Abstract
We aimed to investigate the differential diagnosis of depressive episodes in patients with major depressive disorder (MDD) and bipolar disorder (BD) using peripheral blood cytokine expression levels. The levels of interleukin (IL)-2, IL-6, IL-10, IL-17, IL4, and IL-12; interferon (IFN)-γ; and tumor necrosis factor (TNF)-α were measured in patients with MDD and BD presenting acute episodes in an inpatient psychiatric setting. The expression levels of IL-6, IL-10, IL-17, and IFN-γ in the MDD and BD groups were higher than those in the control group (P < .05), but there was no significant difference between the patient groups and control group. Only the expression levels of TNF-α and IL-4 were higher in both groups than in the control group, and the BD group had higher levels than the MDD group (P < .05). The expression levels of IL-17, IFN-γ, IL-10, and IL-4 were significantly higher in BD-related manic episodes than in BD-related depressive episodes (P < .05). IL-6, IFN-γ, TNF-α, IL-10, and IL-4 levels were higher in BD-related depressive episodes than in MDD-related depressive episodes (P < .05). The receiver operating characteristic curve test for MDD and BD and the area under the curve for IL-4 revealed good clinical predictability. Patients with MDD and BD exhibited different cytokine profiles when experiencing acute episodes; patients with BD exhibited a more severe immune-inflammatory response system–compensatory immunoregulatory response system (CIRS) imbalance. IL-4 was found to have diagnostic value in differentiating between active depressive episodes in MDD and BD.
Keywords: biomarkers, bipolar disorder, cytokines, interleukin-4, major depressive disorder
1. Introduction
Major depressive disorder (MDD) and bipolar disorder (BD) are serious psychiatric disorders. Patients with BD experience multiple depressive episodes during their lifetime, and the symptom profile of this phase is almost identical to that of a monophasic depressive episode. Distinguishing these disorders is important for clinicians as the treatment strategies for the 2 conditions differ, and administering antidepressants to a patient with BD may lead to aggravation of their symptoms.[1] There is an urgent need for improved differentiating factors to better address clinical needs and elucidate disease mechanisms, such as a biological marker that can be used to identify these disease states. In recent years, the neuroimmune inflammatory hypothesis has been considered an important pathogenetic mechanism for both MDD and BD. This hypothesis associates the target disorder with the activation of the immune-inflammatory response system (IRS), which manifests as an increase in pro-inflammatory factors, and the activation of the compensatory immunoregulatory response system (CIRS), exerting negative immunoregulatory effects through the activation of the T helper 2 (Th-2) and T regulatory (Treg) mechanisms and suppresses IRS overreaction. It has been found that the IRS–CIRS imbalance manifests differently in MDD and BD.[2–4] Therefore, more studies are needed to elucidate differences in peripheral immunophenotypes between MDD and BD.
In this study, the 8 cytokines of the IRS and CIRS systems were analyzed: interleukin (IL)-6 and tumor necrosis factor (TNF)-α, released by activated M1 macrophages, interferon (IFN)-γ and IL-2 released by T helper 1 (Th1) cells, IL-17 secreted by T helper 17 (Th17) cells of the IRS and IL4 secreted by Th2 cells and IL-10 secreted by Treg cells of the CIRS. This study examined the differences in inflammatory profiles between MDD and BD by measuring multiple cytokine levels in peripheral blood. The aim of this study was to identify potential biological markers to assist in differentiating MDD from BD.
2. Materials and Methods
2.1. Participant selection
Patients with affective disorders who visited the inpatient psychiatric department of Tongde Hospital of Zhejiang Province from March 2020 to May 2022 were considered for participation in this study. In total, 115 patients were selected: 77 women and 38 men, aged 24 ± 15 years. The patients had the following presentations: 35 patients had manifestations of BD (depressive episodes, 16; manic episodes, 6; mixed or unspecified episodes, 13), and 80 had manifestations of depression. Thirty-five patients were assigned to the untreated group for which medication was not administered before admission or had been stopped for more than 1 month at the time of admission; 78 patients were assigned to the treatment group for which regular medication had been administered before onset. The enrollment criteria conformed to the diagnostic criteria for MDD or BD in the International Classification of Diseases-10, and all participants were experiencing acute episodes at the time of admission.
The exclusion criteria were as follows: clear manifestation of infection, major physical diagnoses such as autoimmune diseases, other severe mental disorders such as schizophrenia or intellectual disability, and pregnancy.
As a control group, 30 healthy individuals (physical examiners or employees of our hospital) were recruited. The control group comprised of 13 men and 17 women aged 25.3 ± 5.3 years. The control group participants had no history of psychiatric disorders, and participation was voluntary in all cases. The same exclusion criteria were used for both the experimental and control groups.
This study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province (Ethical 2022-YAN-NO.154-JY). The Ethics Committee agreed to waive the requirement of informed consent, and the study complied with the Declaration of Helsinki regarding human participation in scientific research.
