Background:
Retrograde type A dissection (RTAD) is a devastating complication of thoracic endovascular repair (TEVAR) with low incidence but high mortality. The objective of this study is to report the incidence, mortality, potential risk factors, clinical manifestation and diagnostic modalities, and medical and surgical treatments.
Methods:
A systematic review and single-arm and two-arm meta-analyses evaluated all published reports of RTAD post-TEVAR through January 2021. All study types were included, except study protocols and animal studies, without time restrictions. Outcomes of interest were procedural data (implanted stent-grafts type, and proximal stent-graft oversizing), the incidence of RTAD, associated mortality rate, clinical manifestations, diagnostic workouts and therapeutic management.
Results:
RTAD occurred in 285 out of 10,600 patients: an estimated RTAD incidence of 2.3% (95% CI: 1.9–2.8); incidence of early RTAD was approximately 1.8 times higher than late. Wilcoxon signed-rank testing showed that the proportion of RTAD patients with acute type B aortic dissection (TBAD) was significantly higher than those with chronic TBAD (P = .008). Pooled meta-analysis showed that the incidence of RTAD with proximal bare stent TEVAR was 2.1-fold higher than with non-bare stents: risk ratio was 1.55 (95% CI: 0.87–2.75; P = .13). Single arm meta-analysis estimated a mortality rate of 42.2% (95% CI: 32.5–51.8), with an I2 heterogeneity of 70.11% (P < .001).
Conclusion:
RTAD is rare after TEVAR but with high mortality, especially in the first month post-TEVAR with acute TBAD patients at greater risk as well as those treated with proximal bare stent endografts.
Keywords: complication, meta-analysis, retrograde type A aortic dissection, TEVAR
1. Introduction
Aortic dissection generally has a high rate of mortality if untreated; with Type A aortic dissection particularly, 30-day mortality can be as high as 90%.[1] The true incidence of aortic dissection is not well known, but with the advent of new diagnostic modalities over the last decade, estimations have dramatically risen.[2,3] Annually, 5 to 10 people per million experience an aortic dissection in the United States with 43,000 to 47,000 lives claimed due to the involvement of the aorta and its branches.[4,5]
The condition is conventionally classified as Stanford Types A or B, with the latter involving the descending aorta. New classifications – such as TEM (Type of dissection, location the primary Entry, and Malperfusion) and the Society for Vascular Surgeons reporting standards – have further clarified the extent of the disease process and improved awareness of the disease mechanism to guide decision making and predict outcomes.[6,7] Acute Type B aortic dissection (TBAD) is an uncommon condition involving the descending aorta that remains a challenging problem for cardiothoracic and vascular surgeons as well as interventional radiologists whereas treatment of chronic TBAD can vary between medical and surgical therapies.[8–10]
Conventionally, patients with uncomplicated TBAD receive medical treatment, while evidence progressively support thoracic endovascular aortic repair (TEVAR) as the preferred treatment for complicated and some high-risk TBAD according to Society for Vascular Surgeons guidelines.[7,11] While endovascular techniques were initially used for patients not indicated for conventional surgery, indications have rapidly expanded owing to recent clinical progress over the last decades.[12] Increasing evidence shows positive TEVAR outcomes with acceptable protection against aorta-related death in mid-term follow-up. TEVAR stabilizes the dissected aorta and prevents late complications by expanding the true lumen, inducing both false lumen thrombosis and aortic wall remodeling. In comparison with traditional open aortic surgery, TEVAR has the benefits of fewer complications, smaller incisions, and shorter length of hospital stay which explains the reason that it is currently the preferred treatment for complicated TBAD.[13]
TEVAR is still linked with major complications such as acute or delayed retrograde Type A dissection (RTAD), stroke, bowel infarction, access-related complications, paraplegia, endoleaks, limb ischemia, or wound infection.[14] RTAD is a devastating complication of this procedure with a low incidence, but mortality rates exceed 40%.[15] A wide range of studies on RTAD post-TEVAR have reported small numbers of patients with unclear diagnostic and therapeutic approaches. Different etiologies have been proposed for RTAD but is essentially due to unfavorable interaction between the stent-graft and dissecting membrane that can produce a new primary entry tear and lead to rupture of the membrane. Interpretation is complicated by heterogeneity of data quality, definitions and the reported parameters; as well by its broader relation to any stent graft-induced aortic wall injury and to other iatrogenic injury in non-dissections.[13,16–18] RTAD is also sometimes referred to as proximal SINE (to complement distal stent-graft-induced new entry).[19]
We conducted this comprehensive systematic review and single-arm and two-arm meta-analyses to identify all published reports on RTAD post-TEVAR with the intention of recording the incidence, mortality, potential risk factors, clinical manifestation and diagnostic modalities, and medical and surgical treatments. The findings might assist in designing appropriate clinical strategies to minimize occurrence and diagnose and treat this complication early and effectively in the hope of improving future procedural safety and outcomes.
2. Methods
This is a systematic review carried out according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Table S1, Supplemental Digital Content, http://links.lww.com/MD/I478, which illustrates PRISMA 2020 checklist).[20] We used the PICOS strategy (population, intervention, comparison, outcomes, and design of studies) to formulate the research question and eligibility criteria:
Population: patients with Type B aortic dissection
Intervention: thoracic endovascular aortic repair
Comparators: none
Outcome: incidence of RTAD, re-intervention and its types, mortality of RTAD
Study design: all study designs except for study protocols, animal studies.
To eliminate the risk of analyzing the same patients more than once, the studies were assessed and duplicate publications and overlapping reports were removed. Extensive effort was made to minimize the impact of covert duplicate or metachronous re-publication from the same groups or patient cohorts; for these cases, only the latest report was included.
The search was conducted in PubMed, Cochrane Central, Embase, and Web of Science databases through January 2021.[21,22] The search terms included “TEVAR,” “retrograde dissection,” “thoracic stent-graft,” “endograft,” and “graft” with the Boolean operator “OR,” was restricted to English-language results and with no limits on date of publication. All retrieved results were assessed and screened to obtain additional relevant articles not indexed in common databases.
To be included in the meta-analysis, publications had to meet all the following inclusion criteria: (1) Articles reporting complications of RTAD post-TEVAR among those who underwent endovascular repair or hybrid repair of thoracic aortic pathology; (2) Diagnosis of aortic pathology made by computed tomography (CT) imaging of the thorax, abdomen, or pelvis; (3) Series with more than 5 patients with TEVAR; (4) Demographic data and comorbidities of the patients; (5) At least one of the basic outcome criteria (number of patients with TEVAR, number of patients with RTAD, or mortality of RTAD).
After first screening of titles and abstracts in selected electronic databases, the full texts of appropriate studies were evaluated and their data were extracted by three investigators (SAHS, NH, and MMO) independently. Discrepancies among these investigators were resolved through discussions with a senior author (HE). The following data for each study were extracted: study characteristics, patient characteristics, studies quality, aortic pathology, procedural data (implanted stent-grafts type, and proximal stent-graft oversizing), mean follow-up period, number of patients with RTAD, re-intervention and its types, and RTAD mortality rate.
Since our study is based on already published literature with no interaction with human subjects, no issues related to medical ethics were needed to be reported.
2.1. Definition of extracted data
Regular and irregular imaging follow-up period was considered as ≥ 3 thoracic CTs after TEVAR and < 3, respectively. Aortic dissection was described as an acute event if it occurred within the first 14 days from the onset of symptoms, and chronic beyond 14 days. Postoperative mortality was defined as all death events occurred during follow-up. Early RTAD or early mortality was considered if occurred within the first 3 months from the TEVAR procedure, while late RTAD or late mortality occurred after 3 months from the TEVAR procedure.[23,24]
2.2. Statistical analysis
For the single-arm meta-analysis, analyses of proportions were conducted for data using a random effects model to calculate pooled incidences of RTAD and mortality rates and their confidence intervals (CI) using per protocol and intention to treat data when available. For the two-arm meta-analysis, dichotomous data were presented as risk ratios (RR) and continuous data as weighted mean differences. Summary effect measures were presented along with their corresponding 95% CIs. Statistical heterogeneity was evaluated with the I2 statistic. I2 value between 0% and 25% indicates insignificant heterogeneity, 26% and 50% low heterogeneity, 51% and 75% moderate heterogeneity, and 76% and 100% high heterogeneity. When I2 was < 50%, a fixed-effects model was used and when it was > 50%, a random-effects model. For the analysis of other data that were not included in the meta-analysis, the data were analyzed using the statistical package IBM SPSS version 26.0 (Statistical Package for the Social Sciences, Chicago, IL). The categorical variables are expressed as proportions and frequencies. The continuous variables are summarized as mean ± standard-deviations. Also, in order to explore the independent nature of some categorical variables, Chi-square or exact Fisher test were used. Normality of numerical variables was checked using the Kolmogorov Smirnov test. t-test or Wilcoxon test were applied for comparing of two related groups. One-way ANOVA, Kruskal Wallis and Friedman tests also were implemented based on the normality test for more than two-group comparisons. A P value less than .05 was considered statistically significant in all analyses.
3. Results
The literature search yielded 1963 potentially eligible articles. After considering our selection criteria, 78 eligible clinical studies[4,5,8,10,12,14,17,25–95] published between 2002 and 2020 were enrolled in the qualitative and quantitative analysis (Fig. 1). Of the total included records, 59, 10, and 9 studies were single-center, national multi-center, and international multi-center studies, respectively (Table 1). Most of the studies were conducted in Europe (31/78 studies; 39.7%) and Asia (26/78 studies; 33.3%). Sixteen studies (20.5%) were conducted in North America, one in South America, and four were multi-continental studies. The studies were assigned into two categories according to the number of TBAD patients undergoing TEVAR during the study period. Thirty-nine studies (50%) with 1321 patients, and 39 (50%) with 9279 cases had < 50 and > 50 cases, respectively.
Figure 1.
PRISMA flow chart of the study.
Table 1.
Details and characteristics of studies reporting retrograde type A dissection after thoracic endovascular aortic repair.
