The Middle East and Africa bear a disproportionate burden of diabetes and associated cardiovascular complications,1,2 yet high-quality contemporary data on the prevalence of cardiovascular risk in people with type 2 diabetes (T2D) are lacking in these regions.3,4
PACT-MEA (An International Chart Review and Survey for the Prevalence and Clinical Management of Atherosclerotic Cardiovascular Diseases in Patients With Type 2 Diabetes Across Countries in the Middle East and Africa; URL: https://www.clinicaltrials.gov; Unique identifier: NCT05317845) is a noninterventional, cross-sectional, observational study conducted at 55 centers in 7 countries. The study was approved by local institutional review boards, and study participants provided informed consent. We estimated the prevalence of established atherosclerotic cardiovascular disease (eASCVD), cardiovascular risk categories (according to the European Society of Cardiology [ESC] 2021 guidelines5), and achievement of guideline-recommended targets in people with T2D. Patients were included if they were ≥18 years of age and were diagnosed with T2D ≥180 days before study entry. Data were collected during a single, routine health visit with a standardized electronic case report form. The primary outcome was the prevalence of eASCVD (coronary, cerebrovascular, and peripheral artery diseases), estimated overall across the regions and stratified by country. The secondary outcome was the proportion of patients who were at high or very high atherosclerotic cardiovascular disease (ASCVD) risk, defined by ESC 2021 guidelines. To account for the differences in population sizes, prevalence and ESC risk estimates were weighted to account for the size of the diabetes population in each country. The data that support the findings of this study are available from the corresponding author on reasonable request.
The overall study sample included 3726 individuals (Bahrain, 366; Egypt, 550; Jordan, 576; Kuwait, 350; Qatar, 346; South Africa, 996; United Arab Emirates, 542). The mean age was 58 years (SD, 12 years), equally represented with male (53%) and female (47%) participants, with 58% of participants between 45 and 64 years of age. Median body mass index was 30 kg/m2 (interquartile range, 27–35 kg/m2; class 1, 2, and 3 obesity, 29%, 15%, and 9%, respectively). Nearly all patients (98%) had coronary risk factors; 84% had at least 2 risk factors. Median duration of T2D was 10 years (interquartile range, 5–17 years), and the mean glycated hemoglobin was 8% (SD, 2%). Hypertension and dyslipidemia were present in 71% and 92% of patients, respectively, and 14% reported a history of current smoking. Most patients (77%) were on statins, (98% on moderate- to high-intensity statins) with a median low-density lipoprotein cholesterol of 85 mg/dL (interquartile range, 65–115 mg/dL). Mean estimated glomerular filtration rate was 82 mL·min−1·1.73 m−2 (SD, 25 mL·min−1·1.73 m−2); 16% had an estimated glomerular filtration rate of <60 mL·min−1·1.73 m−2. Twenty-seven percent of patients had a urinary albumin-to-creatinine ratio of 30 to 300 mg/g, and 7% had a urinary albumin-to-creatinine ratio of >300 mg/g. Microvascular complications of retinopathy, neuropathy, or nephropathy were present in 14%, 25%, and 15% of patients, respectively. Seven percent of patients had heart failure. Inhibitors of the renin-angiotensin system were used by 51% of study participants. In terms of glucose-lowering therapies, 77% were using biguanides, 38% were using insulin, 36% were taking sodium-glucose cotransporter-2 inhibitors, and 13% were using glucagon-like peptide-1 receptor agonists.
