Abstract
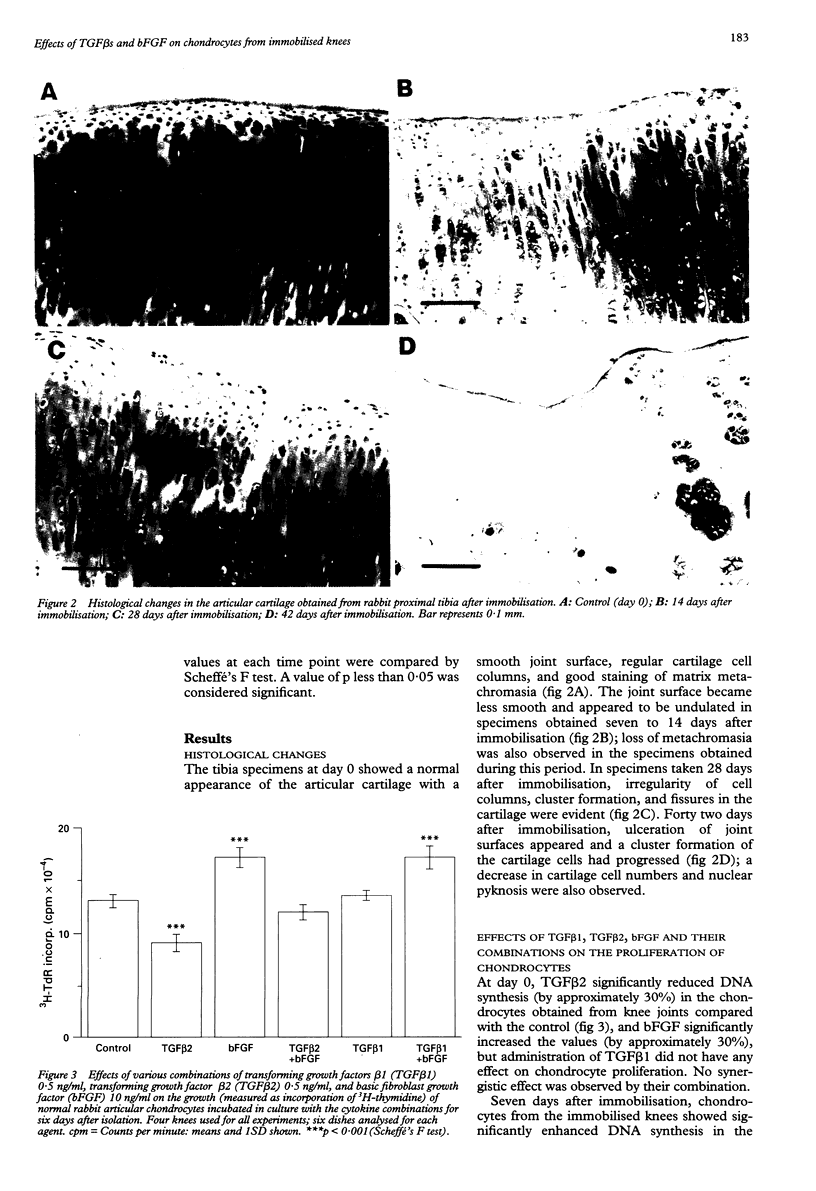
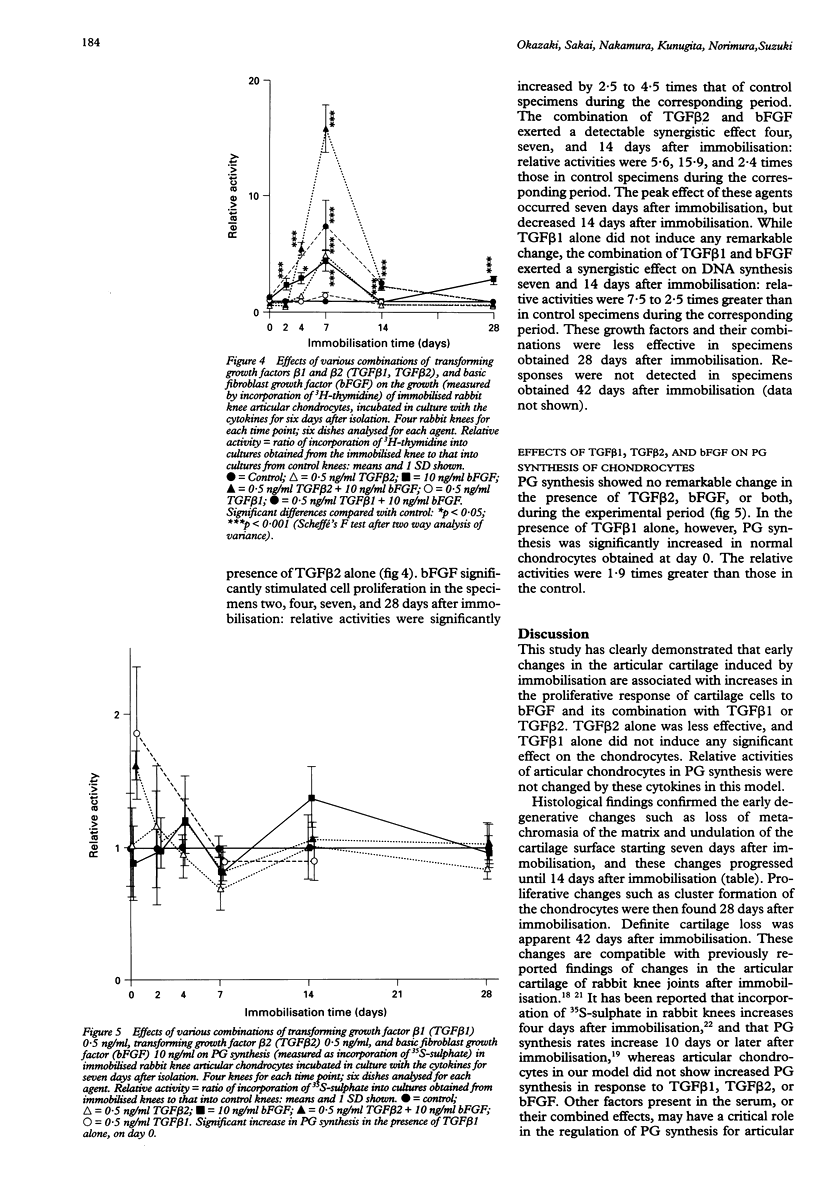
OBJECTIVE: To clarify the effects of transforming growth factor beta 1 (TGF beta 1), TGF beta 2, and basic fibroblast growth factor (bFGF) on cell proliferation and proteoglycan (PG) synthesis in articular chondrocytes obtained from immobilised rabbit knees. METHODS: The right knees of rabbits were immobilised in full extension for up to 42 days using fiberglass casts. Specimens for histology were stained with safranin O. Chondrocytes were isolated from the weight bearing regions of the femur and tibia of the immobilised knees and cultured with combinations of growth factors. Cell proliferation and PG synthesis were determined by 3H-thymidine and 35S-sulphate incorporations. RESULTS: Histological study revealed loss of metachromasia in the articular cartilage at seven days, fissuring and cell clusters at 28 days, and loss of cartilage layers 42 days after immobilisation. Radioisotope assay of the chondrocytes revealed no remarkable change in DNA synthesis in the presence of either TGF beta 1 or TGF beta 2 alone. bFGF markedly stimulated cell proliferation in specimens obtained 0 to seven days after immobilisation. The combination of either TGF beta 1 or TGF beta 2 with bFGF had a synergistic effect, inducing significant increases in DNA synthesis four, seven, and 14 days after immobilisation. PG synthesis by chondrocytes from immobilised joints was not significantly altered by these agents. CONCLUSION: TGF beta 1 or TGF beta 2 in combination with bFGF exert synergistic effects on cell proliferation in articular chondrocytes obtained from the rabbit knee during the early days after immobilisation by a cast. These results suggest a critical role of cytokine combinations in the development of articular cartilage degeneration after immobilisation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
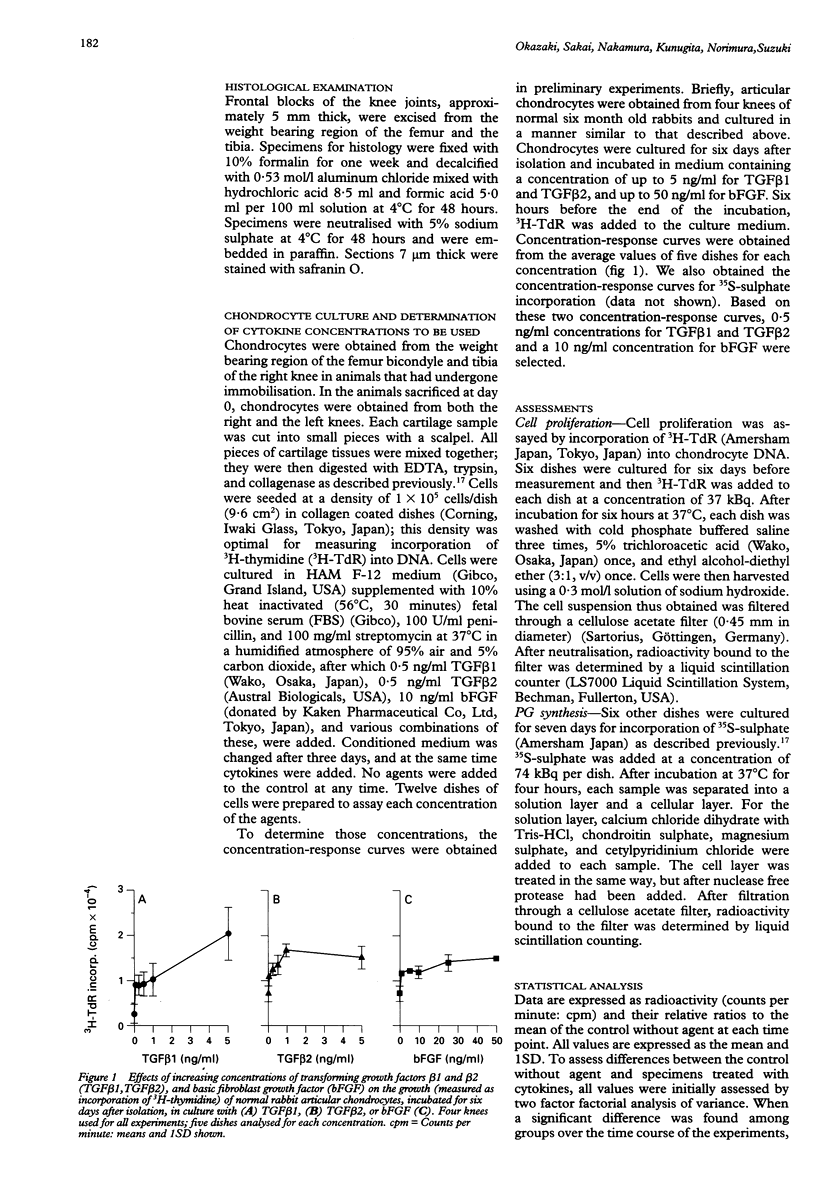
- Candolin T., Videman T. Surface changes in the articular cartilage of rabbit knee during immobilization. A scanning electron microscopic study of experimental osteoarthritis. Acta Pathol Microbiol Scand A. 1980 Sep;88(5):291–297. doi: 10.1111/j.1699-0463.1980.tb02499.x. [DOI] [PubMed] [Google Scholar]