| First author | Year | Duration | Geography | Center | Mean follow-up (mo) | TEVARs (n) | RTAD (n) | Age (yr) | Male sex (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEVAR | RTAD | TEVAR | RTAD | |||||||||
| Czermak[25] | 2002 | (1996–2001) | Austria | Innsbruck | SC | 17.8 | 5 | 1 | 51.12 | 43 | NR | NR |
| Kato[26] | 2002 | (1997–2001) | Japan | Mie, Matsusaka | SC | 27 | 28 | 1 | 66.6 | NR | 22 | NR |
| Palmer[27] | 2002 | (1999–2001) | Germany | Ulm | SC | 14 | 14 | 2 | 60.3 | 47.5 | 12 | 2 |
| Fattori[28] | 2003 | (1997–2002) | Italy | Bologna | SC | 25 | 22 | 2 | NR | NR | NR | NR |
| Grabenwoger[29] | 2004 | (1996–2003) | Austria | Vienna | SC | NR | 20 | 1 | NR | NR | NR | NR |
| Hansen[30] | 2004 | (1998–2003) | USA | Torrance | SC | 24 | 24 | 1 | 69 (43–86) | NR | NR | NR |
| Lee[31] | 2004 | (1994–2002) | South Korea | Seoul | SC | 34 | 37 | 1 | NR | NR | NR | NR |
| Dong Xu[32] | 2005 | (2001–2004) | China | Beijing | SC | 32 | 24 | 3 | NR | NR | NR | NR |
| Fattori[33] | 2006 | (1996–2004) | Italy, Germany, France, Netherlands, etc. | Multicenter | IMC | 24 | 180 | 2 | NR | NR | NR | NR |
| Duebener[34] | 2007 | (2000–2006) | Germany | Luebeck | SC | 38 | 13 | 1 | 59.5 | NR | 10 | NR |
| Zipfel[35] | 2007 | (1999–2005) | Germany | Berlin | SC | 23 | 57 | 1 | 62 | 38 | 43 | 0 |
| Kpodonu[36] | 2008 | (2000–2006) | USA | Pennsylvania | NMC | NR | 91 | 6 | NR | 69 | NR | 3 |
| Neuhauser[14] | 2008 | (1997–2007) | Austria | Innsbruck | SC | 43 | 28 | 5 | NR | 65 | NR | 4 |
| Dong[37] | 2009 | (2000–2007) | China | Shanghai | SC | 26 | 443 | 11 | NR | 43 | NR | NR |
| Chiesa[38] | 2011 | (1999–2011) | Italy | Milan | SC | NR | 188 | 3 | NR | NR | NR | NR |
| Kim[39] | 2011 | (2002–2009) | USA | Torrance | SC | 12.4 | 41 | 3 | 67.6 | NR | 31 | NR |
| Oberhuber[40] | 2011 | (1999–2011) | Germany | Ulm | SC | 12.7 | 19 | 1 | 60 | NR | 17 | NR |
| Parsa[41] | 2011 | (2005–2009) | USA | North Carolina | SC | 27 | 51 | 2 | 57 | NR | 37 | NR |
| Wiedemann[4] | 2013 | (1996–2010) | Austria | Vienna | SC | 52 | 80 | 3 | 59 | NR | 58 | NR |
| Lotfi[42] | 2013 | (1997–2011) | UK | London | SC | 15 | 11 | 3 | NR | NR | NR | NR |
| Wiedemann[5] | 2014 | (1999–2011) | Austria, France, Italy, Spain, USA | Multicenter | IMC | 37 | 110 | 6 | 61 | NR | 86 | NR |
| Faure[43] | 2014 | (2000–2011) | France | Montpellier | SC | 12.2 | 41 | 1 | 66 | NR | 34 | NR |
| Idrees[44] | 2014 | (2000–2012) | USA | Cleveland, Ohio | SC | 48 | 766 | 15 | NR | 65 | NR | NR |
| Zhang[45] | 2014 | (1998–2012) | China | Shanghai | SC | 58.4 | 252 | 2 | 54.1 | NR | 206 | NR |
| Gorlitzer[46] | 2012 | (2005–2011) | Austria, Switzerland | Vienna, Bern | IMC | NR | 29 | 4 | NR | 62 | NR | 2 |
| Huang[47] | 2013 | (2004–2011) | China | Guangzhou | SC | NR | 563 | 4 | 54.09 | 62.75 | 485 | 3 |
| Cochernnec[48] | 2013 | (2004–2011) | France | Creteil | SC | 24.5 | 17 | 4 | 60 | 63.75 | 11 | 2 |
| Shuyang Lu[49] | 2012 | (2006–2011) | China | Shanghai | SC | 34.79 | 419 | 9 | NR | 56.6 | 277 | 6 |
| Yang[50] | 2012 | (2006–2011) | Taiwan | Taipei | SC | 24.1 | 61 | 1 | 62.7 | NR | 51 | NR |
| Bunger[51] | 2013 | (2006–2012) | Germany | Rostock | SC | 27.9 | 45 | 1 | 59.9 | 55 | 38 | 1 |
| Canaud[52] | 2014 | (2002–2012) | UK | London | SC | NR | 309 | 11 | 63.1 | NR | 248 | NR |
| Lombardi[53] | 2012 | (2007–2012) | Italy, Germany, Australia, USA | Multicenter | IMC | 12 | 40 | 3 | 58 | NR | 28 | NR |
| Jia[54] | 2013 | (2007–2010) | China | Beijing, Zhengzhou, Xinxiang | NMC | 28.5 | 208 | 3 | 52.1 | NR | 154 | NR |
| Li[55] | 2014 | (2005–2012) | China | Beijing | NMC | 32.2 | 669 | 6 | NR | 41.2 | NR | 20 |
| Hanna[56] | 2014 | (2005–2012) | USA | North Carolina | SC | 34.1 | 50 | 1 | 59 | NR | 36 | NR |
| De Rango[57] | 2014 | (2005–2013) | Italy | Rome, Perugia | NMC | 29.2 | 104 | 4 | 69.8 | NR | 90 | NR |
| Appoo[58] | 2015 | (2008–2012) | Canada | Alberta | SC | 72 | 16 | 0 | 63.8 | NR | NR | NR |
| Desai[59] | 2015 | (2005–2012) | USA | Philadelphia | SC | 132 | 9 | 64.1 | NR | 80 | NR | |
| Kische[60] | 2015 | (2009–2015) | Germany | Berlin, Rostock | NMC | 25.6 | 35 | 1 | 63 | NR | 27 | NR |
| Bockler[61] | 2016 | (2009–2010) | Germany, UK, Italy, Sweden | Multicenter | IMC | 24 | 24 | 1 | NR | NR | NR | NR |
| Faure[62] | 2016 | (2005–2015) | France | Montpellier | SC | 24.3 | 33 | 1 | 65.1 | 62 | 26 | 1 |
| Wang[63] | 2016 | (2005–2013) | China | Zhengzhou | SC | 32 | 360 | 5 | 52 | 51.8 | 304 | 4 |
| Asaloumidis[64] | 2017 | (2000–2014) | Greece | Thessaloniki | SC | 74 | 40 | 2 | 65 | NR | 33 | NR |
| Zhao Liu[65] | 2017 | (2008–2016) | China | Nanjing | SC | 30.5 | 58 | 6 | 57.3 | NR | 40 | NR |
| Min-Hong Zhang[66] | 2017 | (2011–2013) | China | Beijing | SC | 26.4 | 85 | 3 | 64.3 | NR | 59 | NR |
| Tjaden[67] | 2018 | (2010––2016) | USA, Europe, Brazil and Oceania | Multicenter | IMC | 26 | 264 | 6 | 62 | NR | 211 | NR |
| Tao Ma[68] | 2018 | (2005–2013) | China, UK | Shanghai, London | IMC | 31.2 | 852 | 27 | 55 | NR | 720 | NR |
| Laquian[69] | 2018 | (2011–2014) | USA | Florida, Alabama | NMC | 17.9 | 27 | 1 | 63 | NR | 17 | NR |
| Chen[70] | 2018 | (2007–2014) | China | Hebei, Beijing | NMC | 17.9 | 167 | 1 | NR | NR | 112 | NR |
| Piotr Buczkowski[71] | 2019 | (2007–2017) | Poland | Poznan | SC | 55 | 68 | 2 | NR | NR | NR | NR |
| Eleshra[72] | 2020 | (2010–2017) | Germany | Hamburg | SC | 28 | 64 | 1 | 64.8 | NR | 49 | NR |
| Fukushima[73] | 2019 | (2011–2017) | Japan | Chiba | SC | 14.2 | 24 | 0 | 67.7 | NR | 21 | NR |
| Wang [74] | 2019 | (2013–2014) | USA | Multicenter | IMC | 1 | 397 | 6 | 60.4 | NR | 286 | NR |
| Yammine[17] | 2019 | (2012–2017) | USA | North Carolina | SC | 14.25 | 186 | 15 | 61.6 | 61.5 | 112 | 8 |
| Miura[75] | 2019 | (2013–2017) | Japan | Sapporo | SC | 19.6 | 22 | 0 | 63 | NR | 16 | NR |
| Chassin-Trubert[76] | 2020 | (2013–2019) | France | Montpellier | SC | 26 | 17 | 0 | NR | NR | NR | NR |
| Pellenc[77] | 2019 | (2015–2018) | France | Paris | SC | 22 | 20 | 0 | NR | NR | NR | NR |
| Jiechang Zhu[78] | 2018 | (2015–2016) | China | Tianjin | SC | 6.95 | 20 | 0 | 53 | NR | 16 | NR |
| Riesterer[79] | 2018 | (2002–2017) | Germany | Freiburg | SC | 16 | 34 | 1 | NR | NR | NR | NR |
| Giles[12] | 2019 | (2005–2016) | USA | Gainesville | SC | 17 | 258 | 12 | 61.5 | NR | 203 | NR |
| Kuo[80] | 2019 | (2006–2016) | USA | Los Angeles | SC | 14 | 71 | 2 | 58.6 | NR | 52 | NR |
| Joo[81] | 2019 | (1994–2017) | South Korea | Seoul | SC | NR | 17 | 2 | 50.4 | 42 | 14 | 2 |
| Cao[82] | 2020 | (2015–2018) | China | Beijing | SC | 17.6 | 76 | 4 | 50.3 | NR | 51 | NR |
| El-Beyrouti[83] | 2020 | (2018–2019) | Germany | Mainz, Tübingen | NMC | 11.6 | 5 | 0 | NR | NR | NR | NR |
| Charltonouw[84] | 2018 | (1999–2014) | USA | Houston | SC | 51.6 | 43 | 3 | NR | NR | NR | NR |
| Lou[85] | 2020 | (2012–2018) | USA | South Carolina | SC | 36 | 91 | 3 | 52.6 | NR | 60 | NR |
| Lee[86] | 2020 | (2003–2017) | South Korea | Seoul, Incheon and Cheonan | NMC | 39.4 | 87 | 2 | 58.3 | NR | 62 | NR |
| Oshi[87] | 2020 | (2009–2019) | Japan | Fukuoka | SC | 39.2 | 40 | 1 | 66.5 | NR | 26 | NR |
| Puech-Leao[88] | 2020 | (2004–2017) | Brazil | Sao Paulo | SC | 57 | 42 | 4 | 59.1 | NR | 32 | NR |
| Sobocinski[89] | 2020 | (2005–2015) | Sweden, France | Multicenter | IMC | 1 | 41 | 2 | 58.8 | NR | 32 | NR |
| Shuo Zhao[90] | 2020 | (2009–2018) | China | Shandong | SC | 10.7 | 79 | 1 | 49.9 | NR | 61 | NR |
| Bavaria[91] | 2015 | (2010–2012) | USA | Multicenter | NMC | 12 | 50 | 2 | 57.2 | NR | 40 | NR |
| Peidro[92] | 2018 | (2007–2015) | France | Marseille | SC | 29 | 26 | 2 | NR | NR | NR | NR |
| Ding[93] | 2018 | (2011–2016) | China | Guangzhou | SC | 30.8 | 16 | 1 | 51.3 | 64 | 12 | 1 |
| Nozdrzykowskia[94] | 2015 | (2002–2013) | Germany | Leipzig | SC | NR | 129 | 1 | NR | NR | NR | NR |
| Lei Liu[95] | 2016 | (2013–2014) | China | Shanghai | SC | 15.4 | 203 | 11 | 55 | 52.4 | 167 | 7 |
| Hu[10] | 2019 | (2013–2017) | China | Zhejiang | SC | 25.8 | 571 | 12 | NR | NR | NR | NR |
| Gao[8] | 2019 | (2001–2013) | China | Beijing | SC | 77.7 | 751 | 4 | 52.8 | NR | 619 | NR |
IMC = international multicenter, NMC = national multicenter, NR = not reported, RTAD = retrograde type A dissection, SC = single center, TEVAR = thoracic endovascular aortic repair.
Table 2 summarizes the demographic and perioperative characteristics of 10,600 TBAD patients who underwent TEVAR. Patient populations ranged from 5 to 852, with a mean age of 57.4 years, 77.8% being male. Hypertension (83.4%) and smoking (47.7%) were the leading underlying diseases. Preoperative details are summarized in supporting information (see Table S2, Supplemental Digital Content, http://links.lww.com/MD/I479, which illustrates reported risk factors for RTAD). A majority of cases were acute TBAD: in 61/78 reports (n = 6741), 4049 cases (60%) were specified as acute TBAD; in 59 reports (n = 6686), 2997 cases (44.8%) were chronic TBAD. However, 17 and 19 studies, respectively, did not specify TBAD chronicity (see Table S3, Supplemental Digital Content, http://links.lww.com/MD/I480, which illustrates TBAD chronicity).
Table 2.
