Abstract
Background
Fetal growth is dependent on placental nutrient supply, which is influenced by placental perfusion and transporter abundance. Previous research indicates that adequate choline nutrition during pregnancy improves placental vascular development, supporting the hypothesis that choline may affect placental nutrient transport.
Objective
The present study sought to determine the impact of maternal choline supplementation (MCS) on placental nutrient transporter abundance and nutrient metabolism during late gestation.
Methods
Female non-Swiss albino mice were randomly assigned to the 1×, 2×, or 4× choline diet (1.4, 2.8, and 5.6 g choline chloride/kg diet, respectively) 5 d before mating (n = 16 dams/group). The placentas and fetuses were harvested on gestational day (E) 15.5 and E18.5. The placental abundance of macronutrient, choline, and acetylcholine transporters and glycogen metabolic enzymes, and the placental concentration of glycogen were quantified. Choline metabolites and docosahexaenoic acid (DHA) concentrations were measured in the placentas and/or fetal brains. Data were stratified by gestational day and fetal sex and were analyzed by using mixed linear models.
Results
At E15.5, MCS downregulated the placental transcript and protein abundance of glucose transporter 1 (GLUT1) (−40% to −73%, P < 0.05) and the placental transcript abundance of glycogen-synthesizing enzymes (−24% to −50%, P ≤ 0.05). At E18.5, MCS upregulated GLUT3 protein abundance (+55%, P = 0.016) and the transcript abundance of glycogen-synthesizing enzymes only in the female placentas (+36% to +60%, P < 0.05), resulting in a doubling (P = 0.01) of the glycogen concentration. A higher placental transcript abundance of the transporters for DHA, choline, and acetylcholine was also detected in response to MCS, consequently altering their concentrations in the placentas or fetal brains (P ≤ 0.05).
Conclusions
These data suggest that MCS modulates placental nutrient transporter abundance and nutrient metabolism in late gestation of mouse pregnancy, with subsequent effects on nutrient supply for the developing fetus.
Key words: choline, pregnancy, placenta, macronutrient transporter, glycogen metabolism, acetylcholine, fetal sex
Introduction
The theory of fetal programming posits that the intrauterine environment plays a key role in determining offspring health later in life. By functioning as a nutrient sensor, the placenta is positioned to play an integral role in fetal programming because it actively modifies processes involved in uteroplacental perfusion, placental nutrient transport, and metabolism to modulate the efficiency by which nutrients are transported to the fetus (1). Numerous studies have reported aberrant expression and activity of placental macronutrient transporters and metabolic enzymes in preeclampsia (2, 3, 4), intrauterine growth restriction (5, 6), maternal obesity (7, 8) and gestational diabetes (9)—all of which have adverse consequences on fetal development.
Amino acids are essential for normal fetal growth because they are used in protein synthesis, energy production, and signaling pathways (10). The placental system A amino acid transporters (SNATs) are responsible for the uptake of nonessential amino acids, such as glycine, which can subsequently be used in exchange for the uptake of essential amino acids, such as leucine, via other transporters (1). Therefore, SNATs are important for transporting both nonessential and essential amino acids. The major placental SNATs are SLC38A1 (SNAT1), SLC38A2 (SNAT2), and SLC38A4 (SNAT4) (1).
Long-chain PUFAs (LCPUFAs) are also needed for fetal development. Not only do they provide energy, but they are also components of cell membranes and precursors to signaling molecules, such as eicosanoids (1, 11). A sufficient supply of LCPUFAs, particularly DHA, is critical for the normal development of the fetal nervous system and has been correlated with better cognitive outcomes in postnatal life (11, 12). However, both the placenta and fetus have minimal enzymatic activity to generate LCPUFAs. Therefore, placental transfer of LCPUFAs from the maternal circulation is the major source of fetal LCPUFAs (12). Placentas have several FA transporters (FATPs), but a study conducted in healthy pregnant women indicates that only SLC27A1 (FATP1) and SLC27A4 (FATP4) are correlated with placental and fetal DHA concentrations (13).
Similar to FAs, the fetus has limited ability to synthesize glucose, which is the major energy substrate used in fetal metabolism. Consequently, the fetus relies on the placenta to transport glucose from the maternal circulation (1). Two major glucose transporters (GLUTs) are found in the placenta: SLC2A1 (GLUT1) and SLC2A3 (GLUT3). Placental trophoblasts also store a large amount of glycogen (14) and express major enzymes in the glycogen metabolic pathway (3, 15, 16). As such, placental glycogen can be mobilized to provide glucose to the developing fetus at times when fetal metabolic demand exceeds maternal supply (14).
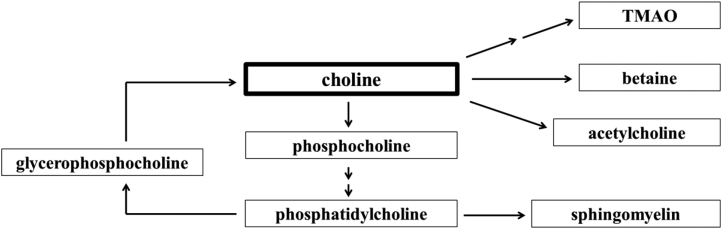
Choline is a bioactive micronutrient often grouped with the B vitamins. Placental transport of choline from the maternal circulation is largely mediated by the choline transporter-like protein 1 (CTL1) (17). Within the placenta, choline and its metabolites (Figure 1) perform many functions essential for normal development. For example, acetylcholine, the acetylated form of choline, regulates amino acid transport (18). Acetylcholine is also delivered to the fetus by placental organic cation transporter 3 (OCT3) (19, 20), where it affects fetal brain development and function.
FIGURE 1.
Choline metabolic pathways. TMAO, trimethylamine N–oxide.
Notably, the abundance of placental nutrient transporters and metabolic enzymes is often altered in pregnancy disorders characterized by abnormal placental vascularization and perfusion (2, 3, 4, 5, 6), possibly serving as a compensatory response to maintain an adequate nutrient supply to the fetus. Because we have shown that maternal choline supplementation (MCS) during pregnancy improves placental vascular development in mice (21), factors that mediate placental nutrient delivery and metabolism may also be affected. As such, the current study was conducted to provide insights into the effect of MCS on placental nutrient transporter abundance and placental nutrient metabolism during late gestation of mouse pregnancy.
Methods
Animals and diets
All animal protocols and procedures used in this study were approved by the Institutional Animal Care and Use Committees at Cornell University and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Adult male and female non-Swiss albino mice were obtained from Harlan and used for breeding. The offspring of the breeding pairs were weaned when they were 3 wk old and provided ad libitum access to water and the 1× choline diet. The 1× choline diet contains the recommended amounts of nutrients by the AIN (22), except for the replacement of choline bitartrate with an equivalent amount of choline chloride (1.4 g choline chloride/kg diet) (Supplemental Table 1; Dyet 103345; Dyets). Five days before mating, female mice were randomly assigned to the 1× choline diet, 2× choline diet, or 4× choline diet (n = 16 dams/diet). The 2× choline and 4× choline diets are identical to the 1× choline diet except that they contain 2.8 and 5.6 g choline chloride/kg diet, respectively (Supplemental Table 1; Dyet 103346 and 103347). These dosages were based on previous studies indicating improved vascular development in the human placenta with 2× choline supplementation (23) and improved brain development in the mouse offspring with 4× choline supplementation (24). Presence of a vaginal plug indicated conception and was designated as gestational day (E) 0.5. The female mice continued to consume their assigned diet until they were euthanized at E15.5 or E18.5. These late gestational time points represent periods of rapid growth and increased fetal nutrient demand.
