Abstract
Nickel-catalyzed reductive cross-electrophile coupling reactions are becoming increasingly important in organic synthesis, but application at scale is limited by three interconnected challenges: a reliance on amide solvents (complicated workup, regulated), the generation of stoichiometric Zn salts (complicated isolation, waste disposal issue), and mixing/activation challenges of zinc powder. We show here an electrochemical approach that addresses these three issues: the reaction works in acetonitrile with diisopropylethylamine as the terminal reductant in a simple undivided cell (graphite(+)/nickel foam(−)). The reaction utilizes a combination of two ligands, 4,4′-di-tert-butyl-2,2′-bipyridine and 4,4′,4′′-tri-tert-butyl-2,2′:6′,2′′-terpyridine. Studies show that, alone, the bipyridine nickel catalyst predominantly forms protodehalogenated aryl and aryl dimer, whereas the terpyridine nickel catalyst predominantly forms bialkyl and product. By combining these two unselective catalysts, a tunable, general system results because excess radical formed by the terpyridine catalyst can be converted to product by the bipyridine catalyst. As the aryl bromide becomes more electron rich, the optimal ratio shifts to have more of the bipyridine nickel catalyst. Lastly, examination of a variety of flow-cell configurations establishes that batch recirculation can achieve higher productivity (mmol product/time/electrode area) than single-pass, that high flow rates are essential to maximizing current, and that two flow cells in parallel can nearly halve the reaction time. The resulting reaction is demonstrated on gram scale and should be scalable to kilogram scale.
Keywords: nickel, electrochemistry, cross-coupling, flow, cross-electrophile coupling, mechanism, multimetallic catalysis
Graphical Abstract

The catalytic formation of C–C bonds is dominated by the coupling of pre-formed organometallic nucleophiles (e.g., organoboronic acids, organozinc reagents, silanes, Grignard reagents) with organic electrophiles.1 Of the two coupling partners, organometallic nucleophiles are less abundant and are often synthesized from carbon electrophiles,2 incurring additional synthetic steps. Methods based upon in situ organometallic reagent generation avoid additional steps,3 but all of these reactions are accompanied by stoichiometric metal salt generation. Cross-electrophile coupling generates the same products as cross-coupling, but directly from two electrophiles and without the need for stoichiometric metal, in theory.4 In practice, the use of metal reductants5 (e.g., Zn, Mn, Mg) or metal sacrificial anodes6,7,8 have been the most general strategies used for C(sp2)–C(sp3) bond formation (Scheme 1). Applications in industry and academia have almost exclusively generated stoichiometric metal waste (e.g., MnX2, ZnX2, or boron or silicon waste). Metal byproducts can be difficult to separate out, the disposal of which is highly regulated,9 and this has forced process changes in pharmaceutical applications.10 As cross-electrophile coupling matures, the realization of its green-chemistry potential on scale has become of increasing interest.
Scheme 1. Electrochemical Cross-Electrophile Coupling for Large Scale Applications.
Besides stoichiometric metal waste, analyses at BMS,5b,6,8 Merck,11 and Pfizer12 have identified the major issues in scale-up to be heterogeneity/mixing, inconsistency of metallic reductant quality, the possibility of strong exotherms, and reliance on amide solvents (Scheme 1).13 While progress has been made, no approach has been able to make progress on all of these challenges without introducing new complexities. For example, mixing issues are avoided by packed-bed Zn columns,14 but still rely upon amide solvents and generate metal salts. Photoredox approaches avoid a heterogeneous reductant, but introduce co-catalysts and the need to shine light into the reactor.15,16,17 The organic reductant tetrakis(dimethylamino)ethylene (TDAE) avoids metal salts,18 but its amidinium salts are highly insoluble and TDAE is costly, air sensitive, and moisture sensitive.19,20 Electrochemical approaches to reductive C–C bond formation were extensively studied in the 1970s and 1980s before seeing a recent renaissance,6,21 but most of these studies rely upon sacrificial metal anodes (generating stoichiometric metal waste) and/or amide solvents. Compared to soluble sacrificial reductants, sacrificial metal anodes avoid complications due to substrate or catalyst oxidation at the anode and the metal salts generated typically do not interfere with catalysis. However, because sacrificial anodes degrade over time, their use on scale or in flow can be more complicated.11,12 An MIT and Snapdragon team was the first to use a sacrificial amine terminal reductant for electrochemical C(sp2)-C(sp3) cross-electrophile coupling.22 We recently reported that amine terminal reductants could allow the use of acetonitrile instead of amide solvents,12 which simplifies workup. However, both cases required the use of a divided cell with a Nafion™ cation exchange membrane which can be more complicated23 to scale up due to chemical and mechanical stresses on the membrane.24 In addition, divided cells typically have high cell resistance, sometimes requiring larger amounts of electrolyte,25 complicating workup.26 Li recently reported on a paired-electrolysis approach in NMP where the anodic oxidation of triphenylphosphine generates phosphonium bromide in situ which converts alcohols to alkyl bromides, the substrate for the cathodic cross-electrophile coupling reaction.27 Rueping demonstrated the arylalkylation of alkynes using electrochemistry and photo-assisted electrochemistry to selectively form the E and Z isomers, respectively, using a terminal amine reductant in DMA.28 These approaches use an undivided cell, but again rely on an amide solvent and high equivalents of coupling partners.
We show here the electrochemical cross-electrophile coupling of alkyl and aryl bromides using a common amine, diisopropylethyl amine (DIPEA),29 as the terminal reductant / base in an undivided cell (graphite(+)/nickel foam(−)) with acetonitrile as the solvent and only 0.1 equiv of electrolyte. We demonstrate the affordable30 scale up of this reaction in flow using two Micro Flow Cells by ElectroCell31 capable of producing 0.27 g of product per hour. Further scaling is possible with larger flow cells (up to 1 m2) from the same company, which should allow for scaling up to ~5 kg product/week.32
Starting from conditions we reported previously for cross-electrophile coupling reactions in divided cells and inspired by some photoredox-driven cross-electrophile coupling reports, we found that by using DIPEA as the terminal reductant, the reaction is selective towards the product even in undivided cells. These reactions were run under constant current, using acetonitrile as the solvent, only 0.1 equiv (20 mM) tetrabutylammonium hexafluorophosphate (TBAPF6) as the electrolyte,33 and a combination of nickel bipyridine and nickel terpyridine catalysts.