2.2. Analytic methods
Prior to analysis, the clinical and demographic information of all the participants were collected, including age, sex, height, weight, and clinical diagnoses.
2.3. Plasma cytokine assays
The results of laboratory examinations for cytokine plasma levels performed at admission were collected retrospectively. Tests for 8 cytokines were performed. First, 2 mL of peripheral blood was collected from all participants and placed in EDTA-K2 anticoagulation tubes. Peripheral blood was centrifuged at 1000g for 30 minutes and the plasma was separated. The calibrator was diluted multiplicatively to obtain 8 final concentrations (10,000 pg/mL; 2500 pg/mL; 625 pg/mL; 156.3 pg/mL; 39.1 pg/mL; 9.8 pg/mL; 2.4 pg/mL; 0 pg/mL) from largest to smallest, respectively. 25 μL of capture microsphere antibody and 25 μL of detection antibody and 25 μL of buffer were added to each concentration of calibrator tubes and patient plasma tubes, and then shaken for 2 hours at room temperature and protected from light. 25 μL SA-PE was added to all sample tubes and shaken for 0.5 hours away from light. Finally, all sample tubes were centrifuged at 250 g with diluent for 5 minutes, and then the supernatant was removed. Subsequently, plasma expression levels of IL-2, IL-4, IL-6, IL-10, IFN-γ, IL-17, IL-12, and TNF-α were measured using a flow cytometry microsphere array. The kit manufacturer own software can plot standard curves and produce sample cytokine results. The procedure was performed in accordance with the instructions of the cytometric bead array kit (Qingdao Raisecare Biotechnology Co., Ltd China), and detection was conducted using a Navious flow cytometer (Beckman Coulter).
2.4. Statistical analysis
SPSS 18.0 and GraphPad software were used for statistical data analysis, and the distribution of continuous variables was evaluated using the Kolmogorov–Smirnov test (K–S test). Data conforming to normal distribution were described as mean ± standard deviation. The t test was used to analyze 2 independent samples, and the Kruskal–Wallis H test was used to compare multiple groups. Non-normally distributed data were described as median (25th percentile, 75th percentile) and analyzed using the non-parametric Mann–Whitney U test or Kruskal–Wallis H test. The diagnostic relevance of cytokine levels was analyzed by the receiver operating characteristic (ROC) curve and cutoff values, and the sensitivity of each cytokine was determined. Statistical significance was set at P < .05.
3. Results
3.1. Demographics and clinical data
Age, sex, height, weight, and preadmission medications were collected from March 2020 to May 2022 for patients admitted with a clinical diagnosis of MMD or BD. There were no significant differences in age, sex, or body mass index between the MDD and BD groups (Table 1). Thirty-two patients with MDD and 5 patients with BD did not use medication before admission or had stopped using medication on their own for more than a month before admission. Details of pre-hospital medication use for the remaining patients are shown in Table 2.
Table 1.
Demographic information of patients with BD and MDD.
| MDD group (A) (n = 80) | BD group (B) (n = 35) | Control group (C) (n = 30) | F/χ2 | P value | |
|---|---|---|---|---|---|
| Age | 24.9 (16.34) | 21.9 (11.41) | 25.3 (5.3) | 0.693 | .502 |
| Sex (men/women) | 18/62 | 10/25 | 13/17 | 4.671 | .097 |
| BMI (kg/m2) | 20.9 (3.44) | 21.9 (4.12) | 22.0 (4.19) | 2.266 | .108 |
BD = bipolar disorder, BMI = body mass index, MDD = major depressive disorder
Table 2.
Patient medication details.
| MDD (person-time) | BD (person-time) | |
|---|---|---|
| Sertraline | 14 | 5 |
| Aripiprazole | 7 | 3 |
| Quetiapine | 12 | 14 |
| Citalopram | 13 | 2 |
| Lithium carbonate | 3 | 10 |
| Fluoxetine | 7 | 2 |
| Olanzapine | 5 | 4 |
| Fluvoxamine | 3 | 3 |
| Venlafaxine | 5 | 2 |
| Lurasidone | 1 | 1 |
| Sulpiride | 3 | 1 |
| Sodium valproate | 2 | 10 |
| Trazodone | 1 | 1 |
| Mirtazapine | 3 | |
| Paroxetine | 1 | |
| Duloxetine | 2 | |
| flupentixol + Melitracen (Deanxit) | 2 | |
| Risperidone | 1 | |
| Oxcarbazepine | 1 |
Some patients were treated with multiple drug combinations. Therefore, each individual unit displayed on the table corresponds to an instance of a specific drug prescribed to a specific person.
BD = bipolar disorder, MDD = major depressive disorder.