- Chen P., Carrington J. L., Hammonds R. G., Reddi A. H. Stimulation of chondrogenesis in limb bud mesoderm cells by recombinant human bone morphogenetic protein 2B (BMP-2B) and modulation by transforming growth factor beta 1 and beta 2. Exp Cell Res. 1991 Aug;195(2):509–515. doi: 10.1016/0014-4827(91)90403-h. [DOI] [PubMed] [Google Scholar]
- Cuevas P., Burgos J., Baird A. Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem Biophys Res Commun. 1988 Oct 31;156(2):611–618. doi: 10.1016/s0006-291x(88)80887-8. [DOI] [PubMed] [Google Scholar]
- Eronen I., Videman T., Friman C., Michelsson J. E. Glycosaminoglycan metabolism in experimental osteoarthrosis caused by immobilization. Acta Orthop Scand. 1978 Aug;49(4):329–334. doi: 10.3109/17453677809050083. [DOI] [PubMed] [Google Scholar]
- Fava R., Olsen N., Keski-Oja J., Moses H., Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989 Jan 1;169(1):291–296. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galéra P., Rédini F., Vivien D., Bonaventure J., Penfornis H., Loyau G., Pujol J. P. Effect of transforming growth factor-beta 1 (TGF-beta 1) on matrix synthesis by monolayer cultures of rabbit articular chondrocytes during the dedifferentiation process. Exp Cell Res. 1992 Jun;200(2):379–392. doi: 10.1016/0014-4827(92)90186-c. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Sublet A., Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994 Mar;158(3):476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- Hill D. J., Logan A. Peptide growth factors and their interactions during chondrogenesis. Prog Growth Factor Res. 1992;4(1):45–68. doi: 10.1016/0955-2235(92)90004-2. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Jr, Higginbotham J. D., Chandrasekhar S. Transforming growth factor-beta and fibroblast growth factor act synergistically to inhibit collagen II synthesis through a mechanism involving regulatory DNA sequences. J Cell Physiol. 1989 Oct;141(1):8–15. doi: 10.1002/jcp.1041410103. [DOI] [PubMed] [Google Scholar]
