Background:
The impact of early implementation of cardiac rehabilitation (CR) in heart failure (HF) patients remains to be elucidated. This study sought to determine whether CR during HF hospitalization could improve prognostic outcomes in patients with acute decompensated HF.
Methods:
We analyzed patients with HF enrolled in the JROADHF (Japanese Registry of Acute Decompensated Heart Failure) registry, a retrospective, multicenter, nationwide registry of patients hospitalized for acute decompensated HF. Eligible patients were divided into 2 groups according to CR during hospitalization. The primary outcome was a composite of cardiovascular death or rehospitalization due to cardiovascular event after discharge. The secondary outcomes were cardiovascular death and cardiovascular event rehospitalization.
Results:
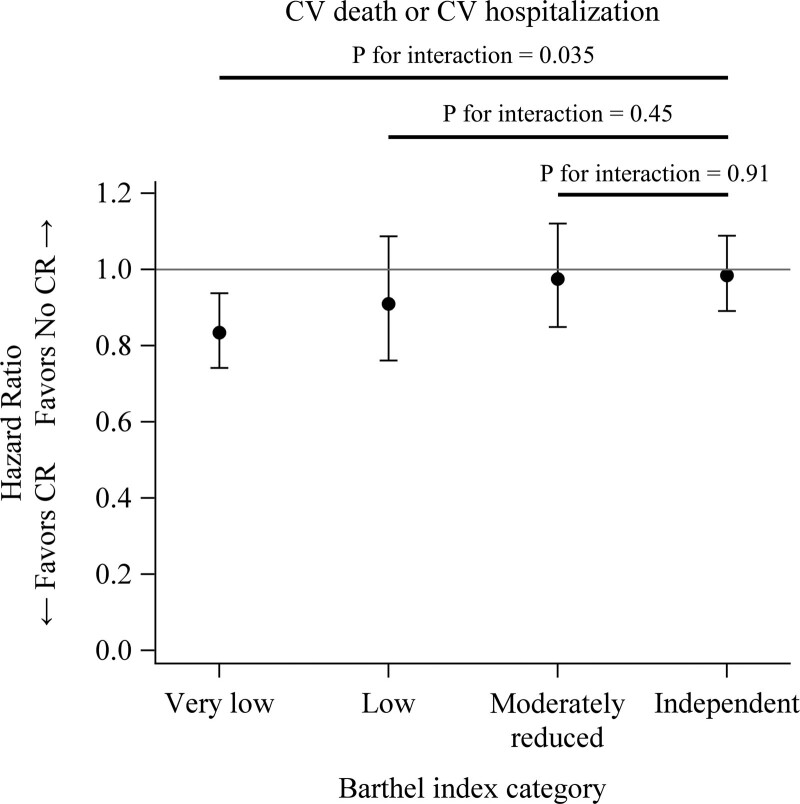
Out of 10 473 eligible patients, 3210 patients underwent CR. Propensity score matching yielded 2804 pairs. Mean age was 77±12 years and 3127 (55.8%) were male. During a mean follow-up of 2.8 years, the CR group had lower incidence rates of the composite outcome (291 versus 327 events per 1000 patient-years; rate ratio, 0.890 [95% CI, 0.830–0.954]; P=0.001) and rehospitalization due to cardiovascular event (262 versus 295 events per 1000 patient-years; rate ratio, 0.888 [95% CI, 0.825–0.956]; P=0.002) than the no CR group. In-hospital CR was associated with an improvement in Barthel index for activities of daily living (P=0.002). Patients with very low Barthel index at admission were benefited by CR in comparison with patients with independent Barthel index (very low; hazard ratio, 0.834 [95% CI, 0.742–0.938]: independent; hazard ratio, 0.985 [95% CI, 0.891–1.088]; P for interaction=0.035).
Conclusions:
CR implementation during hospitalization was associated with better long-term outcomes in patients with acute decompensated HF. These data support the need for a randomized, controlled, adequately powered trial to definitively test the role of early physical rehabilitation in hospitalized patients with HF.
Keywords: cardiac rehabilitation, cardiovascular conditioning, heart failure, prognosis, quality of life
What is New?
In Japan, cardiac rehabilitation is recommended for patients with acute decompensated heart failure during hospitalization. However, the effects of early implementation of cardiac rehabilitation have not been fully elucidated. In this study, cardiac rehabilitation during heart failure hospitalization is associated with improvements in quality of life at discharge and prognosis after discharge.
What Are the Clinical Implications?
Improvement of physical function by early implementation of cardiac rehabilitation is the key to better prognosis in heart failure patients. Cardiac rehabilitation during heart failure hospitalization may be more effective in patients with severe physical deconditioning.
Exercise training was shown to improve quality of life in patients with heart failure (HF) in 1999.1 Thereafter, several studies have reported the effectiveness of cardiac rehabilitation (CR) on quality of life, exercise capacity, and prognosis in patients with HF.2–4 However, HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training), a randomized controlled trial including 2331 stable outpatients with HF, demonstrated that exercise training was not associated with better clinical outcomes.5 ExTraMATCH II (Exercise Training Meta-Analysis of Trials for Chronic Heart Failure II), an individual patient data meta-analysis, failed to show the beneficial effects of exercise-based CR on prognosis in patients with HF.6 In the most recent meta-analysis concluded that low to moderate-quality evidence showed that CR probably reduced the risk of all-cause hospital rehospitalization.7,8 It is still difficult to conclude the association between CR and outcomes with conviction due to lack of evidence. In addition, previous studies which reported the beneficial effects of CR on cardiovascular outcomes mainly focused on outpatients with chronic stable HF but not those with acute decompensated HF.