As shown in Table 1, nickel, L1/L2, DIPEA, and electricity, are all necessary for product formation (entries 3–4,6–7). NiBr2•3H2O is lower cost than other nickel pre-catalysts, is available on large scale, and outperforms other pre-catalysts due to improved solubility (see Supporting Information Figure S1). Although this small amount of water is well tolerated (0.3 equiv H2O from pre-catalyst), larger amounts of water (5% v/v) resulted in lower yield (entry 2). The reactions are also tolerant of air. While initial reactions were set up with exclusion of water and air, we later found that benchtop setup provided similarly good results (Supporting Information Figure S3). We found we could run reactions with nickel stripped from the cathode by reversing the current for 0.2 F/mol before starting the reaction (entry 5), which could be an advantage in jurisdictions where nickel salts are regulated as carcinogens.34 DIPEA was the optimal amine because it does not react with the alkyl halide. Triethyl amine provided lower yields due to N-alkylation (see Supporting Information S2). Several electrode materials were tested, and high yields were obtained with nickel foam as the cathode and graphite rod as the anode (entries 8–9). This is a practical advantage because they are easier to work with than RVC, which has a similarly high surface area, but is brittle.35 The applied current was chosen to be 1.33 mA/cm2. At higher current the selectivity decreased, forming more alkyl dimer and protodehalogenation of the aryl bromide (entry 10). However, lower current did not significantly improve the yield (entry 11). At lower temperatures, alkyl dimer formation increased (entry 12). LiBr can be used as an electrolyte instead of TBAPF6, albeit with a decrease in yield from 89% to 66% (entry 13).
Table 1.
Optimization of Electrochemical Cross-Electrophile Coupling in an Undivided Batch Cell.

|
|||||
|---|---|---|---|---|---|
| Entry | Deviation from Standard Conditions | Yield (%)b | |||
| 1 | 2 | 3 | 4 | ||
| 1 | None | 3 | 5 | 89 | 3 |
| 2 | 5% v/v H2O | 54 | 11 | 38 | 29 |
| 3 | No L1 or L2 | 83 | 43 | 7 | 0 |
| 4 | No NiBr2 • 3 H2O | 98 | 60 | 0 | 0 |
| 5 | No NiBr2 • 3 H2O, reverse current for 0.2 F/mol beforehand | 1 | 8 | 69 | 5 |
| 6 | No DIPEA | >99 | 68 | 0 | 0 |
| 7 | 0 F/molc | 99 | 93 | 0 | 0 |
| 8 | RVC instead of Ni foam and graphite | 45 | 17 | 41 | 18 |
| 9 | Graphite instead of Ni foam | 13 | 26 | 8 | 29 |
| 10 | 0.7 mA/cm2 instead of 1.3 mA/cm2 | 0 | 2 | 80 | 11 |
| 11 | 2.0 mA/cm2 instead of 1.3 mA/cm2 | 0 | 0 | 78 | 12 |
| 12 | 45 °C instead of 70 °C | 35 | 16 | 35 | 31 |
| 13 | LiBr instead of TBAPF6 | 3 | 0 | 66 | 10 |
Ni foam cathode surface area = 7.5 cm2.
Corrected GC yield vs dodecane.
Reaction mixture was stirred and heated for 4.3 h (length of reaction for 4 F/mol at 1.3 mA/cm2).
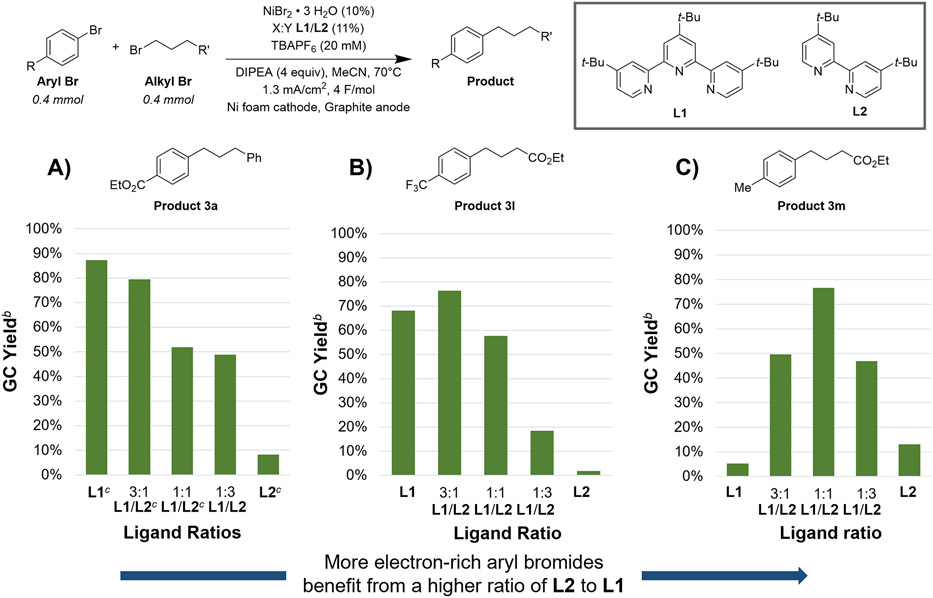
As we have reported in several studies,12,36 a two-catalyst multimetallic system was optimal.37 We found that reactions of electron-poor aryl bromides with only terpyridine nickel catalysts provided reasonable yields of cross-product accompanied by bialkyl and unreacted aryl bromide (Scheme 2A, 2B). In reactions with a less reactive aryl bromide (4-tolyl bromide), the terpyridine-only system produced primarily bialkyl (Scheme 2C). In contrast, reactions with bipyridine nickel catalysts provided a low yield of cross-product along with protodehalogenated aryl (ArH) and aryl dimer with minimal consumption of alkyl bromide regardless of the identity of the aryl bromide (Scheme 2, see Supporting Information Figures S5-7 for full time course data with the different ligand ratios for three example substrate pairs). The addition of bipyridine nickel to reactions catalyzed by terpyridine nickel complexes decreases the amount of alkyl dimer formed and increases product yield. The optimal ratio appears to be dependent upon the relative reactivity of the aryl halide and the alkyl halide (Scheme 2). For very electron deficient aryl bromides, the terpyridine nickel catalyst on its own results in the best selectivity (Scheme 2A). However, for slightly electron deficient aryl bromides, a 3:1 ratio of terpyridine nickel catalyst to bipyridine nickel catalyst provided the best selectivity (Scheme 2B). As the aryl bromide becomes increasing electron-rich (and, therefore, less reactive for oxidative addition), the optimal ratio shifts to 1:1 ratio of the nickel catalysts (Scheme 2C). These findings contrast with cross-electrophile couplings of aryl bromides with alkyl bromides using metal reductants in amide solvents,38 where reactions conducted with terpyridine ligands provide primarily alkyl dimer products and bipyridine ligands are optimal.
