Abstract
Objectives:
To summarize current evidence regarding facility and prescriber characteristics associated with potentially harmful medication (PHM) use by residents in nursing homes (NHs), which could inform the development of interventions to reduce this potentially harmful practice.
Design:
Scoping review.
Setting and Participants:
Studies conducted in the United States that described facility and prescriber factors associated with PHM use in NHs.
Methods:
Electronic searches of PubMed/MEDLINE were conducted for articles published in English between April 2011 and November 2021. PHMs were defined based on the Beers List criteria. Studies testing focused interventions targeting PHM prescribing or deprescribing were excluded. Studies were characterized by the strengths and weaknesses of the analytic approach and generalizability.
Results:
Systematic search yielded 1253 articles. Of these, 29 were assessed in full text and 20 met inclusion criteria. Sixteen examined antipsychotic medication (APM) use, 2 anticholinergic medications, 1 sedative-hypnotics, and 2 overall PHM use. APM use was most commonly associated with facilities with a higher proportion of male patients, younger patients, and patients with severe cognitive impairment, anxiety, depression, and aggressive behavior. The use of APM and anticholinergic medications was associated with low registered nurse staffing ratios and for-profit facility status. No studies evaluated prescriber characteristics.
Conclusions and Implications:
Included studies primarily examined APM use. The most commonly reported facility characteristics were consistent with previously reported indicators of poor NH quality and NHs with patient case mix more likely to use PHMs.
Keywords: Potentially harmful medications, prescribing, nursing homes, antipsychotics
Potentially harmful medication (PHM) use in nursing homes (NHs) has long been a target of regulatory policies and practice-based interventions.1–4 The use of these medications in older adults has been associated with a decline in functional status, emergency department visits, greater risk of hospitalization, and mortality.5,6 Current evidence outside of the NH suggests that the most effective interventions to reduce PHM use are multidisciplinary, multifaceted, patient-centered, and provide educational information directly to the patient and/or caregiver.7,8 However, the implementation of such interventions in the NH setting can be challenging.9 For example, although the National Partnership to Improve Dementia Care in NHs reduced the use of antipsychotic medications (APMs) among patients with dementia by 39.4% between 2011 and 2020,10 substantial facility-level variation in APM prescribing remains, with 8% of residents taking antipsychotics in the bottom quartile of NHs compared with 19% in the top quartile by APM prescribing.11 A better understanding of facility- and prescriber-level factors associated with PHM use in NHs may inform the development of effective interventions to reduce PHM use in NHs.
Previous research on factors associated with the prescribing of PHMs for older adults have focused on broad populations of community-dwelling older adults or patients with select chronic conditions (eg, diabetes, arthritis, or depression).12,13 To inform the design and implementation of interventions to reduce PHM prescribing in NHs, we need a more robust understanding of the factors contributing to PHM use in NHs. Thus, our objectives were to (1) provide a comprehensive overview of the facility- and prescriber-level factors associated with prescribing of PHM to NH residents, and (2) utilize this overview to provide a potential framework to guide future intervention design. To answer these questions, we employed a scoping review approach to synthesize existing evidence in this field to efficiently identify potential gaps in the literature for future research.
Methods
Search Strategy
The “2019 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults” for medications to avoid by older adults that are not specific to a disease state, was used to identify PHMs in this study. The search was limited to studies conducted in the United States, to allow comparison of similar NH characteristics and health care market environment, and to studies written in English. Search terms were selected to cover the 2 dimensions of interest: PHMs and medication use in NHs. Initial keywords included potentially inappropriate medication, potentially inappropriate prescription, Beers criteria, nursing facility, long-term care, nursing care, assisted living facility, and homes for the aged. Articles were manually reviewed to identify additional relevant terms and medical subject headings (MeSH terms) to add to the initial comprehensive article pull. Once a final search strategy was created, a query of PubMed/MEDLINE via Pubmed.gov was conducted on April 7, 2021, for studies published between April 2011 and April 2021. The search was repeated on November 16, 2021, for relevant articles published between April and November 2021. Complete search terms are available in Supplementary Material 1.
Eligibility Criteria
The following inclusion criteria were applied to the titles and abstracts of all citations: (1) the study described facility or prescriber-level factors associated with the use of PHM in NHs, (2) the study sample was from NH(s) [including Veteran Affairs NH(s)], (3) the study was conducted in the United States, and (4) class of medication is included in the AGS Beers Criteria list to avoid in older adults in general. We did not focus specifically on medication classes with conditional recommendations such as avoiding chronic use for nonsteroidal anti-inflammatory drugs and disease-drug interactions such as nitrofurantoin in kidney disease. The following studies were excluded: (1) evaluations of specific interventions targeting PHM prescribing or deprescribing; (2) studies that focused on specific conditions such that results could not be meaningfully compared with other included studies (eg, patients with Parkinson disease); and (3) studies of a single medication formulation (rather than all medications in a class). Studies were also excluded if they had the primary aim of evaluating polypharmacy in general.
Data Extraction
Prior to data extraction, a data charting worksheet was created through careful discussion by the authors regarding information relevant to the review. After the initial review, articles identified for full-text review were read independently by at least 2 authors and relevant information from each of the included articles was extracted using the data charting worksheet. Elements of the data extraction sheet included bibliographic information, study type, study aim, cohort, variables, PHM definition, analytic approach, main findings, strengths, weaknesses, and generalizability. Special consideration was paid to PHM definition and the variables tested in each study for association with PHM use. Any disagreements between the 2 reviewers in data extraction and study interpretation were resolved through discussion with a third reviewer.