3.2. Comparison of cytokine levels in patients from the untreated and treatment group
Eighty patients with MDD were recruited, including 32 patients not under medication and 48 patients with recurrent episodes. Thirty-five patients with BD were recruited, including 5 patients not under medication and 30 patients with recurrent episodes. The patients not under medication had no history of long-term use of antipsychotic medication or were not on drug treatment for more than a month at admission. The relapsed patients had a history of previous use of antipsychotic medication, but the medication had been unable to prevent another acute episode. There was no statistically significant difference between the cytokine levels in peripheral blood between the untreated and the treatment groups.
3.3. Comparison of cytokine levels between the BD, MDD, and control groups
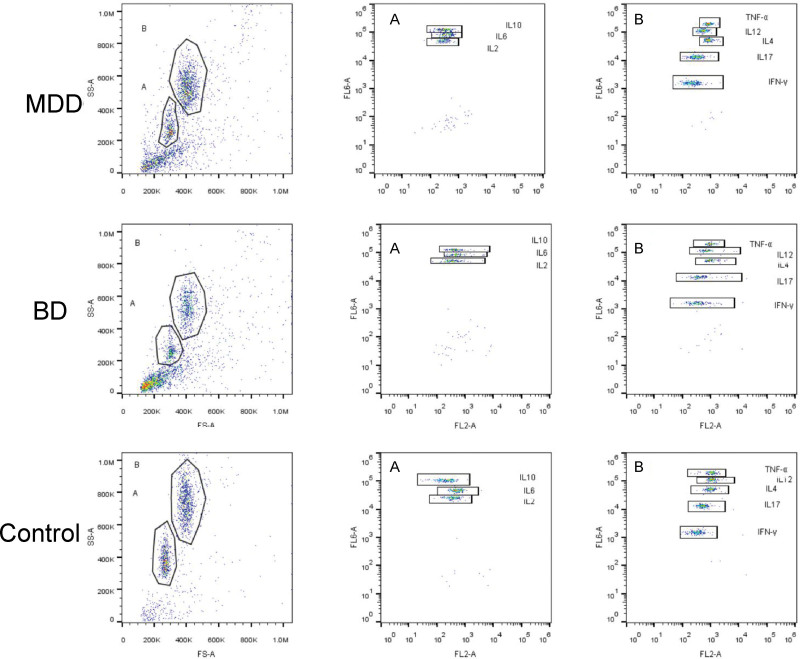
The plasma expression levels of IL-6, IL-10, IL-17, IL-4, IFN-γ, and TNF-α in the MDD, BD, and control groups were statistically different (P < .05), but this was not the case for the levels of IL-2 and IL-12 (P < .05). Further comparison revealed that IL-6, IL-10, IL-17, and IFN-γ expression levels were higher in the MDD and BD groups than in the control group (P < .05); however, there was no significant statistical difference between the patient and control groups. Only the expression levels of TNF-α and IL-4 were higher in the MDD and BD groups than those in the control group, and the levels in the BD group were higher than those in the MDD group (P < .05). (Table 3) (Fig. 1).
Table 3.
Comparison of cytokine levels between BD, MDD, and control groups.
| MDD group (A) (n = 80) | BD group (B) (n = 35) | Control group (C) (n = 30) | P value | P value | |||
|---|---|---|---|---|---|---|---|
| Kruskal–Wallis H test | Mann–Whitney U test | ||||||
| (A vs B) | (A vs C) | (B vs C) | |||||
| IL-6 | 2.68 (0.49,2.53) | 3.97 (0.67,4.42) | 0.49 (0.13,1.31) | .0016 | .063 | .011 | .0005 |
| IL-10 | 0.73 (0.32,1.06) | 1.04 (0.39,1.3) | 0.23 (0.18,0.49) | .0002 | .542 | .0010 | .0002 |
| IFN-γ | 3.94 (0,3.75) | 6.95 (0,3.5) | 0 (0,1.78) | .0016 | .0758 | .0078 | .0005 |
| IL-17 | 1.28 (0,1.39) | 3.11 (0,3.5) | 0. (0,0) | .0004 | .1689 | .0009 | <.0001 |
| IL-4 | 0.68 (0,0.63) | 1.24 (0,1.22) | 0 (0,0) | <.0001 | .011 | .0018 | <.0001 |
| TNF-α | 1.07 (0,1.76) | 2.39 (0,4.10) | 0 (0,0.32) | .0010 | .0182 | .2782 | .0036 |
| IL-2 | 2.04 (0,3.40) | 2.04 (0,3.40) | 0.40 (0.25,1.09) | .4599 | .2448 | .8924 | .3173 |
| IL-12 | 0.61 (0,0.80) | 0.86 (0,1.20) | 0.31 (0.14,0.71) | .7288 | .8280 | .4038 | .7115 |
All results are shown as median (25th percentile, 75th percentile) and expressed as pg/mL.
BD = bipolar disorder, IL = interleukin, IFN = interferon, MDD = major depressive disorder, TNF = tumor necrosis factor.
Figure 1.