Scheme 2. Ligand Effects on Reaction Yield.a.
aStandard Conditions: Aryl Br (0.4 mmol), Alkyl Br (0.4 mmol), NiBr2•3H2O (0.04 mmol), Varying ratios of L1 / L2 (0.044 mmol), TBAPF6 (0.04 mmol), DIPEA (1.6 mmol), MeCN (2 mL), 70 °C, 10 mA, Ni foam cathode (surface area = 7.5 cm2), graphite anode. bCorrected GC yield vs dodecane. cAverage of data from two reactions. A) Aryl Br = ethyl 4-bromobenzoate, Alkyl Br = 1-bromo-3-phenylpropane, B) Aryl Br = 4-bromobenzotrifluoride, Alkyl Br = ethyl 4-bromobutyrate, C) Aryl Br = 4-bromotoluene, Alkyl Br = ethyl 4-bromobutyrate
An open question with two-ligand nickel systems is the origin of the synergistic effect, as exemplified in Scheme 2C: how do two poor catalysts combine to result in high yields of cross-product? Based upon our own studies and literature data,39,40 we propose two connected catalytic cycles for product formation (Scheme 3). The first cycle is catalyzed by (L1)Ni and is poorly selective for product over bialkyl when the aryl bromide is deactivated for oxidative addition compared to the alkyl bromide. The mechanism of this cycle is likely related to the mechanism first proposed by Vannucci.40 Based upon CV studies and analysis of the reaction cell potential, we propose that the accessible (L1)NiI intermediate41 reacts with both Ar-Br and Alkyl-Br, with a preference for Alkyl-Br, resulting in product formation and a large amount of excess free Alkyl• that, on its own, would result in large amounts of Alkyl–Alkyl product.42 The second cycle is catalyzed by (L2)Ni and relies upon excess Alkyl• from the (L1)Ni system for product formation. The (L2)Ni0 catalyst, which should be accessible under these conditions,41 reacts almost exclusively with aryl bromide to form a relatively stable (L2)NiII(Ar)Br species that can capture excess Alkyl• generated by (L1)Ni to form cross-coupled product. While each catalyst is capable of product formation alone, they both suffer from poor selectivity. The combined catalysts complement each other, resulting in a synthetically useful system. This dual-catalyst system contrasts with a different system recently reported by Sevov, where a 2:1 ligand/nickel ratio was optimal, and their studies suggested ligand substitution was occurring during each turnover.7m,43
Scheme 3. Proposed Multimetallic Mechanism for Two-Catalyst Cross-Electrophile Coupling XEC.a.
a Based upon CV data, we depict oxidative addition at (L1)NiIX and at (L2)Ni0. It is important to note that oxidative addition could occur from either oxidation state, but results in the same arylnickel(II) intermediate. Similarly, this proposal does not attempt to account for potential ligand exchange processes.
The substrate scope for this simplified electrochemically-driven cross-electrophile coupling is similarly broad to reactions with metal reductants (Scheme 4). The two-catalyst system allows couplings with a broad array of substrates, such as couplings of aryl bromides with ortho-coordinating groups (3i-j)44 and Lewis-basic nitrogen heterocycles (3q-u).45 Primary and secondary alkyl bromides couple in good yield without the need for iodide additives.46 Sterically hindered substrates such as neopentyl bromide or methyl 2-bromobenzoate resulted in lower yields. While our standard 3:1 L1/L2 catalyst mixture was effective for a wide array of substrate pairs, it was especially useful for aryl bromides with electron-withdrawing groups. For aryl bromides that are slower to react by oxidative addition, such as 4-bromoanisole, yields could be improved by increasing the relative amount of L2 ligand. In general, the selectivity of the reaction can be optimized by increasing the ratio of L1 if there is unreacted alkyl bromide and/or high amounts of aryl H and aryl dimer, or by increasing the ratio of L2 if there is unreacted aryl bromide and/or high amounts of alkyl dimer.
Scheme 4: Substrate Scope in Batcha,b.
aReactions were conducted in an undivided cell and run on 0.4 mmol scale in MeCN (2 mL). bIsolated yields are shown. cContains <2% alkyl dimer that was inseparable. d1:3 ratio of L1/L2., e1:1 ratio of L1/L2. f5:1 ratio of L1/L2.
A key goal of this study was simplifying transition of the conditions to flow for scale up. Nickel-mediated electrochemistry has been reported in both single-pass (with slow flow) and in batch recirculation (with faster flow). The drastically different flow rates employed will impact mass transport and could result in different levels of productivity and selectivity. Further, both setups could be utilized with multiple cells in either parallel or series. We tested these configurations with our system and compared productivity (mmol product/time/electrode area).
Using the conditions we optimized in batch, we tested the two different flow setups (Scheme 5A). General reaction conditions for the flow optimization are shown in Scheme 5B. In most cases, we used 4,4′,4′′-trimethyl-2,2′:6′,2′′-terpyridine (L3) and 4,4′-dimethyl-2,2′-bipyridine (L4) instead of L1 and L2, respectively. These methyl ligands showed similar reactivity to the tert-butyl ligands, but can be synthesized in one step from picoline47 or purchased at lower cost.48 In the single pass setup,22 we screened residence time (average time that a given molecule is in the cell) and current density (Scheme 5C). We attribute ArH formation primarily to over-reduction of arylnickel(II) intermediates, as suggested by Sevov in their recent studies.7i Reduction of arylnickel(II) to form ArH is a complex function of current density, nickel concentration, turnover frequency, and mass transport. To avoid this side reaction in single-pass flow experiments, the catalyst loading was increased (from 5 to 10 mol%), and high selectivity toward the product could be regained (entries 1–3). However, further increasing the current density again resulted in diminished yield (entry 4).