More recently, Kitzman et al9 conducted a randomized controlled trial and demonstrated that the early rehabilitation resulted in greater improvement in physical function than usual care in outpatients with HF. Although this important trial failed to show beneficial effects of CR on cardiovascular outcomes, early implementation of CR is thought to be beneficial for patients with HF. It drives us to assess CR during HF hospitalization because we can provide CR as soon as possible to prevent further physical deconditioning in comparison with outpatient CR in Japan. Moreover, the Japanese nationwide registry has potential to provide us with reliable results.10
The Japanese Circulation Society Guideline on Rehabilitation in Patients with Cardiovascular Disease recommended the standard CR program in patients with acute decompensated HF.11 According to this guideline, around 40% of the patients with acute decompensated HF underwent CR during the index hospitalization in Japan.12 Hospitalized patients with HF are more likely to be complicated by frailty.13 Physical functions, including frailty and activity of daily living (ADL), are closely associated with a prognosis in patients with HF.14–16 Recently, CR has been shown to effectively improve frailty.17 Therefore, improvement in physical function by early CR implementation for acute decompensated HF might contribute to better prognostic outcomes. However, to date, the impact of in-hospital CR for patients with acute decompensated HF on long-term outcomes has not been elucidated.
The JROADHF (Japanese Registry of Acute Decompensated Heart Failure) is a retrospective, multicenter, nationwide registry of hospitalized patients with HF, enrolling 13 238 patients admitted with HF in a web-based registry at 158 participating hospitals in Japan.10 This extensive registry was developed by linking the Japanese Registry Of All cardiac and vascular Diseases-the Japanese Diagnosis Procedure Combination system, a nationwide claim database. JROADHF database is useful for analyzing the characteristics and treatments of patients hospitalized for acute decompensated HF in Japan. Although previous CR studies mainly focused on HF with reduced ejection fraction, we did not exclude HF with preserved ejection fraction patients because we assumed that CR could benefit any types of HF.18 The aim of this study was to determine whether the implementation of CR during the hospitalization could improve long-term outcomes in patients with acute decompensated HF.
Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Japanese Registry of Acute Decompensated Heart Failure
The JROADHF is a multicenter registry of patients hospitalized for the worsening HF in Japan.10 In this registry, HF was defined with International Classification of Diseases, Tenth Revision codes, and medical chart review. First, International Classification of Diseases, Tenth Revision codes related to HF (I50.0, I50.1, and I50.9), and additional diagnostic codes (30 101 or 30 102 representing acute HF or exacerbation of chronic HF) were used. Then, the diagnosis was confirmed by medical chart review. This 2-step method yielded more reliable HF definition. Baseline data were collected during the episode of index hospitalization during 2013 from 158 hospitals. Follow-up data were collected up to 5 years after the hospitalization. The baseline data include demography, cause of HF, precipitating cause, comorbidities, clinical status, electrocardiographic and echocardiographic findings, and treatment including discharge medications.
Study Patients
From the database of JROADHF, patients who were discharged alive were included. Patients were excluded if they had left ventricular assist device or information about CR was missing. The reason why information about CR was missing is that we failed to extract claim data due to technical issues. Hence, the mechanism of missing was at random and not affected by patient information. We included any left ventricular ejection fraction categories as stated in Introduction section. Eligible patients were divided into 2 groups according to the implementation of CR.
In-Hospital CR
Since the database used in our study obtained information from individuals who were hospitalized in 2013, the CR was conducted according to the Japanese Guidelines for rehabilitation in patients with cardiovascular disease published in 2012. This guideline was published in Japanese, so we translated corresponding part into English (Supplemental Methods). Briefly, ambulation program is initiated once hemodynamics is stabilized, even if the patients are still on inotropes. This program includes stretching on the bed, sitting upright, and walking. During the program, symptoms, vital signs, and electrocardiogram are monitored. We increment the strength and time of the program. Upon completion of ambulation program, then patients proceed to the exercise program. This includes walking, ergometer, aerobic exercise, and low-level resistance training under supervision.
Implementation of CR
Implementation of CR was defined as Diagnosis Procedure Combination code (claim database in Japan) 180027410 or 180027510. Institution which can provide CR was defined as institution with at least 1 Diagnosis Procedure Combination code 180027410 or 180027510 in 2013.
Outcomes
The primary outcome was a composite of cardiovascular death or rehospitalization due to cardiovascular event. The secondary outcomes were cardiovascular death and rehospitalization due to cardiovascular event. Cardiovascular event was defined as a composite of HF, myocardial infarction, angina pectoris, arrhythmia, stroke, and pulmonary embolism. We also assessed the primary outcome among subgroups; age (≥80 versus <80 years), sex, left ventricular ejection fraction (≥40 versus <40%), ischemic heart disease, atrial fibrillation, hypertension, diabetes, chronic kidney disease (stage 1–2 versus 3–5), anemia, and, the use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, mineralocorticoid receptor antagonists, and beta-blockers.
Statistical Analysis
Patient characteristics were compared with Pearson χ2 test for categorical variables and Student t test or Wilcoxon rank sum test for continuous variables and were presented as mean±SD or median with interquartile range. A propensity score was estimated by fitting a logistic-regression model which adjusted for age, sex, New York Heart Association functional class (I–II versus III–IV), prior HF hospitalization, ischemic heart disease, hypertension, diabetes mellitus, stroke, chronic obstructive pulmonary disease, malignancy, pacemaker, implantable cardioverter defibrillator, cardiac resynchronization therapy-defibrillator, heart rate, atrial fibrillation, left ventricular ejection fraction, hemoglobin, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, mineralocorticoid receptor antagonist, beta-blockers, loop diuretics, thiazide, digitalis, oral inotrope, and amiodarone. It is possible that physician in charge did not provide CR for patients with low life expectancy, which can be a potential indication bias. We included extracardiac variables (stroke, chronic obstructive pulmonary disease, and malignancy) as covariates to exclude potential indication bias. Malignancy, cardiovascular diseases, and pulmonary diseases accounted for 27.4%, 24.8%, and 12.2% of death, respectively, according to the statistics from the Japanese Ministry of Health, Labour and Welfare. Considering these statistics, stroke (associated with aspiration pneumonia), chronic obstructive pulmonary disease, and malignancy are appropriate to be chosen.