Risk factors in type B aortic dissection patients who underwent thoracic endovascular aortic repair and those who experienced retrograde type A dissection.
| Risk factors | Studies (n) | Total TBAD patients (N) | Patients with risk factor (n) | Patients with risk factor (%) | |
|---|---|---|---|---|---|
| All patients who underwent thoracic endovascular aortic repair | |||||
| Male gender | 52 | 7110 | 5534 | 77.8 | |
| Hypertension | 47 | 6134 | 5118 | 83.4 | |
| Diabetes mellitus | 37 | 4779 | 474 | 9.9 | |
| Coronary artery disease | 38 | 4477 | 668 | 14.9 | |
| Renal impairment | 38 | 3581 | 446 | 12.4 | |
| Pulmonary disease | 35 | 3369 | 446 | 13.2 | |
| Marfan syndrome | 20 | 2925 | 44 | 1.5 | |
| ASA I | 3 | 958 | 42 | 4.3 | |
| ASA II | 11 | 1482 | 279 | 18.8 | |
| ASA III | 13 | 1558 | 565 | 36.2 | |
| ASA IV | 13 | 1558 | 598 | 38.3 | |
| ASA V | 8 | 1126 | 52 | 4.6 | |
| Smoking | 34 | 5283 | 2521 | 47.7 | |
| Age (yr) | 57.4 | NR | |||
| Risk factors | Studies (n) | Patients with risk factor (n) | Total RTAD (n) | Total TBAD patients (N) | Total TBAD patients (%) |
| Patients with retrograde type A dissection | |||||
| Male gender | 16 | 66 | 77 | 2747 | 85.7 |
| Hypertension | 7 | 44 | 51 | 2099 | 86.2 |
| Diabetes mellitus | 4 | 5 | 35 | 1634 | 14.2 |
| Coronary artery disease | 4 | 4 | 32 | 1306 | 12.5 |
| Renal impairment | 5 | 6 | 41 | 1725 | 14.6 |
| Pulmonary disease | 5 | 7 | 41 | 1725 | 17.0 |
| Marfan syndrome | 11 | 9 | 58 | 2444 | 15.5 |
| ASA | NR | NR | NR | NR | NR |
| Smoking | 4 | 21 | 32 | 1306 | 65.6 |
| Age (yr) | 56.6 | NR | |||
ASA = American Society of Anesthesiology physical status classification, NR = not reported, RTAD = retrograde type A dissection, TBAD = type B aortic dissection.
Of patients who experienced RTAD, mean age was 56.6 years and 85.7% were male. Hypertension (86.2%) was the most common comorbidity for RTAD, followed by smoking (65.6%), pulmonary disease (17.0%), Marfan syndrome (15.5%), renal impairment (14.6%), diabetes mellitus (14.2%), and coronary artery disease (12.5%) (Table 2).
Table 3 presents TEVAR details and the stent-grafts used in each study. From 50 enrolled studies, proximal bare stents were used in 3033 (66%) and proximal non-bare stents in 1569 cases (34%).
Table 3.
Details of published reports of thoracic endovascular aortic repair and incidence of retrograde type A dissection.
| First author | Year | Stent-graft detail | Total TEVARs (N) | TEVAR device | RTAD (n) | TEVAR in patients with RTAD | ||
|---|---|---|---|---|---|---|---|---|
| Bare stent | Non-bare stent | Bare stent | Non-bare stent | |||||
| Czermak | 2002 | Talent (Medtronic) | 5 | 5 | 0 | 1 | 1 | 0 |
| Kato | 2002 | Z stents covered with expanded polytetrafluoroethylene (Impra); Z stents covered with woven polyester | 28 | 0 | 28 | 1 | 0 | 1 |
| Palmer | 2002 | Thoracic Excluder (Gore); Talent (Medtronic) | 14 | 3 | 11 | 2 | 1 | NR |
| Fattori | 2003 | Talent (Medtronic); Thoracic Excluder (Gore) | 22 | NR | NR | 2 | NR | NR |
| Grabenwoger | 2004 | Talent (Medtronic) | 20 | 20 | 0 | 1 | 1 | 0 |
| Hansen | 2004 | AneuRx (Medtronic); Talent (Medtronic); and Excluder (Gore) | 24 | NR | NR | 1 | NR | NR |
| Lee | 2004 | Custom-designed stent-grafts (Impra); 2-component system consisted of a 3-part unsupported nitinol wire stents covered with a graft of synthetic polyester fabric (Dacron; Ube) | 37 | NR | NR | 1 | 1 | 0 |
| Dong Xu | 2005 | TALENT (Medtronic); ENDOFIT (Endomed); VASOFLOW (Vascore); AEGIS (Microport); KINPRIDE (Grikin) | 24 | NR | NR | 3 | NR | NR |
| Fattori | 2006 | Talent (Medtronic) | 180 | 180 | 0 | 2 | 2 | 0 |
| Duebener | 2007 | Talent and Valiant (Medtronic) | 13 | 13 | 0 | 1 | 1 | 0 |
| Zipfel | 2007 | Talent (Medtronic), E-vita (Jotec), Zenith TX1 (Cook), Relay (Bolton Medical), Endofit (Endomed), Valiant (Medtronic), and TAG (Gore). | 57 | NR | NR | 1 | 0 | 1 |
| Kpodonu | 2008 | TAG (Gore) | 91 | 0 | 91 | 6 | 0 | 6 |
| Neuhauser | 2008 | Thoracic Excluder (Gore); Talent (Medtronic) | 28 | NR | NR | 5 | 4 | 1 |
| Dong | 2009 | Talent (Medtronic) | 443 | 401 | 42 | 11 | 11 | 0 |
| Chiesa | 2011 | Not reported | 188 | NR | NR | 3 | NR | NR |
| Kim | 2011 | Talent or Valiant (Medtronic) | 41 | 41 | 0 | 3 | 3 | 0 |
| Oberhuber | 2011 | TAG/cTAG (Gore); Captivia and Valiant (Medtronic); Zenith (Cook) | 19 | 10 | 9 | 1 | 0 | 1 |
| Parsa | 2011 | TAG (Gore), Zenith TX2 (Cook), Talent (Medtronic) | 51 | 1 | 50 | 2 | 0 | 2 |
| Wiedemann | 2013 | Talent (Medtronic); Thoracic Excluder (Gore); Relay (Bolton Medical); Endomed (LeMaitre Vascular), Cook | 80 | 52 | 28 | 3 | 3 | 0 |
| Lotfi | 2013 | TAG; 8 C-TAG (Gore); TX2; 4 TX1 (Cook); Talent; Valiant (Medtronic); Relay (Bolton Medical); Endofit (LeMaitre) | 11 | NR | NR | 3 | NR | NR |
| Wiedemann | 2014 | Talent; Thoracic Excluder; Relay; Zenith; Hemashield; Valiant | 110 | 53 | 57 | 6 | 3 | 3 |
| Faure | 2014 | Thoracic Excluder and C-TAG (Gore); Talent and Valiant (Medtronic); Zenith TX2 (Cook) | 41 | 9 | 32 | 1 | NR | NR |
| Idrees | 2014 | Gore, Cook, Medtronic | 766 | NR | NR | 15 | NR | NR |
| Zhang | 2014 | Hercules (Microport); Talent and Valiant (Medtronic); Zenith (Cook); Relay (Bolton Medical) | 252 | NR | NR | 2 | NR | NR |
| Gorlitzer | 2012 | Valiant (Medtronic); Thoracic Excluder (Gore) | 29 | 24 | 5 | 4 | 4 | 0 |
| Huang | 2013 | Talent (Medtronic); Hercules (Microport); Zenith TX2 (Cook) | 563 | 420 | 143 | 4 | 4 | 0 |
| Cochernnec | 2013 | Cook; Medtronic; Gore; Relay | 17 | 7 | 10 | 4 | 2 | 2 |
| Shuyang Lu | 2012 | Talent; Valiant; Hercules; Zenith TX2 | 419 | NR | NR | 9 | 6 | 3 |
| Yang | 2012 | Zenith TX2 (Cook) | 61 | 0 | 61 | 1 | 0 | 1 |
| Bunger | 2013 | Valiant (Medtronic); Zenith TX2 (Cook); Relay (Bolton Medical) | 45 | NR | NR | 1 | NR | NR |
| Canaud | 2014 | Talent, Valiant, AneuRyx (Medtronic); Vasoflow (Weike Medical); Relay (Bolton Medical); Grikin (Grikin); Ankura (Lifetech); E-vita (Jotec); TAG (Gore) | 309 | NR | NR | 11 | 11 | NR |
| Lombardi | 2012 | Zenith TX2 (Cook) | 40 | 0 | 40 | 3 | 0 | 3 |
| Jia | 2013 | Valiant (Medtronic); Zenith TX2 (Cook); Hercules (Microport) | 208 | NR | NR | 3 | NR | NR |
| Li | 2014 | Talent (Medtronic), Relay (Bolton), Zenith TX2 (Cook), Hercules (Microport), TAG (Gore), Valiant (Medtronic) | 669 | 168 | 501 | 6 | 5 | 1 |
| Hanna | 2014 | TAG/cTAG (Gore); Zenith TX2 (Cook); Talent and Valiant with Captivia (Medtronic) | 50 | 17 | 33 | 1 | NR | NR |
| De Rango | 2014 | Zenith (Cook); TAG/cTAG (Gore); Relay (Bolton Medical); Talent and Valiant (Medtronic) | 104 | NR | NR | 4 | NR | NR |
| Appoo | 2015 | TAG and cTAG (Gore); Zenith TX2 (Cook) | 16 | 2 | 14 | 0 | 0 | 0 |
| Desai | 2015 | Valiant Captivia (Medtronic) (2 of 50; 5% at 1 year) and cTAG (Gore) (5 of 50; 10% at 1 year) | 132 | NR | NR | 9 | NR | NR |
| Kische | 2015 | Zenith; Valiant; Talent | 35 | NR | NR | 1 | NR | NR |
| Bockler | 2016 | cTAG (Gore) | 24 | 24 | 0 | 1 | 1 | 0 |
| Faure | 2016 | Excluder (Gore); TAG (Gore); Talent (Medtronic); Valiant (Medtronic), and Zenith TX2 (Cook) | 33 | NR | NR | 1 | NR | NR |
| Wang | 2016 | Talent (Medtronic); Captivia (Medtronic); Zenith TX2 (Cook); TAG (Gore); (Microport) | 360 | NR | NR | 5 | 4 | NR |
| Asaloumidis | 2017 | Talent (14); TAG (13); Excluder (2); Valiant (2); Captivia (6); Relay (2); AneuRx (1) | 40 | 24 | 16 | 2 | 2 | 0 |
| Zhao Liu | 2017 | Talent and Captivia (Medtronic); TX- 1/TX-2 (Cook); Hercules (Microport); Sinus (OptiMed) | 58 | NR | NR | 6 | NR | NR |
| Min-Hong Zhang | 2017 | Not reported | 85 | NR | NR | 3 | NR | NR |
| Tjaden | 2018 | CTAG or TAG (Gore) | 264 | 264 | 0 | 6 | 6 | NR |
| Tao Ma | 2018 | Talent and Valiant (Medtronic), Zenith TX2 (Cook), Hercules and Castor (Microport), Ankura (Lifetech), Relay (Bolton Medical), EndoFit (LeMaitre), E-vita (Jotec), and TAG (Gore). | 852 | NR | NR | 27 | NR | NR |
| Laquian | 2018 | Not reported | 27 | NR | NR | 1 | NR | NR |
| Chen | 2018 | Not reported | 167 | NR | NR | 1 | NR | NR |
| Piotr Buczkowski | 2019 | Zenith (Cook), JOTEC and Gore | 68 | 0 | 68 | 2 | 0 | 2 |
| Eleshra | 2020 | Not reported | 64 | NR | NR | 1 | NR | NR |
| Fukushima | 2019 | Zenith TX2 Pro-Form (Cook Medical), cTAG (Gore), Relay (Terumo Aortic), Najuta (Kawasumi) | 24 | NR | NR | 0 | NR | NR |
| Wang | 2019 | Valiant (Medtronic), CTAG (Gore), and TX2/Alpha (Cook Medical) | 397 | NR | NR | 6 | NR | NR |
| Yammine | 2019 | Valiant (Medtronic) | 186 | 172 | 0 | 15 | 15 | 0 |
| Miura | 2019 | Relay (Terumo Aortic) | 22 | 22 | 0 | 0 | 0 | 0 |
| Chassin-Trubert | 2020 | Valiant Captivia (Medtronic) | 17 | 17 | 0 | 0 | 0 | 0 |
| Pellenc | 2019 | TX2/TX2 alpha (Cook); cTAG (Gore); Relay (Terumo Aortic); Valiant (Medtronic) | 20 | NR | NR | 0 | 0 | 0 |
| Jiechang Zhu | 2018 | Valiant (Medtronic), Relay (Terumo Aortic) and Ankura (Lifetech) | 20 | 20 | 0 | 0 | 0 | 0 |
| Riesterer | 2018 | Relay NBS (non-bare stent) (Bolton Medical/Terumo Aortic) | 34 | 0 | 34 | 1 | 0 | 1 |
| Giles | 2019 | Not reported | 258 | NR | NR | 12 | NR | NR |
| Kuo | 2019 | TAG/cTAG (Gore), TX2/Alpha (Cook), Valiant (Medtronic) | 71 | 40 | 31 | 2 | NR | NR |
| Joo | 2019 | Valiant (using the Captivia delivery system; Medtronic), Seal (S&G Biotech), TX2 (Cook), TAG (Gore), and unidentified | 17 | 13 | 2 | 2 | 2 | 0 |
| Cao | 2020 | Zenith TX2 (Cook), Valiant (Medtronic), CTAG (Gore), Hercules (MicroPort) and Ankura (Lifetech) | 76 | 65 | 11 | 4 | 3 | 1 |
| El-Beyrouti | 2020 | RelayPro NBS (Terumo Aortic) | 5 | NR | NR | 0 | NR | NR |
| Charltonouw | 2018 | Not reported | 43 | NR | NR | 3 | NR | NR |
| Lou | 2020 | Valiant with Captivia (Medtronic), Zenith TX 2 (Cook), and CTAG (Gore) | 91 | 80 | 11 | 3 | NR | NR |
| Lee | 2020 | Seal (S&G Biotech); Valiant (Medtronic); Zenith TX2 (Cook) | 87 | 51 | 36 | 2 | NR | NR |
| Oshi | 2020 | TAG or cTAG (Gore), Valiant (Medtronic), Zenith TX2 (Cook), and Relay Plus (Terumo Aortic) | 40 | NR | NR | 1 | NR | NR |
| Puech-Leao | 2020 | Not reported | 42 | NR | NR | 4 | NR | NR |
| Sobocinski | 2020 | Not reported | 41 | NR | NR | 2 | NR | NR |
| Shuo Zhao | 2020 | Not reported | 79 | NR | NR | 1 | NR | NR |
| Bavaria | 2015 | Valiant Captivia (Medtronic) | 50 | 50 | 0 | 2 | 2 | 0 |
| Peidro | 2018 | TAG/cTAG (Gore); Valiant/Talent (Medtronic); Zenith/Pro-Form (Cook) | 26 | NR | NR | 2 | NR | NR |
| Ding | 2018 | Valiant (Medtronic); Ankura (Lifetech); ZTEG-2PT (Cook) | 16 | 15 | 1 | 1 | 1 | 0 |
| Nozdrzykowskia | 2015 | TAG/cTAG (Gore); Talent/Valiant/Captivia (Medtronic); Zenith (Cook); and Endofit (LeMaitre Vascular) | 129 | NR | NR | 1 | NR | NR |
| Lei Liu | 2016 | Zenith TX2 (Cook); CTAG (Gore); Talent (Medtronic); and Hercules, Aegis, and Ankura (Microport) | 203 | 85 | 118 | 11 | 5 | 6 |
| Hu | 2019 | Valiant (Medtronic), TAG (Gore), Zenith TX2 (Cook), and Ankura (Lifetech). | 571 | NR | NR | 12 | 8 | 4 |
| Gao | 2019 | GRIMED (GRIMED) in 234 patients, Talent (Medtronic) in 20, Valiant (Medtronic) in 173, Hercules (MicroPort) in 125, Zenith TX2 (Cook) in 86, Relay (Bolton Medical) in 76 and E-vita (Jotec) in 37. | 751 | 665 | 86 | 4 | NR | NR |
NR = not reported, RTAD = retrograde type A dissection, TEVAR = thoracic endovascular aortic repair.
