Abstract
OBJECTIVE:
To investigate the effects of shikonin (SKN) on M1 and M2 polarization of macrophages both in vitro and in vivo.
METHODS:
Collagen-induced arthritis (CIA) in male DBA/1 mice were treated with a dose of 4 mg/kg/day of SKN for 23 d (n = 6/group). The histopathology of inflamed joints in CIA mice was evaluated to test the anti-arthritic effect of SKN. M1/M2 polarization of macrophages induced by lipopolysaccharide (LPS) and interferon (IFN)-γ or interleukin (IL)-4 and IL-13, were used to assess the effect of SKN (0.05, 0.1, and 0.2 μM). The effect of SKN on the protein expression of nitric oxide synthase, arginase, CD68, and CD206 was evaluated using western blot analysis.
RESULTS:
The results of this study revealed that SKN delayed the arthritis feet symptom score, reduced the incidence rate of arthritis, and relieved the inflammation of joints in CIA mice. SKN inhibited M1 macrophage polarization but did not affect M2 macrophage polarization in the joints of CIA mice. Moreover, SKN inhibited M1 polarization induced by LPS and IFN-γ, but did not affect M2 polarization induced by IL-4 and IL-13.
CONCLUSION:
These findings suggest that SKN alleviated CIA through inhibiting M1 macrophage polarization and has great potential as a new drug for RA treatment.
Keywords: Shikonin; arthritis, rheumatoid; macrophages; M1 polarization
1. INTRODUCTION
Rheumatoid arthritis (RA) is a chronic and inflammatory disease with unknown etiology, and it is referred to as "Bi syndrome" in Traditional Chinese Medicine (TCM). TCM is popular due to its simplicity, convenience, cheapness, potential curative effects, and little side effects. Shikonin (SKN) is isolated from Zicao (Radix lithospermi), the dried roots of Lithospermum erythrorhizon Sieb. et Zucc. (Boraginaceae), and it is a commonly used herbal medicine in China and other countries.1,2 Large number of studies has shown that SKN exerts anti-inflammatory, anti-tumor, immune-omodulatory, and analgesic effects, and can alleviate the symptoms of cartilage damage induced due to collagen-induced arthritis (CIA) in mice.3,⇓-5 Macrophages play an important role in the pathogenesis of RA. Inflammatory RA synovial tissue and inflammatory vascular reaction revealed more macrophages than in healthy joints.6 Many studies in the literature have focused on the pro-inflammatory effect of M1 and anti-inflammatory effect of M2 in RA, and classified them based on their polarization function in RA, especially in synovial tissue.6 It has been reported that SKN can effectively modulate macrophages.7,8 However, the role of SKN in M1 or M2 polarization of macrophages has not been reported yet, especially in RA.
Since the 1950s, methotrexate (MTX) has been used for the treatment of RA and provided good results. Although there are many new drugs at present, MTX is still widely used for the treatment of RA and is often used as a positive control in animal experiments. Therefore, MTX was selected as a positive control drug in our animal experiments. Therefore, in this study, we aimed to investigate the effects of SKN on M1 and M2 polarization of macrophages both in vitro and in vivo.
2. MATERIALS AND METHODS
2.1. Chemicals
SKN and MTX were purchased from Shanghai Yuanye Biotechnology Co. (Shanghai, China) and the purity of SKN (99.5%) was verified using high performance liquid chromatography as indicated in the assay requirements of SKN in the Chinese Pharmacopoeia. All reagents related to cell culture were purchased from Gibco (Grand Island, NY, USA); diphenylindole (DAPI) was purchased from AAT Bioquest (Sunnyvale, CA, USA); CIA-related reagents were purchased from Chondrex (Chondrex, WA, USA); anti-fluorescence quenching coating agents were purchased from Abcam (Cambridge, England). CD68, CD206, nitric oxide synthase (iNOS), and arginase antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz County, CA, USA). M-CSF, LPS, IFN-γ, IL-4, and IL-13 were purchased from Peprotech (Suzhou, China). All other immunofluorescence related reagents were purchased from Abcam (Cambridge, England).