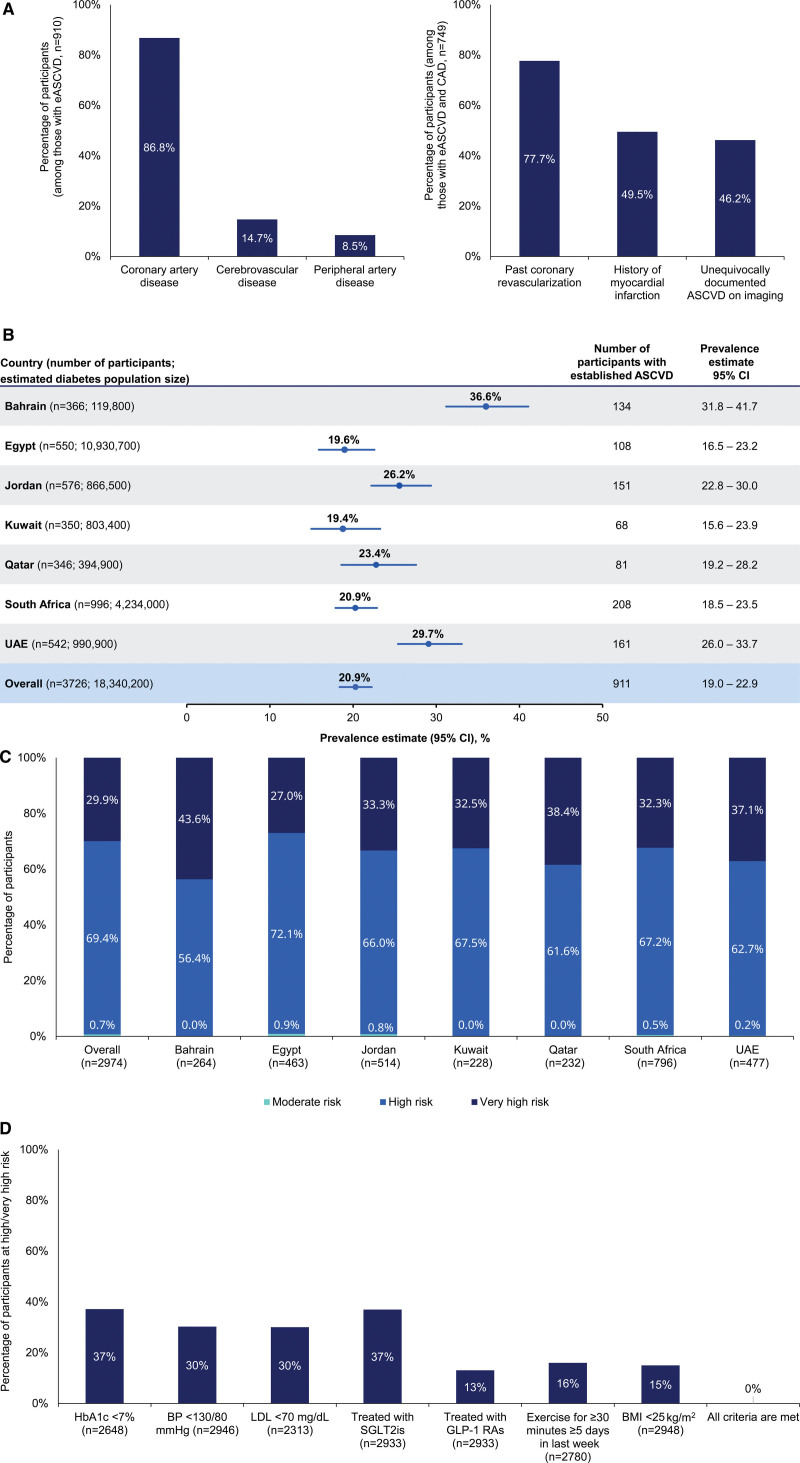
The weighted prevalence of eASCVD was 20.9% (95% CI, 19.0%–22.9%; Figure), higher for men, 26.6% than for women, 16.0%; increasing with age: 6.4%, 20.1%, and 33.2% in the 18- to 44-, 45- to 64-, and ≥65-year-old categories, respectively. The most common type of eASCVD was coronary artery disease (87%; Figure), with 50% having a history of myocardial infarction and 78% having a history of coronary artery revascularization. Bahrain had the highest prevalence of eASCVD (36.6%), followed by the United Arab Emirates, Jordan, Qatar, South Africa, and Egypt. Kuwait had the lowest prevalence among the participating countries (19.4%; Figure).