Critical Appraisal
An element of the data extraction worksheet included strengths and weaknesses of the included studies as well as generalizability of findings. After data extraction was completed, these characteristics of each study were discussed by all authors to establish a consensus regarding the inclusion of the study in the scoping review. In addition, the authors discussed the methods utilized by included studies and their appropriateness for the studies’ proposed research questions and outcomes. Recurrent strengths and weaknesses as well as serious methodological limitations of each study were then synthesized to produce a critical appraisal of the existing literature.
Synthesis of Results
The findings of included studies were summarized organizing the results into categories related to each specific class of PHM use in NHs found in the literature. The factors most commonly associated with PHM prescribing across different studies were evaluated and reported together including categories for patient demographics and patient diagnoses measured at the facility level. Thus, NHs were characterized by the facility-wide prevalence of certain conditions and patient demographics summarized for the entire facility. Findings that were mixed or not consistent across different studies were reported separately.
Results
Selection of Sources of Evidence
Electronic database search retrieved 1253 articles, which were screened by title and abstract. Initial screening resulted in 29 articles for full-text review. During full-text review 9 articles were excluded due to PHM definition (eg, immediate vs extended release bladder anti-muscarinic medications),14,15 lack of NH or prescriber level factors,16,17 narrow clinical population or disease condition,18–20 secondary analysis of a previously published study,21 and methodological issues.22 For example, 1 study that examined the use of antimuscarinics in patients with Parkinson disease was excluded because it was deemed too narrowly focused to be meaningfully compared with other studies. Other examples of excluded studies were (1) a study comparing patients in one NH to another NH, and (2) a study comparing 2 patient populations—one with a diagnosis and one without—both receiving PHMs. Twenty studies were included in the final review (Figure 1).
Fig. 1.
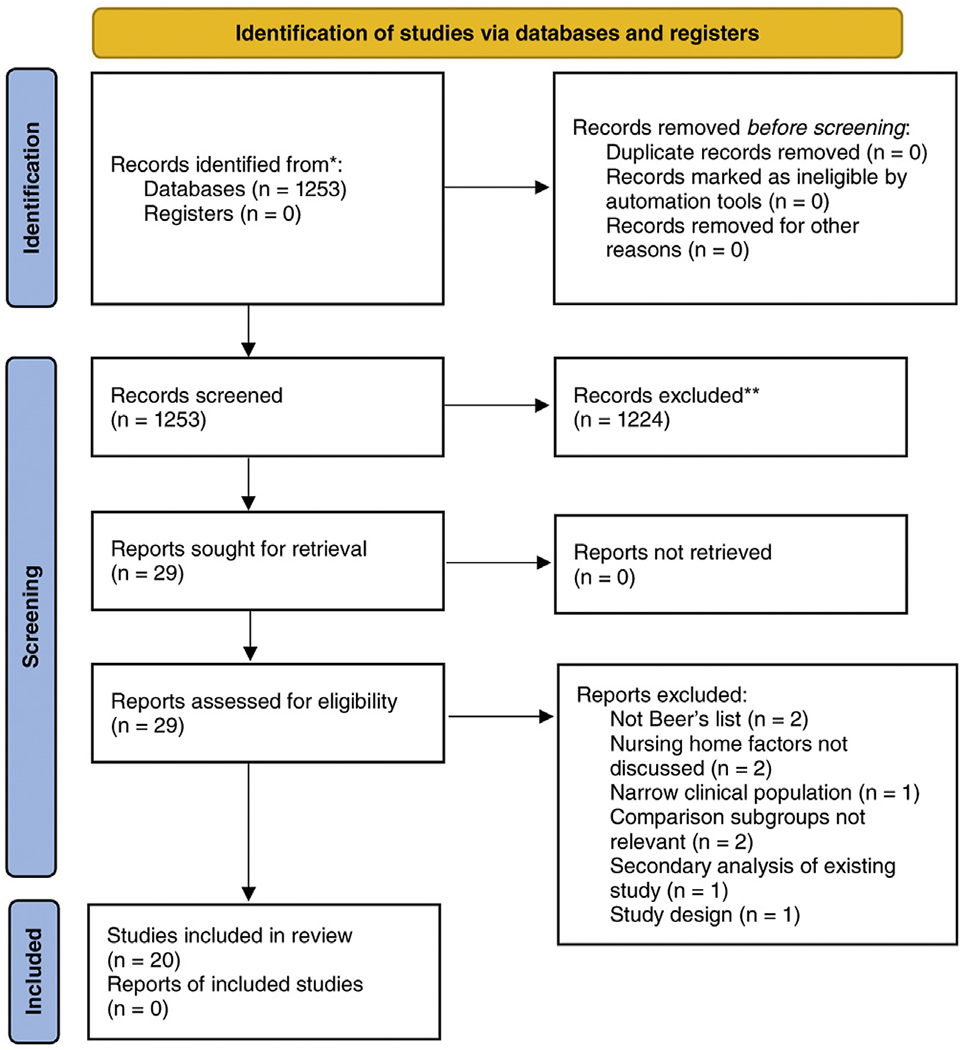
PRISMA diagram of included studies.