2.2. Ethics
This research was conducted with the approval of the Ethics Committee of Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All the experimental procedures were conducted in accordance with the relevant rules and regulations.
2.3. Animals
Male DBA/1 mice, six to eight-week-old, and weighing 16-18 g were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. (production license No. SCXK 2017-0005). Male SD rats weighing 150-170 g were purchased from the Medical Experimental Animal Center of Guangdong Province (production license No. SCXK 2018-0002). Mice and rats were maintained at 12:12 h (L: D) with 60% ± 5% relative humidity at (22 ± 3) °C. The mice and rats had free access to food and water.
2.4. CIA induction
Twenty-four male DBA/1 mice were randomly divided into four groups in equal numbers (n = 6): the control group (Control), CIA model group (CIA), CIA mice treated with SKN group, and CIA mice treated with MTX group. Except for the control group, arithritis was induced in all other mice through intradermally injecting 100 μg bovine type II collagen prepared in complete Freund’s adjuvant (CFA) at the base of the tail.9 Mice were boosted intradermally with 100 μg type II collagen prepared in incomplete Freund’s adjuvant (IFA) on day 21.
2.5. CIA treatment
SKN was then dissolved in phosphate buffered saline (PBS) containing 0.05% dimethyl sulfoxide (DMSO) for administering into the cells and mice. It was intraperitoneally administered daily for 23 d from day 21 after primary immunization. The dosage selection for SKN (4 mg·kg-1·d-1) was based on the results of a previous study.10 MTX was intraperitoneally administered at a dose of 0.5 mg/kg and once two days a week for 23 d from day 21 after primary immunization. Mice in the CIA and control groups were administered an equal volume of vehicle.
2.6. Histological evaluation
On the 43rd day of the first immunization, all mice were sacrificed through cervical spondylectomy, and then, the ankle joints of both lower limbs were removed. After 48-72 d of fix-ation, decalcification was conducted for about one month, and then the tissue blocks were repaired, embedded, sliced, and st-ained. All the data were analyzed as previously described.11,⇓,⇓,⇓-15
2.7. Extraction and culturing of macrophages isolated from bone marrow
For this step, we followed a published study with slight modifications.16 Rats were selected and sacrificed by breaking the cervical vertebra. The rats were soaked in 75% ethanol for 5 min. The legs were separated from the abdominal midline with scissors, and the leg skin was removed obliquely to separate the legs. The leg muscle and other soft tissues were removed, and the fibula was removed to separate and expose the complete femur and tibia. The leg bone was cut off at the joint, the serum-free 1640 medium was aspirated using a 2 mL syringe, the needle was inserted into the bone marrow cavity, the bone marrow was washed repeatedly until it turned white, and the bone marrow washing solution was collected into a 50 mL centrifuge tube. The bone marrow cells were centrifuged at 300 g for 5 min with 10 mL of M-CSF (10 μg/L) solution. The supernatant was discarded and replaced with 5 mL of 1640 medium containing M-CSF (10 μg/L) and 10% serum for 4 d.
Primary bone marrow-derived-macrophages (BMDMs) were induced to M1 using LPS 10 μg/L + IFN-γ 20 μg/L, and M2 using IL-4 20 μg/L + IL-13 20 μg/L.
2.8. Immunofluorescence
The paraffin-embedded sections (5 μm thick) of ankle joints were used for the immunofluorescence analysis. BMDMs were pre-treated with LPS 10 μg/L + IFN-γ 20 μg/L or IL-4 20 μg/L + IL-13 20 μg/L for 1 h, and incubated with or without different concentrations of SKN (0.05, 0.1, and 0.2 μM) for 24 h before cell fixation. For F4/80, iNOS, arginase, CD68, and CD206, the sections and BMDMs were incubated overnight at 4℃. Then sections and BMDMs were incubated for 1 h at room temperature with the secondary antibody. The secondary antibody used against F4/80 was Alexa Fluor®488 goat anti-rabbit IgG (G + L), which emits green fluorescence, while the secondary antibody used against iNOS, arginase, CD68, and CD206 was Alexa Fluor®594 goat anti-mouse IgG (G + L), which emits red fluorescence. Each film was photographed in six random fields. Then, it was analyzed by image pro plus.