Figure.
Summary of primary findings in PACT-MEA. A, Type of atherosclerotic cardiovascular disease (ASCVD) among patients with established ASCVD (eASCVD; left) and type of coronary artery disease (CAD) among patients with eASCVD and CAD (right). Types of ASCVD and CAD are not mutually exclusive; 1 participant may have multiple diagnoses. Data are unweighted. B, Prevalence of eASCVD in people with type 2 diabetes (T2D), by country and overall (across the 7 countries). Prevalence estimates for each country are unweighted. Overall prevalence is calculated as a weighted estimate to account for the size of the diabetes population of each country. Diabetes prevalence data are from the IDF [International Diabetes Federation] Diabetes Atlas, 10th edition, 2021. C, Proportion of people within each ASCVD risk category according to European Society of Cardiology (ESC) 2021 guidelines, overall and by country. Overall risk category proportion (across the 7 countries) was calculated as a weighted estimate to account for the size of the diabetes population of each country. Individual country data are unweighted. There were 752 patients for whom data used to calculate the risk levels were missing; these patients were excluded from the analysis. D, Proportion of patients at high/very high risk achieving ESC 2021 guideline–recommended targets for risk factors by people with T2D. Sample sizes reflect patients for whom data were available in the medical record. PACT-MEA (Prevalence and Clinical Management of Atherosclerotic Cardiovascular Diseases in Patients With Type 2 Diabetes Across Countries in the Middle East and Africa) was conducted in primary and secondary care facilities. Patients were enrolled between April 21, 2022, and August 31, 2022. Study site investigators and their staff were instructed to approach any adult patient diagnosed with T2D attending their clinic for a routine visit. Inclusion/exclusion criteria as follows: age ≥18 years at the time of informed consent; diagnosed with T2D ≥180 days before informed consent; not diagnosed with type 1 diabetes; no known congenital heart disease or malformation; and no mental incapacity, unwillingness, or language barriers precluding adequate understanding or cooperation. Throughout the study, site monitoring was conducted by the clinical research organization to ensure adherence to the study protocol. For the primary outcome, CAD was defined as any of the following: previous acute coronary syndrome, previous myocardial infarction, previous unstable angina, history of stable angina, past coronary revascularization (percutaneous coronary intervention, coronary artery bypass graft), or unequivocally documented ASCVD on imaging (including plaque on coronary angiography or computed tomography angiography). Cerebrovascular disease was defined as either history of stroke that was atherosclerotic in origin or history of transient ischemic attack that was atherosclerotic in origin. Peripheral arterial disease was defined as any of the following: extracranial CAD (unequivocally documented ASCVD on imaging [including plaque on carotid ultrasound or computed tomography angiography] or past arterial revascularization procedure), lower-extremity arterial disease (history of claudication with ankle-brachial index ≤0.90, lesions documented on imaging, past arterial revascularization, history of nontraumatic minor and major amputation), or other peripheral arterial diseases (aortic aneurysm, vertebral artery disease, atherosclerotic upper-extremity artery disease, renal artery disease, mesenteric artery disease). For the secondary outcomes, cardiovascular risk categories were defined as follows: (1) moderate risk: patients with well-controlled short-standing diabetes (eg, <10 years), no evidence of target organ damage (TOD), and no additional ASCVD risk factors; (2) high risk: patients without ASCVD and/or severe TOD (estimated glomerular filtration rate [eGFR] <45 mL.min.1.1.73 m.2 regardless of albuminuria, eGFR 45–59 mL.min.1.1.73 m.2, and microalbuminuria albumin-to-creatinine ratio [ACR] 30–300 mg/g, proteinuria ACR >300 mg/g, or presence of microvascular disease in at least 3 sites), and not fulfilling the moderate-risk criteria; or (3) very high risk: patients with T2D with established ASCVD and/or severe TOD. BMI indicates body mass index; BP, blood pressure; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein; SGLT2i, sodium-glucose cotransporter-2 inhibitor; and UAE, United Arab Emirates.