One-to-one pair matching between the 2 groups was performed by nearest-neighbor matching without replacement. Covariate balances before and after matching were checked by comparison of standardized mean difference (SMD). An SMD <0.1 was considered to indicate a negligible imbalance between the 2 groups. For analysis of associations between CR and outcomes, incidence rates per 1000 patient-years and incidence rate ratio were calculated for each outcome. Hazard ratio (HR) was estimated by Cox regression analysis and Fine and Gray model, and their results were compared for the primary analysis. Others were analyzed with Fine and Gray model. Results were presented with 95% CI and P value. Sensitivity analyses were described in the Supplemental Methods (Sensitivity Analyses section).
To assess ADL related to frailty, the Barthel index was categorized into 4 subcategories: 80 to 100 (independent ADL), 50 to 79 (moderately reduced ADL), 25 to 49 (low ADL), and 0 to 24 (very low ADL).19 The effects of inpatient CR on changes in Barthel index from admission to discharge were examined with generalized estimating equations adjusted for same covariates as used in propensity score matching.
To test whether Barthel index categories were an effect modifier for CR, we did a post-hoc analysis. Association between CR and the primary outcome according to Barthel index at admission was examined with Cox regression model including CR, Barthel index subcategories, interaction term (CR×Barthel index subcategories), and covariates used in the propensity score matching.
All tests were 2-tailed and P<0.05 was considered to be statistically significant. All analyses were performed with the SAS statistical package (version 9.4, SAS Institute, Cary, NC).
Ethic Statements
This study protocol was organized to ensure compliance with the Declaration of Helsinki and the Guidelines for the Epidemiological Research published by the Japanese Ministry of Health, Labour and Welfare. The original study protocol was approved by our Institutional Review Board.
Results
Patient Characteristics
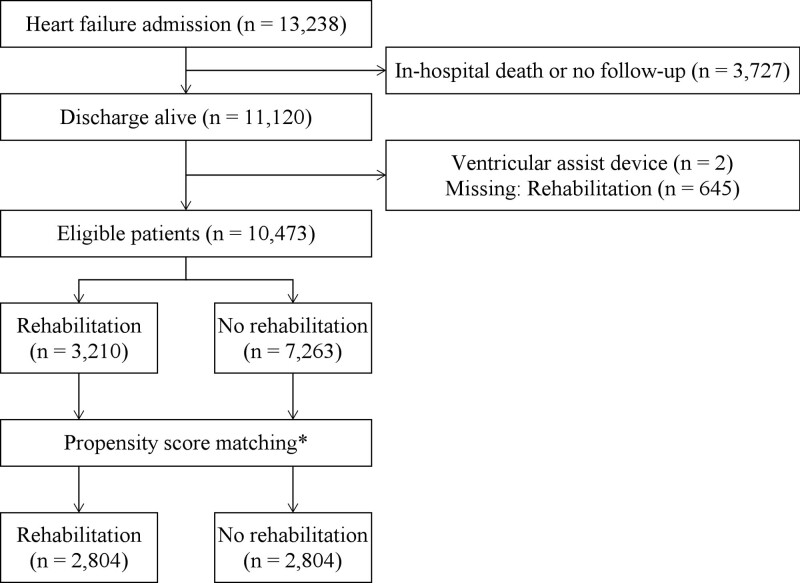
Out of the 13 238 patients in this registry, 11 120 patients were discharged alive (Figure 1). Among them, 645 patients whose information about CR was missing and 2 patients with left ventricular assist device were excluded. The remaining 10 473 patients were finally included in the present analysis. Of them, 3210 patients underwent CR during hospitalization. Propensity score matching yielded each 2804 patients (Figure 1).
Figure 1.
Patient selection. *Adjusted for age, sex, prior heart failure (HF) hospitalization, New York Heart Association functional class, etiology of HF, ischemic heart disease, hypertension, diabetes, stroke, chronic obstructive pulmonary disease, malignancy, heart rate, atrial fibrillation, pacemaker, implantable cardioverter defibrillator, cardiac resynchronization therapy-defibrillator, left ventricular ejection fraction, left ventricular mass index, hemoglobin, estimated glomerular filtration rate, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, mineralocorticoid receptor antagonist, beta-blockers, loop diuretics, thiazide, digitalis, oral inotropes, and amiodarone.
