Abstract
Background:
Despite the use of safe and effective conventional drugs, drug therapy problems (DTPs) pose a threat to the successful management of hypertension. DTPs are of a great concern in health care because of their serious consequences such as poor quality of life, increased health care costs, morbidity and mortality. However, there is no published information regarding the prevalence of DTPs and associated factors among hypertensive patients in Uganda.
Objective:
The aim of the study was to determine the prevalence and factors associated with DTPs among hypertensive patients at the hypertension clinic of Mbarara Regional Referral Hospital (MRRH).
Method:
A cross-sectional study was conducted at the hypertension clinic, MRRH, Uganda among 228 hypertensive patients. Data were collected from medical records using a data abstraction tool and patients were interviewed using a structured questionnaire. Data analysis was done using Statistical Package for Social Sciences (SPSS) version 22.0. Descriptive analysis was used to determine the prevalence of DTPs. Logistic regression was used to determine the association between the independent and dependent variables. Variables were considered statistically significant at p-value <0.05.
Results:
A total of 178 DTPs were identified among 141 hypertensive patients. The prevalence of antihypertensive-related DTPs was 61.8% (95% confidence interval [CI]: 55.3–67.5) with an average of 1.26 ± 0.52 DTPs per patient. Out of 141 participants with DTPs, 109 (77.3%) had one DTP, 27 (19.1%) had 2 DTPs, and 5 (3.5%) had 3 DTPs. The most common types of antihypertensive-related DTPs were ‘dosage too low’ which accounted for 53 (29.8%), followed by ‘adverse drug reactions’ which accounted for 48 (27%). Uncontrolled blood pressure (BP; adjusted odds ratio [AOR]: 4.17; 95% CI: 2.33–7.45, p < 0.001) and routine laboratory test results (AOR: 1.87; 95% CI: 1.04–3.36, p = 0.036) were significantly associated with antihypertensive-related DTPs among hypertensive patients.
Conclusion:
Almost two-thirds of study participants had antihypertensive-related DTPs. The most common DTPs were ‘dosage too low’ and ‘adverse drug reactions’ which both accounted for almost a third of the total DTPs each. Uncontrolled BP and routine laboratory test results were significantly associated with antihypertensive-related DTPs among the study participants. Our study emphasizes the need for improved patient care by clinical pharmacists to identify and prevent DTPs among hypertensive patients.
Keywords: drug therapy problems, hypertension clinic, hypertensive patients, Uganda
Introduction
Hypertension is defined as persistently elevated arterial blood pressure (BP) of ⩾ 140/90 mmHg. 1 It is a serious medical condition that significantly increases the risks of heart, brain, kidney and other illnesses. 2 It is estimated that 1.13 billion people worldwide suffer from hypertension (HTN), and two-thirds live in low- and middle-income countries. 3 Globally, hypertension remains among the leading cause of mortality accounting for approximately 9.4 million deaths annually and is a growing public health problem in sub-Saharan Africa. 4
Drug therapy problem (DTP) can be defined as any undesirable event experienced by a patient that involves drug treatment, which actually or potentially interferes with achieving the desired goals of therapy and requires expert judgment to resolve. 5 DTPs include ‘unnecessary drug therapy’, ‘needs additional drug therapy’, ‘ineffective drug’, ‘dosage too low’, ‘adverse drug reaction’, ‘dosage too high and finally ‘non -adherence’. 5
Despite the use of safe and effective conventional drugs, DTPs pose a threat to the successful management of hypertension. 6 Patients with hypertension are prone to dose and indication-related drug problems that are attributed to co-morbidities and use of multiple drugs.7–10 Globally, DTPs have become far more prevalent over the past years, accounting for 1.3%–41.3% of hospital admissions. 11 It is also reported that worldwide, more than half of all medicines are prescribed and dispensed inappropriately and half of the patients fail to take them correctly. 12 The prevalence of DTPs in the United States is 89.5%. 13 In Africa, studies reported that 52.8% to 82% of ambulatory hypertensive patients experienced at least one DTP.14–19
DTPs are associated with morbidity, mortality, increased health costs as well as poor quality of life of patients. 20 According to a systematic review in the United States (United States of America) and European countries, the cost of treating DTPs especially adverse drug reactions (ADRs) among out patients is about €174 to €8,515. 21 In Nigeria, the economic cost implication of treating DTPs was € 1050.64, equivalent to about 1.9% of the total cost of all medications used by all medical inpatients during admission. 22 According to a study conducted in Spain, drug-related mortality was at 18.4%, 23 whereas a systematic review in Africa indicated that the mortality rate among patients hospitalized due to DTPs was as high as 5.7% with an average rate of 2.7%. 11 Patients with hypertension have a higher risk for DTPs due to multiple co-morbid conditions and use of multiple drugs that predispose them mostly to drug–drug interactions and ADRs. 24 Other factors associated with DTPs among patients with hypertension include polypharmacy, presence of co-morbidities, older age (above 65 years), uncontrolled blood pressure, females and low family income.25,26 The most common antihypertensive-related DTPs among ambulatory hypertensive patients are unnecessary drug therapy, need for additional drug therapy, dosage too high and ineffective drug therapy. 16
In Uganda, the prevalence of hypertension is 31.5% in adults. 27 According to the World Health Organization (WHO) report in 2018, non-communicable diseases (NCDs) are estimated to account for 33% of all deaths in Uganda, and the mortality rate due to cardiovascular diseases is 10% of which hypertension is the most common. 28 Despite the increasing mortality rate, hypertension awareness, treatment and control are unacceptably low across the globe, especially in low- and middle-income countries. 29
Although there are several studies done in other countries on the prevalence of DTPs among ambulatory hypertensive patients, no similar study has been done in Uganda. Therefore, this research was aimed at determining the prevalence, types and factors associated with antihypertensive-related DTPs among patients at the hypertension clinic of Mbarara Regional Referral Hospital (MRRH).