- Inoue H., Kato Y., Iwamoto M., Hiraki Y., Sakuda M., Suzuki F. Stimulation of cartilage-matrix proteoglycan synthesis by morphologically transformed chondrocytes grown in the presence of fibroblast growth factor and transforming growth factor-beta. J Cell Physiol. 1989 Feb;138(2):329–337. doi: 10.1002/jcp.1041380216. [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Sato K., Nakashima K., Fuchihata H., Suzuki F., Kato Y. Regulation of colony formation of differentiated chondrocytes in soft agar by transforming growth factor-beta. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1006–1011. doi: 10.1016/0006-291x(89)92208-0. [DOI] [PubMed] [Google Scholar]
- Joyce M. E., Roberts A. B., Sporn M. B., Bolander M. E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990 Jun;110(6):2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Gospodarowicz D. Sulfated proteoglycan synthesis by confluent cultures of rabbit costal chondrocytes grown in the presence of fibroblast growth factor. J Cell Biol. 1985 Feb;100(2):477–485. doi: 10.1083/jcb.100.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Iwamoto M., Koike T. Fibroblast growth factor stimulates colony formation of differentiated chondrocytes in soft agar. J Cell Physiol. 1987 Dec;133(3):491–498. doi: 10.1002/jcp.1041330309. [DOI] [PubMed] [Google Scholar]
- Lafeber F. P., Vander Kraan P. M., Huber-Bruning O., Vanden Berg W. B., Bijlsma J. W. Osteoarthritic human cartilage is more sensitive to transforming growth factor beta than is normal cartilage. Br J Rheumatol. 1993 Apr;32(4):281–286. doi: 10.1093/rheumatology/32.4.281. [DOI] [PubMed] [Google Scholar]
- Langenskiöld A., Michelsson J. E., Videman T. Osteoarthritis of the knee in the rabbit produced by immobilization. Attempts to achieve a reproducible model for studies on pathogenesis and therapy. Acta Orthop Scand. 1979 Feb;50(1):1–14. doi: 10.3109/17453677909024083. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Nakashima M., Eguchi K., Aoyagi T., Yamashita I., Ida H., Sakai M., Shimada H., Kawabe Y., Nagataki S., Koji T. Expression of basic fibroblast growth factor in synovial tissues from patients with rheumatoid arthritis: detection by immunohistological staining and in situ hybridisation. Ann Rheum Dis. 1994 Jan;53(1):45–50. doi: 10.1136/ard.53.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redini F., Daireaux M., Mauviel A., Galera P., Loyau G., Pujol J. P. Characterization of proteoglycans synthesized by rabbit articular chondrocytes in response to transforming growth factor-beta (TGF-beta). Biochim Biophys Acta. 1991 Jul 10;1093(2-3):196–206. doi: 10.1016/0167-4889(91)90123-f. [DOI] [PubMed] [Google Scholar]