The weighted distribution of cardiovascular risk categories according to the 2021 ESC guidelines revealed that 69.4% were classified as high risk and 29.9% as very high risk (Figure). Pertaining to achievement of guideline-recommended targets, 37% of patients at high/very high risk achieved a glycated hemoglobin <7%, 30% met the blood pressure goal of <130/80 mm Hg, and 30% achieved a low-density lipoprotein cholesterol <70 mg/dL. Thirty-seven percent were on sodium-glucose cotransporter-2 inhibitors; 13% were on glucagon-like peptide-1 receptor agonists; 16% exercised ≥5 times per week; and 15% had a body mass index <25 kg/m2. However, none of the participants achieved all guideline recommendations (Figure).
Study limitations are acknowledged. Prevalence estimates may not be truly representative of an entire country given the modest sample size and convenience sampling approach. However, the population size differences are mitigated by weighting the mean ASCVD prevalence and risk estimates by each country’s diabetes population size with the respective prevalence rates. Interpretation of the clinical findings may be limited by missing laboratory data. Screening for heart failure with natriuretic peptides and echocardiography is low in the region and was not captured; this may have resulted in the lower prevalence of heart failure observed.
In summary, we provide one of the first contemporary prevalence estimates of vascular risk among people with T2D across the Middle East and Africa. One in 5 people with T2D in these regions has eASCVD; 99.3% met ESC high/very high-risk criteria, and none of the patients in our cohort achieved all the guideline-recommended targets for prevention. These data underscore the importance of, and immediate need for, approaches to risk reduction in these vulnerable populations.
Article Information
Acknowledgments
The authors thank Rebecca Hahn, MPH, of KJT Group, Inc, Rochester, NY, for providing editorial support in accordance with Good Publication Practice 2022 guidelines. The authors also thank Andrea Stoltz, MS, and Aaron Woods, BA, of KJT Group, Inc, for statistical analysis support. They thank the PACT-MEA study participants, investigators, coordinators, and study staff. The authors also thank Gaurav Kumar, MPharm, of Novo Nordisk for managing the PACT-MEA study operations. They thank the participating study sites: Awali Cardiac Center; Salmaniya Medical Center; Bahrain Defense Force Hospital; King Hamad University Hospital; Ain Shams University Hospital; Menoufia University Hospital; NIDE; Alexandria University Hospital; Mansoura University Hospital; Bader Medical Complex, private clinic; Ibn Al Haytham Hospital; Islamic Hospital; Abdali Medical Center; Jabal Al Zaytoun Hospital; Dr Munir Abu Al Samen, private clinic; Jordan University Hospital; Istishari Hospital; King Abdullah University Hospital; JUST Center; Farwaniya Hospital; Yarmouk Medical Center; Omariya Clinic; Al Jahra Hospital; West Mishrif Clinic; Mubarak AlKabeer Hospital; Hamad General Hospital; Al Wakra Hospital; Diabetes Life Clinic; Chris Hani Baragwanath Academic Hospital; Cape Town Medical Research Centre; Dr Naeem Moosa private practice; Lenasia Clinical Trial Center; Drs H & V Makan Centre for Diabetes; Diabetes Care Centre; Life Fourways Hospital; Diabetes Care Centre & CDE Centre; Netcare Greenacres Hospital; Sandton Mediclinic; Netcare Alberton Hospital; CDE, Houghton Group Practice; Dr Hilton Kaplan Inc; Langeberg Medical Centre; Rashid Hospital; Dubai Hospital; Thumbay University Hospital; Al Qassimi Hospital; Dubai Diabetes Center; Aster Clinics; Fujairah Hospital; Cleveland Clinic Abu Dhabi; and NMC Specialty Hospital. S.V. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. E.M.C., G.T., G.Y., and L.L.N.H. were responsible for the concept and design. S.V. N.A., F.A., H.A., W.A., S.H.A.-K., J.H., L.L.N.H., L.L., R.A.M., E.M.C., H.S., G.T., G.Y., and S.S. were responsible for the acquisition, analysis, or interpretation of data. S.V. developed the first draft of the manuscript. S.V., N.A., F.A., H.A., W.A., S.H.A.-K., J.H., L.L.N.H., L.L., R.A.M., E.M.C., H.S., G.T., G.Y., and S.S. were responsible for critical revision of the manuscript for important intellectual content. Novo Nordisk funded the study and the editorial assistance provided by KJT Group, Inc.