Scheme 5: Optimization of Electrochemical Cross-Electrophile Coupling in Flow.
aCorrected GC yield vs 1,3,5-trimethoxybenzene. bLigands = 3:1 L1/L2. c4 F/mol, dCorrected GC yield vs dodecane. eIsolated yield.
In our experiments, batch recirculation is a superior approach to scale up electrochemical cross-electrophile coupling reactions in flow (Scheme 5D). In these reactions, each pass through the electrochemical cell is relatively fast and only a small amount of conversion is achieved per pass (Scheme 5A). While lower yields were obtained at low flow rate and nickel loading (Scheme 5D, entries 1 and 6), increasing nickel catalyst loading (entries 1–3) and increasing the rate of mass transport (by increasing the flow rate) improved selectivity and yield (entries 3–5 and 6–8). Increasing nickel concentration by decreasing the amount of solvent used was also effective, albeit limited by the solubility of reaction components (entries 9 and 10). Being able to tolerate higher current density allows recirculation to be more efficient (higher productivity per unit of electrode surface area) than batch or single pass flow (Scheme 5E). Multiple flow cells can be used to increase the electrode surface area and therefore decrease the total reaction time. Combining two flow cells in parallel resulted in slightly higher yield and efficiency than combining them in series (Scheme 5E). We hypothesize that better efficiency in batch recirculation is due to increased mass transport enabling the relatively slow cross-electrophile coupling reaction to primarily proceed outside of the electrochemical cell. The electrochemical cell is only needed to form nickel(0) at a pace sufficient to support the rest of the steps (Scheme 3). Using the optimized conditions for recirculation, we scaled up the reaction to gram-scale with two electrochemical cells in parallel. A smaller tubing size was used to avoid tubing rupture over longer periods of time, resulting in lower flow rates. At a lower flow rate with 11.4 mA/cm2 current density, the yield decreased (as expected from our studies), but this slower flow could be compensated for by decreasing the current density. At 5.7 mA/cm2 current density, we achieved 73% isolated yield (Scheme 5F). See SI for more details on how to set up the flow equipment and practical considerations.
In conclusion, we have developed a simple electrochemical approach for the scale up of C(sp2)-C(sp3) cross-electrophile coupling that avoids the use of metal reductants and amide solvents. By tuning the ligand ratio, a broader range of substrates can be coupled than with a single catalyst because the rate of alkyl radical generation can be adjusted to match the rest of the reaction steps. When translating this reaction to flow, increasing catalyst concentration and flow rate were instrumental in maintaining good yield at current densities practical for up to kg-scale synthesis. However, further scaling will require an improved understanding of the cathodic chemistry to allow for higher turnover frequency so that higher current densities can be supported.7i In addition, while the use of acetonitrile solvent and amine reductant is an improvement over amide solvents and zinc, in principle electrochemical approaches should allow for the use of still greener solvents and reductants. We are presently working on addressing these limitations and will report our results in due course.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH (R01GM097243 to DJW and R35GM134929 to SSS) and Pfizer (ECH, DJW).
Footnotes
Supporting Information. Additional tables of data, electrochemical data, detailed experimental procedures, characterization data for isolated compounds, and copies of NMR data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Magano J; Dunetz JR Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev 2011, 111, 2177–2250. [DOI] [PubMed] [Google Scholar]; (b) Roughley SD; Jordan AM The Medicinal Chemist's Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]; (c) Carey J; Laffan D; Thomson C; Williams M Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem 2006, 4, 2337–2347. [DOI] [PubMed] [Google Scholar]
- 2.(a) Handbook of functionalized organometallics: applications in synthesis; Knochel P, Ed.; Wiley-VCH: Weinheim, 2005, pp 653. [Google Scholar]; (b) Molander GA; Jean-Gérard L Cross-Coupling Reactions of Organotrifluoroborate Salts. Organic React. 2013, 79, 1–316. [Google Scholar]; (c) Haas D; Hammann JM; Greiner R; Knochel P Recent Developments in Negishi Cross-Coupling Reactions. ACS Catal. 2016, 6, 1540–1552. [Google Scholar]
- 3.(a) Knappke CEI; Grupe S; Gärtner D; Corpet M; Gosmini C; Jacobi von Wangelin A, Reductive Cross-Coupling Reactions between Two Electrophiles. Chem.–Eur. J 2014, 20, 6828–6842. [DOI] [PubMed] [Google Scholar]; (b) Sase S; Jaric M; Metzger A; Malakhov V; Knochel P One-Pot Negishi Cross-Coupling Reactions of In Situ Generated Zinc Reagents with Aryl Chlorides, Bromides, and Triflates. J. Org. Chem 2008, 73, 7380–7382. [DOI] [PubMed] [Google Scholar]; (c) Czaplik WM; Mayer M; Jacobi von Wangelin A, Domino Iron Catalysis: Direct Aryl-Alkyl Cross-Coupling. Angew. Chem., Int. Ed 2009, 48, 607–610. [DOI] [PubMed] [Google Scholar]; (d) Krasovskiy A; Duplais C; Lipshutz B, Zn-Mediated, Pd-Catalyzed Cross-Couplings in Water at Room Temperature Without Prior Formation of Organozinc Reagents. J. Am. Chem. Soc 2009, 131, 15592–15593. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Bhonde VR; O'Neill BT; Buchwald SL, An Improved System for the Aqueous Lipshutz–Negishi Cross-Coupling of Alkyl Halides with Aryl Electrophiles. Angew. Chem., Int. Ed 2016, 55, 1849–1853. [DOI] [PubMed] [Google Scholar]; (f) Thakore RR; Takale BS; Gallou F; Reilly J; Lipshutz BH, N,C-Disubstituted Biarylpalladacycles as Precatalysts for ppm Pd-Catalyzed Cross Couplings in Water under Mild Conditions. ACS Catalysis 2019, 9, 11647–11657. [Google Scholar]