Methods and materials
Study design and setting
A cross-sectional study was conducted at the hypertension clinic of Mbarara Regional Referral Hospital for a period of 3 months from 2 November 2021 to 30 January 2022. MRRH is a government aided hospital located in Mbarara district in the South western region of Uganda. The hospital majorly serves over 4 million people from South-Western districts of Uganda. It has 600 beds and provides general out-patient, in-patients and emergency services. Currently, the hypertension clinic serves about 450 hypertensive patients and this clinic is run by 1 physician, 3 medical residents, 1 medical officer, 1 nursing officer and 1 records officer. The hypertension clinic day is every Tuesday of the week and the services offered include; triage (BP measurement, height measurement, weight measurement), health education and treatment. Hypertensive patients are prescribed drugs for 2 months, that is, every 2 months; these patients come back to the clinic for review.
Study population
All ambulatory hypertensive patients on follow up at MRRH, who visited the hypertension clinic during the study period.
Eligibility criteria
Inclusion criteria
All hypertensive patients 18 years and above who visited the hypertension clinic during the study period and had used antihypertensive drugs as well as consented to participate were included in the study.
Exclusion criteria
Patients with incomplete medical records and those that were unable to complete the interview were excluded.
Sample size
The sample size for the study was determined using single population proportion formula (Cochran, 1963)
where n = sample required, Z = critical value for 95% confidence interval (1.96), e = margin of error (5%) = 0.05, P = prevalence rate = 50% since there was no similar study done in Uganda and in East Africa, n = (1.96 x 1.96 x 0.50 x 0.50) / (0.05 x 0.05) = 384 participants.
The hypertension clinic serves a total of 450 hypertensive patients which is less than 10,000 people. Applied the finite population correction formula
where n is the sample size and N the population size, n = 384/1 + ((384 – 1)/450), n = 207 participants.
Additional 10% of the sample size for contingency, which led to a total of 228 participants.
Sampling technique
Systematic random sampling technique was used in this study.
Data collection methods and procedures
The data collection team consisted of the principal investigator (clinical pharmacist) and two trained research assistants (pharmacist and a medical resident). After the systematic random sampling, the selected patient’s details were checked against a list of patients who had previously taken part in the study to ensure that each eligible patient participates once. The patient would then voluntarily consent to participate in the study by writing. Thereafter, patients were interviewed using a structured questionnaire to obtain data on socio-demographics (marital status, religion, monthly income and education level), history of drug allergies, medical history, medication history which included herbal and over-the-counter drugs, medication adherence to prescribed drugs and ADR.
For medication adherence, questions adopted from Hill-Bone medication adherence scale were asked. 30 Non-adherence was considered if the drug therapy was indicated, effective and safe. Males and females were considered non-adherent if they scored <29.1 and <32.3, respectively. 30 Once non-adherence was identified, the Cipolle et al. 5 classification was used to determine the cause.
ADR were suspected when there was a relationship between time of drug administration and the onset of the ADR while excluding other causes. All suspected adverse events were assessed using the Naranjo Scale. Participants with definite (⩾9), probable5–8 and possible1–4 ADR causality scores in the Naranjo ADR causality algorithm were considered to be having ADR related to drug therapy. 31
Data from review of medical records were collected and recorded using a pretested data abstraction tool. Medical records were reviewed to obtain data on vital signs, current medication, results of current laboratory tests, ADR (clinical and laboratory evaluations) and the current medical conditions which were important for identification of DTPs.
Each documented drug therapy was evaluated for appropriateness, effectiveness and safety using the Lexicomp drug interaction checker and the Uganda clinical guideline. 32 According to Lexicomp drug–drug interaction checker, only drug–drug interactions in class C (monitor therapy), D (consider therapy modification) and X (avoid drug combination) are considered clinically significant. Identified DTPs and their causes were broadly classified according to the Cipolle et al. 5 classification system. Enrollment of patients was halted after the estimated sample size of 228 participants was reached.
Operational definitions
Controlled blood pressure
Is BP that is < 140/90 mmHg. 32
Drug therapy problem
Can be defined as any undesirable event experienced by a patient that involves drug treatment, which actually or potentially interferes with achieving the desired goals of therapy and requires professional judgment to resolve. 5
Unnecessary drug therapy
Can be defined as patient not having a clinical indication for drug therapy at a time, multiple drug products are being used for a condition that requires only single drug, non-drug therapy more appropriate, addiction or recreational drug use and treating avoidable adverse reaction. 5
Needs additional therapy
Additional drug is required to initiate therapy for untreated condition or to prevent a new medical condition from developing in the patient or synergistic therapy for a medical condition that requires additional pharmacotherapy. 5
Ineffective drug
The drug not being the most effective for the medical condition and a different drug is needed, the medical condition is refractory to the drug product and a different drug is needed or maybe the dosage form of the drug product is inappropriate, the drug product is contraindicated in this patient, and finally drug is not indicated for condition. 5
Dosage too low
Too low dosage to produce the desired response in the patient, the dosage interval is too infrequent to produce the desired response, incorrect administration, a drug interaction reduces the amount of active drug available resulting in lack of effectiveness in this patient, and then the duration of the drug therapy is too short to produce the desired response. 5
Adverse drug reaction (ADR)
The World Health Organization (WHO) defines an ADR as a response to a drug that is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease or for the modification of physiological function. 33
Dosage too high
Dose too high can be related to toxicity, the dosing frequency being too short for the patient or duration of drug therapy is too long for a patient or a drug interaction increases the amount of active drug available resulting in toxicity in a patient. 5
Quality control
Pre-testing of the data collection tool was done using 10 patients who met the inclusion criteria. This was to help in identifying challenges with the data collection tool and there-after the tool was modified accordingly.