Characteristics of Sources of Evidence
Key characteristics of the included studies are summarized in Table 1. Seventeen of the included studies were retrospective,23–39 and 3 used both retrospective analysis and a prospective questionnaire.12,40,41 There were no randomized controlled trials. The most commonly used dataset for retrospective analysis was the Minimum Data Set (MDS), which was utilized by all 20 included studies; other commonly examined data sets included NH Compare,12,30,39,41 OSCAR,24,26–28,31,33,34 CASPER,23,24,27,29,31,39 and Medicare and Veterans Affairs (VA) claims data.26,35,36 Sample sizes ranged from 17 to 17,286 NHs and from 204 to 3,687,901 patients.
Table 1.
Description of Included Studies
| Studies | Study Interval | Data Source | Study Sample | Analytic Approach | Outcomes | Findings |
|---|---|---|---|---|---|---|
| APM Prescribing | ||||||
| Bonner, 2015 | Feb 2011—Jul 2011 | MDS, NHC, Interviews | 26 NH, 204 patients, 466 interviews | Directed content analysis | High vs low APM use | High APM use was associated with higher proportion of non-White, male, and younger patients, and census tracts with lower education and income. |
| Huybrechts, 2012 | 2001–2005 | MDS, OSCAR, Claims Data | 5751 NH, 65,618 patients* | Mixed-effects logistic regression | % of patients treated with conventional APM vs atypical APM | NHs using conventional APM was associated with lower education level of residents, CHF, hypnotic medication use, lower rates of depression, lower rates of dementia, lack of SCU, lack of team-based physician care, lack of mental health staff, hospital-based facilities, and facilities in the SE region |
| Fashaw, 2020 | 2000–2015 | LTCFocUS† | 12,964 NH | Fixed-effects logistic regression | % of PI APM use‡ | Facilities with higher APM use were associated with higher proportion of residents who were older, Black, female, had greater functional status, worse cognitive impairment, and Medicare enrollment, as well as being part of a chain, for-profit facilities, lower LPN and RN hours, higher CNA hours, and those with SCU. |
| Busch, 2019 | 2004 | MDS, CASPER | 14,699 NH, 115,875 patients§ | Stepwise logistic regression. | Top vs bottom quintile of APM use | Facilities in top quintile of APM use associated with higher proportion of residents who were male, with Alzheimer’s and dementia, other psychotic disorders, anxiety, depression, lower proportion of residents on post-acute stays as well as higher rates of being for-profit and in a rural area. |
| Gellad, 2012 | Jan 2004–Jun 2005 | MDS, VA PBM | 113 NH, 3692 patients | Logistic regression with backwards elimination | APM use | APM use was associated with aggressive behavior, lower CCI score, greater functional status, lower PR use, PTSD, other anxiety, depression, dementia, polypharmacy, hypnotic medication use, SCU admission, and residing in larger facilities. |
| Trinkoff, 2017 | 2004–2010 | MDS, NHC, NH Regulation Plus | 15,508 NH | Linear regression | % of residents with APM use | Lower APM use was associated with states with higher training requirements for CNAs |
| Roberts, 2020 | 2011–2015 | MDS, CASPER, MBSF | 5883 NH, 1,201,096 patients | Linear regression | APM use | Presence of full time QSW associated with lower APM use. |
| White, 2020 | Jan 2015–Dec 2015 | LTCFocUS†, RN4CAST-US, NHC | 245 NH, 674 RNs | Linear regression | % of residents with APM use | Effect of work environment on APM use was non-significant. |
| Lee, 2014 | 2000 | OSCAR, MDS, ARF | 195 NH | Linear regression | % of residents with PI APM use‖ | APM use was associated with higher % of Medicare Residents, but not RN staffing hours. |
| Jung, 2018 | 2000–2013 | MEF, MDS, OSCAR, LTCFocUS† | 15,557 NH | Linear regression | % of residents with APM use | APM use was associated with lower proportion of Medicare Advantage beneficiaries. |
| Tija JF, 2014 | Oct 2009–Sep 2010 | MDS, NHC, Interviews | 60 NH | Linear regression with gamma distribution | % of residents with atypical APM use | APM use was associated with psychiatric consulting group regardless of other facility level factors. |
| Phillips, 2018 | 2015 | MDS, MDHSS cost report data | 458 NH, 29,679 patients** | Stepwise logistic regression, Random-intercept logistic regression | APM use | APM use was associated with being male, younger, lower functional status, greater cognitive impairment, presence of delirium, psychosis indicators, behavioral symptoms, PTDS, anxiety, depression, anxiety and depression medication use. In dementia patients APM use was also associated with wandering. |
| Joyce, 2018 | 2005–2010 | MDS, MedPAR, OSCAR | 704,782 patients | Linear regression | PI APM use†† | Patients admitted to SCUs were less likely to receive APM. |
| Konetzka, 2014 | 1999–2008 | MDS, OSCAR | 4258 NH, 809,645 patients | Difference-in-differences model | APM use | Increase in APM was associated with Black patients and decline in PR use. |
| Mansbach, 2016 | 2014 | MDS, prospective cohort | 17 NH, 216 patients | Logistic regression | APM use for treatment of BPSD | APM use associated with decreased BCAT scores |
| Anticholinergic Medication Prescribing | ||||||
| Niznik, 2017 | Jan 2010–Sep 2010 | MDS, Claims Data | 4720 patients | Multinomial logistic regression | Anticholinergic burden using sum of medication ACB scores | Increased anticholinergic burden was associated depression, hypertension, dementia medication, hospitalization and specialist visits in previous year, LIS, and Medicaid eligibility. |
| Malagaris, 2020 | 2009–2017 | MDS, Claims data | 6703 NH, 299,354 patients | Mixed-effects logistic regression | Use of drug with high anticholinergic properties according to ACB | Anticholinergic drug use was higher in patients who were younger, female, White, residing in for-profit NHs, in rural areas, in the SE region, and with low quality ratings. |
| PHM Prescribing | ||||||