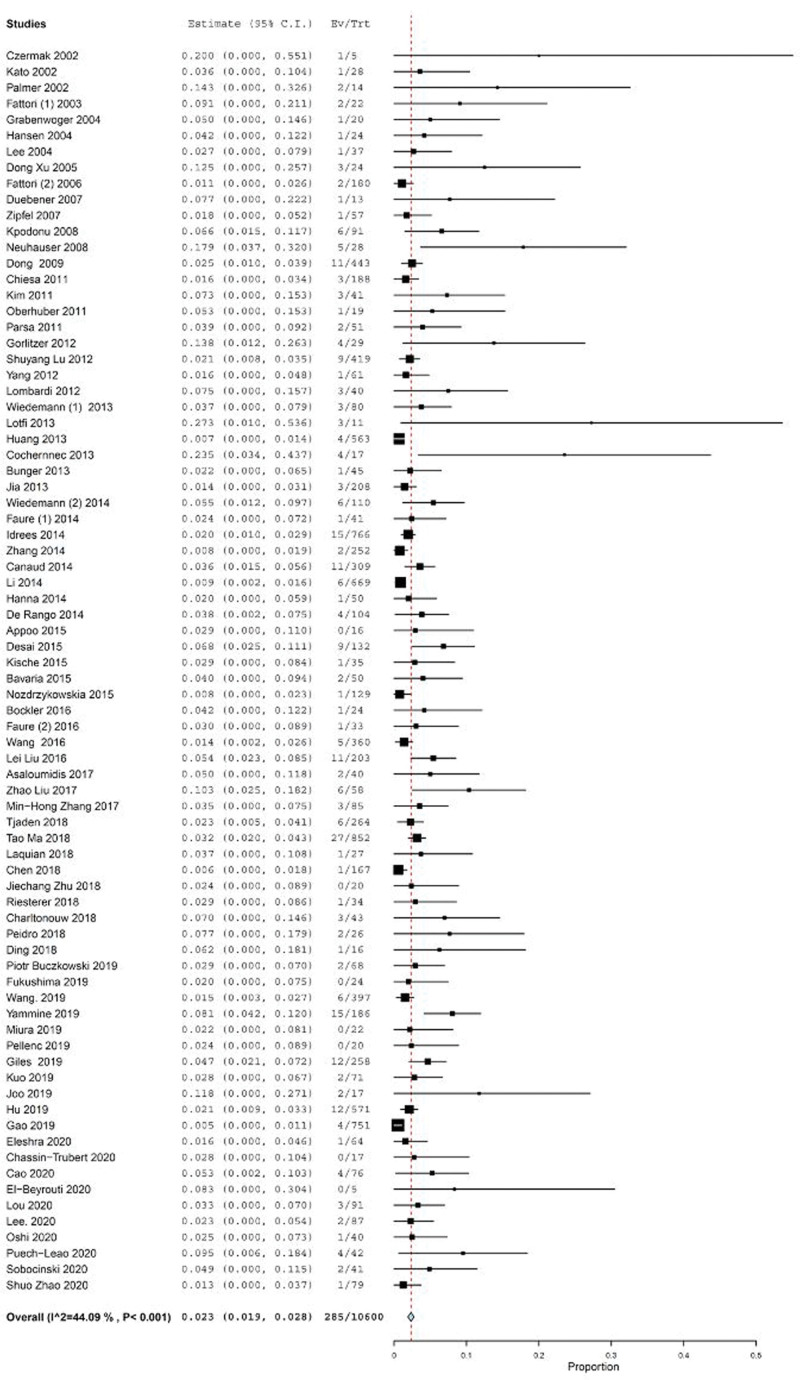
RTAD occurred in 285 cases out of 10,600 patients, representing an estimated RTAD incidence of 2.3% (95% CI: 1.9–2.8), with an I2 heterogeneity of 44.09% (P < .001) (Fig. 2). The incidence of RTAD in the studies conducted in Europe (64/1718 cases; 3.7%), Asia (94/5280 cases; 1.7%), North America (81/2294 cases; 3.5%) as well as multi-continental studies (42/1266 cases; 3.3%) were similar; one smaller study in South America had a higher incidence (4/42 cases; 9.5%). With the exception of one study in South America, no significant difference was found in RTAD incidence among the continents using the Kruskal–Wallis test (P = .08).
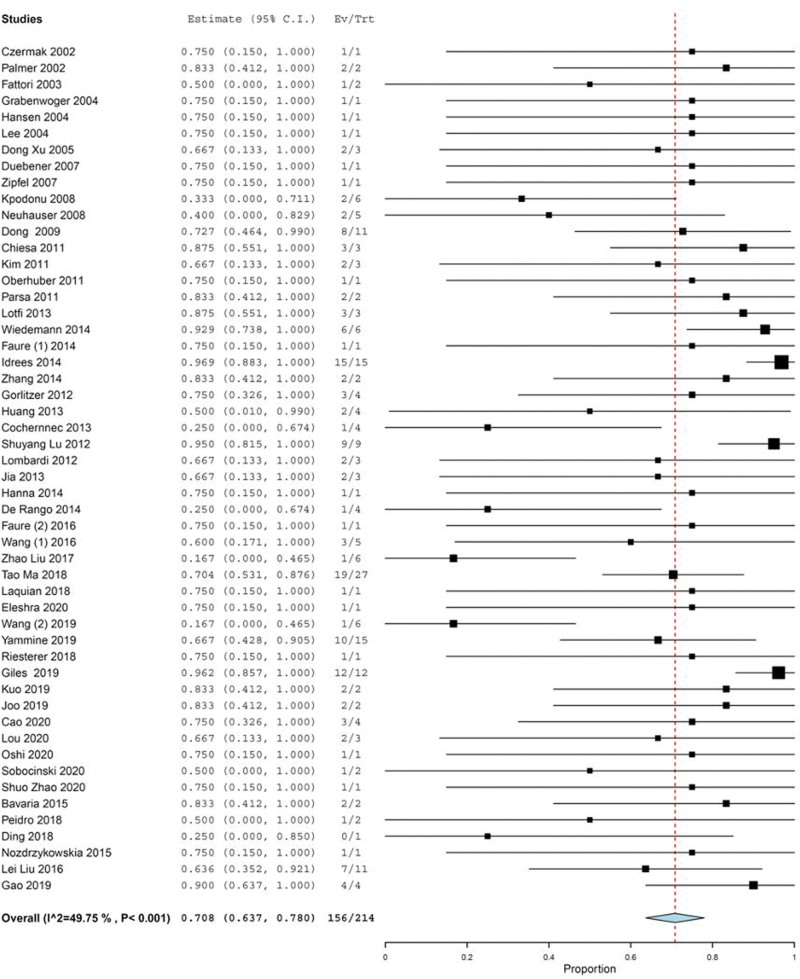
Figure 2.
Forest plot of proportion single-arm meta-analysis for RTAD after TEVAR. RTAD = retrograde type A dissection, TEVAR = thoracic endovascular repair.
Of the overall 285 cases with RTAD, time to occurrence after TEVAR was reported in 147: 89 (60.6%) occurred within 30 days; 43 (29.2%) between 1 and 12 months; 15 (10.2%) later than 1 year. Of the 89 early RTADs (within 30 days), 50 (34.0%) were intraoperative or perioperative (within 15 days of TEVAR) (see Table S4, Supplemental Digital Content, http://links.lww.com/MD/I481, which illustrates time to occurrence of RTAD). The Friedman test showed that the incidence of RTAD was significantly different in these time periods (P = .005). From the enrolled trials, 51 studies with 5058 total cases and 143 RTAD patients reported early RTAD in 94 cases (65.7%). However, 27 studies (5542 total cases and 142 RTAD patients) did not mention any information about the early occurrence of RTAD (Table 4). Forty-seven studies comprising 4592 cases and 128 RTAD patients showed late RTAD in 46 cases (35.9%). However, 31 studies (6008 total and 157 RTAD cases) did not report any information about late RTAD (Table 4). Using Wilcoxon signed-rank test, a significant difference was found in the incidence of RTAD between early and late occurrence (P < .001), i.e., the incidence of early RTAD was 1.8 times higher than that of late RTAD.
Table 4.
Timing of RTAD.
| Time post-TEVAR | Studies (n) | Patients (n) | RTAD (n) | TEVAR (n) |
|---|---|---|---|---|
| 0–14 d | 46 | 50 | 128 | 3730 |
| Early (within 30 d) | 50 | 89 | 153 | 4834 |
| 1—12 mo | 46 | 43 | 138 | 4368 |
| After 1 yr | 47 | 15 | 141 | 4556 |
| Early RTAD | ||||
| Reported | 51 | 94 | 143 | 5058 |
| Not reported | 27 | Not reported | 142 | 5542 |
| Late RTAD | ||||
| Reported | 47 | 46 | 128 | 4592 |
| Not reported | 31 | Not reported | 157 | 6008 |
RTAD = retrograde type A dissection, TEVAR = thoracic endovascular aortic repair.