Sources of Funding
None.
Disclosures
Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Novartis, Novo Nordisk, Pfizer, PhaseBio, S & L Solutions Event Management Inc, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group. Dr Verma is president of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. Dr Alamuddin reports receiving honoraria for Speakers’ Bureau and Advisory Board for Novo Nordisk and MSD. Dr Malik reports receiving honoraria for talks and advisory boards for Novo Nordisk, Lilly, Sanofi Aventis, and Proctor & Gamble. Dr Salek reports receiving unrestricted educational grants from GSK, the European Haematology Association, and Centre for Innovative Regulatory Science. Drs Tombak and Yadav are employees of Novo Nordisk. Drs Husemoen and Mashaki Ceyhan are employees of and own stock in Novo Nordisk. The other authors report no conflicts.
Footnotes
For Sources of Funding and Disclosures, see page 1254.
Circulation is available at www.ahajournals.org/journal/circ
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT05317845.
Contributor Information
Naji Alamuddin, Email: naalamuddin@rcsi.com.
Fatheya Alawadi, Email: ffAlawadi@dha.gov.ae.
Hessa Alkandari, Email: Hessa.Alkandari@dasmaninstitute.org.
Wael Almahmeed, Email: wmahmeed@gmail.com.
Samir H. Assaad-Khalil, Email: assaadkhalil@hotmail.com.
Jihad Haddad, Email: haddad_jihad@yahoo.gr.
Lise Lotte N. Husemoen, Email: lshu@novonordisk.com.
Landman Lombard, Email: landmanlombard@gmail.com.
Rayaz A. Malik, Email: ram2045@qatar-med.cornell.edu.
Emel Mashaki Ceyhan, Email: eemc@novonordisk.com.
Hani Sabbour, Email: hanisabbour1@icloud.com.
Gamze Tombak, Email: GAMT@novonordisk.com.
Gourav Yadav, Email: GOYA@novonordisk.com.
Sam Salek, Email: sssalek52@gmail.com.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 10th ed. 2021. Accessed October 5, 2022. https://www.diabetesatlas.org [Google Scholar]
- 2.Meo SA, Sheikh SA, Sattar K, Akram A, Hassan A, Meo AS, Usmani AM, Qalbani E, Ullah A. Prevalence of type 2 diabetes mellitus among men in the Middle East: a retrospective study. A J Mens Health. 2019;13:155798831984857. doi: 10.1177/1557988319848577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes MB, Rathmann W, Charbonnel B, Khunti K, Kosiborod M, Nicolucci A, Pocock SJ, Shestakova MV, Shimomura I, Tang F, et al. ; DISCOVER Investigators. Treatment of type 2 diabetes mellitus worldwide: baseline patient characteristics in the global DISCOVER study. Diabetes Res Clin Pract. 2019;151:20–32. doi: 10.1016/j.diabres.2019.03.024 [DOI] [PubMed] [Google Scholar]
- 4.Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, Dieuzeide G, Eriksen KT, Hong T, Kaltoft MS, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20: doi: 10.1186/s12933-021-01344-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, et al. ; ESC National Cardiac Societies. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]