- 4.(a) Goldfogel MJ; Huang L; Weix DJ: Cross-Electrophile Coupling. In Nickel Catalysis in Organic Synthesis; Ogoshi S, Ed.; Wiley-VCH: Weinheim, 2020; pp 183–222. [Google Scholar]; (b) Wang X; Dai Y; Gong H Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem 2016, 374, 43. [DOI] [PubMed] [Google Scholar]
- 5.(a) Richmond E; Moran J Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2018, 50, 499–513. [Google Scholar]; (b) Nimmagadda SK; Korapati S; Dasgupta D; Malik NA; Vinodini A; Gangu AS; Kalidindi S; Maity P; Bondigela SS; Venu A; Gallagher WP; Aytar S; González-Bobes F; Vaidyanathan R Development and Execution of an Ni(II)-Catalyzed Reductive Cross-Coupling of Substituted 2-Chloropyridine and Ethyl 3-Chloropropanoate. Org. Process Res. Dev 2020, 24, 1141–1148. [Google Scholar]
- 6.(a) Chaussard J; Folest J-C; Nedelec J-Y; Perichon J; Sibille S; Troupel M Use of Sacrificial Anodes in Electrochemical Functionalization of Organic Halides. Synthesis 1990, 369–381. [Google Scholar]; (b) Gale-Day ZJ Recent Advances in Metal-Catalyzed, Electrochemical Coupling Reactions of sp2 Halides/Boronic Acids and sp3 Centers. Synthesis 2021, 53, 879–888. [Google Scholar]; (c) Novaes LFT; Liu J; Shen Y; Lu L; Meinhardt JM; Lin S Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev 2021, 50, 7941–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ma C; Fang P; Liu D; Jiao K-J; Gao P-S; Qiu H; Mei T-S Transition metal-catalyzed organic reactions in undivided electrochemical cells. Chem. Sci 2021, 12, 12866–12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Conan A; Sibille S; d'Incan E; Périchon J Nickel-catalysed electroreductive coupling of α-halogenoesters with aryl or vinyl halides. J. Chem. Soc., Chem. Commun 1990, 48–49. [Google Scholar]; (b) Durandetti M; Sibille S; Nédélec J-Y; Périchon J A Novel Method of Arylation of α-Chloroketones. Synth. Commun 1994, 24, 145–151. [Google Scholar]; (c) Durandetti M; Nédélec J-Y; Périchon J Nickel-Catalyzed Direct Electrochemical Cross-Coupling between Aryl Halides and Activated Alkyl Halides. J. Org. Chem 1996, 61, 1748–1755. [DOI] [PubMed] [Google Scholar]; (d) Durandetti M; Périchon J; Nédélec J-Y Asymmetric Induction in the Electrochemical Cross-Coupling of Aryl Halides with α-Chloropropionic Acid Derivatives Catalyzed by Nickel Complexes. J. Org. Chem 1997, 62, 7914–7915. [DOI] [PubMed] [Google Scholar]; (e) Urgin K; Barhdadi R; Condon S; Léonel E; Pipelier M; Blot V; Thobie-Gautier C; Dubreuil D Some Mechanistic Aspects of a Nickel-Catalyzed Electrochemical Cross-Coupling between Aryl Halides and Substituted Chloropyridazines. Electrochim. Acta 2010, 55, 4495–4500. [Google Scholar]; (f) Perkins RJ; Pedro DJ; Hansen EC Electrochemical Nickel Catalysis for Sp2-Sp3 Cross-Electrophile Coupling Reactions of Unactivated Alkyl Halides. Org. Lett 2017, 19, 3755–3758. [DOI] [PubMed] [Google Scholar]; (g) Koyanagi T; Herath A; Chong A; Ratnikov M; Valiere A; Chang J; Molteni V; Loren J One-Pot Electrochemical Nickel-Catalyzed Decarboxylative Sp2–Sp3 Cross-Coupling. Org. Lett 2019, 21, 816–820. [DOI] [PubMed] [Google Scholar]; (h) DeLano TJ; Reisman SE Enantioselective Electroreductive Coupling of Alkenyl and Benzyl Halides via Nickel Catalysis. ACS Catal. 2019, 9, 6751–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Truesdell BL; Hamby TB; Sevov CS General C(Sp2)–C(Sp3) Cross-Electrophile Coupling Reactions Enabled by Overcharge Protection of Homogeneous Electrocatalysts. J. Am. Chem. Soc 2020, 142, 5884–5893. [DOI] [PubMed] [Google Scholar]; (j) Kumar GS; Peshkov A; Brzozowska A; Nikolaienko P; Zhu C; Rueping M Nickel-Catalyzed Chain-Walking Cross-Electrophile Coupling of Alkyl and Aryl Halides and Olefin Hydroarylation Enabled by Electrochemical Reduction. Angew. Chem., Int. Ed 2020, 59, 6513–6519. [DOI] [PubMed] [Google Scholar]; (k) Jiao K-J; Liu D; Ma H-X; Qiu H; Fang P; Mei T-S Nickel-Catalyzed Electrochemical Reductive Relay Cross-Coupling of Alkyl Halides to Aryl Halides. Angew. Chem., Int. Ed 2020, 59, 6520–6524. [DOI] [PubMed] [Google Scholar]; (l) Zackasee JLS; Al Zubaydi S; Truesdell BL; Sevov CS Synergistic Catalyst–Mediator Pairings for Electroreductive Cross-Electrophile Coupling Reactions. ACS Catal. 2022, 12, 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Hamby Taylor B; LaLama Matthew J; Sevov Christo S Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling. Science 2022, 376, 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Harwood Stephen J; Palkowitz Maximilian D; Gannett Cara N; Perez P; Yao Z; Sun L; Abruña Héctor D; Anderson Scott L; Baran Phil S Modular terpene synthesis enabled by mild electrochemical couplings. Science 2022, 375, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Zhe Chang JW, Xi Lu, Yao Fu. Synthesis of gem-Difluoroalkenes through Nickel-Promoted Electrochemical Reductive Cross-Coupling. Chin. J. Org. Chem 2022, 42, 147–159. [Google Scholar]; (p) Palkowitz M; Laudadio G; Kolb S; Choi J; Oderinde M; Ewing T; Bolduc P; Chen T; Zhang H; Cheng P; Zhang B; Mandler M; Richter J; Collins M; Schioldager R; Dhar M; Vokits B; Zhu Y; Echeverria P-G; Poss M; Shaw S; Clementson S; Petersen N; Mykhailiuk P; Baran P Overcoming Limitations in Decarboxylative Arylation via Ag-Ni Electrocatalysis. ChemRxiv 2022. DOI: 10.26434/chemrxiv-2022-rpnp8. This content is a preprint and has not been peer-reviewed. Accessed on July 18, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutner GL; Simmons EM; Ayers S; Bemis CY; Goldfogel MJ; Joe CL; Marshall J; Wisniewski SR A Process Chemistry Benchmark for sp2–sp3 Cross Couplings. J. Org. Chem 2021, 86, 10380–10396. [DOI] [PubMed] [Google Scholar]
- 9.EU regulations prohibit the discharge of non-biodegradable material, including heavy metals, to wastewater treatment facilities. Individual site licenses for wastewater effluent restrict metals including zinc, copper and nickel to ppb levels. This results in costly tertiary waste treatment for any waste streams containing these elements.