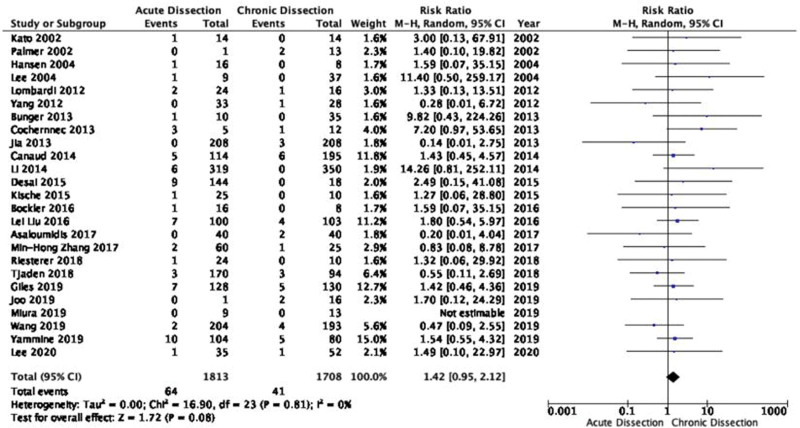
RTAD occurred in 2.2% (114/5230), and 0.9% (45/5169) of the cases in the acute TBAD and chronic TBAD groups, respectively (see Table S4, Supplemental Digital Content, http://links.lww.com/MD/I481, which illustrates time to occurrence of RTAD). Using Wilcoxon signed-rank test revealed that the proportion of RTAD patients with acute TBAD was significantly higher than those with chronic TBAD among all reported RTAD cases (P = .008). Twenty-four studies with 3521 patients provided comparative information on two arms of both acute and chronic TBAD for meta-analysis. The incidence of RTAD in patients with acute TBAD was higher but not statistically significant as compared to the patients with chronic TBAD with a RR of 1.42 (95% CI: 0.95–2.12; P = .08; Fig. 3) using a random model. There was no heterogeneity among the studies (P = .8, Chi2 = 16.9, and I2 = 0%).
Figure 3.
Forest plot for comparing of rates of RTAD post-TEVAR between acute and chronic type of TBAD. CI = confidence interval, RTAD = retrograde type A dissection, TBAD = type B aortic dissection, TEVAR = thoracic endovascular repair.
Although 44 studies described oversizing in TEVAR, most of them provided interval ranges without a detailed numerical description. Of them, stent-graft oversizing was ≤ 10% in 22 studies with 3013 TBAD cases, while it was between 10% to 20% in 20 studies with 2867 TBAD patients, and ≥ 20% in only 2 studies with 350 cases (see Table S5, Supplemental Digital Content, http://links.lww.com/MD/I482, which illustrates stent-graft oversizing). The incidence of RTAD was 3.6% (110/3013), 2.3% (65/2867), and 3.4% (12/350) in the stent-graft oversizing categories of ≤10%, 10% to 20%, and ≥20%.
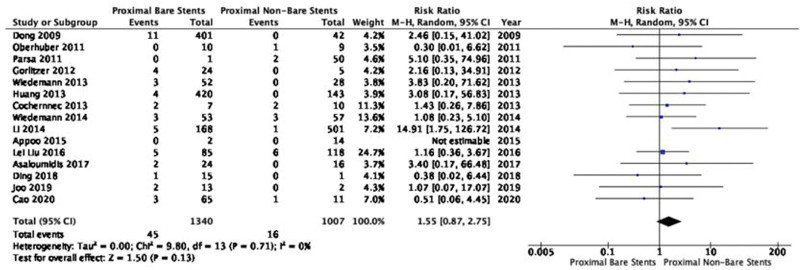
The incidence of RTAD was 2.1% (112/5328) and 0.9% (39/4381) in the proximal bare stents and non-bare stents groups, respectively (Table 2). According to Wilcoxon signed-rank test, among all reported RTAD cases, the proportion of RTAD patients in proximal bare stents group (112/153 cases; 73.2%) was not significantly different from non-bare stents group (39/129 cases; 30.2%) (P = .11). Fourteen studies with 2347 patients provided comparative information on two arms of both proximal bare and non-bare stents for meta-analysis. Pooled meta-analysis showed that the incidence of RTAD in proximal bare stents group was 2.1-fold higher than non-bare stents group with a RR of 1.55 (95% CI: 0.87–2.75; P = .13; Fig. 4) using a random model. There was no heterogeneity among the studies in this meta-analysis (P = .71, Chi2 = 9.80, and I2 = 0%; Fig. 4).
Figure 4.
Forest plot for comparing of rates of RTAD post-TEVAR between implanted proximal bare and non-bare stents. CI = confidence interval, RTAD = retrograde type A dissection, TEVAR = thoracic endovascular repair.
Of 78 selected studies, 14 reported clinical manifestations of RTAD. Chest pain and sudden fluctuations in blood pressure were the main symptoms of RTAD. Four studies described RTAD as asymptomatic after TEVAR. Detailed description of the clinical presentation of RTAD is provided in Table S6, Supplemental Digital Content, http://links.lww.com/MD/I483, which illustrates clinical manifestation of RTAD. Of 285 cases with RTAD, 160 (56.1%) and 29 (10.2%) were diagnosed by CT at regular and irregular imaging follow-up period, respectively. According to Kruskal Wallis Test, cumulative incidence of RTAD did not statistically differ among the studies with regular or irregular imaging follow-up period (P = .63). Twenty-three studies with 4412 TBAD cases and 96 RTAD patients (33.7%) did not share detailed information on imaging follow-up time (see Table S7, Supplemental Digital Content, http://links.lww.com/MD/I484, which illustrates imaging follow-up).
Table 5 shows the surgical and non-surgical treatment of RTAD; 52 studies comprising 7546 TBAD and 214 RTAD cases reported that 156 (72.9%) were treated surgically (Fig. 5). Eight cases (5.1%) were re-operated using the frozen elephant trunk technique. Other total arch repair (including ascending aorta repair and aortic arch repair) was performed in 16.7% (26/156); hemiarch repair or ascending aorta repair alone or Bentall procedure was performed in 19.9% (31/156); and repeated endovascular treatment was performed in 3.8% (6/156). The details of surgical approaches in 61 cases (39.1%) were not reported (see Table S8, Supplemental Digital Content, http://links.lww.com/MD/I485, which illustrates reported treatments of RTAD of enrolled studies). Of 9 studies comprising 73 RTAD cases and 2123 TBAD patients, 17 RTAD patients (23.2%) received non-surgical therapy including conservative wait-and-see and medical treatment (see Table S8, Supplemental Digital Content, http://links.lww.com/MD/I485, which illustrates reported treatments of RTAD of enrolled studies).
Table 5.
Therapeutic options of RTAD.
| Treatment | Nr of studies | Nr of treatment | Total RTAD | Total TEVAR | |
|---|---|---|---|---|---|
| A)Theraputic options | |||||
| Non-surgical | Reported | 9 | 17 | 73 | 2123 |
| ND | 69 | ND | 212 | 8477 | |
| Surgical | Reported | 52 | 156 | 214 | 7546 |
| ND | 26 | ND | 71 | 3054 | |
| Interventions | Nr of studies | Nr of treatment | Percentage | ||
| B)Surgical interventions | |||||
| Surgical treatment | No exact data about open repair | 22 | 61 | 39.10 | |
| Total arch repair (Ascending Aorta + aortic arch replacement) | 12 | 26 | 16.67 | ||
| Ascending repair or hemiarch repair or Bentall procedure or aortic root | 22 | 31 | 19.87 | ||
| Ascending TEVAR or Re-Stent or Stent-Dilatation | 4 | 6 | 3.85 | ||
| Frozen Elephant Trunck | 2 | 8 | 5.13 | ||
| * Undifferentiated | 2 | 24 | 15.38 | ||
RTAD = retrograde type A dissection, TEVAR = thoracic endovascular aortic repair.
Figure 5.
Forest plot of proportion single-arm meta-analysis for surgical re-intervention as therapeutic option of RTAD after TEVAR. CI = confidence interval, RTAD = retrograde type A dissection, TEVAR = thoracic endovascular repair.
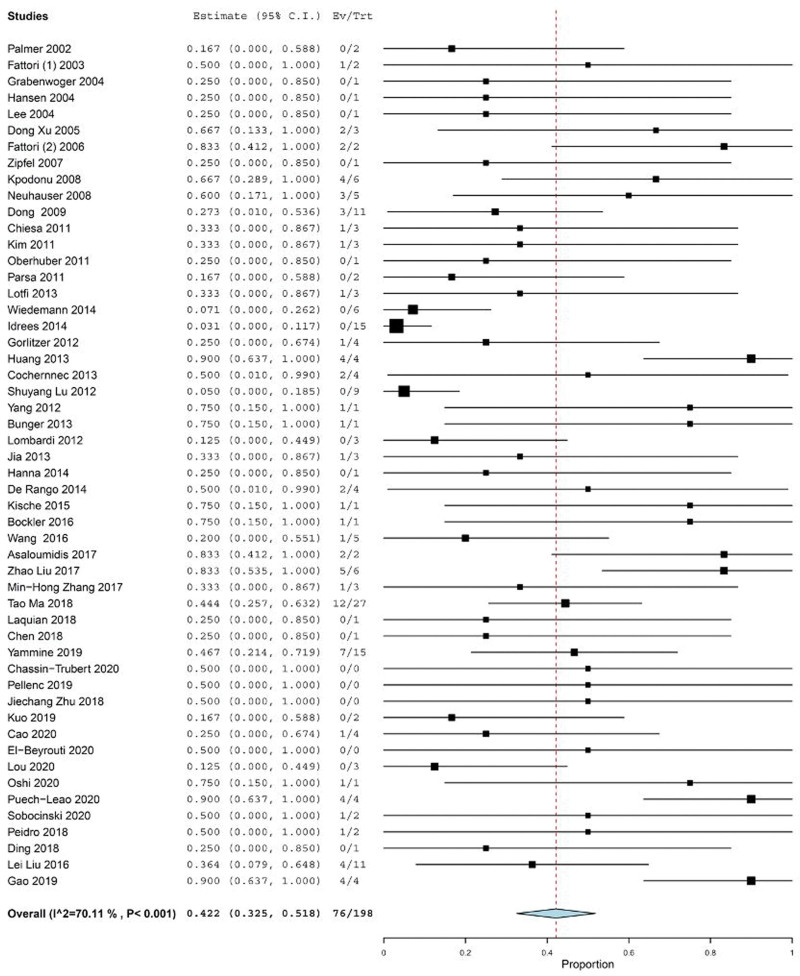
Death among RTAD cases was reported in 76 out of 198 RTAD cases in 52 studies with different follow-up periods (Table 6) and (see Table S9, Supplemental Digital Content, http://links.lww.com/MD/I486, which illustrates time and reasons of mortality of RTAD). Single arm meta-analysis estimated a mortality rate of 42.2% (95% CI: 32.5–51.8), with an I2 heterogeneity of 70.11% (P < .001) (Fig. 6).
Table 6.
Mortality of RTAD.
| Situation of report | Nr of studies | Nr of dead | Total RTAD | Total TEVAR of these Studies | Mortality rate | ||||
|---|---|---|---|---|---|---|---|---|---|
| A)Report of mortality | |||||||||
| Reported | 52 | 76 | 198 | 6915 | 38.30% | ||||
| Not reported | 26 | 43 | 87 | 3685 | |||||
| Time of mortality | Nr of studies | Nr of dead | Total RTAD | Total TEVAR | Mortality rate | ||||
| B)Time interval of mortality | |||||||||
| 0–14 d | 38 | 19 | 107 | 3473 | 17.76 | ||||
| Early 30 d | 39 | 24 | 109 | 3514 | 22.02 | ||||
| 1–12 mo | 37 | 7 | 106 | 3446 | 6.60 | ||||
| After 1 yr | 36 | 8 | 104 | 3375 | 7.69 | ||||
| C)Early or late mortality | |||||||||
| Early mortality | Reported | 39 | 25 | 109 | 3514 | 22.94 | |||
| ND | 39 | ND | 176 | 7086 | |||||
| Late mortality | Reported | 36 | 11 | 104 | 3375 | 10.58 | |||
| ND | 42 | ND | 181 | 7225 | |||||
RTAD = retrograde type A dissection, TEVAR = thoracic endovascular aortic repair.
Figure 6.
Forest plot of proportion single-arm meta-analysis for the mortality rate of RTAD after TEVAR. CI = confidence interval, RTAD = retrograde type A dissection, TEVAR = thoracic endovascular repair.