| Dosa, 2013 | 2004–2009 | VA admin data, MDS, DSS | 176,168 patients | Fixed-effects logistic regression | HEDIS medication use | HEDIS medication use was associated with patients who were older, female, had COPD, DM, renal disease, cancer, and greater cognitive impairment. |
| Crystal, 2020 | 2011–2016 | MDS, NHC, CASPER | 17,289 NH, 3,687,901 patients | Logistic regression | PI APM and sedative-hypnotic use‡‡ | APM use was associated with bipolar disorder, physical/ verbal aggression, Medicaid eligibility, facilities with lower RN staffing, higher LPN staffing, and higher proportion of Medicaid residents. Sedative-hypnotic use was associated with patients who were Hispanic, with verbal aggression, and symptoms of depression, facilities with lower RN staffing, higher LPN staffing, and higher CNA staffing. |
| Tija JB, 2014 | Oct 2009–Sep 2010 | Prescription data, §§ MDS | 460 NH, 5406 patients | Generalized estimating equations | Use of medication of questionable benefit‖‖ | PHM use associated with decreased oral issues, feeding tubes, DNR orders, and hospice. |
ABS, anticholinergic burden scale; ARF, area resource file; BCAT, Brief Cognitive Assessment Tool; BPSD, behavioral and psychological symptoms of dementia; CASPER, Certification and Survey Provider Enhanced Reporting; CCI, Charlson comorbidity index; CHF, congestive heart failure; CNA, certified nursing assistant; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DNR, do not resuscitate; DSS, Decision Support Services; FDA, Food and Drug Administration; HEDIS, Healthcare Effectiveness Data and Information Set; LIS, low income subsidy; LPN, licensed practical nurse; LTCFocUS, Long Term Care Facts on Care in the US; MBSF, Medicare Beneficiary Summary File; MDHSS, Missouri Department of Health and Senior Services; MedPAR, Medicare Provider and Analysis Review File; MEF, Medicare Enrollment File; NHC, Nursing Home Compare; OSCAR, Online Survey Certification and Reporting; PBM, pharmacy benefits management; PI, potentially inappropriate; PR, physical restraint; QSW, qualified social worker; RN4CAST-US, Registered Nurse Forecasting Study; SE, South Eastern.
Cohort excludes patients with diagnosis of schizophrenia or bipolar disorder.
LTCFocUS includes OSCAR, CASPER, and MDS.
Defined as APM use in patients without diagnosis of schizophrenia or bipolar disorder.
Cohort excludes patients with diagnosis of schizophrenia, Tourette, or Huntington disease.
Defined as APM use in patients without diagnosis of schizophrenia, Tourette, Huntington disease, bipolar disorder, or other psychotic disorder.
Cohort excludes patients with diagnosis of schizophrenia, Tourette, Huntington disease, bipolar disorder, or other psychotic disorder.
Defined as patients without FDA approved indication for APM use.
Defined as APM or sedative-hypnotic use in patients without schizophrenia, Tourette, or Huntington disease.
Taken from prescription dispensing database of a national long-term care pharmacy operating in 47 states with distribution similar to OSCAR population.
Defined according to a Delphi consensus process.
Although the search included terms related to all PHM for older adults, the included studies focused primarily on classes of PHM contraindicated for patients with a history of falls or dementia. The majority of studies focused on APMs. Sixteen studies examined APM use,12,23–26,29–31,33,34,37,39–41 2 examined anticholinergic medications,35,36 one sedative-hypnotics,39 and 2 all classes of PHM.32,38 Only 1 study separately analyzed multiple classes of PHM; APM use and sedative hypnotic use were evaluated in separate analyses.39 The most common analytic technique was regression modeling, with only 1 study using qualitative analysis (directed content analysis of interviews).12 The studies were published between 2012 and 2020 using data collected from 1999 to 2017. Only 2 of the studies analyzed data from within the past 5 years.35,39
Critical Appraisal
A main strength of many of the included studies was the use of large, nationally representative data samples. The use of MDS as a data source in multiple studies took advantage of the standardized and comprehensive assessments of individual patients at multiple time points available from this data source.42,43 Of the studies evaluating APM prescribing, 11 studies did not distinguish clinically appropriate and inappropriate prescription of APM use,12,23,25–27,29–31,37,41 while 6 defined inappropriate prescription based on the exclusion of preexisting diagnoses such as schizophrenia and Huntington disease.23,24,28,33,37,39 All studies focused on other classes of PHM did not distinguish appropriate and inappropriate prescribing. Although studies that focused on VA NHs had a high percentage of male participants limiting generalizability to other populations, these studies reported findings generally consistent with the non-VA facilities.25,32 All of the studies were observational and were not designed to evaluate a causal relationship between facility or prescriber characteristics and PHM use. The 3 studies, which included prospective elements were limited by small sample sizes, self-report by facilities or prescribers, and low response rates (as low as 30%).12,40,41
Synthesis of Results
Patient characteristics associated with APM use
Patient characteristics and case mix including demographics and clinical characteristics reported at the facility level were the most consistently reported factors associated with PHM use. The majority of studies (n = 16) examined APM use.12,23–26,29–31,33,34,37,39–41 Factors commonly associated with APM use are summarized in Table 2. Patient demographics most commonly associated with APM use in included studies were a higher proportion of patients in the facility who were male,12,23,37 non-White,12,24,34 younger,12,37 and a Medicare beneficiary.24,28 However, these findings are in conflict with the findings of other included studies, which reported that APM use was associated with a higher prevalence of female,24 older,24 and non-Medicare insured patients.27
Table 2.