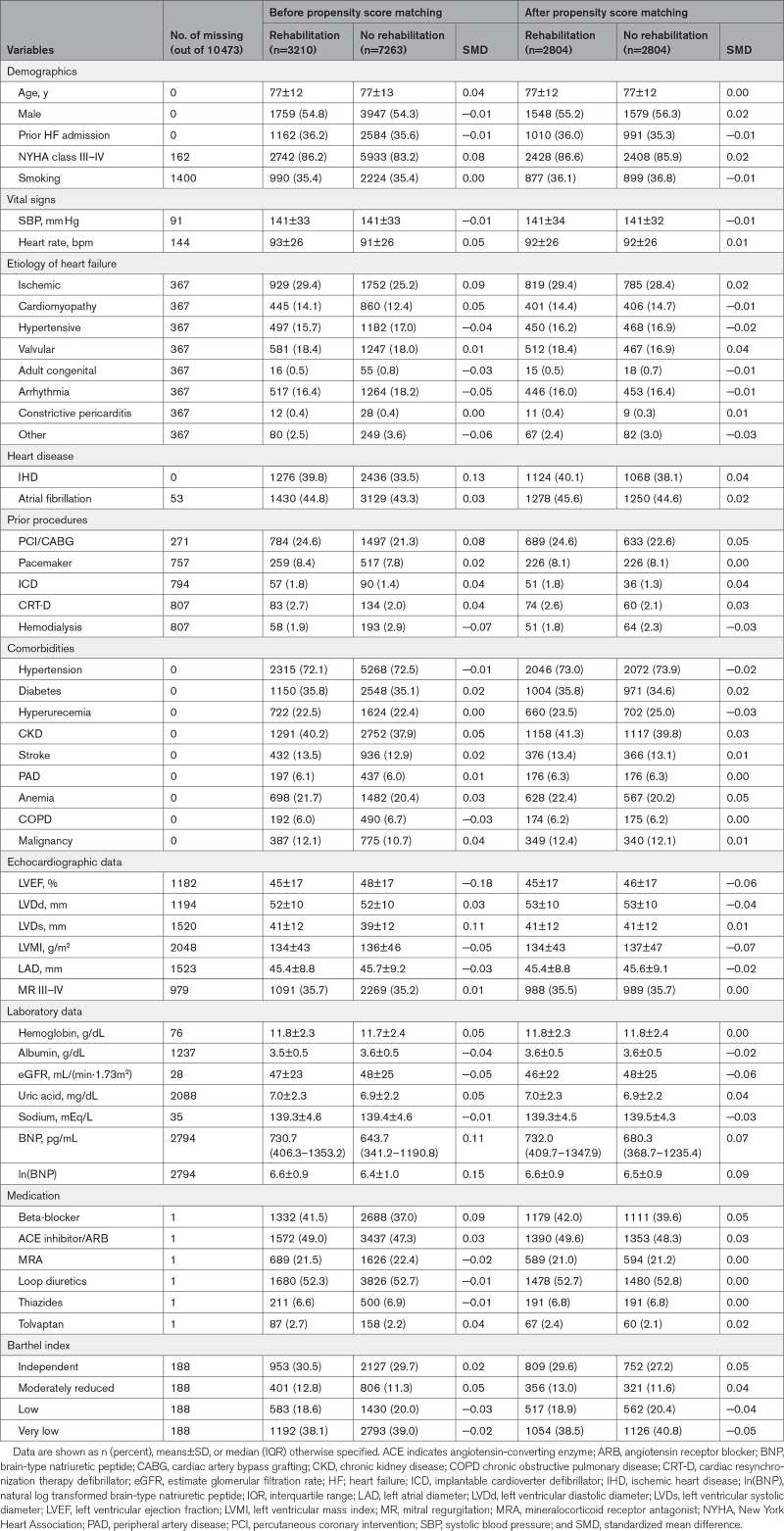
After propensity score matching, variables were considered to be well-balanced (Table 1). In matching cohort, mean age was 77±12 years, and 3127 (55.8%) were male. Echocardiography demonstrated that left ventricular ejection fraction (45±17 versus 46±17%, SMD=−0.06), left ventricular diastolic diameter (53±10 versus 53±10 mm, SMD=−0.04), left ventricular mass index (134±43 versus 137±47 g/m2, SMD=−0.07), left atrial diameter (45.4±8.8 versus 45.6±9.1mm, SMD=−0.02) and the grade III to IV of mitral regurgitation (35.5 versus 35.7%, SMD=0.00) were comparable between the 2 groups. Furthermore, the distribution of Barthel index categories was similar between the 2 groups.
Table 1.
Patient Characteristics
Clinical Outcomes
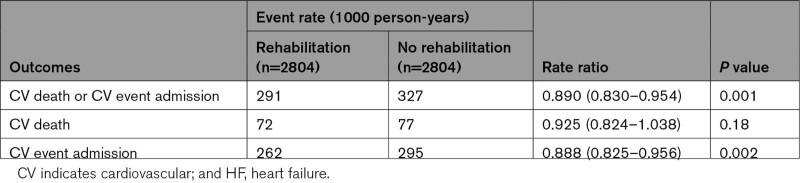
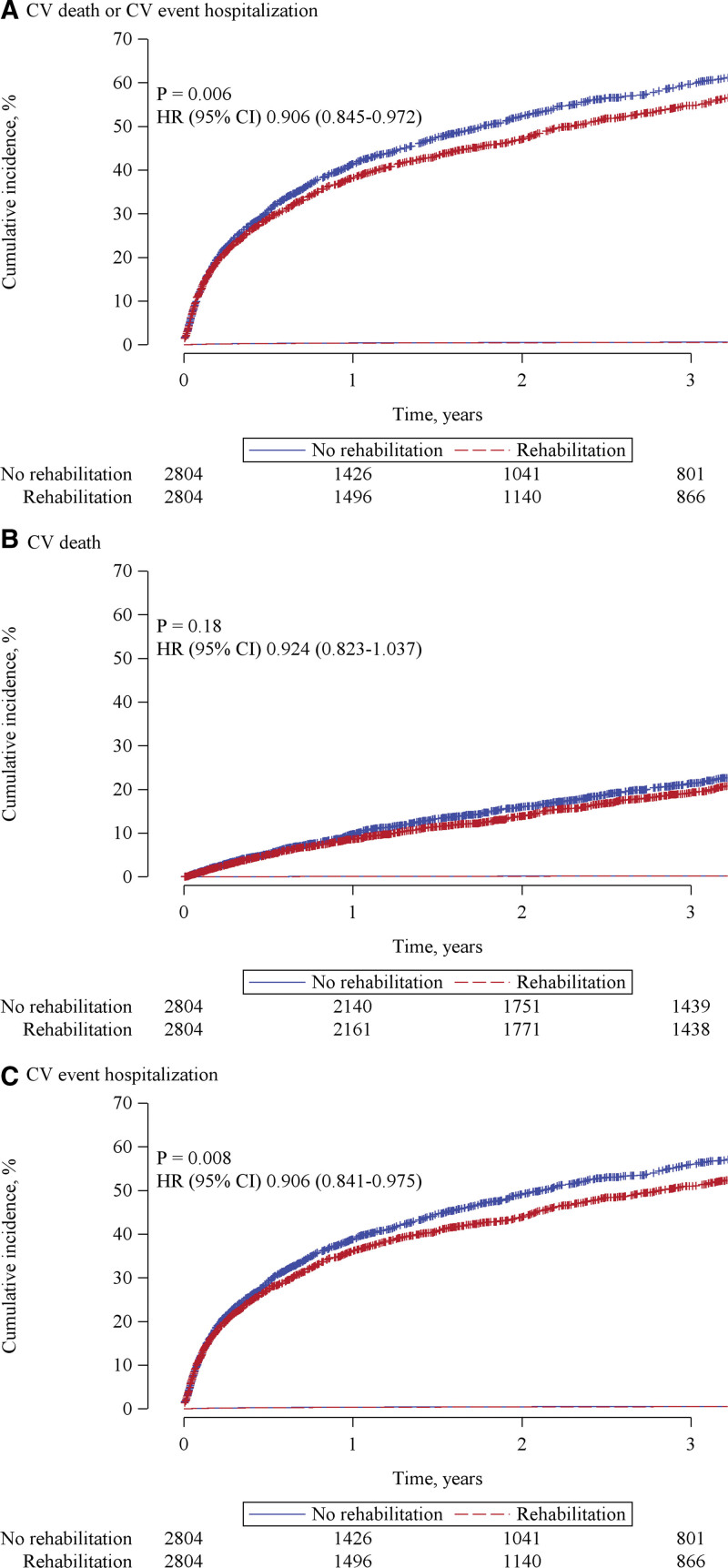
During a mean follow-up of 2.8 years, patients with CR had a lower incidence rate of the composite of cardiovascular death or rehospitalization due to cardiovascular event (291 versus 327 events per 1000 patient-years; rate ratio, 0.890 [95% CI, 0.830–0.954]; P=0.001) and rehospitalization due to cardiovascular event (262 versus 295 events per 1000 patient-years; rate ratio, 0.888 [95% CI, 0.825–0.956]; P=0.002) than those without CR (Table 2). The incidence rate of cardiovascular death was not statistically significantly different between the 2 groups (rate ratio, 0.925 [95% CI, 0.824–1.038]; P=0.18; Table 2). Cox regression analyses for each outcome demonstrated that CR was associated with a lower incidence rate of the composite event (HR, 0.906 [95% CI, 0.845–0.972]; P=0.006) and rehospitalization due to cardiovascular event (HR, 0.906 [95% CI, 0.841–0.975]; P=0.008; Figure 2). Fine and Gray model yielded similar results: incidence rate of the composite event (HR, 0.901 [95% CI, 0.840–0.966]; P=0.003) and rehospitalization due to cardiovascular event (HR, 0.905 [95% CI, 0.841–0.974]; P=0.008; Table S1). Multivariable Cox regression analysis also showed that CR was associated with reduced composite event (HR, 0.935 [95% CI, 0.879–0.995]; P=0.035) and cardiovascular rehospitalization (HR, 0.935 [95% CI, 0.876–0.998]; P=0.042; Table S2). The multiple imputation analysis showed CR was associated with reduced rate of composite of cardiovascular death or rehospitalization (HR, 0.941 [95% CI, 0.907–0.977]; P=0.001) and cardiovascular rehospitalization (HR, 0.948 [95% CI, 