW, Zhang R, Chen S. 2021. Carbapenem resistance-encoding and virulence-encoding conjugative plasmids in Klebsiella pneumoniae. Trends Microbiol 29:65–83. doi: 10.1016/j.tim.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Chen CL, Su LH, Janapatla RP, Lin CY, Chiu CH. 2020. Genetic analysis of virulence and antimicrobial-resistant plasmid pOU7519 in Salmonella enterica serovar Choleraesuis. J Microbiol Immunol Infect 53:49–59. doi: 10.1016/j.jmii.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Wong MH, Xie M, Xie L, Lin D, Li R, Zhou Y, Chan EW, Chen S. 2016. Complete sequence of a F33:A-:B- conjugative plasmid carrying the oqxAB, fosA3, and blaCTX-M-55 elements from a foodborne Escherichia coli strain. Front Microbiol 7:1729. doi: 10.3389/fmicb.2016.01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Studentova V, Dobiasova H, Hedlova D, Dolejska M, Papagiannitsis CC, Hrabak J. 2015. Complete nucleotide sequences of two NDM-1-encoding plasmids from the same sequence type 11 Klebsiella pneumoniae strain. Antimicrob Agents Chemother 59:1325–1328. doi: 10.1128/AAC.04095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong MH, Chan EW, Chen S. 2017. IS26-mediated formation of a virulence and resistance plasmid in Salmonella Enteritidis. J Antimicrob Chemother 72:2750–2754. doi: 10.1093/jac/dkx238. [DOI] [PubMed] [Google Scholar]
- 13.Wailan AM, Paterson DL, Kennedy K, Ingram PR, Bursle E, Sidjabat HE. 2016. Genomic characteristics of NDM-producing Enterobacteriaceae isolates in Australia and their blaNDM genetic contexts. Antimicrob Agents Chemother 60:136–141. doi: 10.1128/AAC.01243-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeifer Y, Wilharm G, Zander E, Wichelhaus TA, Gottig S, Hunfeld KP, Seifert H, Witte W, Higgins PG. 2011. Molecular characterization of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J Antimicrob Chemother 66:1998–2001. doi: 10.1093/jac/dkr256. [DOI] [PubMed] [Google Scholar]
- 15.Toleman MA, Bennett PM, Walsh TR. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol Mol Biol Rev 70:296–316. doi: 10.1128/MMBR.00048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partridge SR, Iredell JR. 2012. Genetic contexts of blaNDM-1. Antimicrob Agents Chemother 56:6065–6067. doi: 10.1128/AAC.00117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janvier F, Jeannot K, Tesse S, Robert-Nicoud M, Delacour H, Rapp C, Merens A. 2013. Molecular characterization of NDM-1 in a sequence type 235 Pseudomonas aeruginosa isolate from France. Antimicrob Agents Chemother 57:3408–3411. doi: 10.1128/AAC.02334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Du X, Ji J, Chen Y, Jiang Y, Yu Y. 2012. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J Antimicrob Chemother 67:2114–2122. doi: 10.1093/jac/dks192. [DOI] [PubMed] [Google Scholar]
- 19.Mataseje LF, Boyd DA, Lefebvre B, Bryce E, Embree J, Gravel D, Katz K, Kibsey P, Kuhn M, Langley J, Mitchell R, Roscoe D, Simor A, Taylor G, Thomas E, Turgeon N, Mulvey MR, Canadian Nosocomial Infection Surveillance P . 2014. Complete sequences of a novel blaNDM-1-harbouring plasmid from Providencia rettgeri and an FII-type plasmid from Klebsiella pneumoniae identified in Canada. J Antimicrob Chemother 69:637–642. doi: 10.1093/jac/dkt445. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Yuan M, Chen H, Chen X, Jia YC, Zhu X, Bai L, Bai X, Fanning S, Lu JX, Li J. 2017. First report of Klebsiella oxytoca strain simultaneously producing NDM-1, IMP-4, and KPC-2 carbapenemases. Antimicrob Agents Chemother 61:e00877-17. doi: 10.1128/AAC.00877-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugawara Y, Akeda Y, Sakamoto N, Takeuchi D, Motooka D, Nakamura S, Hagiya H, Yamamoto N, Nishi I, Yoshida H, Okada K, Zin KN, Aye MM, Tonomo K, Hamada S. 2017. Genetic characterization of blaNDM-harboring plasmids in carbapenem-resistant Escherichia coli from Myanmar. PLoS One 12:e0184720. doi: 10.1371/journal.pone.0184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:e34752. doi: 10.1371/journal.pone.0034752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Li XP, Yang RS, Fang LX, Huo W, Li SM, Jiang P, Liao XP, Liu YH. 2016. Complete nucleotide sequence of an IncI2 plasmid coharboring blaCTX-M-55 and mcr-1. Antimicrob Agents Chemother 60:5014–5017. doi: 10.1128/AAC.00774-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, He D, Lv L, Liu W, Chen X, Zeng Z, Partridge SR, Liu JH. 2015. blaCTX-M-1/9/1 hybrid genes may have been generated from blaCTX-M-15 on an IncI2 plasmid. Antimicrob Agents Chemother 59:4464–4470. doi: 10.1128/AAC.00501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Geng S, Chan EW, Chen S. 2019. Increased prevalence of Escherichia coli strains from food carrying blaNDM and mcr-1-bearing plasmids that structurally resemble those of clinical strains, China, 2015 to 2017. Eurosurveillance 24:1800113. doi: 10.2807/1560-7917.ES.2019.24.13.1800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLSI. 2017. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement. CLSI document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 27.Lin D, Xie M, Li R, Chen K, Chan EW, Chen S. 2017. IncFII conjugative plasmid-mediated transmission of blaNDM-1 elements among animal-borne Escherichia coli strains. Antimicrob Agents Chemother 61:e02285-16. doi: 10.1128/AAC.02285-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 29.Li R, Xie M, Dong N, Lin D, Yang X, Wong MHY, Chan EW, Chen S. 2018. Efficient generation of complete sequences of MDR-encoding plasmids by rapid assembly of MinION barcoding sequencing data. Gigascience 7:gix132. doi: 10.1093/gigascience/gix132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia FF, Stevens R. 2014. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.02820-22-s0001.pdf, PDF file, 0.9 MB (897.9KB, pdf)
Data Availability Statement
The completed plasmid sequences for p1108-NDM, p1108-MCR, p1108-emrB, p1108-CMY2, p1108-IncY, p1108-IncFIB, and p1108-Col were deposited in NCBI with accession numbers MG825381, MG825380, MG825377, MG825376, MG825379, MG825378, and MH425140, respectively.