Tissue collection and processing
Both the placentas and fetuses were collected. The placentas were weighed, cut in half, and placed in RNAlater (Invitrogen) or flash-frozen in liquid nitrogen. The fetuses were weighed and flash-frozen in liquid nitrogen. All tissues were stored at −80°C until analytical measurements were performed. One male and 1 female placenta and fetus from each dam in each treatment group at each gestational time point were used for each of the following measurements.
Analytical measurements
Sex genotyping
DNA was extracted from the fetuses, and PCR was performed with the use of a commercial kit (catalog no. 206152; Qiagen) to determine fetal sex (Supplemental Table 2).
mRNA abundance of placental transporters and enzymes
Placental RNA extraction, reverse transcription, and RT-qPCR assays were performed as described previously (21). Genes of interest include Snat1, Snat2, Snat4, Fatp1, Fatp4, Glut1, Glut3, glycogen synthase 1 (Gys1), glycogen branching enzyme 1 (Gbe1), glycogen phosphorylase, muscle (Pygm), glycogen synthase kinase 3 β (Gsk3β), Ctl1, and Oct3. TATA box–binding protein (Tbp) was used as the housekeeping gene because its expression is stable in placental tissue (25) and under different choline intake amounts (26). Data are expressed by the ΔΔCt method where the expression of the targeted gene is normalized by the expression of Tbp as fold change before comparison between samples. All primers were designed by using Primer-BLAST available on the National Center for Biotechnology Information website (Supplemental Table 2).
Transporter protein abundance in the placental membrane
Placental tissues were homogenized in 10 volumes of Buffer A [50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 1 mM EDTA, and protease inhibitor cocktails (Sigma-Aldrich)] and centrifuged at 800 × g for 5 min at 4°C. The supernatant was then centrifuged again at 17,000 × g for 15 min at 4°C. After centrifugation, the pellet, which contained the membrane-bound proteins, was resuspended in 10 volumes of Buffer B [50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 1 mM EDTA, 2% IPEGAL CA-630 (Santa Cruz Biotechnology), and protease inhibitor cocktails (Sigma-Aldrich)]. Total membrane protein concentration was determined by the Bradford assay (Thermo Scientific Pierce). Forty micrograms of the extracts was subjected to SDS-PAGE electrophoresis and transferred onto Immobilon-FL polyvinylidene difluoride membranes (EMD Millipore). Membranes were blocked in LI-COR blocking buffer and then incubated overnight with primary antibodies for GLUT1 (catalog no. 21829–1, 1:200; ProteinTech), GLUT3 (catalog no. AB1344, 1:1000; EMD Millipore), and β-actin (catalog no. 3700, 1:5000; Cell Signaling Technology). Secondary antibodies [IRDye 800CW goat anti-rabbit (catalog no. 925–32211, 1:10,000; LI-COR) and IRDye 680RD goat anti-mouse (catalog no. 925–68070, 1:10,000; LI-COR)] were added to the membranes and incubated for 1 h. Protein bands were visualized and quantified by the Odyssey imaging system (LI-COR). Data are expressed as the ratio of the intensity of targeted protein to the intensity of β-actin before comparison between samples.
Glycogen concentration in the placenta
The placental glycogen concentration was quantified by using a commercial glycogen assay kit (catalog no. KA0861; Abnova) following the manufacturer's protocol. Data are expressed as mg glycogen/g tissue.
Placental concentration of choline metabolites
The concentrations of choline, betaine, phosphocholine, phosphatidylcholine, glycerophosphocholine, and sphingomyelin in the placentas were determined by using LC/MS-MS according to Koc et al. (27). Placental acetylcholine and trimethylamine N–oxide (TMAO) concentrations were determined by using LC/MS-MS according to Holm et al. (28) with modifications based on our equipment (29).
Concentration of choline metabolites in the fetal brain
The choline, betaine, acetylcholine, phosphocholine, phosphatidylcholine, and glycerophosphocholine concentrations in the fetal brain were determined by using LC/MS-MS according to Koc et al. (27).
Concentration of DHA in the fetal brain
Fetal brain DHA analysis was performed by using GC-MS as described previously (30). The DHA concentration was directly calibrated to the internal standard, heptadecanoic acid (17:0), and expressed as mg DHA/mg tissue.
Statistical analysis
All data were stratified by gestational day and fetal sex and then analyzed by using a mixed linear model followed by post hoc Fisher's least significant difference test. Choline treatment was included as an independent fixed effect, maternal identification as an independent random effect, and litter size as a covariate if it had a P value ≤0.05. All statistical analyses were performed by using SPSS software, version 23 (SPSS, Inc.). For the mRNA and protein abundance data, the mean value of 1× choline group for each sex was assigned a value of 1 after normalization, and the mean values of the other choline groups were presented as fractions of this value. All data represent means ± SEMs, and differences were considered statistically significant at P ≤ 0.05.
Results
Placental amino acid transporters
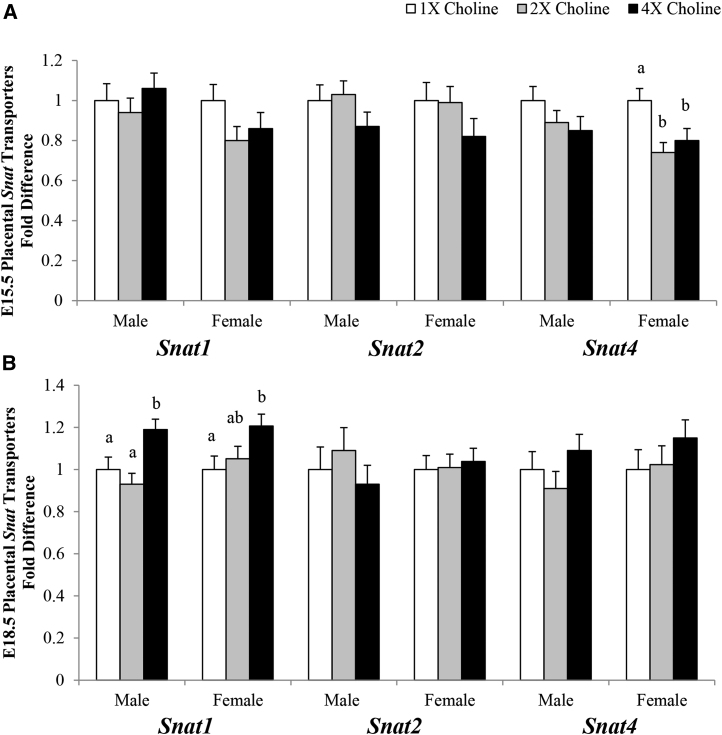
At E15.5, in comparison with the 1× choline group, a lower transcript abundance of Snat4 was observed in the female placentas of the 2× (−26%; P = 0.005) and 4× (−20%; P = 0.025) choline groups, which did not differ from each other. At E18.5, a higher transcript abundance of Snat1 was detected in the male placentas from the 4× choline group compared with the 1× (+19%; P = 0.023) and 2× (+28%; P = 0.002) choline groups, which did not differ from each other. A higher abundance (+21%; P = 0.028) of Snat1 was also detected at E18.5 in the female placentas of the 4× choline group compared with the 1× choline group, whereas no differences were observed when comparing the 2× with the 1× choline group or the 4× with the 2× choline group. The placental Snat2 transcript abundance was unaffected by MCS (Figure 2).