From 79 RTAD cases who died after TEVAR, the time of death was reported in 39 cases. Of whom, 24 cases (61.5%) died within the first 30 days, 7 (17.9%) died between 1–12 months after TEVAR, and 8 (10.2%) deaths occurred one year after TEVAR. From 24 RTAD cases who died in early first month, the time of death was intraoperatively until first two weeks after TEVAR in 19 cases (20.5%). The rate of early mortality was 25 out of total 109 RTAD cases (22.9%) in 39 studies. The rate of late mortality was 11 out of a total 104 RTAD cases (10.5%) from 36 studies. However, 39 (176 cases with RTAD) and 42 studies (181 cases with RTAD) did not report any information about the early and late mortality rate of RTAD, respectively (Table 6) and (see Table S9, Supplemental Digital Content, http://links.lww.com/MD/I486, which illustrates time and reasons of mortality of RTAD). The rate of early mortality of RTAD was 2.1 times higher than that of late mortality. Using Wilcoxon signed-rank Test, no significant difference was found in RTAD incidence between early and late mortality of RTAD (P = .44).
4. Discussion
During the past decade, TEVAR has become one of the most common surgical procedure in many thoracic aortic pathologies.[14,96–99] This method is less invasive than open surgery but still has several complications, including some new ones that are only now being characterized and understood. Some recognized complications that can occur after TEVAR include aneurysm development, aortic rupture, stroke, bowel infarction, paraplegia, limb ischemia, endoleak, and access-related complications.[14,98,100,101] There are also important device-related complications such as stent-graft induced aortic wall injury incurred by TBAD patients after TEVAR, which can require secondary intervention if distal but can be fatal if proximal (RTAD). Although the risk of proximal SINE is low, the fatality of this complication requires vigilance in patients who develop new onset symptoms in the early period after TEVAR treatment. Careful technique, minimal oversizing, and use of disease specific stent-grafts may reduce the risk for RTAD. Distally, SINE is more frequently seen during follow-up in patients treated for chronic dissection. The most important risk factor is oversizing of the stent-graft compared to the true lumen distal landing zone.[102] Therefore, procedure and device-related factors, the natural progression of initial aortic dissection, and unfavorable aortic-dissection anatomy are among the etiological factors mentioned.[13,103]
The RTAD rate after TEVAR might be reduced by improving stent-graft design (non-bare stents and tapering, for example), limited oversizing, and more careful manipulation during deployment.[13] It can also be argued that most of the information and hypotheses about this complication are not well-cited because RTAD has been reported as a rare complication with limited information in each study. To this end, we decided to thoroughly evaluate and analyze all available information about RTAD after TEVAR in TBAD patients.
Our single-arm meta-analysis estimated that the incidence of RTAD after TEVAR in patients with TBAD to be 2.3%. Therefore, it is not a very common complication. There is probably a difference in the incidence of RTAD after TEVAR on different continents. There are also several factors affecting it, such as the genetic background of connective tissue diseases, stents that have been used before, and differences in procedure-related factors. However, it cannot be ignored that the incidence of RTAD has been less pronounced in Asian studies. On the other hand, most Asian studies have been conducted in China. Besides, the incidence of RTAD is similar on the continents of America and Europe and higher than the reported incidences in Asian studies. Consequently, although this complication is considered rare, it needs to be greater attention in European and American countries. Centers with < 50 TBAD cases undergoing TEVAR were 2.26 times more likely to incur RTAD compared to centers with > 50 TBAD cases. As a result, it can be acknowledged that highly experienced centers reported a lower incidence of RTAD, suggesting the important hypothesis that this complication was significantly related to the procedure and postoperative management, strongly dependent on the surgeon’s experience. The decline in RTAD incidence from the introduction of TEVAR to the present may support the hypothesis that the incidence of RTAD decreases with increased experience and better technique. In general, it may be concluded that in China, due to the large population and existence of certain TEVAR centers with a certain number of surgeons, the surgeons have probably more experience in performing TEVAR. European and American countries, while being less populated, have more centers performing TEVAR. For this reason, most surgeons may not yet have reached their full potential. For instance, the risk of RTAD occurrence can increase when surgeons pass a guide wire through a tortuous aortic arch. The risk is exacerbated when getting it through anatomically abnormal areas or when the aorta is distorted or very thin, meaning that any friction from catheter or guide wire can damage the wall. Such risks can be effectively mitigated by more experienced centers and surgeons.[13,16]
Our findings also showed that RTAD occurred primarily as a hyperacute or acute condition rather than a chronic condition. Thus, the first month after TEVAR was the maximum duration for RTAD incidence; in addition, from the moment of TEVAR operation until the first two weeks, the probability of its occurrence was the highest. Our estimates revealed that the incidence of early RTAD was approximately 1.8 times higher than that of late RTAD. One hypothesis is that patients with acute TBAD are more likely to have urgent or emergent TEVAR which may be less accurate in preoperative assessments compared with chronic TBAD patients. Moreover, acute pathological changes in the aorta may increase the probability of extension of dissection and therefore predispose to RTAD. Having said that, Tjaden et al[67] found no significant difference between the risk of RTAD in acute compared to chronic TBAD. In this meta-analysis, the number of RTAD patients with acute TBAD was significantly higher than the number of RTAD patients with chronic TBAD. However, the corresponding risk ratio of 1.42 was not statistically significant. Although the findings were borderline, clinically, it can be accepted that RTAD could be more incurred by patients with acute TBAD. Therefore, more accurate diagnostic and therapeutic evaluation should be adopted to prevent this complication in acute TBAD cases. After evaluating the data, it was shown that there were no significant regional differences in the availability of follow-up data and imaging data.
Some other studies reported that proximal bare stent configuration was associated with an increased risk of RTAD.[104] Chen et al claimed that with a risk ratio of 2.06, the incidence of RTAD in TEVAR was higher in the proximal bare stent than the proximal non-bare stent.[103] This meta-analysis found that risk of RTAD in the proximal bare stents group was 2.1-fold more than in the proximal non-bare stents group. According to our comparative meta-analysis, the difference in the incidence of RTAD was not significant in the two groups of proximal bare stents and non-bare stents with a risk ratio of 1.50. This finding can be interpreted in the way that the quality of proximal bare stents design and the experience of surgeons working with these stents’ models have probably increased in recent years. However, it cannot be ignored that according to previous studies, the percentage of RTAD in the proximal bare stent group was higher, even though it was not significant. Besides, it is clinically significant that if the patient is at risk of RTAD after TEVAR, such as patients with Marfan syndrome, connective tissue diseases, and acute TBAD who want to undergo non-elective TEVAR, proximal non-bare stents might be the best choice.
Dong et al explained that using angiotensin-converting enzyme inhibitors, B-blockers, calcium antagonists, or angiotensin receptor blockers was only suggested as medical management procedures when RTAD was limited, and the patient’s situation was clinically stable.[105] In the present study, 11.5% of the studies with 73 patients reported non-surgical treatment with RTAD, implying that conservative wait-and-see treatment or re-surgical treatment was not accepted by patients, hence the use of non-surgical treatments. It is clear that surgical treatment should be applied in patients with unstable and limited progression since using drug treatment is not sufficient. Our findings suggested that some of the most common surgical reinterventions could treat RTAD, including ascending aorta repair alone, hemiarch replacement, and Bentall procedure. Clinically, after RTAD diagnosis followed by TEVAR, it is recommended to make treatment decisions by an interdisciplinary aortic team including vascular surgeons, cardiac surgeons, radiologists, intensive-care specialists, and anesthesiologists to evaluate the re-intervention carefully and to manage clinical and radiological follow-ups and postoperative care.
The RTAD mortality rate post-TEVAR, although low, was significantly higher than spontaneous type A aortic dissection.[106,107] This was clearly more significant during the first month post-TEVAR compared to 1 to 12 months, and one year after TEVAR. Of those who died due to RTAD during the first month after TEVAR, 79.1% died during surgery or in the first hours and days after surgery. Due to the significant and high mortality rate of this uncommon complication, RTAD should be considered as one of the differential diagnoses with high risk during ICU stay or hospital stay after surgery and even after discharge. If the patient suddenly suffers from any chest pain, back pain, chest discomfort, sudden changes in blood pressure, syncope, or any other sudden clinical signs, appropriate radiological evaluations should be performed to perform appropriate reintervention as soon as possible and to avoid sudden death. Numerous studies have also suggested that the occurrence of RTAD coincides with the onset of multi-organ failure and eventual death.[108,109] It should be mentioned that most research done on RTAD had a small sample size, and the mortality rate varied according to various treatment strategies applied.[108,109] Hence further well-designed, large scale clinical trials with longer-term follow-up are needed to accurately evaluate mortality rate of RTAD after TEVAR and its diagnostic workout and surgical management. We recommend that future studies investigate the correlation between genetic parameters and incidence for RTAD, as well as patients who die due to RTAD.
Author contributions
Conceptualization: Sadeq Ali-Hasan-Al-Saegh, Salvatore Scali, Mohammad Bashar Izzat, Hazem El Beyrouti.
Data curation: Sadeq Ali-Hasan-Al-Saegh, Nancy Halloum, Hazem El Beyrouti.
Formal analysis: Sadeq Ali-Hasan-Al-Saegh, Nancy Halloum, Mohannad Abualia, Mohammad Bashar Izzat.
Investigation: Sadeq Ali-Hasan-Al-Saegh, Davor Stamenovic.
Methodology: Sadeq Ali-Hasan-Al-Saegh, Nancy Halloum, Salvatore Scali, Mohannad Abualia, Mohammad Bashar Izzat, Patrick Bohan, Hazem El Beyrouti.
Project administration: Davor Stamenovic.
Software: Sadeq Ali-Hasan-Al-Saegh, Davor Stamenovic.
Supervision: Marc Kriege, Hazem El Beyrouti.
Writing – original draft: Sadeq Ali-Hasan-Al-Saegh, Nancy Halloum, Hazem El Beyrouti.
Writing – review & editing: Sadeq Ali-Hasan-Al-Saegh, Salvatore Scali, Marc Kriege, Mohannad Abualia, Davor Stamenovic, Mohammad Bashar Izzat, Patrick Bohan, Roman Kloeckner, Mehmet Oezkur, Bernhard Dorweiler, Hendrik Treede, Hazem El Beyrouti.
Supplementary Material
Abbreviations:
- CI
- confidence interval
- CT
- computed tomography
- ICU
- intensive care unit
- RR
- risk ratio
- RTAD
- retrograde type A dissection
- TBAD
- type B aortic dissection
- TEVAR
- thoracic endovascular repair
SA-H-A-S and NH contributed equally to this work.
Since our study is based on already published literature with no interaction with human subjects, no issues related to medical ethics were needed to be reported.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Ali-Hasan-Al-Saegh S, Halloum N, Scali S, Kriege M, Abualia M, Stamenovic D, Bashar Izzat M, Bohan P, Kloeckner R, Oezkur M, Dorweiler B, Treede H, El Beyrouti H. A systematic review and meta-analysis of retrograde type A aortic dissection after thoracic endovascular aortic repair in patients with type B aortic dissection. Medicine 2023;102:15(e32944).
Contributor Information
Sadeq Ali-Hasan-Al-Saegh, Email: Sadeq.AlSaegh@unimedizin-mainz.de.
Nancy Halloum, Email: nancy.halloum@unimedizin-mainz.de.
Salvatore Scali, Email: salvatore.scali@surgery.ufl.edu.
Marc Kriege, Email: Marc.Kriege@unimedizin-mainz.de.
Mohannad Abualia, Email: Mohannad.Abualia@unimedizin-mainz.de.
Davor Stamenovic, Email: davorstamenovic@gmail.com.
Mohammad Bashar Izzat, Email: mbizzat@gmail.com.
Roman Kloeckner, Email: Roman.kloeckner@gmail.com.
Mehmet Oezkur, Email: Mehmet.Oezkur@unimedizin-mainz.de.
Bernhard Dorweiler, Email: bernhard.dorweiler@googlemail.com.
Hendrik Treede, Email: Hendrik.Treede@unimedizin-mainz.de.