The expression of cytokines in MDD, BD, control groups as dot plot by flow cytometry. The A-gate contains 3 cytokines (IL-10, IL-6, IL-2), The B-gate contains 5 cytokines (IL-4, IFN-γ, IL-17, IL-12, and TNF-α). BD = bipolar disorder, IL = interleukin, IFN = interferon, MDD = major depressive disorder, TNF = tumor necrosis factor.
3.4. Comparison of cytokine levels between patients presenting with depressive and manic episodes in the BD group
The expression levels of IL-17, IFN-γ, IL-10, and IL-4 were significantly higher in patients presenting with depressive episodes than in those presenting with manic episodes (P < .05) (Table 4).
Table 4.
Comparison of cytokine levels between patients with depressive and manic episodes in the BD group.
| Depressive episode group (n = 16) | Manic episode group (n = 6) | P value | |
|---|---|---|---|
| Mann–Whitney U test | |||
| IL-10 | 1.163 (0.57, 1.52) | 0.37 (0.22, 0.51) | .0041 |
| IFN-γ | 7.69 (0.38, 11.63) | 0.59 (0, 1.22) | .0266 |
| IL-17 | 2.34 (0.01, 2.72) | 0.12 (0, 0.17) | .0131 |
| IL-4 | 2.12 (0.21, 4.38) | 0.13 (0, 0.20) | .0092 |
| IL-6 | 5.97 (0, 1.52) | 1.06 (0, 2.05) | .0604 |
| IL-2 | 2.67 (0, 4.83) | 0.73 (0.14, 1.27) | .6913 |
| TNF-α | 2.71 (0, 3.48) | 0.49 (0, 0.85) | .1244 |
| IL-12 | 0.68 (0, 1.18) | 0.19 (0, 0.31) | .8971 |
All results are shown as median (25th percentile, 75th percentile) and expressed as pg/mL.
BD = bipolar disorder, IL = interleukin, IFN = interferon, TNF = tumor necrosis factor
*12 patients presented with mixed or unrecognized episode types.
3.5. Comparison of cytokine levels in patients experiencing depressive episodes in the MDD and BD groups
IL-6, IFN-γ, TNF-α, IL-10, and IL-4 levels in patients presenting with depressive episodes in the BD group were significantly higher than the levels in those with depressive episodes in the MDD group (P < .05) (Table 5).
Table 5.
Comparison of cytokine levels between MDD- and BD-associated depressive episodes.
| MDD group (n = 60) | BD group (n = 16) | P value | |
|---|---|---|---|
| Mann–Whitney U test | |||
| IL-6 | 2.68 (0.49, 2.53) | 5.97 (0.73, 8.66) | .0369 |
| IL-10 | 0.73 (0.32, 1.06) | 1.163 (0.57, 1.52) | .0241 |
| IFN-γ | 3.94 (0, 3.75) | 7.69 (0.38, 11.63) | .02 |
| IL-4 | 0.68 (0, 0.63) | 2.12 (0.21, 4.38) | .0027 |
| TNF-α | 1.07 (0, 1.76) | 2.71 (0.1, 3.48) | .0396 |
| IL-2 | 1.56 (0, 1.37) | 2.67 (0, 4.83) | .4556 |
| IL-17 | 1.28 (0, 1.39) | 2.34 (0, 2.73) | .1185 |
| IL-12 | 0.61 (0, 0.80) | 0.68 (0, 1.18) | .9318 |
All results are shown as a median (25th percentile, 75th percentile) and expressed as pg/ml.
BD = bipolar disorder, IL = interleukin, IFN = interferon, MDD = major depressive disorder, TNF = tumor necrosis factor
3.6. Diagnostic efficacy of cytokine levels in diagnosing BD
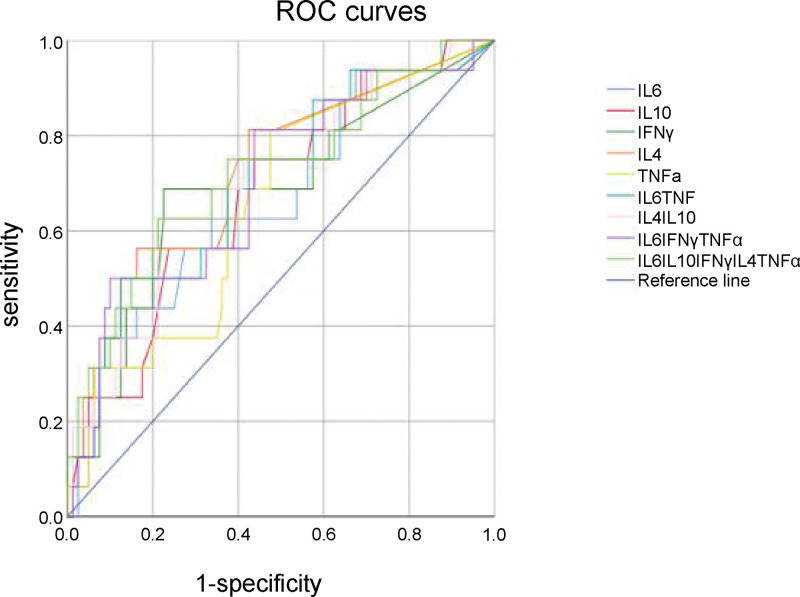
IL-6, IFN-γ, TNF-α, IL-10, and IL-4 levels were selected for a follow-up study based on previous statistical results. To further analyze the diagnostic efficacy of cytokine levels in discriminating depressive episodes between patients with MDD and BD, ROC curves were used to compare individual cytokine levels (IL-6, IFN-γ, TNF-α, IL-10, and IL-4) and multiple cytokine levels (IL-6 + TNF-α, IL-4 + IL-10, IL-6 + IFN-γ+TNF-α, and IL-6 + IFN-γ+TNF-α+IL-10 + IL-4) between the groups (Fig. 1, Table 6). The area under the curve (AUC) for IL-4 was 0.7223, indicating a potential diagnostic value (cutoff value, 0.81 pg/mL; sensitivity, 0.563; specificity, 0.828; positive predictive value, 0.434; negative predictive value, 0.918; positive likelihood ratio, 3.27; negative likelihood ratio, 0.53) (Table 6, Fig. 2).