FIGURE 2.
Transcript abundance of the amino acid transporters in the placentas from dams in the 1×, 2×, and 4× choline groups at (A) E15.5 and (B) E18.5. Values are given as means ± SEMs, n = 6–7 dams/group for E15.5, and n = 7–8 dams/group for E18.5. Means without a common letter differ, P ≤ 0.05. E, gestational day; Snat, system A amino acid transporter.
Placental FA transporters
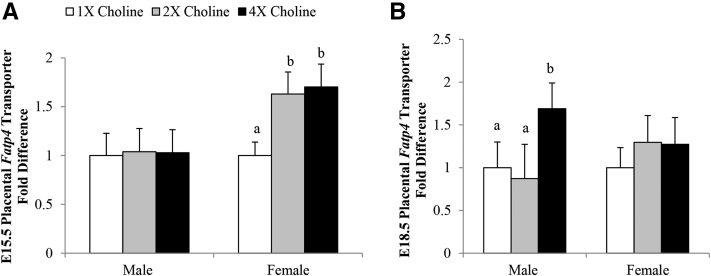
At E15.5, in comparison with the 1× choline group, a higher transcript abundance of Fatp4 was observed in the female placentas of both the 2× (+63%; P = 0.017) and 4× (+70%; P = 0.011) choline groups, which did not differ from each other. At E18.5, a higher transcript abundance of Fatp4 was detected in the male placentas from the 4× choline group than in those from the 1× (+69%; P = 0.05) and 2× (+93%; P = 0.035) choline groups, which did not differ from each other (Figure 3). The placental Fatp1 transcript abundance was unaffected by MCS (data not shown).
FIGURE 3.
Transcript abundance of the FA transporter, Fatp4, in the placentas from dams in the 1×, 2×, and 4× choline groups at (A) E15.5 and (B) E18.5. Values are given as means ± SEMs, n = 6 dams/group for each gestational day. Means without a common letter differ, P ≤ 0.05. E, gestational day; Fatp4, FA transporter 4.
Placental glucose transporters
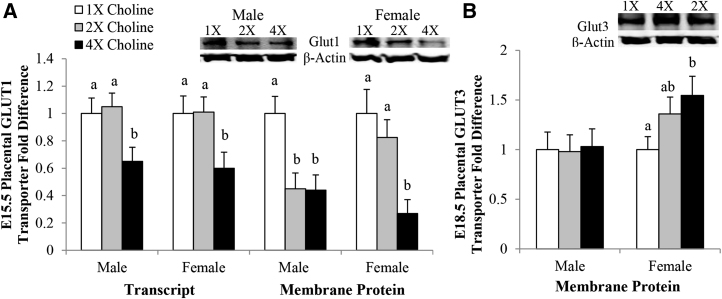
At E15.5, an ∼40% lower Glut1 transcript abundance was detected in the male and female placentas of the 4× choline group than in those of the 1× (P = 0.028 and 0.038, respectively) and 2× (P = 0.008 and 0.024, respectively) choline groups, which did not differ from each other. In comparison with the 1× choline group, 55% fewer GLUT1 transporter proteins were detected in the 4× (P = 0.041) and 2× (P = 0.043) choline groups of the male placentas, which did not differ from each other. The GLUT1 protein abundance in the membrane of the female placentas from the 4× choline group was also lower than those from the 1× (−73%; P = 0.023) and 2× (−67%; P = 0.039) choline groups, whereas no differences were detected between the 2× and 1× choline groups (Figure 4A). At E18.5, the placental transcript and protein abundance of GLUT1 was unaffected by MCS (data not shown).
FIGURE 4.
Transcript and membrane protein abundance of GLUT1 in the placentas from dams in the 1×, 2×, and 4× choline groups at E15.5 (A). Membrane protein abundance of GLUT3 in the placentas from dams in the 1×, 2×, and 4× choline groups at E18.5 (B). Values are given as means ± SEMs, n = 6–7 dams/group for each gestational day. Means without a common letter differ, P ≤ 0.05. E, gestational day; GLUT, glucose transporter.
At E15.5, the placental GLUT3 transcript and protein expression did not differ among the choline groups (data not shown). Although no changes in the Glut3 transcript abundance were detected at E18.5, a 55% higher GLUT3 protein abundance (P = 0.016) was found in the membrane of the female placentas from the 4× choline group than in those from the 1× choline group. There were no detectable differences in the GLUT3 protein abundance between the 2× and 1× choline groups or between the 4× and 2× choline groups (Figure 4B). No similar changes were observed in the male placentas at this time point.
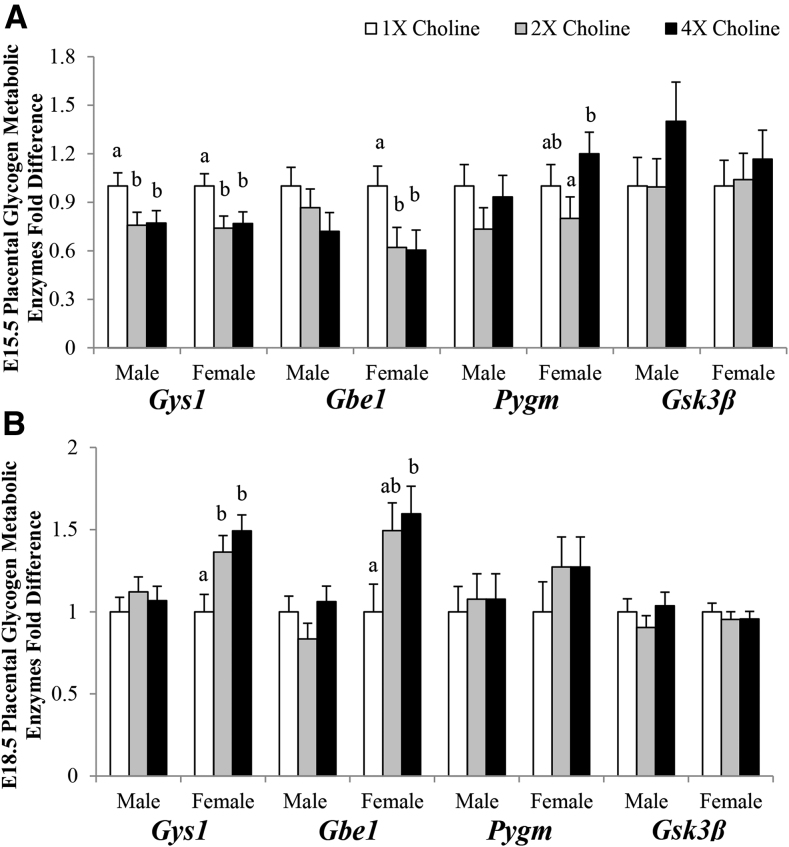
Placental glycogen metabolic enzymes
At E15.5, in comparison with the 1× choline group, a 24% lower transcript expression of the glycogen-synthesizing enzyme Gys1 was found in the male placentas from both the 4× (P = 0.05) and 2× (P = 0.046) choline groups, which did not differ from each other. Moreover, in comparison with the 1× choline group, a 25% lower transcript expression of Gys1 and a 40% lower transcript expression of Gbe1 were found in the female placentas from both 4× (P = 0.04 and P = 0.04, respectively) and 2× (P = 0.024 and P = 0.047, respectively) choline groups, which did not differ from each other. The transcript expression of Pygm, an enzyme involved in glycogen breakdown, was higher (+50%; P = 0.027) only when comparing the 4× with the 2× choline group. No difference in the placental transcript expression of Gsk3β was detected in response to MCS (Figure 5A).