2.9. Western-blot analysis
This experiment was conducted as described previously.11,⇓,⇓-14 Synovium tissue from mice was collected. BMDM were pre-treated with LPS 10 μg/L + IFN-γ 20 μg/L or IL-4 20 μg/L + IL-13 20 μg/L for 1 h, and incubated with or without different concentrations of SKN (0.05, 0.1, and 0.2 μM) for 24 h before cell preparation. The membranes were blocked at room temperature for 2 h and incubated overnight at 4°C with primary antibodies against iNOS (dilution 1:250), arginase (dilution 1:250), CD68 (dilution 1:200), CD206 (dilution 1:1000), and glyceraldehyde-phosphate dehydrogenase (dilution 1:2500). After adding a secondary antibody (dilution 1:4000), the membrane was incubated at room temperature for 2 h. Then, it was developed in dark and analyzed using Image-Pro Plus software.
2.10. Statistical analysis
SPSS version 11.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. Continuous variables were expressed as mean ± standard error of mean. Pathological scores were analyzed using non-parametric Kruskal-Wallis tests. Other data were analyzed using analysis of variance followed by the post hoc test or Student’s t-test. Differences were considered statistically significant at P < 0.05.
3. RESULTS
3.1. SKN relieves arthritis in CIA mice
SKN and MTX were administered intraperitoneally from days 21 to 43 of the first immunization in DBA/1 mice with CIA. As shown as previously described,15 SKN and MTX alleviated the degree of swelling in CIA mice, and SKN exerted a better effect than MTX as showed in the histopathological evaluation. Altogether, these results indicate that SKN relieved arthritis in CIA mice. Moreover, the effect of SKN is better than that of the positive control, MTX.
3.2. SKN inhibits M1 macrophage polarization in the joints of CIA mice
Immunofluorescence was used to assess the expression of F4/80 (the marker of macrophage), CD68, and iNOS (the marker of M1 macrophage) in the synovium. A significant amount of M1 macrophage staining was observed in the inflamed joints of CIA mice, and this was significantly relieved in the SKN- and MTX-treated groups compared to the control and CIA groups (Figure 1A-1C).
Figure 1. SKN inhibits M1 macrophage polarization in the joints of CIA mice (via immunofluorescence, ×40) .