Variables Most Commonly Associated with APM Use
| Independent Variables | Number of Studies |
|---|---|
| Male | 3*,12,23,37 |
| Non-White | 312,24,34 |
| Younger | 2†,12,37 |
| Medicare beneficiary | 2‡,24,28 |
| Severe cognitive Impairment | 324,37,40 |
| Anxiety | 323,25,37 |
| Depression | 323,25,37 |
| Aggressive behavior | 325,37,39 |
| PTSD | 225,37 |
| Other psychotic disorder | 223,37 |
| Greater functional status | 2§,24,25 |
| Lower RN h | 224,39 |
| For profit | 223,24 |
| Presence of/admission to SCU | 2‖,24,25 |
ADL, activities of daily living scale; PTSD, post-traumatic stress disorder.
One study found the opposite, that female individuals had higher APM use.24
One study found the opposite, that older patients had higher APM use.24
One study found the opposite that Medicare beneficiaries received fewer APM prescriptions.27
One study found the opposite, that lower ADL score was associated with higher APM use.37
One study found the opposite, that admission to SCU was associated with lower APM use.33
Other patient characteristics commonly associated with APM use were clinical characteristics including severe cognitive impairment,24,37,40 anxiety,23,25,37 depression,23,25,37 aggressive behavior,25,37,39 post-traumatic stress disorder,25,37 other psychotic disorders,23,37 and greater functional status.24,25 One study was in conflict with these results, finding that lower functional status was associated with increased APM use.37 This same study found that greater cognitive impairment was associated with increased APM use.
Other factors associated with APM use
Nine studies investigated the role of facility characteristics in APM use.23–26,28,29,31,33,39 Multiple included studies found that lower registered nurse (RN) hours per resident day,24,39 for-profit status,23,24 and the presence of a special care unit (SCU) for the treatment of patients with dementias24,25 were associated with greater APM use. However, 1 study found that SCUs were associated with decreased APM use.33
One study reported prescriber level differences in PHM use. Tija et al found that, regardless of other factors, APM prescribing was associated with the psychiatric consulting group contracted with the facility.41 However, the study did not examine specific prescriber characteristics to explain the differences in APM prescribing between the groups.41
Other classes of PHM use: anticholinergics, sedative hypnotics, and PHM use overall
Five studies investigated factors associated with other classes of PHM (Table 1).32,35,36,38,39 Two studies measured anticholinergic prescribing rates.35,36 One reported increased anticholinergic use was associated with higher rates of depression, hypertension, recent hospitalization, receipt of low-income subsidy, and eligibility for Medicaid.36 Another found an association between anticholinergic use and a higher prevalence of younger, female, and White patients, for-profit ownership, location in a rural area, South East region of the United States, and lower NH quality ratings.35
One study examined factors related to sedative-hypnotic use in the NHs.39 It found that sedative-hypnotic use was higher among patients who are Hispanic, in facilities with lower RN staffing and higher licensed practical nursing or certified nursing assistant staffing. The same study also reported PHM use was associated with staff reporting verbal aggression by the residents and in NHs with a higher prevalence of depression among the residents.
Dosa et al examined factors associated with PHM use in general and found it was associated with patients who were older, female, had chronic obstructive pulmonary disease, diabetes, renal disease, cancer, and greater cognitive impairment.32 Tija et al found that PHM use among NH residents with dementia was more frequently observed in residents without feeding difficulties or in facilities that used feeding tubes at higher rates, or had a lower prevalence of patients with a Do Not Resuscitate order or in hospice care.38
Discussion
Summary of Evidence
This scoping review identified 20 relevant studies of patient and facility characteristics associated with PHM prescribing in NHs. The majority of studies examined a single class of PHMs (antipsychotics) and used retrospective analyses of MDS. Facility characteristics that were commonly associated with antipsychotic use and consistent across studies included facilities with higher proportions of non-White patients as well as facilities with a higher prevalence of severe cognitive impairment, anxiety, depression, aggressive behavior, and post-traumatic stress disorder among the residents. Higher proportions of younger patients, male patients, and Medicare beneficiaries, as well as a higher prevalence of patients with less functional impairment were also associated with APM use in some studies; however, other studies did not find these characteristics to be associated with APM use. Additional facility level characteristics associated with APM use were lower RN hours, for-profit status, and the presence of a SCU. There was a relative dearth of information on prescriber level characteristics in the literature specific to PHMs on the Beers list.
The majority of studies that produced consistent results regarding APM prescribing focused on patient race and sex and case-mix in the facilities. Those characteristics are associated with APM use in other settings. Some studies have reported similar findings to those in our review while others have reported an inverse relationship. For example a 2015 study of community-dwelling older adults with dementia found that antipsychotic use was lower among patients who were male, non-White and Hispanic.44 This finding was contradicted by a 2015 study by Xiong et al, which reported that among community-dwelling adults with dementia, Hispanic patients were significantly more likely to receive APMs.45 In addition, a 2014 study conducted in Finland found that APM use was associated with younger patients, patients who were male, and patients with a history of psychiatric disorders.46 The findings that were mixed across studies included younger patients, more Medicare beneficiaries, and patients with less functional impairment. These results contradicted other reviewed studies, which found that older patients, non-Medicare beneficiaries, and patients of lower functional status were more likely to be prescribed APMs. In addition, NHs with more patients with severe cognitive impairment and those with a SCU (typically a dementia care unit) were associated with APM use in a separate study. Few studies evaluated facility characteristics associated with APM use that did not reflect patient case mix or demographics.