FIGURE 5.
Transcript abundance of the enzymes involved in glycogen metabolism in the placentas from dams in the 1×, 2×, and 4× choline groups at (A) E15.5 and (B) E18.5. Values are given as means ± SEMs, n = 8 dams/group for each gestational day for Gys1, Pygm, and Gsk3β; n = 6 dams/group for each gestational day for Gbe1. Means without a common letter differ, P ≤ 0.05. E, gestational day; Gbe1, glycogen branching enzyme 1; Gsk3β, glycogen synthase kinase 3 β Gys1, glycogen synthase 1; Pygm, glycogen phosphorylase, muscle.
At E18.5, in comparison with the 1× choline group, the Gys1 transcript abundance was higher in female placentas of the 4× (+49%; P = 0.004) and 2× (+36%; P = 0.025) choline groups, which did not differ from each other. The placental transcript abundance of Gbe1 was also higher (+60%; P = 0.024) in response to 4× compared with 1× choline, but no differences were observed when comparing 2× with 1× choline or 4× with 2× choline. MCS had no impact on the transcript expression of these enzymes in the male placentas or on the placental Pygm and Gsk3β transcript abundance in either sex at E18.5 (Figure 5B).
Placental glycogen concentration
At E15.5, a trend for a reduced glycogen concentration in the female placentas was noted in response to MCS, but it did not reach statistical significance (P ≥ 0.06). At E18.5, the glycogen concentration doubled (P = 0.01) in the female placentas of the 4× compared with the 1× choline group. Borderline higher (P = 0.051) concentrations of glycogen were also detected in the female placentas from the 2× choline group than in those in the 1× choline group. No significant difference in the glycogen concentration in the female placentas was found between the 4× and 2× choline groups. The glycogen concentration in the male placentas remained largely unaffected by MCS at either time point (Table 1).
TABLE 1.
Effect of maternal choline supplementation on placental glycogen concentration at E15.5 and E18.51
| Glycogen, mg/g tissue | Male | Female | ||||
|---|---|---|---|---|---|---|
| 1× choline | 2× choline | 4× choline | 1× choline | 2× choline | 4× choline | |
| E15.5 | 19.2 ± 2.6a | 17.1 ± 2.2a | 15.8 ± 2.6a | 19.1 ± 2.0a | 13.9 ± 1.9a | 14.4 ± 2.0a |
| E18.5 | 9.8 ± 2.8a | 8.9 ± 2.8a | 10.4 ± 2.8a | 6.0 ± 2.0a | 10.9 ± 2.0a,b | 12.7 ± 2.0b |
Values are means ± SEMs, n = 5 dams/group at E15.5 and n = 6 dams/group at E18.5. Means in a row for the same fetal sex without a common superscript letter differ, P ≤ 0.05. E, gestational day.
Placental choline transporters and choline metabolites concentration
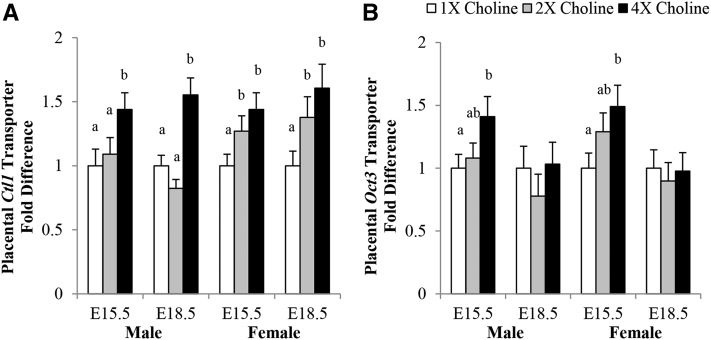
In comparison with the 1× and 2× choline groups, male placentas from 4× choline group had a higher Ctl1 transcript abundance at E15.5 (+44% and +32%; P = 0.036 and P = 0.05, respectively) and E18.5 (+55% and +88%; P = 0.002 and P < 0.001, respectively). No differences were found between the 2× and 1× choline groups at either time point. The female placentas of the 4× and 2× choline (compared with 1×) choline groups had a higher Ctl1 transcript abundance at E15.5 (+44% and +27%; P = 0.015 and P = 0.055, respectively) and E18.5 (+61% and +38%; P = 0.007 and P = 0.049, respectively), but no differences were found between the 4× and 2× choline groups at either gestational day (Figure 6A). The Oct3 transcript abundance in both male (P = 0.038) and female (P = 0.023) placentas of the 4× choline group was nearly 50% higher compared with the 1× choline group, whereas no differences were found between the 2× and 1× choline group or the 4× and 2× choline group (Figure 6B).
FIGURE 6.
Transcript abundance of (A) Ctl1 and (B) Oct3 in the placentas from dams in the 1×, 2×, and 4× choline groups at E15.5 and E18.5. Values are given as means ± SEMs, n = 6 dams/group for each gestational day. Means without a common letter differ, P ≤ 0.05. Ctl1, choline transporter-like protein 1; E, gestational day; Oct3, organic cation transporter 3.
In the male placentas at E15.5, the acetylcholine concentration was lower (−57% to −70%; P < 0.001) whereas the TMAO concentration was higher (+151% to +980%; P < 0.001) in response to 4× choline compared with 1× and 2× choline. Although no differences in the placental acetylcholine concentration were observed between the 2× and 1× choline groups, the placental TMAO concentration was 330% higher (P = 0.007) in the 2× choline group. At E18.5, the male placentas of the 4× (compared with the 1×) choline group had a higher concentration of acetylcholine (+533%; P < 0.001) and TMAO (+971%; P < 0.001) but lower concentrations of choline (−25%; P = 0.032) and phosphocholine (−22%; P = 0.028). The male placentas of the 2× (compared with the 1×) choline group also had higher acetylcholine (+150%; P = 0.034) and TMAO (+300%; P = 0.025) concentrations and a lower phosphocholine concentration (−26%; P = 0.01). Higher acetylcholine (+153%; P = 0.029) and TMAO (+168%; P < 0.001) concentrations were also detected in the male placentas of the 4× (compared with the 2×) choline group. MCS did not affect the concentrations of phosphatidylcholine, glycerophosphocholine, sphingomyelin, and betaine at either study time point (Table 2).