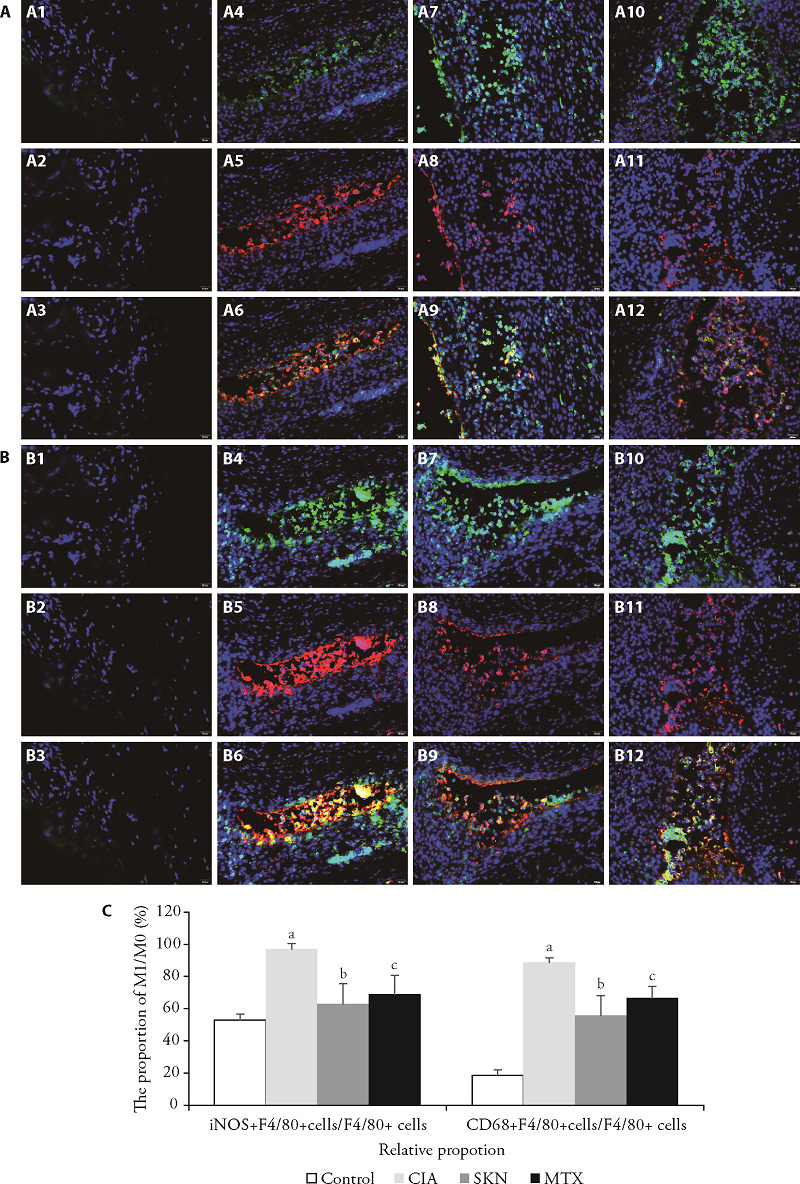
A, C: photomicrographs of iNOS+ M1 macrophage immunofluorescence stained synovial membrane tissues in knee joints of control, CIA, 4 mg/kg SKN-treated, and 0.5 mg/kg MTX-treated CIA mice are shown, respectively;the timing of the dosage for 23 d from day 21 after primary immunization. A1-A3: the F4/80+ cells, iNOS+ cells, iNOS+ F4/80+ cells in control group; A4-A5: the F4/80+ cells, iNOS+ cells, iNOS+ F4/80+ cells in CIA group; A6-A9: the F4/80+ cells, iNOS+ cells, iNOS+ F4/80+ cells in SKN group; A10-A12: the the F4/80+ cells, iNOS+ cells, iNOS+ F4/80+ cells in MTX group; B, C: photomicrographs of CD68+ M1 macrophage immunofluorescence-stained ankle joints of control, CIA, SKN-treated, and MTX-treated CIA mice are shown, respectively. B1-B3: the F4/80+ cells, CD68+ cells, CD68+ F4/80+ cells in control group; B4-B5: the F4/80+ cells, CD68+ cells, CD68+ F4/80+ cells in CIA group; B6-B9: the F4/80+ cells, CD68+ cells, CD68+ F4/80+ cells in SKN group; B10-B12: the the F4/80+ cells, CD68+ cells, CD68+ F4/80+ cells in MTX group. All data are present as mean ± standard error of mean (n = 6). aP < 0.001, compared to the control group; bP < 0.01, and cP < 0.05, compared to the CIA group. SKN: shikonin; CIA: collagen-induced arthritis; iNOS: nitric oxide synthase; MTX: methotrexate.
3.3. SKN did not affect M2 macrophage polarization in synovium tissue of the joints in CIA mice
Immunofluorescence was used to assess the expression of F4/80 (the marker of macrophage), CD206, and arginase (the marker of M2 macrophage) in the synovium. Except for the normal group, there was no significant difference in the number of M2 macrophages among different groups.
3.4. Morphology of BMDM cells
The adherent primary BMDMs were cultured and subcultured. The morphology of BMDMs was observed under an inverted microscope. Under the action of M-CSF, bone marrow-derived monocytes differentiate into BMDMs, and the cells were fusiform, round, or oval "fried egg-like" (Figure 2A-2C). The purity of macrophages was detected using immunofluorescence, and the positivity of macrophage-specific marker-F4/80 reached 99% (Figure 2D).
Figure 2. Cell morphology of BMDM.