References
- [1].Ahlsson A, Wickbom A, Geirsson A, et al. Is there a weekend effect in surgery for type a dissection?: results from the nordic consortium for acute type A aortic dissection database. Ann Thorac Surg. 2019;108:770–6. [DOI] [PubMed] [Google Scholar]
- [2].McClure RS, Brogly SB, Lajkosz K, et al. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: a population-based study. J Thorac Cardiovasc Surg. 2018;155:2254–64.e4. [DOI] [PubMed] [Google Scholar]
- [3].Sacks D, Baxter B, Campbell BCV, et al.; From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–32. [DOI] [PubMed] [Google Scholar]
- [4].Wiedemann D, Mahr S, Vadehra A, et al. Thoracic endovascular aortic repair in 300 patients: long-term results. Ann Thorac Surg. 2013;95:1577–83. [DOI] [PubMed] [Google Scholar]
- [5].Wiedemann D, Ehrlich M, Amabile P, et al. Emergency endovascular stent grafting in acute complicated type B dissection. J Vasc Surg. 2014;60:1204–8. [DOI] [PubMed] [Google Scholar]
- [6].Sievers HH, Rylski B, Czerny M, et al. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact Cardiovasc Thorac Surg. 2020;30:451–7. [DOI] [PubMed] [Google Scholar]
- [7].Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020;71:723–47. [DOI] [PubMed] [Google Scholar]
- [8].Gao HQ, Xu SD, Ren CW, et al. Analysis of perioperative outcome and long-term survival rate of thoracic endovascular aortic repair in uncomplicated type B dissection: single-centre experience with 751 patients. Eur J Cardiothorac Surg. 2019;56:1090–6. [DOI] [PubMed] [Google Scholar]
- [9].Gao Z, Qin Z, An Z, et al. Prognostic value of preoperative hemoglobin levels for long-term outcomes of acute type B aortic dissection post-thoracic endovascular aortic repair. Front Cardiovasc Med. 2020;7:588761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu W, Zhang Y, Guo L, et al. A graft inversion technique for retrograde type A aortic dissection after thoracic endovascular repair for type B aortic dissection. J Cardiothorac Surg. 2019;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].MacGillivray TE, Gleason TG, Patel HJ, et al. The Society of Thoracic Surgeons/American Association for thoracic surgery clinical practice guidelines on the management of type B aortic dissection. J Thorac Cardiovasc Surg. 2022;163:1231–49. [DOI] [PubMed] [Google Scholar]
- [12].Giles KA, Beck AW, Lala S, et al. Implications of secondary aortic intervention after thoracic endovascular aortic repair for acute and chronic type B dissection. J Vasc Surg. 2019;69:1367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang L, Zhao Y, Zhang W, et al. Retrograde type A aortic dissection after thoracic endovascular aortic repair: incidence, time trends and risk factors. Semin Thorac Cardiovasc Surg. 2020. [DOI] [PubMed] [Google Scholar]
- [14].Neuhauser B, Greiner A, Jaschke W, et al. Serious complications following endovascular thoracic aortic stent-graft repair for type B dissection. Eur J Cardiothorac Surg. 2008;33:58–63. [DOI] [PubMed] [Google Scholar]
- [15].Sirignano P, Pranteda C, Capoccia L, et al. Retrograde type B aortic dissection as a complication of standard endovascular aortic repair. Ann Vasc Surg. 2015;29:127.e5–9. [DOI] [PubMed] [Google Scholar]
- [16].Chen Y, Zhang S, Liu L, et al. Retrograde type A aortic dissection after thoracic endovascular aortic repair: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yammine H, Briggs CS, Stanley GA, et al. Retrograde type A dissection after thoracic endovascular aortic repair for type B aortic dissection. J Vasc Surg. 2019;69:24–33. [DOI] [PubMed] [Google Scholar]
- [18].Doberne JW, Sabe AA, Vekstein AM, et al. Stent Graft-induced aortic wall injury (SAWI) - incidence, risk factors, and outcomes. Ann Thorac Surg. 2022. [DOI] [PubMed] [Google Scholar]
- [19].Pantaleo A, Jafrancesco G, Buia F, et al. Distal Stent Graft-induced new entry: an emerging complication of endovascular treatment in aortic dissection. Ann Thorac Surg. 2016;102:527–32. [DOI] [PubMed] [Google Scholar]
- [20].Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [21].Goossen K, Tenckhoff S, Probst P, et al. Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg. 2018;403:119–29. [DOI] [PubMed] [Google Scholar]
- [22].Kalkum E, Klotz R, Seide S, et al. Systematic reviews in surgery-recommendations from the Study Center of the German society of surgery. Langenbecks Arch Surg. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jormalainen M, Raivio P, Biancari F, et al. Late Outcome after Surgery for Type-A aortic dissection. J Clin Med. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hirji S, McGurk S, Kiehm S, et al. Utility of 90-Day Mortality vs 30-Day mortality as a quality metric for transcatheter and surgical aortic valve replacement outcomes. JAMA Cardiol. 2020;5:156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Czermak BV, Waldenberger P, Perkmann R, et al. Placement of endovascular stent-grafts for emergency treatment of acute disease of the descending thoracic aorta. Am J Roentgenol. 2002;179:337–45. [DOI] [PubMed] [Google Scholar]
- [26].Kato N, Shimono T, Hirano T, et al. Midterm results of stent-graft repair of acute and chronic aortic dissection with descending tear: the complication-specific approach. J Thorac Cardiovasc Surg. 2002;124:306–12. [DOI] [PubMed] [Google Scholar]
- [27].Pamler RS, Kotsis T, Görich J, et al. Complications after endovascular repair of type B aortic dissection. J Endovasc Ther. 2002;9:822–8. [DOI] [PubMed] [Google Scholar]
- [28].Fattori R, Napoli G, Lovato L, et al. Descending thoracic aortic diseases: stent-graft repair. Radiology. 2003;229:176–83. [DOI] [PubMed] [Google Scholar]
- [29].Grabenwoger M, Fleck T, Ehrlich M, et al. Secondary surgical interventions after endovascular stent-grafting of the thoracic aorta. Eur J Cardiothorac Surg. 2004;26:608–13. [DOI] [PubMed] [Google Scholar]
- [30].Hansen CJ, Bui H, Donayre CE, et al. Complications of endovascular repair of high-risk and emergent descending thoracic aortic aneurysms and dissections. J Vasc Surg. 2004;40:228–34. [DOI] [PubMed] [Google Scholar]
- [31].Lee K-H, Won JY, Lee DY, et al. Elective stent-graft treatment of aortic dissections. J Endovasc Ther. 2004;11:667–75. [DOI] [PubMed] [Google Scholar]
- [32].Dong Xu S, Zhong Li Z, Huang FJ, et al. Treating aortic dissection and penetrating aortic ulcer with stent graft: thirty cases. Ann Thorac Surg. 2005;80:864–8. [DOI] [PubMed] [Google Scholar]
- [33].Fattori R, Nienaber CA, Rousseau H, et al.; Talent Thoracic Retrospective Registry. Results of endovascular repair of the thoracic aorta with the Talent Thoracic stent graft: the Talent Thoracic Retrospective Registry. J Thorac Cardiovasc Surg. 2006;132:332–9. [DOI] [PubMed] [Google Scholar]
- [34].Duebener L, Hartmann F, Kurowski V, et al. Surgical interventions after emergency endovascular stent-grafting for acute type B aortic dissections. Interact Cardiovasc Thorac Surg. 2007;6:288–92. [DOI] [PubMed] [Google Scholar]
- [35].Zipfel B, Hammerschmidt R, Krabatsch T, et al. Stent-grafting of the thoracic aorta by the cardiothoracic surgeon. Ann Thorac Surg. 2007;83:441–8; discussion 448. [DOI] [PubMed] [Google Scholar]
- [36].Kpodonu J, Preventza O, Ramaiah VG, et al. Retrograde type A dissection after endovascular stenting of the descending thoracic aorta. Is the risk real? Eur J Cardiothorac Surg. 2008;33:1014–8. [DOI] [PubMed] [Google Scholar]
- [37].Dong ZH, Fu WG, Wang YQ, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation. 2009;119:735–41. [DOI] [PubMed] [Google Scholar]
- [38].Chiesa R, Tshomba Y, Logaldo D, et al. Hybrid repair of aortic aneurysms and dissections: the European perspective. Tex Heart Inst J. 2011;38:687–90. [PMC free article] [PubMed] [Google Scholar]
- [39].Kim KM, Donayre CE, Reynolds TS, et al. Aortic remodeling, volumetric analysis, and clinical outcomes of endoluminal exclusion of acute complicated type B thoracic aortic dissections. J Vasc Surg. 2011;54:316–24; discussion 324. [DOI] [PubMed] [Google Scholar]
- [40].Oberhuber A, Winkle P, Schelzig H, et al. Technical and clinical success after endovascular therapy for chronic type B aortic dissections. J Vasc Surg. 2011;54:1303–9. [DOI] [PubMed] [Google Scholar]
- [41].Parsa CJ, Williams JB, Bhattacharya SD, et al. Midterm results with thoracic endovascular aortic repair for chronic type B aortic dissection with associated aneurysm. J Thorac Cardiovasc Surg. 2011;141:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lotfi S, Clough RE, Ali T, et al. Hybrid repair of complex thoracic aortic arch pathology: long-term outcomes of extra-anatomic bypass grafting of the supra-aortic trunk. Cardiovasc Intervent Radiol. 2013;36:46–55. [DOI] [PubMed] [Google Scholar]
- [43].Faure EM, Canaud L, Agostini C, et al. Reintervention after thoracic endovascular aortic repair of complicated aortic dissection. J Vasc Surg. 2014;59:327–33. [DOI] [PubMed] [Google Scholar]
- [44].Idrees J, Arafat A, Johnston DR, et al. Repair of retrograde ascending dissection after descending stent grafting. J Thorac Cardiovasc Surg. 2014;147:151–4. [DOI] [PubMed] [Google Scholar]
- [45].Zhang L, Zhou J, Lu Q, et al. Potential risk factors of re-intervention after endovascular repair for type B aortic dissections. Catheter Cardiovasc Interv. 2015;86:E1–10. [DOI] [PubMed] [Google Scholar]
- [46].Gorlitzer M, Weiss G, Moidl R, et al. Repair of stent graft-induced retrograde type A aortic dissection using the E-vita open prosthesis. Eur J Cardiothorac Surg. 2012;42:566–70. [DOI] [PubMed] [Google Scholar]
- [47].Huang WH, Luo SY, Luo JF, et al. Perioperative aortic dissection rupture after endovascular stent graft placement for treatment of type B dissection. Chin Med J (Engl). 2013;126:1636–41. [PubMed] [Google Scholar]
- [48].Cochennec F, Tresson P, Cross J, et al. Hybrid repair of aortic arch dissections. J Vasc Surg. 2013;57:1560–7. [DOI] [PubMed] [Google Scholar]
- [49].Lu S, Lai H, Wang C, et al. Surgical treatment for retrograde type A aortic dissection after endovascular stent graft placement for type B dissection. Interact Cardiovasc Thorac Surg. 2012;14:538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang C-PO, Hsu C-P, Chen W-Y, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type B aortic dissection. J Vasc Surg. 2012;55:1600–10. [DOI] [PubMed] [Google Scholar]
- [51].Bünger CM, Kische S, Liebold A, et al. Hybrid aortic arch repair for complicated type B aortic dissection. J Vasc Surg. 2013;58:1490–6. [DOI] [PubMed] [Google Scholar]
- [52].Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg. 2014;260:389–95. [DOI] [PubMed] [Google Scholar]
- [53].Lombardi JV, Cambria RP, Nienaber CA, et al.; STABLE investigators. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55:629–40.e2. [DOI] [PubMed] [Google Scholar]
- [54].Jia X, Guo W, Li TX, et al. The results of stent graft versus medication therapy for chronic type B dissection. J Vasc Surg. 2013;57:406–14. [DOI] [PubMed] [Google Scholar]
- [55].Li B, Pan XD, Ma WG, et al. Stented elephant trunk technique for retrograde type A aortic dissection after endovascular stent graft repair. Ann Thorac Surg. 2014;97:596–602. [DOI] [PubMed] [Google Scholar]
- [56].Hanna JM, Andersen ND, Ganapathi AM, et al. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2014;59:96–106. [DOI] [PubMed] [Google Scholar]
- [57].De Rango P, Cao P, Ferrer C, et al. Aortic arch debranching and thoracic endovascular repair. J Vasc Surg. 2014;59:107–14. [DOI] [PubMed] [Google Scholar]
- [58].Appoo JJ, Herget EJ, Pozeg ZI, et al. Midterm results of endovascular stent grafts in the proximal aortic arch (zone 0): an imaging perspective. Can J Cardiol. 2015;31:731–7. [DOI] [PubMed] [Google Scholar]