Table 6.
AUC area, cutoff, sensitivity, and specificity for each cytokine.
| AUC | Cutoff value (pg mL) | Sensitivity | Specificity | P value | 95% CI | |
|---|---|---|---|---|---|---|
| IL-6 | 0.665 | 1.81 | 0.625 | 0.662 | .038 | 0.514–0.816 |
| IL-10 | 0.678 | 0.70 | 0.750 | 0.562 | .025 | 0.538–0.818 |
| IFN-γ | 0.680 | 4.25 | 0.688 | 0.775 | .023 | 0.526–0.834 |
| IL-4 | 0.722 | 0.81 | 0.563 | 0.838 | .005 | 0.579–0.865 |
| TNF-α | 0.655 | 0.24 | 0.688 | 0.575 | .050 | 0.513–0.798 |
| IL-6 + TNF-α | 0.698 | 0.13 | 0.750 | 0.575 | .013 | 0.554–0.842 |
| IL-4 + IL-10 | 0.701 | 0.18 | 0.625 | 0.787 | .011 | 0.558–0.844 |
| IL-6 + IFN-γ+TNF-α | 0.699 | 0.13 | 0.813 | 0.562 | .012 | 0.554–0.845 |
| IL-6 + IFN-γ+TNF-α + IL-4 + IL-10 | 0.719 | 0.185 | 0.625 | 0.787 | .006 | 0.571–0.866 |
AUC = area under the curve, BD = bipolar disorder, IL = interleukin, IFN = interferon, MDD = major depressive disorder, TNF = tumor necrosis factor.
Figure 2.
ROC curves for each cytokine. The largest value for the area under the curve corresponded to the IL-4 curve (0.722). IL-4 = interleukin-4, ROC = receiver operating characteristic curve. L = interleukin, ROC = receiver operating characteristic.
4. Discussion
Numerous studies have confirmed the involvement of neuroimmune inflammatory pathway activation in the pathogenesis of MDD and BD, particularly the T cell-mediated adaptive immune response, which releases soluble cytokines and chemokines that are key regulatory factors of neuroimmunity, with some exerting pro- or anti-inflammatory effects.[5] A previous meta-analysis found that after effective treatment of acute depression, the disturbed immune response was stabilized, and the elevated IL-6, IL-1RA, and TNF-α levels had reduced. Effective treatments for BD yielded a similar pattern.[6]
In this study, we first compared the cytokine levels of patients with affective disorders between those with no history of drug treatment at the onset and those with a previous history of drug treatment who were experiencing a relapse and found no statistically significant differences between these 2 groups. Some meta-analyses have shown that certain antidepressants, such as selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors, can reduce the levels of IL-6, IL-1β, and TNF-α after effective treatment.[7,8] This indicates that some antidepressant drugs have a slight anti-inflammatory effect, and patients experiencing a relapse exhibit a disordered immune response similar to that of patients experiencing the first episode without concomitant medication. It has been hypothesized that the cytokine levels would change again when MDD and BD patients experience a relapse, and indeed, the levels were not significantly different from those in patients experiencing their first episode.
The levels of pro-inflammatory (IL-6, IL-17, IFN-γ, and TNF-α) and anti-inflammatory factors (IL-4 and IL-10) were significantly elevated in patients with MDD and BD, suggesting that both the IRS and CIRS were activated and involved in maintaining immune system homeostasis. However, only TNF-α and IL-4 levels were significantly different between patients with MDD and with BD, with significantly higher levels observed in the BD group.