- 10.(a) Acemoglu M; Baenziger M; Krell CM; Marterer W: Experiences with Negishi Couplings on Technical Scale in Early Development. In Transition Metal-Catalyzed Couplings in Process Chemistry; Magano J, Dunetz JR, Eds.; Wiley-VCH: Weinheim, 2013; pp 15–23. [Google Scholar]; (b) COMMISSION IMPLEMENTING DECISION (EU) 2016/902 of 30 May 2016 establishing best available techniques (BAT) conclusions, under Directive 2010/75/EU of the European Parliament and of the Council, for common waste water and waste gas treatment/ management systems in the chemical sector. Official Journal of the European Union, L: Legislation 2016, 59 (L152), 23–42. [Google Scholar]
- 11.Lovato K; Fier PS; Maloney KM The application of modern reactions in large-scale synthesis. Nat. Rev. Chem 2021, 5, 546–563. [DOI] [PubMed] [Google Scholar]
- 12.Perkins RJ; Hughes AJ; Weix DJ; Hansen EC Metal-Reductant-Free Electrochemical Nickel-Catalyzed Couplings of Aryl and Alkyl Bromides in Acetonitrile. Org. Process Res. Dev 2019, 23, 1746–1751. [Google Scholar]
- 13.For a summary of current challenges, see: Diorazio LJ; Hose DRJ; Adlington NK Toward a More Holistic Framework for Solvent Selection. Org. Process Res. Dev 2016, 20, 760–773. [Google Scholar]
- 14.Watanabe E; Chen Y; May O; Ley SV A Practical Method for Continuous Production of sp3-Rich Compounds from (Hetero)Aryl Halides and Redox-Active Esters. Chem.–Eur. J 2020, 26, 186–191. [DOI] [PubMed] [Google Scholar]
- 15.Chan AY; Perry IB; Bissonnette NB; Buksh BF; Edwards GA; Frye LI; Garry OL; Lavagnino MN; Li BX; Liang Y; Mao E; Millet A; Oakley JV; Reed NL; Sakai HA; Seath CP; MacMillan DWC Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev 2022, 122, 1485–1542. [DOI] [PubMed] [Google Scholar]
- 16.(a) Using silanes as the terminal reductant. Zhang P; Le CC; MacMillan DWC Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc 2016, 138, 8084–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bacauanu V; Cardinal S; Yamauchi M; Kondo M; Fernández DF; Remy R; MacMillan DWC Metallaphotoredox Difluoromethylation of Aryl Bromides. Angew. Chem., Int. Ed 2018, 57, 12543–12548. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen TQ; MacMillan DWC A Metallaphotoredox Strategy for the Cross-Electrophile Coupling of α-Chloro Carbonyls with Aryl Halides. Angew. Chem., Int. Ed 2019, 58, 14584–14588. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sakai HA; Liu W; Le CC; MacMillan DWC Cross-Electrophile Coupling of Unactivated Alkyl Chlorides. J. Am. Chem. Soc 2020, 142, 11691–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kölmel DK; Ratnayake AS; Flanagan ME Photoredox cross-electrophile coupling in DNA-encoded chemistry. Biochem. Biophys. Res. Commun 2020, 533, 201–208. [DOI] [PubMed] [Google Scholar]; (f) Faraggi TM; Rouget-Virbel C; Rincón JA; Barberis M; Mateos C; García-Cerrada S; Agejas J; de Frutos O; MacMillan DWC Synthesis of Enantiopure Unnatural Amino Acids by Metallaphotoredox Catalysis. Org. Process Res. Dev 2021, 25, 1966–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) González-Esguevillas M; Fernández DF; Rincón JA; Barberis M; de Frutos O; Mateos C; García-Cerrada S; Agejas J; MacMillan DWC Rapid Optimization of Photoredox Reactions for Continuous-Flow Systems Using Microscale Batch Technology. ACS Cent. Sci 2021, 7, 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) A promising alternative to the generation of silicon waste is the use of amines as terminal reductants. This approach is less-well developed thus far. See: Duan Z; Li W; Lei A Nickel-Catalyzed Reductive Cross-Coupling of Aryl Bromides with Alkyl Bromides: Et3N as the Terminal Reductant. Org. Lett 2016, 18, 4012–4015. [DOI] [PubMed] [Google Scholar]; (b) Paul A; Smith MD; Vannucci AK Photoredox-Assisted Reductive Cross-Coupling: Mechanistic Insight into Catalytic Aryl–Alkyl Cross-Couplings. J. Org. Chem 2017, 82, 1996–2003. [DOI] [PubMed] [Google Scholar]; (c) Mou Z-D; Wang J-X; Zhang X; Niu D Stereoselective Preparation of C-Aryl Glycosides via Visible-Light-Induced Nickel-Catalyzed Reductive Cross-Coupling of Glycosyl Chlorides and Aryl Bromides. Adv. Synth. Catal 2021, 363, 3025–3029. [Google Scholar]; (d) Badir SO; Sim J; Billings K; Csakai A; Zhang X; Dong W; Molander GA Multifunctional Building Blocks Compatible with Photoredox-Mediated Alkylation for DNA-Encoded Library Synthesis. Org. Lett 2020, 22, 1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Anka-Lufford LL; Huihui KMM; Gower NJ; Ackerman LKG; Weix DJ Nickel-Catalyzed Cross-Electrophile Coupling with Organic Reductants in Non-Amide Solvents. Chem.–Eur. J 2016, 22, 11564–11567. [DOI] [PubMed] [Google Scholar]; (b) Suzuki N; Hofstra JL; Poremba KE; Reisman SE Nickel-Catalyzed Enantioselective Cross-Coupling of N-Hydroxyphthalimide Esters with Vinyl Bromides. Org. Lett 2017, 19, 2150–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Charboneau DJ; Barth EL; Hazari N; Uehling MR; Zultanski SL A Widely Applicable Dual Catalytic System for Cross-Electrophile Coupling Enabled by Mechanistic Studies. ACS Catal. 2020, 10, 12642–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Charboneau DJ; Huang H; Barth EL; Germe CC; Hazari N; Mercado BQ; Uehling MR; Zultanski SL Tunable and Practical Homogeneous Organic Reductants for Cross-Electrophile Coupling. J. Am. Chem. Soc 2021, 143, 21024–21036. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Charboneau DJ; Hazari N; Huang H; Uehling MR; Zultanski SL Homogeneous Organic Electron Donors in Nickel-Catalyzed Reductive Transformations. J. Org. Chem 2022, 87, 7589–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiberg N Tetraaminoethylenes as Strong Electron Donors. Angew. Chem. Int. Ed 1968, 7, 766–779. [Google Scholar]
- 20.Further advances in this area may be able to address these limitations. Recent advances in TDAE derivatives have addressed the moisture and air stability somewhat, but they are still relatively high cost, and the oxidized salts still suffer from low solubility. See references 18d and 18e.