TABLE 2.
Effect of maternal choline supplementation on the concentration of choline metabolites in the male placentas at E15.5 and E18.51
| E15.5 | E18.5 | |||||
|---|---|---|---|---|---|---|
| 1× choline | 2× choline | 4× choline | 1× choline | 2× choline | 4× choline | |
| Choline, μmol/g tissue | 0.93 ± 0.06a | 1.02 ± 0.06a | 1.02 ± 0.06a | 1.14 ± 0.09a | 1.05 ± 0.09a,b | 0.85 ± 0.09b |
| Acetylcholine, pmol/g tissue | 97 ± 21a | 67 ± 14a | 29 ± 15b | 6 ± 3a | 15 ± 6b | 38 ± 8c |
| TMAO, nmol/g tissue | 10 ± 7a | 43 ± 7b | 108 ± 7c | 14 ± 12a | 56 ± 12b | 150 ± 12c |
| Phosphocholine, nmol/g tissue | 541 ± 24a | 564 ± 24a | 591 ± 24a | 382 ± 25a | 281 ± 25b | 299 ± 25b |
| Phosphatidylcholine, μmol/g tissue | 24 ± 1a | 25 ± 1a | 25 ± 1a | 20 ± 1a | 20 ± 1a | 21 ± 1a |
| Glycerophosphocholine, nmol/g tissue | 93 ± 20a | 114 ± 20a | 128 ± 20a | 183 ± 36a | 97 ± 36a | 156 ± 36a |
| Sphingomyelin, μmol/g tissue | 7.76 ± 0.30a | 8.08 ± 0.30a | 8.32 ± 0.30a | 6.36 ± 0.34a | 6.71 ± 0.34a | 6.70 ± 0.34a |
| Betaine, μmol/g tissue | 7.19 ± 0.40a | 6.68 ± 0.40a | 6.66 ± 0.40a | 7.01 ± 0.68a | 6.08 ± 0.68a | 5.81 ± 0.68a |
Values are means ± SEMs, n = 7 dams/group for each gestational day. Means in a row for the same gestational day without a common superscript letter differ, P ≤ 0.05. E, gestational day; TMAO, trimethylamine N–oxide.
In the female placentas at E15.5, the acetylcholine concentration was lower (−75%; P < 0.001) whereas concentrations of TMAO (+1700%; P < 0.001) and glycerophosphocholine (+93%; P = 0.02) were higher in response to 4× compared with 1× choline. The placental TMAO concentration was also higher (+186%; P < 0.001) when comparing 4× with 2× choline. Similarly, a lower placental acetylcholine concentration (−69%; P = 0.034) and higher placental TMAO concentration (+529%; P = 0.003) were detected when comparing 2× with 1× choline. At E18.5, female placentas from the 4× and 2× (compared with the 1×) choline groups had higher concentrations of acetylcholine (+529% and +100%; P < 0.001 and P = 0.032, respectively), TMAO (+694% and +235%; P < 0.001 and P = 0.009, respectively), phosphocholine (+26% and +23%; P = 0.008 and P = 0.015, respectively), and phosphatidylcholine (+21% and +16%; P < 0.001 and P = 0.006, respectively). When comparing the 4× with 2× choline group, higher placental acetylcholine (+214%; P < 0.001) and TMAO (+137%; P < 0.001) concentrations were observed. The placental concentrations of choline, sphingomyelin, and betaine were unaffected by MCS at either time point (Table 3).
TABLE 3.
Effect of maternal choline supplementation on the concentration of choline metabolites in the female placentas at E15.5 and E18.51
| E15.5 | E18.5 | |||||
|---|---|---|---|---|---|---|
| 1× choline | 2× choline | 4× choline | 1× choline | 2× choline | 4× choline | |
| Choline, μmol/g tissue | 1.30 ± 0.12a | 1.07 ± 0.12a | 1.12 ± 0.12a | 1.33 ± 0.09a | 1.13 ± 0.09a | 1.18 ± 0.09a |
| Acetylcholine, pmol/g tissue | 89 ± 18a | 28 ± 17b | 22 ± 13b | 7 ± 3a | 14 ± 4b | 44 ± 7c |
| TMAO, nmol/g tissue | 7 ± 7a | 44 ± 7b | 126 ± 7c | 17 ± 10a | 57 ± 10b | 135 ± 10c |
| Phosphocholine, nmol/g tissue | 525 ± 40a | 554 ± 40a | 600 ± 40a | 248 ± 15a | 306 ± 15b | 312 ± 15b |
| Phosphatidylcholine, μmol/g tissue | 23 ± 1a | 24 ± 1a | 25 ± 1a | 19 ± 1a | 22 ± 1b | 23 ± 1b |
| Glycerophosphocholine, nmol/g tissue | 84 ± 22a | 133 ± 21a,b | 162 ± 21b | 153 ± 29a | 123 ± 29a | 199 ± 29a |
| Sphingomyelin, μmol/g tissue | 8.03 ± 0.47a | 7.90 ± 0.46a | 8.07 ± 0.46a | 6.94 ± 0.33a | 6.71 ± 0.33a | 7.61 ± 0.33a |
| Betaine, μmol/g tissue | 6.38 ± 0.56a | 6.22 ± 0.56a | 6.66 ± 0.56a | 6.55 ± 0.45a | 7.05 ± 0.45a | 5.71 ± 0.45a |
Values are means ± SEMs, n = 7 dams/group for each gestational day. Means in a row for the same gestational day without a common superscript letter differ, P ≤ 0.05. E, gestational day; TMAO, trimethylamine N–oxide.
Placental and fetal weights
The number of viable offspring did not differ between the choline treatment groups at either gestational day (data not shown). Although no difference was detected in the placental weight, a trend (P = 0.07) for a higher weight at E18.5 was detected in the female fetuses from the 4× choline group compared with those from the 1× choline group. There were no differences in the weight of the female fetuses and their placentas when comparing the 2× and 1× choline groups or the 4× and 2× choline groups at this time point. The weights of the male fetuses and their placentas were also unaffected by MCS at both gestational days (Table 4).
TABLE 4.
Effect of maternal choline supplementation on fetal and placental weights at E15.5 and E18.51
| Male | Female | |||||
|---|---|---|---|---|---|---|
| 1× choline | 2× choline | 4× choline | 1× choline | 2× choline | 4× choline | |
| Weight on E15.5, g | ||||||
| Fetus | 0.39 ± 0.02a | 0.39 ± 0.01a | 0.41 ± 0.02a | 0.39 ± 0.01a | 0.39 ± 0.01a | 0.41 ± 0.02a |
| Placenta | 0.09 ± 0.004a | 0.08 ± 0.004a | 0.08 ± 0.004a | 0.08 ± 0.004a | 0.08 ± 0.004a | 0.08 ± 0.005a |
| Weight on E18.5, g | ||||||
| Fetus | 1.18 ± 0.03a | 1.25 ± 0.03a | 1.21 ± 0.03a | 1.13 ± 0.03a | 1.17 ± 0.03a,b | 1.20 ± 0.03(b) |
| Placenta | 0.09 ± 0.005a | 0.09 ± 0.005a | 0.09 ± 0.005a | 0.08 ± 0.004a | 0.09 ± 0.004a | 0.09 ± 0.004a |
Values are means ± SEMs. Averages of all placentas or fetuses of the same sex from each dam (n = 8/group for each gestational day) were determined and used in the analysis. Means in a row for the same fetal sex without a common superscript letter differ, P ≤ 0.05. Means in a row for the same fetal sex with a superscript letter in parentheses indicate P = 0.07. E, gestational day.