Previous studies have reported increased expression of various pro- and anti-inflammatory factors in MDD and BD. Evidence from a meta-analysis showed that the levels of IL-6, TNF-α, IL-10, sIL-2R, CCL-2, IL-12, IL-13, IL-18, and IL-1 were elevated in patients with depression, and antidepressant treatment significantly reduced the levels of IL-6, IL-10, TNF-α, and CCL-2.[9] Another meta-analysis on BD indicated that concentrations of sIL-2R, TNF-α, and sTNF-R1 were significantly elevated in patients with BD during an episode. Moreover, it was found that the levels of various cytokines in patients with BD in the stable phase were not significantly different from those in healthy controls.[10] Elevated serum IL-6 levels in patients with MDD and BD compared with the levels in the control group have also been reported in other studies.[11] The increase in IL-6 levels may lead to increased resistance to antidepressant medication and is associated with a high risk of suicidal behavior.[3,6] Recent studies found elevated levels of TNF-α in patients with MDD and BD as well as elevated concentrations of sTNF-R1 and sTNF-R2 during acute episodes,[12,13] A meta-analysis showed that TNF-α levels were also increased in patients with BD.[14] Another meta-analysis on depression found that patients with MDD also had significantly increased IL4 and IL-10 levels compared to the control group.[9] These cytokines are involved in activating Treg cells and exert a negative immunomodulatory effect. All of the above studies demonstrated that patients with MDD and BD exhibit activation of both the IRS and CIRS, in line with the results observed in the present study.
Macrophages and microglia play a major role in the innate immune system of the CNS and can perform different functions by changing their phenotype. The main role of M1 macrophages is to defend the body against pathogens in the acute phase and promote the release of pro-inflammatory factors, whereas M2 macrophages are mainly involved in the repair and removal of damaged tissue.[15] There is evidence that M1 macrophages (including microglia and CNS macrophages) play a role in the development of MDD.[5,16] Although clinical studies directly investigating the role of M1 macrophages in the CNS of patients with MDD are lacking, pro-inflammatory cytokine production was reduced when the transport of peripheral blood monocytes to the brain was blocked in a rodent stress model, accompanied by a reduction in depression-like behavior. Thus, peripheral blood M1 macrophages may induce an increase in cytokine levels in MDD.[17]
M1 macrophages and Th-1 cells can release large amounts of various cytokines such as IL-1β, IL-6, TNF-α, and IFN-γ, which can activate the IL-6 trans-signaling pathway, promote the proliferation of Th-17 cells, and stimulate the release of IL-17 to maintain a chronic inflammatory state.[18] In such a state, the levels of C-reactive protein and of various other positive acute temporal proteins are increased. However, when the inflammatory response reaches a certain threshold, immune regulation is activated, and M2 macrophages and Treg cells exert a negative immunoregulatory effect by participating in Th-2 activation, producing the anti-inflammatory factors IL-4 and IL-10 to exert complex immunoregulatory effects, inhibiting IL-1 release, and increasing IL-1RA production to maintain immune stability.[2]
Animal experiments have demonstrated that peripheral inflammatory signals can be transmitted to the central nervous system through multiple pathways. Cytokines can leak through the circumventricular organs between the third and fourth ventricles to the blood-brain barrier or bind to saturated transporter molecules in the blood-brain barrier. In addition, cytokines can bind to peripheral afferent nerve fibers, such as the vagus nerve, to transmit signals to the CNS,[19] and immune cells such as monocytes can enter the brain.[20] These data support the hypothesis that peripheral inflammation can lead to inflammation in the brain and induce a variety of psychiatric disorders.
In this study, the cytokine profiles of patients with BD experiencing manic and depressive episodes were comparatively analyzed, and it was found that the levels of pro-inflammatory cytokines IL-17 and IFN-γ and anti-inflammatory cytokines IL-10 and IL-4 were significantly higher in patients with BD experiencing depressive episodes than in those experiencing manic episodes. This indicates that the peripheral immune profiles of patients with BD vary when different types of episodes are experienced. A previous study found that the levels of pro-inflammatory factors such as IL-6 and TNF-α reduced in patients with BD in remission and were not significantly different from those of the control group, and only IL-4 remained highly expressed in BD patients in remission,[13] suggesting that the CIRS is continuously activated in this group of patients. A meta-analysis found that IL-4 and IL-10 levels remained high in patients with BD in the chronic phase compared with those in the control group, but IL-10 levels returned to normal in the acute manic phase.[6]
In clinical practice, MDD and BD can be difficult to diagnose. It has been found that depressive episodes associated with MDD and BD have different peripheral immune profiles. In particular, IL-1β, TNF-α, sTNFR1, IL-12, and IL-10 levels have been found to be significantly higher in patients with MDD than in those with BD, while IL-6, sTNF-R2, IL-18, and IL-33 levels have been found to be significantly higher in patients with BD.[4] This finding is in contrast with the results of previous studies. The results of the present study partially differed from the results of previous studies: for example, the present study concluded that the expression levels of IL-10 and TNF-α in patients with MDD were higher than those in patients with BD, and no statistical difference was found in the levels of IL-12 between MDD and BD. These results are inconsistent with the results from previous studies and may be related to the heterogeneity of patients with BD, but patients with manic episodes were predominant in previous studies. According to the results of this study, the expression profiles of cytokines in patients with BD during manic episodes and depressive episodes are different. The expression of the pro-inflammatory factors IL-17 and IFN-γ and the anti-inflammatory factors IL-10 and IL-4 were significantly higher during depressive episodes of BD than during manic episodes. On comparing the cytokine profiles between MDD and BD depressive episodes, we found that the levels of pro-inflammatory factors IL-6, IFN-γ, and TNF-α and the anti-inflammatory factors IL-10 and IL-4 were higher in patients with depressive episodes of BD than in patients with MDD, indicating that the IRS and CIRS activation responses were stronger during depressive episodes of BD, as demonstrated by a pronounced IRS–CIRS imbalance.