Of the other classes of PHM, 2 studies examined anticholinergics, 1 sedatives/benzodiazepines, and 2 evaluated overall PHM use. These studies reported inconsistent findings and their small number limits their interpretation in the context of this review.
Implications for Practice, Policy, and Research
This review identified facility-level factors impacting the prescription of PHM. Prescriber characteristics, which could be targeted by interventions, have not been evaluated in US-based and NH-based studies. For example, to design behavioral interventions which have been effective in reducing the use of inappropriate medications in other settings (eg, antibiotics for upper respiratory infections in children),47,48 we need more information on NH prescribers. Future research should evaluate the role of interdisciplinary teams including pharmacists who perform medication review and education as well as nurse practitioners and physician assistants who prescribe to NH residents alongside physicians. For example, a study in Canada found that medication regimen reviews and a multidisciplinary approach improved medication appropriateness in NH residents with severe dementia.49 Furthermore, the role of consulting prescribers in PHM use could be better elucidated. The finding by Tija et al documenting wide variation in psychiatric consulting groups’ prescribing practices highlights the need to better understand prescriber characteristics in PHM prescribing.41 Efforts in this area may be limited by a dearth of data on NH prescribers in public NH data sets. Although some pre-scriber characteristics are collected in claims datasets, those sources are expensive and challenging to use. Although our definition of PHM use, which used the AGS Beers Criteria did not include antibiotic prescribing in NHs, antibiotic stewardship in the NH is an important topic which warrants a separate review.
The design of such interventions to reduce PHM use in the NH setting for all residents requires a better understanding of the drivers behind variation in prescribing. Overall, we found scant information relevant to potential targets for intervention design. Much of the evidence attributed variation between facilities to differences in patient case mix. Furthermore, the majority of existing evidence focused on APM use with little research on the other classes of PHM. There is anecdotal evidence that suggests that benzodiazepine prescribing in NHs is on the rise and benzodiazepines are being used as substitutes to APMs to modify resident behaviors in the NH. Future studies should evaluate all classes of psychoactive PHMs simultaneously, considering evidence suggests that as APM use in NHs declines, the use of other classes of PHMs is increasing.50 In addition, NH characteristics that explain variation between facilities in PHM use may inform future research on deprescribing. For example, we found that the presence of a dementia unit was associated with APM use. This setting may represent an amenable target for interventions focused on PHM deprescribing.
Nevertheless, several facility level characteristics were consistently associated with PHM use across the studies. Inadequate staffing has been linked to poor outcomes in the NH. High PHM use may represent one of many mechanisms by which insufficient staffing may lead to poor patient outcomes. For-profit ownership of NHs has also been associated with poor NH quality across many domains. For-profit facilities may be more sensitive to the incentives to cut costs and increase revenue, which may lead to trade offs between staffing and overuse of sedating medications, for example.
Strengths and Limitations
We conducted a review of PubMed/MEDLINE to create a comprehensive overview of the available literature on facility and prescribing provider-level factors that may be modified to reduce PHM use in NHs. Our search revealed no prior reviews on factors influencing PHM use over the past decade. The focus on facility and prescribers enabled us to systematically review a narrow number of factors that could inform facility or prescriber level interventions to reduce PHM use. The scoping approach allowed flexibility to address a broad research question and draw from research focused on several related classes of PHM.
Despite these strengths, scoping reviews are subject to limitations. These include possible bias from a less rigid search strategy than that found in systematic reviews.51 Nevertheless, we aimed to conduct our scoping review in as systematic a manner as possible, using a well-defined, comprehensive search strategy, and a detailed discussion of potential bias in the included studies. In addition, scoping reviews because of the diverse nature of included studies, limit the ability to synthesize the results of multiple studies. We aimed to limit the introduction of bias from the search strategy and assessment of quality of evidence by implementing a rigorous search strategy and evaluating methodological elements of included studies as part of the inclusion criteria of the review. Furthermore, most of the studies in our review used retrospective secondary data analyses, using regression to analyze MDS data from a sample of NHs, allowing for a more cohesive synthesis of results. Our review does not cover several important tools for deprescribing PHM (such as the Stopp/Start criteria52), which have been shown to be effective but did not evaluate facility or prescriber characteristics directly.
Conclusions and Implications
This scoping review examined literature published over the past decade on facility characteristics associated with PHM in NHs. The literature focused on APM use, patient case mix and demographic characteristics, with few specific facility-level and no prescriber-level characteristics examined. Higher rates of behavioral issues and patients with dementia as well as facility characteristics previously associated with poor NH care quality overall (such as low staffing levels) were associated with PHM use. One possible explanation of these findings is that these factors may reflect facility need due to facility strain from limited resources including direct care staffing, access to subspecialty mental health care, and limited external or informal caregiver support. Further these resource needs may be compounded due to the presence of more complex patients requiring more direct care and mental health intervention. In summary, the evidence on potentially modifiable facility level factors is scant with few studies and conflicting findings. Future research should evaluate modifiable facility and prescriber level factors that are more relevant to intervention design previously shown to reduce PHM use in other settings, including multidisciplinary team dynamics, behavioral economics approaches, patient-centered care models, and interventions that leverage family caregiver engagement.