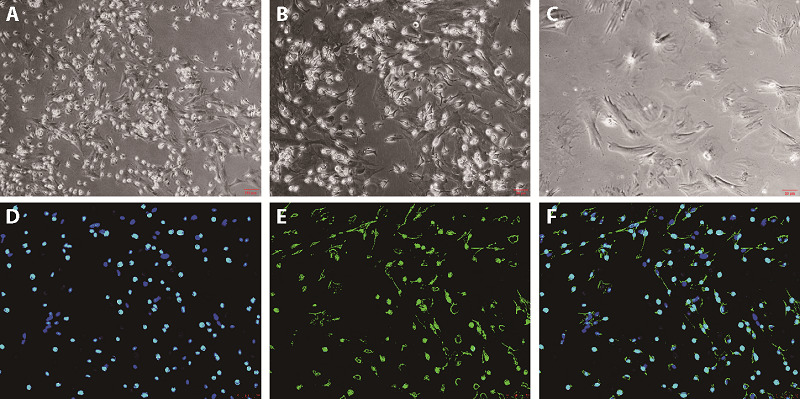
A: the morphology of BMDMs at 10 × magnification; B: the morphology of BMDMs at 20× magnification; C: the morphology of BMDMs at 40× magnification; D: DAPI in BMDMs via immunofluorescence; E: F4/80 in BMDMs via immunofluorescence; F: the positive rate of F4/80 in BMDMs via immunofluorescence. BMDM: bone marrow-derived-macrophages; DAPI: diphenylindole.
3.5. SKN inhibits M1 polarization induced by LPS + IFN-γ in BMDMs
Compared with the undifferentiated M0 macrophages, LPS + IFN- γ induced the polarization of M1 macrophages that were labeled with CD68 and iNOS (both red), and F4/80 (green) for M0 undifferentiated macrophages. The results of immunofluorescence experiments are shown in Figure 3. Compared with the blank group, the number of M0 (F4/80+) cells in LPS and IFN-γ group increased significantly. Compared with the LPS + IFN- γ group, the number of M1 (CD68+ and iNOS+) cells in SKN group, was significantly reduced.
Figure 3. SKN inhibits M1 polarization induced by LPS and IFN-γ in bone marrow-derived macrophages (via immunofluorescence, ×20) .
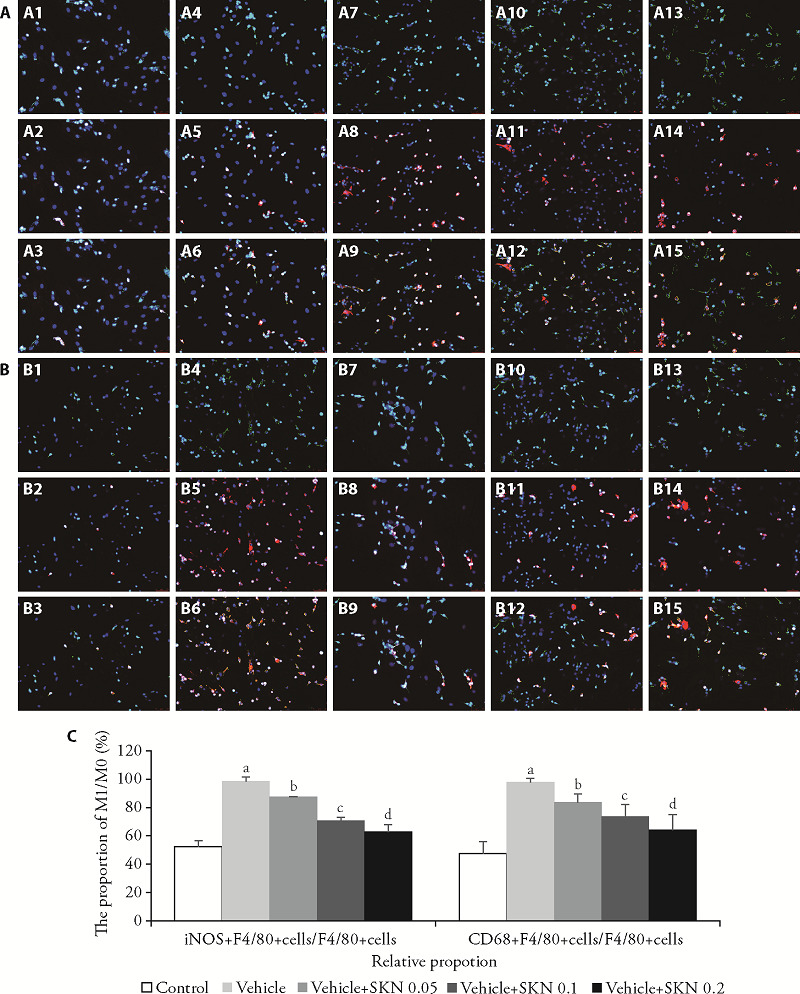
BMDMs were pre-treated with LPS 10 μg/L + IFN-γ 20 μg/L or IL-4 20 μg/L + IL-13 20 μg/L for 1 h, and incubated with or without different concentrations of SKN (0.05, 0.1, and 0.2 μM) for 24 h. A, C: effect of SKN on polarization of iNOS+M1 induced by LPS and IFN-γ.A1-A3: the F4/80+ cells, iNOS+ cells, the iNOS+F4/80+ cells in BMDMs; A4-A6: the F4/80+ cells, iNOS+ cells, the iNOS+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs; A7-A9: the F4/80+ cells, iNOS+ cells, the iNOS+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs with SKN 0.05 μM; A10-A12: the F4/80+ cells, iNOS+ cells, the iNOS+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs with SKN 0.1 μM; A13-A15: the F4/80+ cells, iNOS+ cells, the iNOS+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs with SKN 0.05 μm. ; B, C: effect of SKN on polarization of CD68+M1 induced by LPS and IFN-γ. B1-B3: the F4/80+ cells, CD68+ cells, CD68+F4/80+ cells in BMDMs; B4-B6: the F4/80+ cells, CD68+ cells, CD68+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs; B6-B9: the F4/80+ cells, CD68+ cells, CD68+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs with SKN 0.05 μM; B10-B12: the F4/80+ cells, CD68+ cells, CD68+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs with SKN 0.1 μM; B13-B15: the F4/80+ cells, CD68+ cells, CD68+F4/80+ cells in LPS 10 μg/L + IFN-γ 20 μg/L induced BMDMs with SKN 0.2 μm. All data are expressed as mean ± standard error of mean (n = 12). aP < 0.001, compared with the control group. bP < 0.05, cP < 0.01, and dP < 0.001, compared to the vehicle group. SKN: shikonin; iNOS: nitric oxide synthase; LPS: lipopolysaccharide; IFN: interferon; BMDMs: bone marrow-derived-macrophages.
3.6. SKN did not affect M2 polarization induced by IL4 + IL13 in BMDMs
Compared with the undifferentiated M0 macrophages, IL-4 + IL-13 induced polarization of M2 macrophages that were labeled with CD206 and arginase (both red), and F4/80 (green) for M0 undifferentiated macrophages. The results of immunofluorescence experiments are shown in Figure6. Compared with the blank group, the number of M0 (F4/80+) cells in IL-4 + IL-13 group increased significantly (P < 0.01). However, compared with the IL-4 + IL-13 group, the number of M2 (CD206+ and Arginase+) cells did not change significantly.
3.7. SKN inhibits protein expression of M1 polarization mediators
The protein expression levels of iNOS, arginase, CD68, and CD206 in the joints of CIA mice and LPS + IFN-γ- or IL4 + IL13-induced BMDMs modulated in a dose-dependent manner. The levels of iNOS (Figure 4A) and CD68 (Figure 4B), but not arginase (Figure 4C) and CD206 (Figure 4D) treated with SKN were significantly decreased. Additionally, SKN significantly inhibited the expression of iNOS (Figure 4E) and CD68 (Figure 4F), but not arginase (Figure 4G) and CD206 (Figure 4H) in LPS + IFN-γ- or IL4 + IL13-induced BMDMs.
Figure 4. SKN inhibits M1 polarization in CIA mice and LPS + IFN-γ- or IL4 + IL13-induced BMDMs .