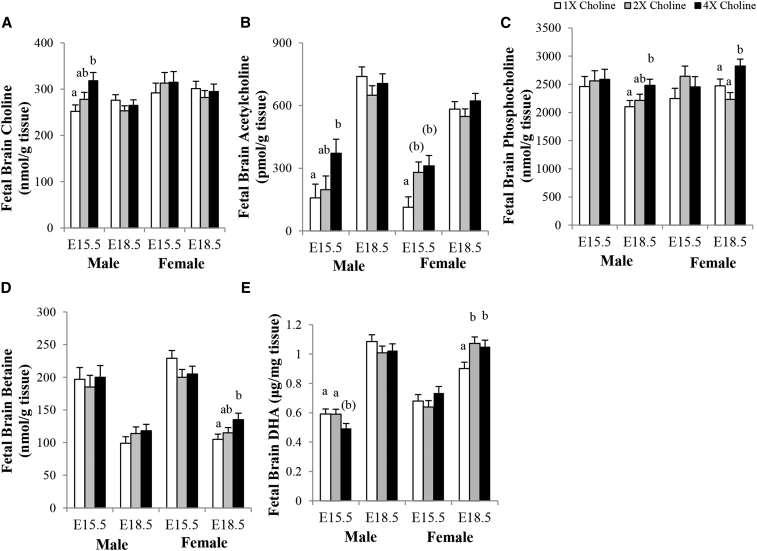
Choline metabolites in the fetal brain
At E15.5, the brains of the male fetuses from the 4× (compared with the 1×) choline group had higher concentrations of choline (+26%; P = 0.007; Figure 7A) and acetylcholine (+135%; P = 0.021; Figure 7B), but no differences were observed when comparing the 2× and 1× choline groups or the 4× and 2× choline groups. In comparison with the 1× choline group, the brain acetylcholine concentration also tended to be higher for female fetuses of the 4× (+175%; P = 0.052) and 2× (+148%; P = 0.08) choline groups, which did not differ from each other. At E18.5, the male fetuses from the 4× (compared with the 1×) choline group had a higher brain phosphocholine concentration (+18%; P = 0.026) but not when comparing the 2× and 1× choline groups or the 4× and 2× choline groups. The female fetuses of the 4× choline group also had more phosphocholine in their brains than in those from the 1× (+14%; P = 0.05) and 2× (+27%; P = 0.003) choline groups, which did not differ from each other (Figure 7C). The betaine concentration in the brains of the female fetuses from the 4× (compared with the 1×) choline group at E18.5 was 29% higher (P = 0.018) but not when comparing the 2× and 1× choline groups or the 4× and 2× choline groups (Figure 7D). MCS did not affect the brain concentrations of phosphatidylcholine and glycerophosphocholine in the male and female fetuses at either study time point (data not shown).
FIGURE 7.
Concentrations of (A) choline, (B) acetylcholine, (C) phosphocholine, (D) betaine, and (E) DHA in the brains of fetuses whose mothers were in the 1×, 2×, and 4× choline groups at E15.5 and E18.5. Values are given as means ± SEMs, n = 7 dams/group for each gestational day. Means without a common letter differ, P ≤ 0.05. Means with a letter in parentheses indicate 0.05 < P < 0.1. E, gestational day.
DHA concentration in the fetal brain
Because the placental Fatp4 transcript expression was altered by maternal choline treatments, we measured the concentration of DHA in the fetal brain. The DHA concentration in the male fetal brains tended to be lower at E15.5 in the 4× compared with the 1× (P = 0.054) and 2× (P = 0.056) choline groups, which did not differ from each other. These differences were not detected at E18.5 among the choline groups. In comparison with 1× choline, the DHA concentration in the female fetal brains at E18.5 was significantly higher in response to 4× (+17%; P = 0.028) and 2× (+19%; P = 0.013) choline, which did not differ from each other (Figure 7E). Similar results were obtained with the inclusion of fetal brain weight as a covariate in the statistical model (data not shown).
Discussion
In the present study, we demonstrate effects of supplementing the maternal diet with additional choline before conception on the placental abundance of nutrient transporters and placental nutrient metabolism (i.e., glucose and choline) during late gestation of mouse pregnancy. Differences were noted between the 2× and 1× choline groups for some outcome variables but were more frequently detected between the 4× and 1× choline groups. As such, our discussion focuses on the effects of 4× choline supplementation.
MCS alters the placental abundance of macronutrient transporters and the placental metabolism of glucose
Supplementing the maternal diet with additional choline decreased the number of Glut1 transporters at E15.5 in both male and female placentas. A significant reduction in Snat4, an amino acid transporter, was also detected in female placentas at E15.5. Importantly, however, no adverse effects of extra choline were detected on placental or fetal weight at this time point, suggesting that another source of glucose, such as placental glycogen, was used to meet fetal glucose demands. Indeed, we found that MCS downregulated the expression of glycogen-synthesizing enzymes Gys1 in both male and female placentas and Gbe1 in female placentas, whereas an upregulated expression of the glycogen-degrading enzyme Pygm was detected in female placentas. Although not statistically significant (P ≥ 0.06), we also detected a numerically lower glycogen concentration in the female placentas, indicating that placental glycogen was being broken down to maintain a constant glucose supply to the fetus. The mechanism by which choline reduced Glut1 and Snat4 abundance is unclear but may be a secondary response to enhanced placental perfusion (which would enhance nutrient delivery) in the choline-supplemented dams. In this regard, we previously reported that MCS significantly increased the luminal area of the maternal spiral arteries in mice fed a diet containing the same choline concentration (21). The subsequent changes in glycogen metabolism are consistent with the tendency for regulatory systems to temporarily overshoot or overcompensate to maintain homeostasis (31) and to increase body capability to deal with more difficult metabolic challenges in the future (32).
At E18.5, MCS upregulated the Snat1 transporter in both male and female placentas, increasing the availability of substrates, such as glycine, which can be used to generate glucose via gluconeogenesis (33, 34). In response to MCS, the number of GLUT3 transporters in the female placentas also increased, which would be expected to increase the amount of glucose supplied to the fetus. However, an upregulation in the glycogen-synthesizing genes, Gys1 and Gbe1, was detected along with more glycogen in these placentas. The greater glycogen storage in choline-supplemented mice may be due to a glucose-sparing effect of glycine (33, 34). Alternatively, transport of glucose via the high-affinity GLUT3 transporter may be reversible when the fetus is hyperglycemic (35). Although not statistically significant (P = 0.07), female fetuses in the 4× choline group tended to weigh more at E18.5 than did those from the 1× choline group, which is consistent with a hyperglycemic environment in the fetal compartment. Collectively, these data suggest that the transport of glucose from fetal circulation back into the placenta at E18.5 was favored in an attempt to avoid glucose surplus in the fetal compartment and fetal overgrowth. Additional studies using labeled glucose methodology to measure glucose flux between the maternal, placental, and fetal compartments will be needed to verify this hypothesis.