- 21.(a) Budnikova Y Metal complex catalysis in organic electrosynthesis. Russ. Chem. Rev 2002, 71, 111–139. [Google Scholar]; (b) Yoshida J-i.; Kataoka K; Horcajada R; Nagaki A Modern Strategies in Electroorganic Synthesis. Chem. Rev 2008, 108, 2265–2299. [DOI] [PubMed] [Google Scholar]; (c) Yan M; Kawamata Y; Baran PS Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev 2017, 117, 13230–13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H; Breen CP; Seo H; Jamison TF; Fang Y-Q; Bio MM Ni-Catalyzed Electrochemical Decarboxylative C–C Couplings in Batch and Continuous Flow. Org. Lett 2018, 20, 1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pletcher D; Green RA; Brown RCD Flow Electrolysis Cells for the Synthetic Organic Chemistry Laboratory. Chem. Rev 2018, 118, 4573–4591. [DOI] [PubMed] [Google Scholar]
- 24.(a) Editors, W. V. A. L. H. A. G.: Fuel cell technology and applications; John Wiley and Sons Ltd: Chichester, England, 2003; Vol. 3. [Google Scholar]; (b) Healy J; Hayden C; Xie T; Olson K; Waldo R; Brundage M; Gasteiger H; Abbott J Aspects of the Chemical Degradation of PFSA Ionomers used in PEM Fuel Cells. Fuel Cells 2005, 5, 302–308. [Google Scholar]; (c) Kusoglu A; Weber AZ New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev 2017, 117, 987–1104. [DOI] [PubMed] [Google Scholar]
- 25.For example, reference 22 used 0.8 equiv of tetrabutylammonium hexafluorophosphate (MW = 387.4 g/mol) in the cathodic compartment, but to balance charge the final solution will contain 2.8 equiv of this salt.
- 26.(a) Yoo SJ; Li L-J; Zeng C-C; Little RD Polymeric Ionic Liquid and Carbon Black Composite as a Reusable Supporting Electrolyte: Modification of the Electrode Surface. Angew. Chem., Int. Ed 2015, 54, 3744–3747. [DOI] [PubMed] [Google Scholar]; (b) Herold S; Waldvogel SR; Little RD; Yoo SJ Applicability of a Polymerized Ionic Liquid/Carbon Nanoparticle Composite Electrolyte to Reductive Cyclization and Dimerization Reactions. Electrochim. Acta 2016, 196, 735–740. [Google Scholar]; (c) Broese T; Francke R Electrosynthesis Using a Recyclable Mediator–Electrolyte System Based on Ionically Tagged Phenyl Iodide and 1,1,1,3,3,3-Hexafluoroisopropanol. Org. Lett 2016, 18, 5896–5899. [DOI] [PubMed] [Google Scholar]
- 27.Li Z; Sun W; Wang X; Li L; Zhang Y; Li C Electrochemically Enabled, Nickel-Catalyzed Dehydroxylative Cross-Coupling of Alcohols with Aryl Halides. J. Am. Chem. Soc 2021, 143, 3536–3543. [DOI] [PubMed] [Google Scholar]
- 28.Zhu C; Yue H; Rueping M Nickel Catalyzed Multicomponent Stereodivergent Synthesis of Olefins Enabled by Electrochemistry, Photocatalysis and Photo-Electrochemistry. Nat Commun 2022, 13, 3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.For evidence that DIPEA is the terminal oxidant in these reactions, see Supporting Information page S35. Analysis of a large-scale reaction allowed isolation of i-Pr2NH salts that would be derived from DIPEA oxidation followed by hydrolysis and protonation in workup.
- 30.The total cost of goods for our flow setup, including specialized pumps, two flow cells, electrodes, and power supplies, is $6,254 without any special discounts. This is comparable to the cost of a single potentiostat for CV measurements. See Supporting Information Section 1.1 for details.
- 31.See Supporting Information Section 4.6 for large scale flow procedure.
- 32.Efficiency calculation for 1 m2 electrode surface area scale-up using values from Scheme 5F:
- 33.Reactions with no added electrolyte started out with a high voltage due to the poor conductivity of the solution, but as the reaction progressed (and salts were formed) the voltage decreased, and final yields were similar to those with electrolyte added.
- 34.The use of nickel salts is increasingly regulated in Europe, for example (REACH). https://echa.europa.eu/substance-information/-/substanceinfo/100.239.198 and https://echa.europa.eu/substance-information/-/substanceinfo/100.202.593 and https://echa.europa.eu/documents/10162/13641/nickel_bg_annex1_en.pdf/12d24cbf-8f7e-0f1f-64c3-4992df4d00e8 (accessed on June 9, 2022).