- [59].Desai ND, Gottret JP, Szeto WY, et al. Impact of timing on major complications after thoracic endovascular aortic repair for acute type B aortic dissection. J Thorac Cardiovasc Surg. 2015;149(2 Suppl):S151–6. [DOI] [PubMed] [Google Scholar]
- [60].Kische S, D’Ancona G, Belu IC, et al. Perioperative and mid-term results of endovascular management of complicated type B aortic dissection using a proximal thoracic endoprosthesis and selective distal bare stenting. Eur J Cardiothorac Surg. 2015;48:e77–84. [DOI] [PubMed] [Google Scholar]
- [61].Böckler D, Brunkwall J, Taylor PR, et al.; CTAG registry investigators. Thoracic endovascular aortic repair of aortic arch pathologies with the conformable thoracic aortic graft: early and 2 year results from a european multicentre registry. Eur J Vasc Endovasc Surg. 2016;51:791–800. [DOI] [PubMed] [Google Scholar]
- [62].Faure EM, Canaud L, Marty-Ané C, et al. Hybrid aortic arch repair for dissecting aneurysm. J Thorac Cardiovasc Surg. 2016;152:162–8. [DOI] [PubMed] [Google Scholar]
- [63].Wang G, Zhai S, Li T, et al. Mechanism and management of retrograde type A aortic dissection complicating TEVAR for type B aortic dissection. Ann Vasc Surg. 2016;32:111–8. [DOI] [PubMed] [Google Scholar]
- [64].Asaloumidis N, Karkos CD, Trellopoulos G, et al. Outcome after endovascular repair of subacute type B aortic dissection: a combined series from two greek centers. Ann Vasc Surg. 2017;42:136–42. [DOI] [PubMed] [Google Scholar]
- [65].Liu Z, Zhang Y, Liu C, et al. Treatment of serious complications following endovascular aortic repair for type B thoracic aortic dissection. J Int Med Res. 2017;45:1574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang MH, Du X, Guo W, et al. Early and midterm outcomes of thoracic endovascular aortic repair (TEVAR) for acute and chronic complicated type B aortic dissection. Medicine (Baltimore). 2017;96:e7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tjaden BL, Jr., Sandhu H, Miller C, et al. Outcomes from the gore global registry for endovascular aortic treatment in patients undergoing thoracic endovascular aortic repair for type B dissection. J Vasc Surg. 2018;68:1314–23. [DOI] [PubMed] [Google Scholar]
- [68].Ma T, Dong ZH, Fu WG, et al. Incidence and risk factors for retrograde type A dissection and stent graft-induced new entry after thoracic endovascular aortic repair. J Vasc Surg. 2018;67:1026–33.e2. [DOI] [PubMed] [Google Scholar]
- [69].Laquian L, Scali ST, Beaver TM, et al. Outcomes of thoracic endovascular aortic repair for acute type B dissection in patients with intractable pain or refractory hypertension. J Endovasc Ther. 2018;25:220–9. [DOI] [PubMed] [Google Scholar]
- [70].Chen L, Yang SJ, Guo FL, et al. Experience with thoracic endovascular aortic repair applied in treating Stanford type B aortic dissection: an analysis of 98 cases. Adv Clin Exp Med. 2018;27:1259–62. [DOI] [PubMed] [Google Scholar]
- [71].Buczkowski P, Puślecki M, Majewska N, et al. Endovascular treatment of complex diseases of the thoracic aorta-10 years single centre experience. J Thorac Dis. 2019;11:2240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Eleshra A, Kölbel T, Tsilimparis N, et al. Candy-plug generation II for false lumen occlusion in chronic aortic dissection: feasibility and early results. J Endovasc Ther. 2019;26:782–6. [DOI] [PubMed] [Google Scholar]
- [73].Fukushima S, Ohki T, Toya N, et al. Initial results of thoracic endovascular repair for uncomplicated type B aortic dissection involving the arch vessels using a semicustom-made thoracic fenestrated stent graft. J Vasc Surg. 2019;69:1694–703. [DOI] [PubMed] [Google Scholar]
- [74].Wang GJ, Cambria RP, Lombardi JV, et al. Thirty-day outcomes from the society for vascular surgery vascular quality initiative thoracic endovascular aortic repair for type B dissection project. J Vasc Surg. 2019;69:680–91. [DOI] [PubMed] [Google Scholar]
- [75].Miura S, Kurimoto Y, Maruyama R, et al. Thoracic endovascular aortic repair on zone 2 landing for type B aortic dissection. Ann Vasc Surg. 2019;60:120–7. [DOI] [PubMed] [Google Scholar]
- [76].Chassin-Trubert L, Mandelli M, Ozdemir BA, et al. Midterm follow-up of fenestrated and scalloped physician-modified endovascular grafts for zone 2 TEVAR. J Endovasc Ther. 2020;27:377–84. [DOI] [PubMed] [Google Scholar]
- [77].Pellenc Q, Roussel A, De Blic R, et al. False lumen embolization in chronic aortic dissection promotes thoracic aortic remodeling at midterm follow-up. J Vasc Surg. 2019;70:710–7. [DOI] [PubMed] [Google Scholar]
- [78].Zhu J, Zhao L, Dai X, et al. Fenestrated thoracic endovascular aortic repair using physician modified stent grafts for acute type B aortic dissection with unfavourable landing zone. Eur J Vasc Endovasc Surg. 2018;55:170–6. [DOI] [PubMed] [Google Scholar]
- [79].Riesterer T, Beyersdorf F, Scheumann J, et al. Accuracy of deployment of the Relay non-bare stent graft in the aortic arch. Interact Cardiovasc Thorac Surg. 2019;28:797–802. [DOI] [PubMed] [Google Scholar]
- [80].Kuo EC, Veranyan N, Johnson CE, et al. Impact of proximal seal zone length and intramural hematoma on clinical outcomes and aortic remodeling after thoracic endovascular aortic repair for aortic dissections. J Vasc Surg. 2019;69:987–95. [DOI] [PubMed] [Google Scholar]
- [81].Joo HC, Youn YN, Kwon JH, et al. Late complications after hybrid aortic arch repair. J Vasc Surg. 2019;70:1023–30.e1. [DOI] [PubMed] [Google Scholar]
- [82].Cao L, Ge Y, He Y, et al. Association between aortic arch angulation and bird-beak configuration after thoracic aortic stent graft repair of type B aortic dissection. Interact Cardiovasc Thorac Surg. 2020;31:688–96. [DOI] [PubMed] [Google Scholar]
- [83].El Beyrouti H, Lescan M, Doemland M, et al. Early results of a low-profile stent-graft for thoracic endovascular aortic repair. PLoS One. 2020;15:e0240560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Charlton-Ouw KM, Sandhu HK, Leake SS, et al. New type A dissection after acute type B aortic dissection. J Vasc Surg. 2018;67:85–92. [DOI] [PubMed] [Google Scholar]
- [85].Lou XY, Duwayri YM, Jordan WD, et al. The safety and efficacy of extended TEVAR in acute type B aortic dissection. Ann Thorac Surg. 2020;110:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lee SJ, Kang WC, Ko YG, et al. Aortic remodeling and clinical outcomes in type B aortic dissection according to the timing of thoracic endovascular aortic repair. Ann Vasc Surg. 2020;67:322–31. [DOI] [PubMed] [Google Scholar]
- [87].Oishi Y, Yamashita Y, Kimura S, et al. Preoperative distal aortic diameter is a significant predictor of late aorta-related events after endovascular repair for chronic type B aortic dissection. Gen Thorac Cardiovasc Surg. 2020;68:1086–93. [DOI] [PubMed] [Google Scholar]
- [88].Puech-Leao P, Estenssoro AEV, Wakassa TB, et al. Long-term results of endovascular treatment of chronic type B aortic dissection by closure of the primary tear. Ann Vasc Surg. 2020;66:179–82. [DOI] [PubMed] [Google Scholar]
- [89].Sobocinski J, Dias NV, Hongku K, et al. Thoracic endovascular aortic repair with stent grafts alone or with a composite device design in patients with acute type B aortic dissection in the setting of malperfusion. J Vasc Surg. 2020;71:400–7.e2. [DOI] [PubMed] [Google Scholar]
- [90].Zhao S, Gu H, Chen B, et al. Dynamic indicators that impact the outcomes of thoracic endovascular aortic repair in complicated type B aortic dissection. J Vasc Interv Radiol. 2020;31:760–8.e1. [DOI] [PubMed] [Google Scholar]
- [91].Bavaria JE, Brinkman WT, Hughes GC, et al. Outcomes of thoracic endovascular aortic repair in acute type B aortic dissection: results from the valiant United States investigational device exemption study. Ann Thorac Surg. 2015;100:802–8; discussion 808. discussion 89. [DOI] [PubMed] [Google Scholar]
- [92].Peidro J, Boufi M, Loundou AD, et al. Aortic anatomy and complications of the proximal sealing zone after endovascular treatment of the thoracic aorta. Ann Vasc Surg. 2018;48:141–50. [DOI] [PubMed] [Google Scholar]
- [93].Ding H, Luo S, Liu Y, et al. Outcomes of hybrid procedure for type B aortic dissection with an aberrant right subclavian artery. J Vasc Surg. 2018;67:704–11. [DOI] [PubMed] [Google Scholar]
- [94].Nozdrzykowski M, Luehr M, Garbade J, et al. Outcomes of secondary procedures after primary thoracic endovascular aortic repair. Eur J Cardiothorac Surg. 2016;49:770–7. [DOI] [PubMed] [Google Scholar]
- [95].Liu L, Zhang S, Lu Q, et al. Impact of oversizing on the risk of retrograde dissection after TEVAR for acute and chronic type B dissection. J Endovasc Ther. 2016;23:620–5. [DOI] [PubMed] [Google Scholar]
- [96].Usai MV, Nugroho NT, Oberhuber A, et al. Influence of TEVAR on blood pressure in subacute type B aortic dissection (TBAD) patients with refractory and non-refractory arterial hypertension. Int Angiol. 2021;40:60–6. [DOI] [PubMed] [Google Scholar]
- [97].Bondesson J, Suh GY, Marks N, et al. Influence of thoracic endovascular aortic repair on true lumen helical morphology for stanford type B dissections. J Vasc Surg. 2021. [DOI] [PubMed] [Google Scholar]
- [98].Czerny M, Pacini D, Aboyans V, et al. Current options and recommendations for the use of thoracic endovascular aortic repair in acute and chronic thoracic aortic disease: an expert consensus document of the European Society for Cardiology (ESC) Working Group of Cardiovascular Surgery, the ESC Working Group on Aorta and Peripheral Vascular Diseases, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothoracic Surg. 2021;59:65–73. [DOI] [PubMed] [Google Scholar]
- [99].Khayat M, Cooper KJ, Khaja MS, et al. Endovascular management of acute aortic dissection. Cardiovasc Diagn Ther. 2018;8(Suppl 1):S97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Neuhauser B, Czermak BV, Fish J, et al. Type A dissection following endovascular thoracic aortic stent-graft repair. J Endovasc Ther. 2005;12:74–81. [DOI] [PubMed] [Google Scholar]
- [101].Eggebrecht H, Nienaber CA, Neuhäuser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489–98. [DOI] [PubMed] [Google Scholar]
- [102].Burdess A, Mani K, Tegler G, et al. Stent-graft induced new entry tears after type B aortic dissection: how to treat and how to prevent? J Cardiovasc Surg (Torino). 2018;59:789–96. [DOI] [PubMed] [Google Scholar]
- [103].Chen YQ, Zhang SM, Liu L, et al. Retrograde type A aortic dissection after thoracic endovascular aortic repair: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Williams JB, Andersen ND, Bhattacharya SD, et al. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J Vasc Surg. 2012;55:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Dong Z, Fu W, Wang Y, et al. Stent graft-induced new entry after endovascular repair for Stanford type B aortic dissection. J Vasc Surg. 2010;52:1450–7. [DOI] [PubMed] [Google Scholar]
- [106].Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothoracic Surg. 2016;49:e44–52. [DOI] [PubMed] [Google Scholar]
- [107].Ghoreishi M, Sundt TM, Cameron DE, et al. Factors associated with acute stroke after type A aortic dissection repair: an analysis of the society of thoracic surgeons national adult cardiac surgery database. J Thorac Cardiovasc Surg. 2020;159:2143–54.e3. [DOI] [PubMed] [Google Scholar]
- [108].An Z, Song Z, Tang H, et al. Retrograde type A dissection after thoracic endovascular aortic repair: surgical strategy and literature review. Heart Lung Circ. 2018;27:629–34. [DOI] [PubMed] [Google Scholar]
- [109].An Z, Tan MW, Song ZG, et al. Retrograde type a dissection after ascending aorta involved endovascular repair and its surgical repair with stented elephant trunk. Ann Vasc Surg. 2019;58:198–204.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.