The data collection tool was translated to Runyankole by a professional and then results were back translated to English to check for consistency. The principal investigator selected and recruited research assistants who were trained on the aim of the study, data collection techniques and how to use the data collection tool. The recruited research assistants were fluent in both English and Runyankole which facilitated patient interview. The principal investigator also closely supervised research assistants during the data collection period.
Data management
The data abstraction form for each patient was assigned an identification code. The filled forms were checked for accuracy, consistency and completeness by the principal investigator on a daily basis. Completed forms were kept under restricted access to protect patient confidentiality and protect data from alteration. Data were later stored in a computer with a password.
Data analysis
All filled data collection forms were checked for completeness in preparation for data entry into statistical package for social sciences (SPSS) version 22.0. Data cleaning was done to detect errors and validate the data. Data analysis was done using the same software. Patient, drug and disease-related factors were presented using descriptive statistics including mean, standard deviation, frequencies and percentages.
The prevalence of DTPs related to antihypertensives was determined by the number of patients with at least one DTP divided by the total number of the sample size. The results were expressed as a percentage.
The association between the independent variables and occurrence of DTPs was determined using univariate and multivariate logistic regression. Variables with p-value < 0.25 during the univariate analysis were further subjected to multivariate logistic regression analysis to control for confounders. Variables were considered statistically significant if p-value was less than 0.05 measured with odds ratio at 95% confidence interval.
Ethical considerations
Approval to conduct the study was obtained from Mbarara University of Science and Technology Research Ethics Committee (Reference number: MUST-2021-187). Written informed consent was obtained from the patients prior to participating in the study and use of their medical records. The purpose, objectives, benefits and risks as well as the impact of the study on the total time spent in the hospital were clearly explained to the patients. Completed forms were kept under restricted access and thus data were processed and presented anonymously to prevent anyone from tracing back the patient’s identity which preserved confidentiality.
Ministry of health COVID-19 guidelines were observed during the data collection process. These guidelines included social distancing, wearing face masks all the time and sanitizing before and after handling patient medical records.
Results
Socio-demographic characteristics of patients
About 230 hypertensive patients were recruited for the study, and 2 were excluded due to incomplete data giving us a total of 228 hypertensive patients that participated in this study. Out of these, majority were females accounting for 173 (75.9%) and 113 (49.6%) were in the age bracket 40–59 years. The mean age of the study participants was 58.13 ± 12.16 years ranging from 23 years to 86 years. In addition, 133 (58.3%) were married, 104 (45.6%) were Anglican by religion, 114 (50%) attended primary education, and 117 (51.3%) were unemployed. Twenty (8.8%) participants were currently using alcohol, 4 (1.8%) were still smoking and 62 (27.2%) were currently using herbal drugs (Table 1).
Table 1.
Socio-demographics characteristics of the study participants at the hypertension clinic, MRRH, from November 2021 to January 2022.
| Variable | Category | Frequency n (%) |
|---|---|---|
| Sex | Male | 55 (24.1) |
| Female | 173 (75.9) | |
| Age (years) | 18–39 | 16 (7) |
| 40–59 | 113 (49.6) | |
| ⩾60 | 99 (43.4) | |
| Marital status | Single | 5 (2.2) |
| Married | 133 (58.3) | |
| Separated/divorced | 14 (6.1) | |
| Widow/widower | 76 (33.3) | |
| Religion | Anglican | 104 (45.6) |
| Catholic | 76 (33.3) | |
| Moslem | 32 (14) | |
| Others | 16 (7) | |
| Educational status | No formal education | 59 (25.9) |
| Primary | 114 (50) | |
| Secondary | 35 (15.4) | |
| Tertiary | 20 (8.8) | |
| Employment status | Employed | 111 (48.7) |
| Unemployed | 117 (51.3) | |
| Monthly income (UGX) | <200,000 | 206 (90.4) |
| ⩾200,000 | 22 (9.6) | |
| Family history of hypertension | Yes | 115 ( 50.4) |
| Alcohol use | Yes | 20 (8.8) |
| Smoking status | Yes | 4 (1.8) |
| Herbal medicine on use | Yes | 62 (27.2) |
MRRH: Mbarara Regional Referral Hospital.
Drug and disease characteristics of study participants
Eighty-three (36.4%) of the patients were overweight and almost two-thirds (149, 65.4%) had uncontrolled BP. In this study, the mean duration of hypertension since diagnosis and initiation of antihypertensive was 5.08 ± 4.41 years and 4.79 ± 4.13 years, respectively. The majority (133, 58.5%) of the participants had at least one co-morbid condition. The most frequently diagnosed co-morbidities were diabetes mellitus 74 (32.5%), and neuropathy 39 (17.1%). The average number of prescribed drugs was 3.85 ± 1.61 whereas an average of 2.18 ± 0.89 antihypertensive drugs was used. Among the study participants, 192 (84.2%) were prescribed calcium channel blockers (CCB) such as immediate release Nifedipine and Amlodipine, 126 (55.3%) were prescribed diuretics such as Bendroflumethiazide, and 119 (52.2%) were prescribed angiotensin receptor blockers (ARB) such as Losartan and Telmisartan (Table 2).