Supplementary Material
Acknowledgments
This work was supported by the NIH grant 2P01AG027296-11.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Ailabouni N, Mangin D, Nishtala PS. DEFEAT-polypharmacy: deprescribing anticholinergic and sedative medicines feasibility trial in residential aged care facilities. Int J Clin Pharm. 2019;41:167–178. [DOI] [PubMed] [Google Scholar]
- 2.Bayliss EA, Shetterly SM, Drace ML, et al. The OPTIMIZE patient- and family-centered, primary care-based deprescribing intervention for older adults with dementia or mild cognitive impairment and multiple chronic conditions: study protocol for a pragmatic cluster randomized controlled trial. Trials. 2020;21:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon SE, Dufour AB, Monti SM, et al. Impact of a videoconference educational intervention on physical restraint and antipsychotic use in nursing homes: results from the EcHO-AGE Pilot Study. J Am Med Dir Assoc. 2016;17:553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fralick M, Bartsch E, Ritchie CS, Sacks CA. Estimating the use of potentially inappropriate medications among older adults in the United States. J Am Geriatr Soc. 2020;68:2927–2930. [DOI] [PubMed] [Google Scholar]
- 5.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157:1531–1536. [PubMed] [Google Scholar]
- 6.Cateau D, Bugnon O, Niquille A. Evolution of potentially inappropriate medication use in nursing homes: retrospective analysis of drug consumption data. Res Social Adm Pharm. 2021;17:701–706. [DOI] [PubMed] [Google Scholar]
- 7.Clyne B, Fitzgerald C, Quinlan A, et al. Interventions to address potentially inappropriate prescribing in community-dwelling older adults: a systematic review of randomized controlled trials. J Am Geriatr Soc. 2016;64:1210–1222. [DOI] [PubMed] [Google Scholar]
- 8.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174:890–898. [DOI] [PubMed] [Google Scholar]
- 9.Tjia J, Gurwitz JH, Briesacher BA. Challenge of changing nursing home prescribing culture. Am J Geriatr Pharmacother. 2012;10:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Partnership to Improve Dementia Care in Nursing Homes: Antipsychotic Medication Use Data Report In: CMS, ed2021. [Google Scholar]
- 11.Minimum Data Set Quality Measures Dataset. In: CMS, ed2021. [Google Scholar]
- 12.Bonner AF, Field TS, Lemay CA, et al. Rationales that providers and family members cited for the use of antipsychotic medications in nursing home residents with dementia. J Am Geriatr Soc. 2015;63:302–308. [DOI] [PubMed] [Google Scholar]
- 13.Swanoski MT, Little MM, St Hill CA, Ware KB, Chapman S, Lutfiyya MN. Potentially inappropriate medication prescribing in U.S. older adults with selected chronic conditions. Consult Pharm. 2017;32:525–534. [DOI] [PubMed] [Google Scholar]
- 14.Hanlon JT, Aspinall SL, Handler SM, et al. Potentially suboptimal prescribing for older veteran nursing home patients with dementia. Ann Pharmacother. 2015;49:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moga DC, Wu Q, Doshi P, Goodin AJ. An investigation of factors predicting the type of bladder antimuscarinics initiated in Medicare nursing homes residents. BMC Geriatr. 2017;17:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare and Medicaid Services’ National Partnership to Improve Dementia Care with the Use of Antipsychotics and Other Psychotropics in Long-term Care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryskina KL, Lam C, Jung HY. Association between clinician specialization in nursing home care and nursing home clinical quality scores. J Am Med Dir Assoc. 2019;20:1007–1012.e1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chekani F, Holmes HM, Johnson ML, Chen H, Sherer JT, Aparasu RR. Use of atypical antipsychotics in long-term care residents with Parkinson’s disease and comorbid depression. Drug Healthc Patient Saf. 2020;12:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jester DJ, Hyer K, Molinari V, Andel R, Rozek E. Age-dependent determinants of antipsychotic use among newly admitted residents of skilled nursing facilities: a population-based study. Int J Geriatr Psychiatry. 2018;33:1370–1382. [DOI] [PubMed] [Google Scholar]
- 20.Covington LP, McCarrell J, Hoerster NS. Prevalence of anticholinergic medication use in the program of all-inclusive care for the elderly. Consult Pharm. 2016;31:168–174. [DOI] [PubMed] [Google Scholar]
- 21.Resnick B, Kolanowski A, Van Haitsma K, et al. Current psychotropic medication use and contributing factors among nursing home residents with cognitive impairment. Clin Nurs Res. 2021;30:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daly JM, Bay CP, Levy BT, Carnahan RM. Caring for people with dementia and challenging behaviors in nursing homes: a needs assessment geriatric nursing. Geriatr Nurs. 2015;36:182–191. [DOI] [PubMed] [Google Scholar]
- 23.Busch SH, Cohen MS, Konetzka RT. Assessing the relative contribution of resident versus facility characteristics associated with antipsychotic medication receipt among nursing facility residents. Med Care. 2019;57:822–829. [DOI] [PubMed] [Google Scholar]
- 24.Fashaw S, Chisholm L, Mor V, et al. Inappropriate antipsychotic use: the impact of nursing home socioeconomic and racial composition. J Am Geriatr Soc. 2020;68:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huybrechts KF, Rothman KJ, Brookhart MA, et al. Variation in antipsychotic treatment choice across US nursing homes. J Clin Psychopharmacol. 2012;32:11–17. [DOI] [PubMed] [Google Scholar]