0.911–0.986]; P=0.007; Table S2). To exclude potential bias caused by quality of treatment which differed between institution, we conducted multivariable analysis after excluding institutions which did not provide CR. CR was still associated with a reduced composite of cardiovascular death or rehospitalization (HR, 0.930 [95% CI, 0.868–0.996]; P=0.038; Table S3). We conducted multivariable analysis including outpatient CR as a covariate to distinguish effects of inpatient CR from that of outpatient CR. Inpatient CR was associated with reduced composite of cardiovascular death or rehospitalization (HR, 0.924 [95% CI, 0.859–0.994]; P=0.035) and cardiovascular rehospitalization (HR, 0.921 [95% CI, 0.853–0.993]; P=0.033; Table S4).
Table 2.
Incidence Rate and Incidence Rate Ratio
Figure 2.
Cumulative incidence curves for each outcome. Cumulative incidence curves for (A) cardiovascular (CV) death or CV event hospitalization, (B) CV death, and (C) CV event hospitalization in a propensity-matched cohort. HF indicates heart failure; and HR, hazard ratio.
For comparison, we conducted a univariate analysis. While several adjusted analyses consistently showed significant association between CR and cardiovascular outcomes, it did not reach a statistical significance in the univariate analysis (HR, 0.954 [95% CI, 0.897–1.015]; P=0.13). This is called a suppression effect. Suppression effects can occur even when there are little or no association between suppressor variables and the variable of interest when suppressor variables share some variance with response variables.20 Given that there were no strong correlation between CR and other baseline variables (Table S5), it was less likely that there was a multicollinearity and it biased results. As a negative control analysis, we assessed the malignancy and other noncardiac deaths in several methods. None of them reached a statistical significance (Table S6).
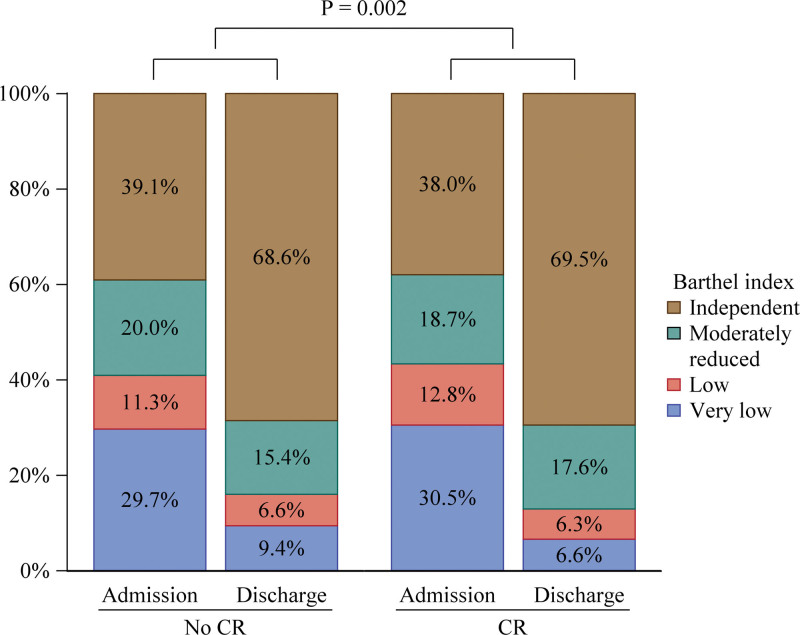
Subgroup analysis did not show specific subgroups who were especially benefited by CR (Figure S1). Improvement in Barthel index from admission to discharge was greater in CR group (P=0.002) after adjustment for Barthel index at admission and other covariates (Figure 3). Patients with lower Barthel index at admission were benefited by CR (very low; HR, 0.834 [95% CI, 0.742–0.938]: independent; HR, 0.985 [95% CI, 0.891–1.088], P value for interaction [very low versus independent]=0.035; Figure 4).