- 35.Heard DM; Lennox AJJ Electrode Materials in Modern Organic Electrochemistry. Angew. Chem., Int. Ed 2020, 59, 18866–18884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.(a) Everson DA; Shrestha R; Weix DJ Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2010, 132, 920–921; [DOI] [PubMed] [Google Scholar]; (b) Zhao Y; Weix DJ Nickel-Catalyzed Regiodivergent Opening of Epoxides with Aryl Halides: Co-Catalysis Controls Regioselectivity. J. Am. Chem. Soc 2014, 136, 48–51; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ackerman LKG; Anka-Lufford LL; Naodovic M; Weix DJ Cobalt co-catalysis for cross-electrophile coupling: diarylmethanes from benzyl mesylates and aryl halides. Chem. Sci 2015, 6, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(a) Multimetallic Catalysis in Organic Synthesis; Wiley VCH: Weinheim, 2004. [Google Scholar]; (b) Trost BM; Luan X Contemporaneous Dual Catalysis by Coupling Highly Transient Nucleophilic and Electrophilic Intermediates Generated in Situ. J. Am. Chem. Soc 2011, 133, 1706–1709. [DOI] [PubMed] [Google Scholar]; (c) Allen AE; MacMillan DWC Synergistic catalysis: A powerful synthetic strategy for new reaction development. Chem. Sci 2012, 3, 633–658. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Pérez-Temprano MH; Casares JA; Espinet P Bimetallic Catalysis using Transition and Group 11 Metals: An Emerging Tool for C–C Coupling and Other Reactions. Chem.–Eur. J 2012, 18, 1864–1884. [DOI] [PubMed] [Google Scholar]; (e) Kim UB; Jung DJ; Jeon HJ; Rathwell K; Lee S.-g. Synergistic Dual Transition Metal Catalysis. Chem. Rev 2020, 120, 13382–13433. [DOI] [PubMed] [Google Scholar]
- 38.(a) Everson DA; Jones BA; Weix DJ Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc 2012, 134, 6146–6159; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Johnson KA; Biswas S; Weix DJ Cross-Electrophile Coupling of Vinyl Halides with Alkyl Halides. Chem.–Eur. J 2016, 22, 7399–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishi and Umehara proposed completely separated radical generation and radical capture for a related combination of ligands. See: Umehara A; Kishi Y Further Studies on Ni/Zr-mediated One-pot Ketone Synthesis: Use of a Mixture of NiI- and NiII-catalysts Greatly Improves the Molar Ratio of Coupling Partners. Chem. Lett 2019, 48, 947–950. [Google Scholar]
- 40.Vanucci studied the reactivity of (tpy)Ni complexes in photoredox-assisted cross-electrophile coupling of aryl halides with alkyl halides and noted that (tpy)Ni0 and not (tpy)NiI is needed for oxidative addition of both Ar-X and Alkyl-X. Our electrochemical data is consistent with their proposed catalytic cycle. See: Paul A; Smith MD; Vannucci AK Photoredox-Assisted Reductive Cross-Coupling: Mechanistic Insight into Catalytic Aryl–Alkyl Cross-Couplings. J. Org. Chem 2017, 82, 1996–2003. [DOI] [PubMed] [Google Scholar]
- 41.At this time, we cannot conclusively determine the oxidation state from which oxidative addition occurs from the two nickel complexes. CV studies on the individual complexes, the mixtures of complexes, and the cell potential of a constant-current reaction suggest (L1)NiIBr and (L2)Ni0 are the most accessible. See Supporting Information pages S14 to S25 and S36 to S39. Studies on oxidative addition with various ligands and electrophiles are in progress and will be reported in due course.
- 42.Toste and Chang have reported on how spin delocalization in terpyridine nickel complexes leads to halogen atom transfer pathways that generate free alkyl radicals. See: Wuttig A; Derrick JS; Loipersberger M; Snider A; Head-Gordon M; Chang CJ; Toste FD Controlled Single-Electron Transfer via Metal–Ligand Cooperativity Drives Divergent Nickel-Electrocatalyzed Radical Pathways. J. Am. Chem. Soc 2021, 143, 6990–7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.While ligand exchange certainly is possible in this system (see Supporting Information pages S40-42), we do not currently think it plays an important role in this catalyst system because 1:1 [Ni]:[total L] is optimal, (L1)NiX2 is competent for product formation on its own, and there is a continuous, predictable variation in yield and side-product formation with varying ratios of the two catalysts. In Sevov’s study,7m where ligand exchanged was determined to be operative, a 1:2 [Ni]:[total L] was optimal and leaving out either ligand resulted in ≤5% product formation.
- 44.Hansen EC; Pedro DJ; Wotal AC; Gower NJ; Nelson JD; Caron S; Weix DJ New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem 2016, 8, 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.(a) Everson DA; Buonomo JA; Weix DJ Nickel-catalyzed cross-electrophile coupling of 2-chloropyridines with alkyl bromides. Synlett 2014, 25, 233–238; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hansen EC; Li C; Yang S; Pedro D; Weix DJ Coupling of Challenging Heteroaryl Halides with Alkyl Halides via Nickel-Catalyzed Cross-Electrophile Coupling. J. Org. Chem 2017, 82, 7085–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.(a) Everson DA; Jones BA; Weix DJ Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc 2012, 134 (14), 6146–6159. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Johnson KA; Biswas S; Weix DJ Cross-Electrophile Coupling of Vinyl Halides with Alkyl Halides. Chem.–Eur. J 2016, 22 (22), 7399–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.We have found that 4-picoline ($0.01/mmol) can be reliably converted into a mixture of 4,4’-dimethylbipyridine and 4,4’,4’’-trimethyl-2,2’:6’,2’’-terpyridine that can be separated by sublimation. The corresponding reactions with 4-tert-butylpyridine ($0.49/mmol) are not as reliable. Robo MT; Prinsell MR; Weix DJ 4,4′,4″-Trimethyl-2,2′:6′,2″-Terpyridine by Oxidative Coupling of 4-Picoline. J. Org. Chem 2014, 79, 10624–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Currently, 4,4′-di-tert-butyl-2,2′-bipyridine (L2) is $3.63/mmol, 4,4′,4′′-tri-tert-butyl-2,2′:6′,2′′-terpyridine (L1) is $65-84/mmol, and 4,4’-dimethylbipyridine (L4) is $0.34/mmol. 4,4’,4’’-Trimethyl-2,2’:6’,2’’-terpyridine (L3) is $30/mmol (Strem), but we make both the bipyridine and the terpyridine in one step from picoline.47 Data sources from REAXYS chemical source database. https://www.reaxys.com/ (accessed on June 20, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.