- 27.Jung HY, Li Q, Rahman M, Mor V. Medicare Advantage enrollees’ use of nursing homes: trends and nursing home characteristics. Am J Manag Care. 2018;24:e249–e256. [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HY, Blegen MA, Harrington C. The effects of RN staffing hours on nursing home quality: a two-stage model. Int J Nurs Stud. 2014;51:409–417. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AR, Smith AC, Bowblis JR. Nursing Home social services and post-acute care: does more qualified staff improve behavioral symptoms and reduce antipsychotic drug use? J Am Med Dir Assoc. 2020;21:388–394. [DOI] [PubMed] [Google Scholar]
- 30.Trinkoff AM, Storr CL, Lerner NB, Yang BK, Han K. CNA training requirements and resident care outcomes in nursing homes. Gerontologist. 2017;57:501–508. [DOI] [PubMed] [Google Scholar]
- 31.White EM, Aiken LH, Sloane DM, McHugh MD. Nursing home work environment, care quality, registered nurse burnout and job dissatisfaction. Geriatr Nurs. 2020;41:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dosa D, Cai S, Gidmark S, Thomas K, Intrator O. Potentially inappropriate medication use in veterans residing in community living centers: have we gotten better? J Am Geriatr Soc. 2013;61:1994–1999. [DOI] [PubMed] [Google Scholar]
- 33.Joyce NR, McGuire TG, Bartels SJ, Mitchell SL, Grabowski DC. The impact of dementia special care units on quality of care: an instrumental variables analysis. Health Serv Res. 2018;53:3657–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konetzka RT, Brauner DJ, Shega J, Werner RM. The effects of public reporting on physical restraints and antipsychotic use in nursing home residents with severe cognitive impairment. J Am Geriatr Soc. 2014;62:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malagaris I, Mehta HB, Li S, Goodwin JS. Decrease of anticholinergic drug use in nursing home residents in the United States, 2009 to 2017. J Am Geriatr Soc. 2020;68:2797–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niznik J, Zhao X, Jiang T, et al. Anticholinergic Prescribing in Medicare Part D beneficiaries residing in nursing homes: results from a retrospective cross-sectional analysis of Medicare Data. Drugs Aging. 2017;34:925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips LJ, Birtley NM, Petroski GF, Siem C, Rantz M. An observational study of antipsychotic medication use among long-stay nursing home residents without qualifying diagnoses. J Psychiatr Ment Health Nurs. 2018;25:463–474. [DOI] [PubMed] [Google Scholar]
- 38.Tjia J, Briesacher BA, Peterson D, Liu Q, Andrade SE, Mitchell SL. Use of medications of questionable benefit in advanced dementia. JAMA Intern Med. 2014;174:1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crystal S, Jarrin OF, Rosenthal M, Hermida R, Angell B. National partnership to improve dementia care in nursing homes campaign: state and facility strategies, impact, and antipsychotic reduction outcomes. Innov Aging. 2020;4:igaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansbach WE, Mace RA, Clark KM, Firth IM, Breeden JK. Predicting off-label antipsychotic medication use in a randomly selected nursing home sample based on resident and facility characteristics. Res Gerontol Nurs. 2016;9:257–266. [DOI] [PubMed] [Google Scholar]
- 41.Tjia J, Field T, Lemay C, et al. Antipsychotic use in nursing homes varies by psychiatric consultant. Med Care. 2014;52:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doupe MB, Poss J, Norton PG, et al. How well does the minimum data set measure healthcare use? a validation study. BMC Health Serv Res. 2018;18:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruber-Baldini AL, Zimmerman SI, Mortimore E, Magaziner J. The validity of the minimum data set in measuring the cognitive impairment of persons admitted to nursing homes. J Am Geriatr Soc. 2000;48:1601–1606. [DOI] [PubMed] [Google Scholar]
- 44.Maust DT, Strominger J, Bynum JPW, et al. Prevalence of psychotropic and opioid prescription fills among community-dwelling older adults with dementia in the US. JAMA. 2020;324:706–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong GL, Filshtein T, Beckett LA, Hinton L. Antipsychotic use in a diverse population with dementia: a retrospective review of the National Alzheimer’s Coordinating Center Database. J Neuropsychiatry Clin Neurosci. 2015;27:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taipale H, Koponen M, Tanskanen A, Tolppanen AM, Tiihonen J, Hartikainen S. Antipsychotic polypharmacy among a nationwide sample of community-dwelling persons with Alzheimer’s disease. J Alzheimers Dis. 2014;41:1223–1228. [DOI] [PubMed] [Google Scholar]
- 47.Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA. 2014;312:2569–2570. [DOI] [PubMed] [Google Scholar]
- 48.Patel MS. Nudges for influenza vaccination. Nat Hum Behav. 2018;2:720–721. [DOI] [PubMed] [Google Scholar]
- 49.Kroger E, Wilchesky M, Marcotte M, et al. Medication use among nursing home residents with severe dementia: identifying categories of appropriateness and elements of a successful intervention. J Am Med Dir Assoc. 2015;16:629.e1–629.e17. [DOI] [PubMed] [Google Scholar]
- 50.Maust DT, Strominger J, Kim HM, et al. Prevalence of central nervous system-active polypharmacy among older adults with dementia in the US. JAMA. 2021;325:952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sucharew H, Macaluso M. Progress notes: methods for research evidence synthesis: the scoping review approach. J Hosp Med. 2019;14:416–418. [DOI] [PubMed] [Google Scholar]
- 52.O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