Table 2.
Drug and disease characteristics of the study participants at MRRH from November 2021 to January 2022.
| Variable | Category | Frequency n (%) |
|---|---|---|
| Time since diagnosis of hypertension | 1–5 years | 153 (67.1) |
| 6–10 years | 47 (20.6) | |
| >10 years | 28 (12.3) | |
| Time since hypertension treatment | 1–5 years | 156 (68.4) |
| 6–10 years | 47 (20.6) | |
| >10 years | 25 (11.0) | |
| Presence of co-morbidity | Yes | 133 (58.3) |
| Number of co-morbidity | 1 | 94 (41.2) |
| 2 | 37 (16.2) | |
| 3 | 2 (0.9) | |
| Co-morbid conditions | Diabetes mellitus | 74 (32.5) |
| Neuropathy | 39 (17.1) | |
| HIV | 8 (3.5) | |
| Dyslipidemia | 7 (3.1) | |
| Others a | 46 (20.2) | |
| BP status | Controlled | 79 (34.6) |
| Uncontrolled | 149 (65.4) | |
| Total number of drugs | <5 | 158 (69.3) |
| ⩾5 | 70 (30.7) | |
| Drug allergy status | Yes | 13 (5.7) |
| Laboratory tests b | Yes | 98 (43) |
| Potential drug interaction | Yes | 97 (42.5) |
| Body mass index | Underweight | 9 (3.9) |
| Normal weight | 56 (24.6) | |
| Over weight | 83 (36.4) | |
| Obese | 80 (35.1) | |
| Antihypertensive drugs | Calcium channel blockers | 192 (84.2) |
| Diuretics | 126 (55.3) | |
| ARB | 119 (52.2) | |
| Beta blockers | 35 (15.4) | |
| ACEI | 12 (5.3) | |
| Others c | 6 (2.6) |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; BP, blood pressure; HIV, human immunodeficiency virus.
Other co-morbidities; chronic kidney disease, arthritis, gastritis, asthma, peptic ulcer disease.
Availability of at least one routine laboratory test result obtained during the current encounter which include; renal function tests, urinalysis, blood glucose, thyroid stimulating hormone and lipid profile tests that are routinely ordered in hypertensive patients.
Other antihypertensive drugs; Alpha agonists.
Prevalence of antihypertensive-related DTPs
A total of 178 DTPs were identified among 141 hypertensive patients and the prevalence of antihypertensive-related DTPs was 61.8% (95% confidence interval [CI]: 55.3–67.5]. The average number of DTPs was 1.26 ± 0.52 DTPs per patient. Out of the 141 study participants with DTPs, 109 (77.3%) had one DTP, 27 (19.1%) had two DTPs and 5 (3.5%) had three DTPs.
Types and causes of antihypertensive-related DTPs
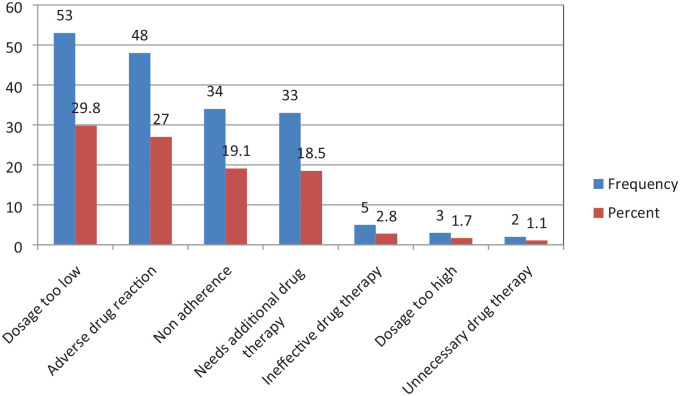
Dosage too low was the most common antihypertensive-related DTP accounting for 53 (29.8%), followed by ADRs, and non-adherence which accounted for 48 (27.0%) and 34 (19.1%) respectively. The least identified DTP was unnecessary drug therapy and it accounted for 2 (1.1%), (Figure 1).
Figure 1.
Types of antihypertensive-related drug therapy problems identified among patients at MRRH from November 2021 to January 2022.
Of the dosage too low, ineffective dose was the major cause and it contributed 41 (23%) while ADRs were caused by undesirable effects which accounted for 35 (19.7%). Non-adherence was mainly caused by inability to afford drug therapy which accounted for 18 (10.1%), (Table 3). Immediate release Nifedipine contributed the most DTPs accounting for 63 (27.6%), followed by Amlodipine 22 (9.6%) and then Losartan 16 (7%).
Table 3.
Types of antihypertensive-related drug therapy problems and their causes among patients at the hypertension clinic, MRRH from November 2021 to January 2022.
| Type of DTP | Cause of DTP | Frequency n (%) |
|---|---|---|
| Needs additional drug therapy | Untreated condition | 1 (0.6) |
| Synergistic therapy | 31 (17.4) | |
| Others | 1 (0.6) | |
| Unnecessary drug therapy | Duplicate therapy | 2 (1.1) |
| Dosage too low | Ineffective dose | 41 (23.0) |
| Dosing too infrequent | 12 (6.7) | |
| Ineffective drug therapy | More effective drug available | 5 (2.8) |
| Dosage too high | Drug interaction | 2 (1.1) |
| Frequency too short | 1 (0.6) | |
| Adverse drug reaction | Undesirable effect | 35 (19.7) |
| safer drug required due to risk factors | 12 (6.7) | |
| Drug interaction | 1 (0.6) | |
| Non-adherence | Cannot afford drug product | 18 (10.1) |
| Patient forgets to take | 9 (5.1) | |
| Patient prefers not to take | 5 (2.8) | |
| Does not understand instructions | 2 (1.1) |
DTP, drug therapy problems.