Figure 3.
The effects of cardiac rehabilitation (CR) on changes of Barthel index from admission to discharge.
Figure 4.
Effects of cardiac rehabilitation (CR) on cardiovascular (CV) death or hospitalization according to Barthel index at admission.
Discussion
The major finding of the present study was that the CR during hospitalization was associated with better postdischarge prognosis among patients with acute decompensated HF. The benefit was significant in patients with lower Barthel index. To our best knowledge, this study is the first report demonstrating beneficial effects of CR implementation during hospitalization on long-term outcomes in patients with acute decompensated HF.
Although a lot of previous studies demonstrated the beneficial effects of CR in patients with HF,1–4,9 most of them focused on outpatients CR. The impact of in-hospital CR for acute decompensated HF has not been elucidated. In comparison with stable HF, patients with acute decompensated HF are known to be frail with impairments in physical function.21 Frailty is an independent predictor of long-term mortality and rehospitalization in patients with HF.15 CR program is related to improving frailty levels, especially in patients who are the frailest.17 In the present study, in-hospital CR was associated with improvement in not only long-term outcomes but also Barthel index, an index for frailty, during hospitalization (Figure 3). This result was consistent with the secondary analysis of the REHAB-HF trial (Rehabilitation Therapy in Older Acute Heart Failure Patients). The REHAB-HF trial results showed that a novel, early physical rehabilitation intervention that included balance and mobility training as well as strength and endurance significantly improved frailty, as measured by the modified Fried criteria.22 Lower Barthel index is known to be associated with poor postdischarge prognosis in patients with acute HF.16 Importantly, in the present study, generalized estimating equations showed that the survival benefit of CR was significant in those with lower Barthel index at admission (Figure 4). These data suggest that improvement of physical function by in-hospital CR could lead to better outcomes in patients with HF and CR should be implemented even if physical deconditioning was worse.
Length of hospital stay for HF is longer in Japan than western countries (18 [12–28] versus 7 [4–11] days).10 Thus, cohort studies in Japan, such as JROADHF study, are ideal to investigate the effect of in-hospital CR among patients with HF. Standard CR program for HF during hospitalization in Japan includes creation of a postdischarge exercise program.11 In the present study, the length of hospital stay in CR group was longer than that in no CR group (25.1±18.5 versus 20.7±16.6 days; P<0.001). CR implementation might prolong hospital stay. The causal relationship between CR implementation and length of hospital stay remains unclear due to observational and retrospective nature of the present study. However, our results suggest that in-hospital CR implementation could lead to better prognosis in spite of further increase in-hospital stay.
The HF-ACTION trial was a randomized controlled trial that established the safety and efficacy of standard endurance exercise training in patients with chronic stable HF with reduced ejection fraction.5 The REHAB-HF trial is the most rigorous trial in hospitalized patients with HF; however, it was insufficiently powered to assess clinical events.9 Furthermore deaths appeared to be increased in the patients with reduced EF. In addition, standard CR was not used due to the impaired balance in around 90% of the older frail patients because it has been shown to increase falls and injuries in these frail patients. An ongoing trial, REHAB-HFpEF (Physical Rehabilitation for Older Patients With Acute Heart Failure With Preserved Ejection Fraction), is designed to assess safety, efficacy, and potential for reducing clinical events of early physical rehabilitation for hospitalization for acute HF (https://www.clinicaltrials.gov; Unique identifier: NCT05525663). Given these safety signals, clinical care should be guided by results of adequately powered, rigorously designed trials.
Previous studies have demonstrated that, although around 40% of the patients with acute decompensated HF underwent in-hospital CR, limited patients participated in the outpatient CR program in Japan.12,23 Consistent with these findings, in the present study, the implementation rate of in-hospital CR was 30% in eligible patients and only 106 patients (1.0%) participated in outpatient CR. It is unlikely that outpatient CR critically affects the results of the present study because the rate of outpatient CR is very low and the multivariable analysis including outpatient CR as a covariate also showed significant association between inpatient CR and cardiovascular outcomes (Table S4). Outpatient CR or in-hospital CR after discharge was associated with better prognosis among patients with stable HF.4,24 Therefore, the combination of in-hospital CR and consecutive outpatient CR might provide more beneficial effects than each of them for patients with acute decompensated HF. Frailty is related to lower implementation rate of outpatient CR.25 In-hospital CR is expected to facilitate implementation and continuation of outpatient CR by improving physical function at discharge.