IRS–CIRS homeostasis is maintained by the involvement of various functional immune cells as well as other factors. Psychosocial stressors such as early trauma may lead to IRS–CIRS destabilization, resulting in inflammatory and neurotoxic responses.[21] Activation of the IRS results in a massive proliferation of inflammatory cells and pro-inflammatory factors, followed by the activation of CIRS and the release of anti-inflammatory cytokines and Treg cells, resulting in negative regulation of the immune response to maintain immune homeostasis. CIRS can facilitate recovery from acute disease by modulating primary immune inflammation. Sustained low activation of IRS and CIRS was observed during periods of stability in patients with MDD and with BD, suggesting that the immune response does not return to a healthy baseline after an acute episode but is maintained at a dynamic steady state; therefore, subsequent episodes result in sensitive IRS and CIRS overreaction.[2]
The IRS–CIRS imbalance has been found to be more severe in patients with BD than in those with MDD, which may be related to abnormal time-dependent sensitization (TDS) in BD. Additionally, an exponential response was also found upon re-exposure to stress.[22] However, the exact mechanism by which this sensitization occurs remains unclear.
Based on the above results, we determined the most useful diagnostic markers and plotted the corresponding ROC curves. In addition to individual cytokines, we also combined multiple cytokines for ROC curve diagnosis, such as IL-6 and TNF-α, which jointly represent M1 macrophage function, IL-4 and IL-10, which jointly represent CIRS function, IL-6, TNF-α and IFN-γ, which jointly represent IRS function, and all 5 cytokines, which jointly represent the IRS–CIRS balance. IL-4 was found to have a diagnostic value with an AUC of 0.7223 and a cutoff value of 0.81 pg/mL. It demonstrated high specificity and negative predictive value, thus exhibiting potential as a valuable biological indicator for differentiating MDD from BD in patients presenting with depressive episodes.
IL-4 is an important anti-inflammatory factor that selectively activates M2 macrophages, thereby reducing the inflammatory response by promoting the release of TGF-β, sIL-1RA, and IL-1.[23] TGF-β and IL-10 can initiate differentiation of T0 into Treg cells, which regulate the immune system, prevent excessive autoimmune responses, and suppress strong immune responses caused by the Th-1, Th-17, and Th-2 cells.[24] IL-4 also directly inhibits the production of the pro-inflammatory factors IL-β, IL-6, and TNF-α. Previous studies have found that stress causes depressive behavior in mice, and recovery may be impaired when they are deficient in IL-4, but by artificially increasing the IL-4 levels, the depressive-like behavior can be reduced.[25] Another recent study found that IL-4 reprogrammed the microglia to an Arg1+ phenotype, which activated the brain-derived-neurotrophic factor signaling pathways to protect hippocampal nerves in stressed mice[26] and maintain brain homeostasis, neuroprotection, and tissue repair.[27] These results indicate that IL-4 plays an important neuroimmunomodulatory role in the brain. Moreover, IL-4 was recently found to have beneficial effects on cognitive performance, which may be related to its ability to induce astrocytes to produce brain-derived-neurotrophic factor or nerve growth factor.
Patients with MDD and BD exhibited IRS–CIRS activation during episodes, but the cytokine expression profiles were slightly different between the groups. Patients with BD exhibited significantly higher IL-4 levels than patients with MDD. Moreover, the depressive episodes of patients with BD had different cytokine expression profiles compared to those of patients with MDD, and BD-associated depressive episodes were characterized by more intense IRS–CIRS activation. Based on the ROC curve, IL-4 may be a promising clinical therapeutic target and biomarker. Although some differences in cytokine levels between the 2 diseases were observed in this study, the affective disorder is a disease caused by a combination of biological, psychological, and social factors. The identification of MDD and BD using only biological indicators may lead to biased results. Therefore, in clinical practice, it is still necessary to make a comprehensive judgment based on the patient medical history, psychological status, social environment, and other factors. This was a cross-sectional study with a small, single-center sample. The effect of patients’ medication status on cytokine levels was ignored, which may limit the generalizability of the results. All patients enrolled in this study presented with acute episodes, and disruption of the immune response as a consequence of the disease itself may have outweighed the effect of the medication.