Factors associated with occurrence of antihypertensive-related drug therapy problems
Univariate logistic regression of the factors associated with occurrence of antihypertensive-related DTPs
At univariate logistic regression, treatment of hypertension for > 10 years (COR: 3.46; 95% CI: 1.13–10.57; p-value = 0.029), BP control (COR, 4.37; 95% CI: 2.44–7.80; p-value < 0.001) and availability of routine laboratory tests results (COR, 1.92; 95% CI: 1.10–3.34; p-value = 0.022) were significantly associated with antihypertensive-related DTP occurrence (Table 4).
Table 4.
Univariate and multivariate analysis of factors associated with antihypertensive-related DTPs among patients at MRRH from November 2021 to January 2022.
| Independent variables | Dependent variable | COR(95% CI) | p-value | AOR(95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| variable | Category | DTP | |||||
| No n(%) | Yes n(%) | ||||||
| 87 (38.2) |
141 (61.8) |
||||||
| Herbal medicine use | Yes | 18 (29.0) | 44 (71.0) | 1.74(0.93–3.26) | 0.085 | 1.73 (0.87–3.42) | 0.118 |
| No | 69 (47.3) | 77 (52.7) | 1 | 1 | |||
| Duration of HTN treatment | 1–5 years | 62 (39.7) | 94 (60.3) | 1 | 1 | ||
| 6–10 years | 21 (44.7) | 26 (55.3) | 0.82 (0.42–1.58) | 0.546 | 0.84 (0.41–1.71) | 0.627 | |
| > 10 years | 4 (16.0) | 21 (84.0) | 3.46 (1.13–10.57) | 0.029 | 3.22 (1.00–10.4) | 0.050 | |
| BP control | Controlled | 48 (60.8) | 31 (39.2) | 1 | 1 | ||
| Uncontrolled | 39 (26.2) | 110 (73.8) | 4.37 (2.44–7.80) | < 0.001 | 4.17 (2.33–7.45) | < 0.001 | |
| Availability of routine Laboratory test results | Yes | 29 (29.6) | 69 (70.4) | 1.92 (1.10–3.34) | 0.022 | 1.87 (1.04–3.36) | 0.036 |
| No | 58 (44.6) | 72 (55.4) | 1 | 1 | |||
AOR: adjusted odds ratio; BP, blood pressure; CI, confidence interval; COR: crude odds ratio; DTP, drug therapy problems; HTN, hypertension.
Multivariate logistic regression of the factors associated with antihypertensive-related DTPs
All variables with p-value < 0.25 at univariate analysis were considered for multivariate logistic regression analysis. Multivariate analysis presented in Table 4 showed that study participants with uncontrolled BP were 4.17 times (adjusted odds ratio [AOR]: 4.17; 95% CI: 2.33–7.45; p-value < 0.001) more likely to experience DTPs compared to participants with controlled BP. Patients with routine laboratory test results were 1.87 times (AOR: 1.87; 95% CI: 1.04–3.36; p-value = 0.036) more associated with antihypertensive-related DTPs than those who didn’t have them.
Discussion
This study was conducted at the hypertension clinic with the aim of establishing the prevalence, types and factors associated with antihypertensive-related DTPs among hypertensive patients. During the study period, 178 DTPs were identified among 141 (61.8%) hypertensive patients with an average of 1.26 ± 0.52 DTPs per patient. The most common types of DTPs were ‘dosage too low’ which accounted for 53 (29.8%), followed by ‘adverse drug reactions’ which accounted for 48 (27%). The multivariate logistic regression analysis showed that uncontrolled BP and availability of routine laboratory test results were significantly associated with DTPs among hypertensive patients.
The current prevalence of antihypertensive-related DTPs of 61.8% is comparable to other studies in Northwest, Ethiopia (62.4%), Eastern Ethiopia (60.5%), Northern Ethiopia (55.6%) and Indonesia (57%).7,14,26,34 However, this finding is lower than 71.2% and 80.7% both in Eastern Ethiopia as well as 82% in Southwest Ethiopia.15,19,25 In addition, studies conducted in Malaysia showed that 88.8% and 90.5% participants had at least one DTP.35,36 The contrast in results could be attributed to difference in assessment of DTPs; our study excluded DTPs related to other drugs other than antihypertensives unlike the other studies that included DTPs related to all drugs. The variation in findings could also be due to difference in study setting, study population, study design and DTP classification; the study by Huri and Wee used retrospective study design and used in-patients, the two studies in Malaysia also used the pharmaceutical care network Europe foundation to classify DTPs whereas our study used the Cipolle classification.
Our study found out that the most common DTP was ‘dosage too low’ at 29.8%. This finding is higher than that reported in Indonesia, Malaysia and Nigeria; ranging from 5.2% to 7.7%.35,37,38 Furthermore, the dosage too low in our study is also higher than 18.5% and 16.8% in two studies conducted in Ethiopia.14,26 The discrepancy in findings could be due to variations in study population and medical practitioner’s expertise and experience. The study by Kefale et al. 26 had 100% patients having both hypertension and diabetes mellitus, whereas our study had only 32.5% having both conditions. The high proportion of ‘dosage too low’ in our setting could be attributed to prescribing an ineffective dose and too infrequent dosing. The failure to control BP adequately among two-thirds (149, 65.4%) of our study participants could be attributed to ‘dosage too low’. This implies the need to adhere to standard treatment guidelines in order to achieve the desired BP goal.