Study Limitations
There are several potential limitations to be acknowledged in the present study. First, the protocol and adaptation of CR in each institute was not recorded in this registry. Second, this study has all the limitations of a retrospective registry combined with a propensity-matching analysis. Propensity matching is known to have multiple limitations, and we cannot be certain there were no significant unaccounted confounding variables despite the extensive adjustment for cardiac and noncardiac variables. Indeed, it is possible that there were unmeasured relevant characteristics that led to a clinical decision of whether to provide early CR. Specifically, patients with low life expectancy were unlikely to be provided CR. To elucidate these issues, further investigation such as prospective studies are needed. Third, as this was a single-country study in Japan, this may limit generalizability of the results, particularly compared to the United States where there are multiple differences in HF hospitalization practices, including shorter average length of stay. Fourth, given that the length of stay was observed to be longer in the early CR group, this could limit acceptance by third-party payers, particularly in the United States where this is a key performance metric. Furthermore, the longer length of stay is a potentially significant confounder. The signals for better outcomes in the early CR group could be explained at least partly by longer length of stay, since multiple studies have shown an inverse correlation between length of stay and rate of subsequent clinical events, including death. Thus, the observed lower events rates could be due to longer length of initial hospitalization, and not to the early CR. Finally, although we assumed that rehabilitation program was based on the guidelines, no specific intervention protocol was utilized in this retrospective, observational study. Thus, relatively few specific details of the intervention are available such that there may have been considerable heterogeneity, limited reproducibility, and generalizability. There were likely substantial differences in the intervention used in these acute hospitalized patients compared to that used in chronic HF which is primarily standard endurance training-based CR (treadmill based; walking, etc, as established by the landmark HF-ACTION trial). Further, there was no formal prospective assessment of frailty status. It has been shown in multiple studies that applying standard endurance training to frail, older, acutely ill patients have been shown to produce falls, injuries, and limited efficacy. Thus, the results of this study should be viewed only as hypothesis-generating and not practice changing, at least on other settings and countries. Furthermore, there are known limitations to propensity matching, so that we cannot exclude potential for significant unknown confounding variables. Ultimately, the optimal role of physical rehabilitation, including type of intervention, patient selection, will require an adequately powered, prospective randomized clinical trial.26 Indeed, the recently launched REHAB-HFpEF trial is designed to answer these questions.
Conclusions
The implementation of CR during HF hospitalization was associated with better postdischarge outcomes in patients with acute decompensated HF.
Article Information
Acknowledgments
This study was conducted on behalf of all the JROADHF (Japanese Registry of Acute Decompensated Heart Failure) investigators. JROADHF was supported by the Japanese Circulation Society and the Japanese Society of Heart Failure. Drs Enzan and Matsushima contributed to research inception and study design. Dr Tsutsui contributed to leadership. Drs Matsushima and Tsutsui contributed to critical review of study design and oversight of study conduct. Drs Kaku and Tohyama contributed to the analysis plan. Drs Enzan, Kaku, Fujino, and Hashimoto and T. Nezu, T. Higuchi, and Y. Nagatomi contributed to data acquisition. Drs Kaku and Tohyama contributed to statistical analysis. Drs Enzan and Matsushima contributed to article preparation. Drs Enzan, Matsushima, Kaku, Tohyama, Fujino, and Tsutsui and T. Nezu, T. Higuchi, and Y. Nagatomi contributed to drafting and revision of the article. Each author contributed important intellectual content during article drafting or revision and has approved the final articles.
Sources of Funding
This work was supported by the Japan Agency for Medical Research and Development Grant (19ek0210080h0003 [Dr Tsutsui]) and Health Sciences Research Grants from the Japanese Ministry of Health, Labour, and Welfare (Comprehensive Research on Cardiovascular Diseases; 20FC1051 [Dr Tsutsui]). The funders had no role in the design and conduct of the study or in collection, management, analysis, and interpretation of the data, the preparation, review, and submission of the article for publication.
Disclosures
Dr Ide is an endowed chair funded by Actelion Pharmaceuticals. Dr Tsutsui reports personal fees from MSD, Astellas, Pfizer, Bristol Myers Squibb, Otsuka Pharmaceutical, Daiichi-Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Bayer Yakuhin, Novartis Pharma, Kowa Pharmaceutical, Teijin Pharma, Medical Review Co, and Japanese Journal of Clinical Medicine; nonfinancial support from Actelion Pharmaceuticals, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Daiichi-Sankyo, IQVIA Services Japan, and Omron Healthcare Co; and grants from Astellas, Novartis Pharma, Daiichi-Sankyo, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, and MSD, outside the submitted work. The other authors declare no conflicts of interest associated with this article.
Supplemental Material
Supplemental Methods
Figure S1
Table S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ADL
- activity of daily living
- CR
- cardiac rehabilitation
- HF
- heart failure
- HR
- hazard ratio
- SMD
- standardized mean difference
For Sources of Funding and Disclosures, see page 320.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.122.010320.
Contributor Information
Hidetaka Kaku, Email: kakuhide@med.kyushu-u.ac.jp.
Takeshi Tohyama, Email: tohyama.takeshi.344@m.kyushu-u.ac.jp.
Tae Higuchi, Email: higuchi.tae.752@m.kyushu-u.ac.jp.
Yuta Nagatomi, Email: nagatomi.yuta.532@m.kyushu-u.ac.jp.
Takeo Fujino, Email: fujino.takeo.982@m.kyushu-u.ac.jp.
Toru Hashimoto, Email: hashimoto.toru.655@m.kyushu-u.ac.jp.
Tomomi Ide, Email: tomomi_i@cardiol.med.kyushu-u.ac.jp.
Hiroyuki Tsutsui, Email: tsutsui.hiroyuki.691@m.kyushu-u.ac.jp.