In addition to the altered expression of amino acid and glucose transporters, MCS increased the placental abundance of Fatp4 transporters, which mediates the placental transfer of DHA to fetal circulation (1). The metabolism of choline and DHA intersects at the phosphatidylethanolamine N-methyltransferase pathway, which utilizes a phosphatidylethanolamine molecule enriched in DHA to make a phosphatidylcholine molecule enriched in DHA (i.e., phosphatidylcholine-DHA). Notably, the phosphatidylethanolamine N-methyltransferase pathway can be enhanced by choline supplementation (36, 37), subsequently increasing the production of phosphatidylcholine-DHA to generate a supply of DHA for placental uptake and transport to the fetus by FATP4. Indeed, others have reported a strong correlation between DHA in maternal plasma phospholipids, placental phospholipids, and cord blood phospholipids and placental FATP4 transcript abundance (13). Our finding of the choline-induced upregulation of Fatp4 transporter prompted us to measure the DHA concentration in the fetal brain, which was significantly higher among female fetuses from the choline-supplemented groups at E18.5. Taken together, these data suggest that supplementing the maternal diet with extra choline upregulates placental Fatp4 in response to the choline-induced increase in phosphatidylcholine-DHA production, ultimately increasing DHA supply to the developing fetus.
Compared with the female placentas, it is worth noting that most of the choline-induced changes were less pronounced in the male placentas. This sexual dimorphism was also detected in our previous study whereby choline-induced changes in inflammatory and angiogenic markers differed between male and female placentas (21). Collectively, these data indicate that there are developmental differences between male and female fetuses, with female fetuses investing more resources in developing their placentas (38).
MCS affects placental transport of choline and its metabolic derivatives
Supplementing the maternal diet with extra choline increased the placental abundance of the choline transporter Ctl1 at E15.5 and E18.5, a finding indicative of enhanced placental choline uptake. Paradoxically, however, the placental concentrations of several choline metabolites were lower in the choline-supplemented groups. For example, diminished concentrations of acetylcholine were detected at E15.5 in the placentas obtained from the choline-supplemented groups. Nonetheless, this lower placental concentration of acetylcholine coincided with a higher placental abundance of Oct3, which transports acetylcholine from the placenta to the fetus (19, 20) and a higher acetylcholine concentration in the fetal brain. As such, these data collectively suggest that MCS increased acetylcholine delivery to the developing fetal brain during the early stages of late gestation, which would be expected to enhance neuron functioning (39) and may contribute to the neuroprotective effects of prenatal choline supplementation (24).
Choline and its metabolic derivatives (Figure 1) have important regulatory roles in placental development and function. As an example, acetylcholine modulates amino acid transport (18), which may explain the concurrent choline-induced changes of placental acetylcholine concentration and placental Snat abundance. Notably, the placental TMAO concentration was significantly higher in response to MCS. TMAO can be synthesized in the maternal liver, which was significantly higher in response to MCS in these mice (21), and then transported into the placenta. Alternatively, it may be produced by microbiota residing within the placental tissue (40). Because TMAO is an osmolyte that maintains cell volume and stabilizes proteins and nucleic acids (41), a higher concentration of this metabolite in the placenta may be beneficial, rather than harmful, for normal placental development and function. Additional studies are needed to fully elucidate the functions of TMAO in the placenta.
Strengths and limitations
The present study has several strengths and weaknesses. Strengths include using 2 different supplemental choline dosages and examining the outcomes at 2 different gestational days. This approach allows us to better capture the effects of choline at different concentrations and at different developmental stages. Unlike some other similar studies examining the impact of maternal diets on placental nutrient transporters and metabolic enzymes, both the placental and fetal tissues were used in this study, allowing us to discern the downstream consequences of the choline-induced alterations in the placental nutrient transport system. One major weakness of this study was insufficient statistical power to account for multiple testing corrections; hence, only unadjusted P values are presented. Because the availability of the placental samples was limited, we were also unable to analyze the protein abundance of all the nutrient transporters. It is also possible that some transporters expressed in human placentas are not found in mouse placentas. As an example, the human placenta expresses both Oct3 and Oct1 acetylcholine transporters (19), but the mouse placenta expresses only Oct3 (20). Nevertheless, the majority of the macronutrient transporters, metabolic enzymes, and metabolites examined in this study are present in both mouse and human placentas and are shown to be altered in human pregnancy disorders. We therefore view the study findings as relevant to human populations and supportive of future work designed to assess the impact of MCS on these placental markers in human pregnancy.
In conclusion, MCS modulates placental nutrient transporter abundance as well as glucose and choline metabolism in late gestation of mouse pregnancy in a manner that is dependent on fetal sex and gestational day. Many of the choline-induced effects appear to be secondary responses that could be related to choline's beneficial effects on placental perfusion and vascularization (21). We also provide evidence of altered nutrient availability in the fetal brain in response to these choline-induced placental changes. As uteroplacental perfusion, placental nutrient transporters, and placental metabolism all affect nutrient supply to the fetus, these choline-induced placental responses may have lasting effects on fetal organ development and functioning with potential long-term health implications.
Acknowledgments
The authors' responsibilities were as follows—ST(C)K, MAC, MSR, and XJ: designed the study; ST(C)K, JHK, and JY: conducted the feeding study and tissue collection; ST(C)K, ZW, JSH, and HRK: conducted the laboratory analyses; ST(C)K: analyzed the data; ST(C)K, JTB, MSR, and MAC: interpreted the data; ST(C)K: wrote the manuscript with input from MSR and MAC; MAC: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript.
Author Disclosures
ST(C)K, JHK, JY, ZW, XJ, JSH, HRK, JTB, MSR, and MAC, no conflicts of interest.
Footnotes
Supported by USDA/National Institute of Food Agriculture grant USDA 2012-67017-30176 (to MAC) and Egg Nutrition Center Dissertation Fellowship [to ST(C)K].