In this study, ‘adverse drug reactions’ accounted for 27%. This finding is comparable with 31.6% reported in a cross-sectional study conducted in Indonesia. 34 However, our results are higher than 19% reported in a retrospective cross-sectional study conducted in Eastern Ethiopia. 17 In addition, the finding also differs from 2.4% reported in a prospective cross-sectional study conducted in Southwestern Ethiopia. 19 The variation could be explained by the presence of a high prevalence of co-morbidities and drug-drug interactions in our setting. In addition, ADRs in our setting were caused by drugs causing undesirable effects and need for safer drugs especially for older adults. Furthermore, Nifedipine contributed the most (77.1%) to the ADRs in our setting. Clinical trials recommend use of long acting CCBs because they have proven efficacy and safety in older adult patients with hypertension.39,40
Non-adherence accounted for almost one-fifth (19.1%) of the antihypertensive-related DTPs. This was mainly (52.9%) attributed to inability to afford drug therapy since patients must use their ‘out-of-pocket money’ to buy drugs from private pharmacies. This finding is lower than 32.8% reported in study conducted in Ethiopia. 10 The difference in findings could be due to variations in socio-economic status, provision of adherence counseling services and adherence assessment tools used. Our study used the hill-bone medication adherence tool which is specific to hypertensive patients whereas the later used the Morisky medication adherence tool. Although uncontrolled BP could be attributed to other different factors such as lifestyle characteristics of patients, ‘dosage too low’; non-adherence could be an important factor among our study participants. Non-adherence to antihypertensive drugs accounts for 30%-50% and is often linked to uncontrolled blood pressure. 41 This therefore, implies that healthcare professionals should strengthen adherence counseling to raise awareness and knowledge in relation to BP control.
The multivariate logistic regression analysis showed that study participants that had uncontrolled BP were 4.17 times (AOR,4.17; 95%CI, 2.33-7.45, P-value < 0.001) more likely to have DTPs as compared to those with controlled BP, which was in agreement to findings in a study conducted by Hussen and Daba who reported that patients with uncontrolled BP were 7.68 times more likely to have DTPs. 25 The most likely cause of uncontrolled BP could be non-adherence to antihypertensive drugs. From our study, non-adherence contributed to 19.1% of the total number of DTP related to antihypertensives. A study conducted in Iran reported that there was a significant relationship between adherence level and BP control. 42 A systematic review and meta-analysis in low- and middle-income countries also reported a similar finding that adherence was related to BP control. 43
In addition, patients who had routine laboratory test results were 1.87 times (AOR, 1.87; 95%CI, 1.04-3.36, P-value = 0.036) more likely to have DTPs than those that didn’t have them. The more the laboratory tests are performed, the more the safety profile of a drug in a patient is known and the more we are able to identify DTPs. Bolivar et al. 44 also emphases that laboratory monitoring helps to detect the risk of unnecessary ADRs.
In Contrary to the present study, other studies identified total number of drugs, low family income, age, presence of co-morbidity and substance use as factors associated with DTPs among hypertensive patients.7,14,16,26 This difference in findings could be attributed to variations in study population, underlying co-morbid conditions, patient characteristics and DTP assessment. In our study, only antihypertensive-related DTPs were assessed unlike other studies. Our study reported a prevalence of 58.3% for co-morbidities while other studies reported 43% and 34.4%.14,16 The current study had 43.4% study participants who were 60 years and above which was similar to 42.7% by Mahammedsied et al. 16 However, this finding was different from 5.4% and 23.4% among adults above 65 years reported by Weldegebreal et al. 14 and Kefale et al. 26 respectively.
Study limitation and strength
Conducting this study at a single health facility was one limitation identified as the findings may not generate sufficient evidence that could be generalized to the larger population.
Our study strength was involving a team of pharmacists and a medical resident in data collection which might have contributed to the quality of data. In addition, the study used a standardized DTP identification criteria as well as standard tools to assess for both ADRs and adherence.
Conclusion
This study reports that almost two-thirds of study participants had antihypertensive-related DTPs. The most common antihypertensive-related DTPs were ‘dosage too low’ and ‘adverse drug reactions’ which each accounted for almost a third of the total DTPs. Uncontrolled BP and availability of routine laboratory test results were significantly associated with antihypertensive-related DTPs among the study participants. Our study emphasizes the need for improved patient care by devoted healthcare professionals such as clinical pharmacists to identify and prevent DTPs among hypertensive patients.
Supplemental Material
Supplemental material, sj-docx-1-tak-10.1177_17539447231160319 for Prevalence and factors associated with drug therapy problems among hypertensive patients at hypertension clinic of Mbarara Regional Referral Hospital, Uganda: a |cross-sectional study by Merab Babirye, Tadele Mekuriya Yadesa, Robert Tamukong and Paul Stephen Obwoya in Therapeutic Advances in Cardiovascular Disease
Acknowledgments
First of all, we would like to thank Pharm-Biotechnology and Tradiotional Medicince Center, Word Bank’s ACE-II project at Mbarara University of Science and Technology, for funding this study. We would also like to express our sincere gratitude to the research assistants, who were involved in data collection, the hypertensive patients that participated in the study and the staff of the hypertension clinic, Mbarara Regional Referral hospital for their support and guidance during the data collection period. The authors would also like to express their heartfelt gratitude to Dr. Eriwala William for all support rendered.