References
- 1.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173 [DOI] [PubMed] [Google Scholar]
- 2.Mueller L, Myers J, Kottman W, Oswald U, Boesch C, Arbrol N, Dubach P. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil. 2007;21:923–931. doi: 10.1177/0269215507079097 [DOI] [PubMed] [Google Scholar]
- 3.Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail. 2005;7:411–417. doi: 10.1016/j.ejheart.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Kamiya K, Sato Y, Takahashi T, Tsuchihashi-Makaya M, Kotooka N, Ikegame T, Takura T, Yamamoto T, Nagayama M, Goto Y, et al. Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ Heart Fail. 2020;13:e006798. doi: 10.1161/CIRCHEARTFAILURE.119.006798 [DOI] [PubMed] [Google Scholar]
- 5.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. ; Investigators H-A. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, O’Connor C, Whellan D, Keteyian SJ, Coats A, et al. ; ExTra MIIC. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail. 2018;20:1735–1743. doi: 10.1002/ejhf.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, Lough F, Rees K, Singh S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;2014:CD003331. doi: 10.1002/14651858.CD003331.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1:CD003331. doi: 10.1002/14651858.CD003331.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ide T, Kaku H, Matsushima S, Tohyama T, Enzan N, Funakoshi K, Sumita Y, Nakai M, Nishimura K, Miyamoto Y, et al. ; Investigators J. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese Registry of Acute Decompensated Heart Failure (JROADHF). Circ J. 2021;85:1438–1450. doi: 10.1253/circj.CJ-20-0947 [DOI] [PubMed] [Google Scholar]
- 11.Izawa H, Yoshida T, Ikegame T, Izawa KP, Ito Y, Okamura H, Osada N, Kinugawa S, Kubozono T, Kono Y, et al. ; Japanese Association of Cardiac Rehabilitation Standard Cardiac Rehabilitation Program Planning Committee. Standard cardiac rehabilitation program for heart failure. Circ J. 2019;83:2394–2398. doi: 10.1253/circj.CJ-19-0670 [DOI] [PubMed] [Google Scholar]
- 12.Kamiya K, Yamamoto T, Tsuchihashi-Makaya M, Ikegame T, Takahashi T, Sato Y, Kotooka N, Saito Y, Tsutsui H, Miyata H, et al. Nationwide survey of multidisciplinary care and cardiac rehabilitation for patients with heart failure in japan- an analysis of the AMED-CHF study. Circ J. 2019;83:1546–1552. doi: 10.1253/circj.CJ-19-0241 [DOI] [PubMed] [Google Scholar]
- 13.Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE-HF cohort study. Eur J Heart Fail. 2020;22:2112–2119. doi: 10.1002/ejhf.1926 [DOI] [PubMed] [Google Scholar]
- 14.Piepoli MF, Conraads V, Corra U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, et al. Exercise training in heart failure: from theory to practice. A consensus document of the heart failure association and the European association for cardiovascular prevention and rehabilitation. Eur J Heart Fail. 2011;13:347–357. doi: 10.1093/eurjhf/hfr017 [DOI] [PubMed] [Google Scholar]
- 15.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–875. doi: 10.1002/ejhf.518 [DOI] [PubMed] [Google Scholar]
- 16.Chivite D, Formiga F, Corbella X, Conde-Martel A, Aramburu O, Carrera M, Davila MF, Perez-Silvestre J, Manzano L, Montero-Perez-Barquero M; Investigators R. Basal functional status predicts one-year mortality after a heart failure hospitalization in elderly patients - The RICA prospective study. Int J Cardiol. 2018;254:182–188. doi: 10.1016/j.ijcard.2017.10.104 [DOI] [PubMed] [Google Scholar]
- 17.Kehler DS, Giacomantonio N, Firth W, Blanchard CM, Rockwood K, Theou O. Association between cardiac rehabilitation and frailty. Can J Cardiol. 2020;36:482–489. doi: 10.1016/j.cjca.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 18.Mentz RJ, Whellan DJ, Reeves GR, Pastva AM, Duncan P, Upadhya B, Nelson MB, Chen H, Reed SD, Rosenberg PB, et al. Rehabilitation intervention in older patients with acute heart failure with preserved versus reduced ejection fraction. JACC Heart Fail. 2021;9:747–757. doi: 10.1016/j.jchf.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryg J, Engberg H, Mariadas P, Pedersen SGH, Jorgensen MG, Vinding KL, Andersen-Ranberg K. Barthel Index at hospital admission is associated with mortality in geriatric patients: a Danish nationwide population-based cohort study. Clin Epidemiol. 2018;10:1789–1800. doi: 10.2147/CLEP.S176035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey S, Elliott W. Suppressor variables in social work research: Ways to identify in multiple regression models. Journal of the Society for Social Work and Research. 2010;1:28–40. doi: 10.5243/jsswr.2010.2 [Google Scholar]
- 21.Reeves GR, Whellan DJ, Patel MJ, O’Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva AM, Kitzman DW. Comparison of frequency of frailty and severely impaired physical function in patients >/=60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection Fraction. Am J Cardiol. 2016;117:1953–1958. doi: 10.1016/j.amjcard.2016.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey A, Kitzman DW, Nelson MB, Pastva AM, Duncan P, Whellan DJ, Mentz RJ, Chen H, Upadhya B, Reeves GR. Frailty and effects of a multidomain physical rehabilitation intervention among older patients hospitalized for acute heart failure: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2023;8:167–176. doi: 10.1001/jamacardio.2022.4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golwala H, Pandey A, Ju C, Butler J, Yancy C, Bhatt DL, Hernandez AF, Fonarow GC. Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: findings from get with the guidelines-heart failure registry. J Am Coll Cardiol. 2015;66:917–926. doi: 10.1016/j.jacc.2015.06.1089 [DOI] [PubMed] [Google Scholar]
- 24.Scalvini S, Grossetti F, Paganoni AM, La Rovere MT, Pedretti RF, Frigerio M. Impact of in-hospital cardiac rehabilitation on mortality and readmissions in heart failure: a population study in Lombardy, Italy, from 2005 to 2012. Eur J Prev Cardiol. 2019;26:808–817. doi: 10.1177/2047487319833512 [DOI] [PubMed] [Google Scholar]
- 25.Flint KM, Stevens-Lapsley J, Forman DE. Cardiac rehabilitation in frail older adults with cardiovascular disease: a new diagnostic and treatment paradigm. J Cardiopulm Rehabil Prev. 2020;40:72–78. doi: 10.1097/HCR.0000000000000492 [DOI] [PubMed] [Google Scholar]
- 26.Vandana S, Kavita S, Steven J, Keteyian Charina F, Alcain Patrice D-N, Jerome L, Fleg Viorel G, et al. ; on behalf of the American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and American College of Cardiology. Supervised exercise training for chronic heart failure with preserved ejection fraction: a scientific statement from the American heart association and American college of cardiology [published online ahead of print March 21, 2023]. Circulation. doi: 10.1161/CIR.0000000000001122 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.