- Sakai A., Suzuki K., Nakamura T., Norimura T., Tsuchiya T. Effects of pulsing electromagnetic fields on cultured cartilage cells. Int Orthop. 1991;15(4):341–346. doi: 10.1007/BF00186874. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Jr, Oegema T. R., Jr Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am. 1979 Apr;61(3):407–416. [PubMed] [Google Scholar]
- Videman T., Michelsson J. E., Rauhamäki R., Langenskiöld A. Changes in 35S-sulphate uptake in different tissues in the knee and hip regions of rabbits during immobilization, remobilization the development of osteoarthritis. Acta Orthop Scand. 1976 Jun;47(3):290–298. doi: 10.3109/17453677608991993. [DOI] [PubMed] [Google Scholar]
- Villiger P. M., Lotz M. Differential expression of TGF beta isoforms by human articular chondrocytes in response to growth factors. J Cell Physiol. 1992 May;151(2):318–325. doi: 10.1002/jcp.1041510213. [DOI] [PubMed] [Google Scholar]
- Vivien D., Boumedienne K., Galera P., Lebrun E., Pujol J. P. Flow cytometric detection of transforming growth factor-beta expression in rabbit articular chondrocytes (RAC) in culture--association with S-phase traverse. Exp Cell Res. 1992 Nov;203(1):56–61. doi: 10.1016/0014-4827(92)90039-b. [DOI] [PubMed] [Google Scholar]
- Vivien D., Galera P., Loyau G., Pujol J. P. Differential response of cultured rabbit articular chondrocytes (RAC) to transforming growth factor beta (TGF-beta)-evidence for a role of serum factors. Eur J Cell Biol. 1991 Apr;54(2):217–223. [PubMed] [Google Scholar]
- Vogel K. G., Hernandez D. J. The effects of transforming growth factor-beta and serum on proteoglycan synthesis by tendon fibrocartilage. Eur J Cell Biol. 1992 Dec;59(2):304–313. [PubMed] [Google Scholar]
- van der Kraan P., Vitters E., van den Berg W. Differential effect of transforming growth factor beta on freshly isolated and cultured articular chondrocytes. J Rheumatol. 1992 Jan;19(1):140–145. [PubMed] [Google Scholar]