Footnotes
ORCID iDs: Merab Babirye  https://orcid.org/0000-0001-7675-0615
https://orcid.org/0000-0001-7675-0615
Tadele Mekuriya Yadesa  https://orcid.org/0000-0001-5151-2610
https://orcid.org/0000-0001-5151-2610
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Merab Babirye, Department of Pharmacy, Mbarara University of Science and Technology, P.O.Box 1410, Mbarara, Uganda.
Tadele Mekuriya Yadesa, Department of Pharmacy, Mbarara University of Science and Technology, Mbarara, Uganda; World Bank, ACE II, Pharm-Biotechnology and Traditional Medicine Center, Mbarara University of Science and Technology, Mbarara, Uganda; Department of Pharmacy, College of Medicine and Health Sciences, Ambo University, Ambo, Ethiopia.
Robert Tamukong, Department of Pharmacy, Mbarara University of Science and Technology, Mbarara, Uganda; World Bank, ACE II, Pharm-Biotechnology and Traditional Medicine Center, Mbarara University of Science and Technology, Mbarara, Uganda.
Paul Stephen Obwoya, Department of Internal Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Declarations
Ethics approval and consent to participate: Approval to conduct the study was obtained from Mbarara University of Science and Technology Research Ethics Committee (Reference number: MUST-2021-187). Written informed consent was obtained from the patients prior to participating in the study and use of their medical records. The purpose, objectives, benefits and risks as well as the impact of the study on the total time spent in the hospital were clearly explained to the patients.
Consent for publication: All authors agreed to the submission of this manuscript for publication and approved the final draft.
Author contributions: Merab Babirye: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Writing – original draft; Writing – review & editing.
Tadele Mekuriya Yadesa: Formal analysis; Supervision; Writing – original draft; Writing – review & editing.
Robert Tamukong: Supervision.
Paul Stephen Obwoya: Supervision.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Pharm-Biotechnology and Traditional Medicine Center at Mbarara University of Science and Technology.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets of the current study are available from the corresponding author on reasonable request.
References
- 1.DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: a pathophysiologic approach. 10 ed. New York: McGraw Hill, 2017. [Google Scholar]
- 2.World Health Organization. A global brief on hypertension: silent killer, global public health crisis: World Health Day 2013. Geneva: World Health Organization, 2013. [Google Scholar]
- 3.Zhou B, Bentham J, Di Cesare M, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017; 389: 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendriks ME, Wit FW, Roos MT, et al. Hypertension in sub-Saharan Africa: cross-sectional surveys in four rural and urban communities. PLoS ONE 2012; 7: e32638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipolle RJ, Strand LM, Morley PC.Pharmaceutical care practice: the patient-centered approach to medication management. New York: McGraw Hill, 2012. [Google Scholar]
- 6.Ikeda N, Sapienza D, Guerrero R, et al. Control of hypertension with medication: a comparative analysis of national surveys in 20 countries. Bull World Health Organ 2013; 92: 10–19C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelchu T, Abdela J.Drug therapy problems among patients with cardiovascular disease admitted to the medical ward and had a follow-up at the ambulatory clinic of Hiwot Fana Specialized University Hospital: the case of a tertiary hospital in eastern Ethiopia. SAGE Open Med 2019; 7: 2050312119860401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Kardiologia Polska 2019; 77: 71–159. [DOI] [PubMed] [Google Scholar]
- 9.Tegegne GT, Gaddisa T, Kefale B, et al. Drug therapy problem and contributing factors among ambulatory hypertensive patients in Ambo General Hospital, West Shoa, Ethiopia. Hypertension 2020; 2: 3–4. [Google Scholar]
- 10.Husene A.Drug therapy problems and their predictors among hypertensive patients on follow up in Dill Chora Referral Hospital, Dire Dawa, Eastern Ethiopia, 2015, https://repository.ju.edu.et/bitstream/handle/123456789/3612/abadir%20final%20thesis.pdf?sequence=1&isAllowed=y
- 11.Ayalew MB, Tegegn HG, Abdela OA.Drug related hospital admissions; a systematic review of the recent literatures. Bull Emerg Trauma 2019; 7: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Hernandez P, Abegunde D, et al. The world medicines situation 2011. Medicine expenditures. Geneva: World Health Organization, 2011, pp. 33–36. [Google Scholar]
- 13.Westberg SM, Derr SK, Weinhandl ED, et al. Drug therapy problems identified by pharmacists through comprehensive medication management following hospital discharge. J Pharm Technol 2017; 33: 96–107. [Google Scholar]
- 14.Weldegebreal AS, Tezeta F, Mehari AT, et al. Assessment of drug therapy problem and associated factors among adult hypertensive patients at Ayder Comprehensive Specialized Hospital, Northern Ethiopia. Afr Health Sci 2019; 19: 2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein M, Lenjisa J, Woldu M, et al. Assessment of drug related problems among hypertensive patients on follow up in Adama Hospital Medical College, East Ethiopia. Clinic Pharmacol Biopharmaceut 2014; 3: 2. [Google Scholar]
- 16.Mahammedsied W, Feyissa M, Shibeshi W.Assessment of drug therapy problems and contributing factors among adult ambulatory hypertensive patients in Ayder Referral Hospital, Mekelle Northern Ethiopia. J Pharma Care Health Sys 2020; 7: 2376-0419.20. [Google Scholar]