The funders had no role in the study design, data collection, data analysis and interpretation, or preparation of this manuscript.
nutrition256107SupplementaryData1
S0
REFERENCES
- 1.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:179827. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwani N, Patil V, Pisal H, Joshi A, Mehendale S, Gupte S, Wagh G, Joshi S. Altered maternal proportions of long chain polyunsaturated fatty acids and their transport leads to disturbed fetal stores in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2014;91:21–30. doi: 10.1016/j.plefa.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Tsoi SC, Cale JM, Bird IM, Kay HH. cDNA microarray analysis of gene expression profiles in human placenta: up-regulation of the transcript encoding muscle subunit of glycogen phosphorylase in preeclampsia. J Soc Gynecol Investig. 2003;10:496–502. doi: 10.1016/s1071-5576(03)00154-0. [DOI] [PubMed] [Google Scholar]
- 4.Arkwright PD, Rademacher TW, Dwek RA, Redman CW. Pre-eclampsia is associated with an increase in trophoblast glycogen content and glycogen synthase activity, similar to that found in hydatidiform moles. J Clin Invest. 1993;91:2744–2753. doi: 10.1172/JCI116515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janzen C, Lei MY, Cho J, Sullivan P, Shin BC, Devaskar SU. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta. 2013;34:1072–1078. doi: 10.1016/j.placenta.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandò C, Tabano S, Pileri P, Colapietro P, Marino MA, Avagliano L, Doi P, Bulfamante G, Miozzo M, Cetin I. SNAT2 expression and regulation in human growth-restricted placentas. Pediatr Res. 2013;74:104–110. doi: 10.1038/pr.2013.83. [DOI] [PubMed] [Google Scholar]
- 7.Brett KE, Ferraro ZM, Holcik M, Adamo KB. Placenta nutrient transport-related gene expression: the impact of maternal obesity and excessive gestational weight gain. J Matern Fetal Neonatal Med. 2016;29:1399–1405. doi: 10.3109/14767058.2015.1049522. [DOI] [PubMed] [Google Scholar]
- 8.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1224–31. doi: 10.1152/ajpregu.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ericsson A, Saljo K, Sjostrand E, Jansson N, Prasad PD, Powell TL, Jansson T. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J Physiol. 2007;581:1323–1332. doi: 10.1113/jphysiol.2007.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battaglia FC, Regnault TR. Placental transport and metabolism of amino acids. Placenta. 2001;22:145–161. doi: 10.1053/plac.2000.0612. [DOI] [PubMed] [Google Scholar]
- 11.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res. 2009;48:52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Hanebutt FL, Demmelmair H, Schiessl B, Larqué E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr. 2008;27:685–693. doi: 10.1016/j.clnu.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Larqué E, Demmelmair H, Gil-Sánchez A, Prieto-Sánchez MT, Blanco JE, Pagán A, Faber FL, Zamora S, Parrilla JJ, Koletzko B. Placental transfer of fatty acids and fetal implications. Am J Clin Nutr. 2011;94(6 Suppl):1908S–1913S. doi: 10.3945/ajcn.110.001230. [DOI] [PubMed] [Google Scholar]
- 14.Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 2006;235:3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- 15.Gårdebjer EM, Cuffe JS, Pantaleon M, Wlodek ME, Moritz KM. Periconceptional alcohol consumption causes fetal growth restriction and increases glycogen accumulation in the late gestation rat placenta. Placenta. 2014;35:50–57. doi: 10.1016/j.placenta.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 16.O'Connell BA, Moritz KM, Walker DW, Dickinson H. Treatment of pregnant spiny mice at mid gestation with a synthetic glucocorticoid has sex-dependent effects on placental glycogen stores. Placenta. 2013;34:932–940. doi: 10.1016/j.placenta.2013.06.310. [DOI] [PubMed] [Google Scholar]
- 17.Lee NY, Choi HM, Kang YS. Choline transport via choline transporter-like protein 1 in conditionally immortalized rat syncytiotrophoblast cell lines TR-TBT. Placenta. 2009;30:368–374. doi: 10.1016/j.placenta.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Sastry BV. Human placental cholinergic system. Biochem Pharmacol. 1997;53:1577–1586. doi: 10.1016/s0006-2952(97)00017-8. [DOI] [PubMed] [Google Scholar]
- 19.Wessler I, Roth E, Deutsch C, Brockerhoff P, Bittinger F, Kirkpatrick CJ, Kilbinger H. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br J Pharmacol. 2001;134:951–956. doi: 10.1038/sj.bjp.0704335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006;34:477–482. doi: 10.1124/dmd.105.006932. [DOI] [PubMed] [Google Scholar]
- 21.Kwan STC, King JH, Yan J, Jiang X, Wei E, Fomin VG, Roberson MS, Caudill MA. Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta. 2017;53:57–65. doi: 10.1016/j.placenta.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Bar HY, Yan J, Jones S, Brannon PM, West AA, Perry CA, Ganti A, Pressman E, Devapatla S, et al. A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1) FASEB J. 2013;27:1245–1253. doi: 10.1096/fj.12-221648. [DOI] [PubMed] [Google Scholar]
- 24.Blusztajn JK, Mellott TJ. Neuroprotective actions of perinatal choline nutrition. Clin Chem Lab Med. 2013;51:591–599. doi: 10.1515/cclm-2012-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta. 2005;26:601–607. doi: 10.1016/j.placenta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Mehedint MG, Craciunescu CN, Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci USA. 2010;107:12834–12839. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74:4734–4740. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- 28.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem. 2003;49:286–294. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- 29.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Stabler SP, Allen RH, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012;95:1060–1071. doi: 10.3945/ajcn.111.022772. [DOI] [PubMed] [Google Scholar]
- 30.Wang DH, Jackson JR, Twining C, Rudstam LG, Zollweg-Horan E, Kraft C, Lawrence P, Kothapalli K, Wang Z, BrennaJTSaturated branched chain, normal odd-carbon-numbered, and n-3 (Omega-3) polyunsaturated fatty acids in freshwater fish in the Northeastern United States. J Agric Food Chem2016 Oct 4 (Epub ahead of print; DOI: 10.1021/acs.jafc.6b03491). [DOI] [PMC free article] [PubMed]
- 31.Houk JC. Control strategies in physiological systems. FASEB J. 1988;2:97–107. doi: 10.1096/fasebj.2.2.3277888. [DOI] [PubMed] [Google Scholar]
- 32.Heylighen F. Cybernetic principles of aging and rejuvenation: the buffering- challenging strategy for life extension. Curr Aging Sci. 2014;7:60–75. doi: 10.2174/1874609807666140521095925. [DOI] [PubMed] [Google Scholar]
- 33.Gaudichon C, Azzout-Marniche D, Tomé D. In: The molecular nutrition of amino acids and proteins. Dardevet D, editor. Academic Press; Cambridge (MA): 2016. Dietary protein and hepatic glucose production; pp. 233–240. [Google Scholar]
- 34.Braverman ER, Pfeiffer CC. 1st ed. Keats Publishing, Inc.; New Canaan (CT): 1987. The healing nutrients within: facts, findings and new research on amino acids. [Google Scholar]
- 35.Schneider H, Reiber W, Sager R, Malek A. Asymmetrical transport of glucose across the in vitro perfused human placenta. Placenta. 2003;24:27–33. doi: 10.1053/plac.2002.0869. [DOI] [PubMed] [Google Scholar]
- 36.West AA, Yan J, Jiang X, Perry CA, Innis SM, Caudill MA. Choline intake influences phosphatidylcholine DHA enrichment in nonpregnant women but not in pregnant women in the third trimester. Am J Clin Nutr. 2013;97:718–727. doi: 10.3945/ajcn.112.050211. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Brenna JT, Stabler SP, Allen RH, Gregory JF, III, Caudill MA. Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am J Clin Nutr. 2013;98:1459–1467. doi: 10.3945/ajcn.113.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol Metab. 2017;28:121–130. doi: 10.1016/j.tem.2016.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S0