Acknowledgments
The author would like to thank all the individuals who participated in the study. The authors would like to thank Editage for providing editorial support in the writing of this manuscript.
Author contributions
Conceptualization: Lingna Lu.
Data curation: Xin Jin.
Funding acquisition: Lingna Lu, Xiwen Hu.
Investigation: Xiwen Hu.
Methodology: Xiwen Hu.
Project administration: Xiwen Hu.
Resources: Xiwen Hu.
Validation: Xin Jin.
Visualization: Xin Jin.
Writing – original draft: Lingna Lu.
Writing – review & editing: Lingna Lu.
Abbreviations:
- BD
- bipolar disorder
- CIRS
- compensatory immunoregulatory response syndrome
- IL
- interleukin
- IFN
- interferon
- IRS
- immune-inflammatory response system
- MDD
- major depressive disorder
- ROC
- receiver operating characteristic
- Th-2
- T helper 2
- TNF
- tumor necrosis factor
- Treg
- T regulatory
This research was supported by the Medical and Health Science and Technology Project of Zhejiang Province (2022KY124, Lingna Lu; 2020KY747, Xiwen Hu) and the Hangzhou Agricultural and Social Development Pilot Project (20211231Y055, Xiwen Hu).
The authors have no conflicts of interest to disclose.
The Ethics Committee waived the requirement for informed consent, and the study complied with the Declaration of Helsinki regarding human participation in scientific research.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Lu L, Hu X, Jin X. IL-4 as a potential biomarker for differentiating major depressive disorder from bipolar depression. Medicine 2023;102:15(e33439).
Contributor Information
Lingna Lu, Email: lulingnalab@163.com.
Xin Jin, Email: goodjinxin@163.com.
References
- [1].Shim I, Woo Y, Kim M, et al. Antidepressants and mood stabilizers: novel research avenues and clinical insights for bipolar depression. Int J Mol Sci. 2017;18:2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maes M, Carvalho AF. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol. 2018;55:8885–903. [DOI] [PubMed] [Google Scholar]
- [3].Kohler CA, Freitas TH, Stubbs B, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55:4195–206. [DOI] [PubMed] [Google Scholar]
- [4].Brunoni AR, Supasitthumrong T, Teixeira AL, et al. Differences in the immune-inflammatory profiles of unipolar and bipolar depression. J Affect Disord. 2020;262:8–15. [DOI] [PubMed] [Google Scholar]
- [5].Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacol. 2011;36:2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-alpha (TNF-alpha) in major depressive disorder: a systematic review. Int J Mol Sci. 2016;17:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kohler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87. [DOI] [PubMed] [Google Scholar]
- [10].Munkholm K, Brauner JV, Kessing LV, et al. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47:1119–33. [DOI] [PubMed] [Google Scholar]
- [11].Sayana P, Colpo GD, Simoes LR, et al. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res. 2017;92:160–82. [DOI] [PubMed] [Google Scholar]
- [12].Sowa-Kuma M, Styczeń K, Siwek M, et al. Lipid peroxidation and immune biomarkers are associated with major depression and its phenotypes, including treatment-resistant depression and melancholia. Neurotox Res. 2018;33:448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brietzke E, Stertz L, Fernandes BS, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214–7. [DOI] [PubMed] [Google Scholar]
- [14].Modabbernia A, Taslimi S, Brietzke E, et al. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. [DOI] [PubMed] [Google Scholar]
- [15].Kalkman HO, Feuerbach D. Antidepressant therapies inhibit inflammation and microglial M1-polarization. Pharmacol Ther. 2016;163:82–93. [DOI] [PubMed] [Google Scholar]
- [16].Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–58. [DOI] [PubMed] [Google Scholar]
- [17].Wohleb ES, Mckim DB, Sheridan JF, et al. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014;8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maes M, Anderson G, Kubera M, et al. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin Ther Targets. 2014;18:495–512. [DOI] [PubMed] [Google Scholar]
- [19].Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor alpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Debnath M, Berk M, Maes M. Translational evidence for the Inflammatory Response System (IRS)/Compensatory Immune Response System (CIRS) and neuroprogression theory of major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110343. [DOI] [PubMed] [Google Scholar]
- [22].Antelman SM, Levine J, Gershon S. Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry. 2000;5:350–6. [DOI] [PubMed] [Google Scholar]
- [23].Woodward EA, Prêle CM, Nicholson SE, et al. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology. 2010;131:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol. 2013;4:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wachholz S, Knorr A, Mengert L, et al. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res. 2017;326:165–72. [DOI] [PubMed] [Google Scholar]
- [26].Zhang J, Rong P, Zhang L, et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv. 2021;7:eabb9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xia Y, Zhang Z, Lin W, et al. Modulating microglia activation prevents maternal immune activation induced schizophrenia-relevant behavior phenotypes via arginase 1 in the dentate gyrus. Neuropsychopharmacol. 2020;45:1896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]