- 17.Ayele Y, Melaku K, Dechasa M, et al. Assessment of drug related problems among type 2 diabetes mellitus patients with hypertension in Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia. BMC Res Notes 2018; 11: 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaila A, Biambo AA, Usman N, et al. Drug related problems and implications for pharmaceutical care interventions in hypertensive outpatients in a Nigerian hospital. J Sci Pract Pharm 2018; 5: 281–286. [Google Scholar]
- 19.Yimama M, Jarso H, Desse TA.Determinants of drug-related problems among ambulatory type 2 diabetes patients with hypertension comorbidity in Southwest Ethiopia: a prospective cross sectional study. BMC Research Notes 2018; 11: 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belayneh YM, Amberbir G, Agalu A.A prospective observational study of drug therapy problems in medical ward of a referral hospital in northeast Ethiopia. BMC Health Serv Res 2018; 18: 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Formica D, Sultana J, Cutroneo PM, et al. The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert Opin Drug Saf 2018; 17: 681–695. [DOI] [PubMed] [Google Scholar]
- 22.Akhideno PE, Fasipe OJ, Isah AO, et al. Economic burden, impact, and consequence of adverse drug reactions among medical inpatients in clinical practice. J Clin Sci 2018; 15: 186. [Google Scholar]
- 23.Pardo Cabello AJ, Del Pozo Gavilán E, Gómez Jiménez FJ, et al. Drug-related mortality among inpatients: a retrospective observational study. Eur J Clin Pharmacol 2016; 72: 731–736. [DOI] [PubMed] [Google Scholar]
- 24.Patel PS, Rana DA, Suthar JV, et al. A study of potential adverse drug-drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J Basic Clin Pharm 2014; 5: 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussen A, Daba FB.Drug therapy problems and their predictors among hypertensive patients on follow up in Dil-Chora Referral Hospital, Dire-Dawa, Ethiopia. Hypertension 2017; 5: 2712–2719. [Google Scholar]
- 26.Kefale B, Tegegne GT, Kefale Y, et al. Magnitude and determinants of drug therapy problems among type 2 diabetes mellitus patients with hypertension in Ethiopia. SAGE Open Med 2020; 8: 2050312120954695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunyera J, Kirenga B, Stanifer JW, et al. Geographic differences in the prevalence of hypertension in Uganda: results of a national epidemiological study. PLoS ONE 2018; 13: e0201001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Noncommunicable diseases country profiles 2018, 2018, https://www.who.int/publications/i/item/9789241514620 [Google Scholar]
- 29.Mills KT, Stefanescu A, He J.The global epidemiology of hypertension. Nat Rev Nephrol 2020; 16: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert EV, Steyn K, Stender S, et al. Cross-cultural validation of the hill-bone compliance to high blood pressure therapy scale in a South African, primary healthcare setting. Ethn Dis 2006; 16: 286–291. [PubMed] [Google Scholar]
- 31.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 32.MOH. Uganda clinical guidelines 2016, 2016, https://www.prb.org/wp-content/uploads/2018/05/Uganda-Clinical-Guidelines-2016-National-Guidelines-for-Management-of-Common-Conditions.pdf
- 33.WHO. Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action. Geneva: World Health Organization, 2002. [Google Scholar]
- 34.Kusumawardani LA, Andrajati R, Nusaibah A.Drug-related problems in hypertensive patients: a cross-sectional study from Indonesia. J Res Pharm Pract 2020; 9: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redzuan A, Ramli A, Pheng M.Drug-related problems in hypertensive patients with multiple comorbidities. J Pharm Res 2017; 1: 000113. [Google Scholar]
- 36.Huri HZ, Wee HF.Drug related problems in type 2 diabetes patients with hypertension: a cross-sectional retrospective study. BMC Endocrine Disorders 2013; 13: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zazuli Z, Rohaya A, Adnyana IK.Drug-related problems in type 2 diabetic patients with hypertension: a prospective study. J Basic Clin Pharma 2017; 8: 251–254. [Google Scholar]
- 38.Ukoha-kalu BO, Adibe MO, Ukwe CV.Identification and resolution of drug therapy problems among hypertensive patients receiving care in a Nigerian Hospital –a pilot study. Ann Clin Hypertens 2020; 4: 20–23. [Google Scholar]
- 39.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The antihypertensive and lipid-lowering treatment to prevent heart attack trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and Lipi. JAMA 2002; 288: 2981–2997. [DOI] [PubMed] [Google Scholar]
- 40.Staessen JA, Richart T, Verdecchia P.Reducing blood pressure in people of different ages. BMJ 2008; 336: 1080–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane D, Lawson A, Burns A, et al. Nonadherence in hypertension: how to develop and implement chemical adherence testing. Hypertension 2022; 79: 12–23. [DOI] [PubMed] [Google Scholar]
- 42.Moharamzad Y, Saadat H, Shahraki BN, et al. Validation of the Persian version of the 8-item Morisky Medication Adherence Scale (MMAS-8) in Iranian hypertensive patients. Glob J Health Sci 2015; 7: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen JØ, Shrestha AD, Neupane D, et al. Non-adherence to anti-hypertensive medication in low-and middle-income countries: a systematic review and meta-analysis of 92443 subjects. J Hum Hypertens 2017; 31: 14–21. [DOI] [PubMed] [Google Scholar]
- 44.Bolívar JFC, Martínez-Martínez F, Ferrit-Martin M.Drug therapy monitoring in patients with type 2 diabetes and hypertension. J Pharm Pharmacol 2017; 5: 169–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tak-10.1177_17539447231160319 for Prevalence and factors associated with drug therapy problems among hypertensive patients at hypertension clinic of Mbarara Regional Referral Hospital, Uganda: a |cross-sectional study by Merab Babirye, Tadele Mekuriya Yadesa, Robert Tamukong and Paul Stephen Obwoya in Therapeutic Advances in Cardiovascular Disease