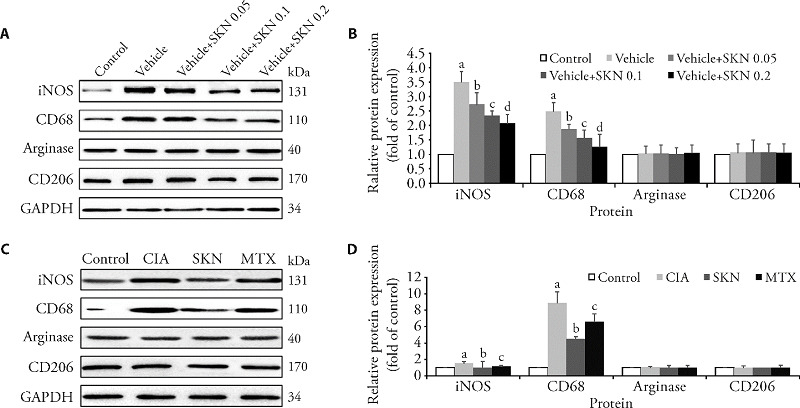
The mice were divided into four groups: the control group (Control), CIA model group (CIA), CIA mice treated with 4 mg/kg SKN group, and CIA mice treated with 0.5 mg/kg MTX group. The timing of the dosage for 23 d from day 21 after primary immunization. BMDMs were pre-treated with LPS 10 μg/L + IFN-γ 20 μg/L or IL-4 20 μg/L + IL-13 20 μg/L for 1 h, and incubated with or without different concentrations of SKN (0.05, 0.1, and 0.2 μM) for 24 h. SKN significantly inhibited the expression of iNOS and CD68, but not arginase and CD206 in LPS + IFN-γ- or IL4 + IL13-induced BMDMs (A and B) (aP < 0.001, compared with the control group. bP < 0.05, cP < 0.01, and dP < 0.001, compared to the vehicle group). Additionally, after SKN and MTX treatment, the expression levels of iNOS and CD68, rather than arginase and CD206, were significantly decreased (C and D). All of the experiments were performed in triplicate. Mean ± standard error of mean was calculated for independent experiments (aP < 0.001, compared to the control group; bP < 0.01, and cP < 0.05, compared to the CIA group.). SKN: shikonin; iNOS: nitric oxide synthase; LPS: lipopolysaccharide; IFN: interferon; IL: interleukin; BMDMs: bone marrow-derived-macrophages; MTX: methotrexate.
4. DISCUSSION
RA is an autoimmune disease characterized by chronic synovitis and cartilage erosion, which eventually leads to the destruction of bones and joints. Due to the continuous and repeated attack of the disease and high disability rate, it is considered a clinically refractory disease.17,18 In the early stage of RA, synovitis is the main lesion, which is characterized by the infiltration of mononuclear macrophages and lymphocytes in the synovial tissue, secretion of a large number of inflammatory cytokines, induction of vascular proliferation in synovial tissue, formation of pannus, and corrosion of the cartilage and destruction of bone tissue, which eventually lead to joint deformity and loss of function.19 Synovitis occurs at the initiation of RA, and its pathogenesis is not fully clear yet, but the role of synovial macrophages in RA has been confirmed by a large number of studies.20,⇓,⇓- 23 Several studies have demon-strated that the activated synovial macrophages release a large amount of tumor necrosis factor alpha, interleukin (IL)-1β, IL-6, IL-23, and so on. Conversely, they promote synovial inflammation and act as a liaison with other lymphoid cells, such as T helper cells (Th) 1, Th17, and fibroblast ties or stimulators. They further promote the production of other inflammatory factors, and then induce pannus formation and bone and joint destruction, and a series of other pathological reactions.24,⇓-26 Additionally, the distribution of synovial macrophages throughout the synovial layer in the joint is also more conducive to its role in mediating synovitis.27 It is noteworthy that some other studies also found that synovial cells are the most reliable biomarkers to assess the severity of RA disease and response to the treatment.28,29 Haringman J found that the number of synovial macrophages in RA patients can not only reflect the degree of joint destruction but also predict the progression of joint destruction in RA.30 It can be seen that synovial macrophages play a central role in the process of RA synovitis, and can also be used as an ideal target for the treatment of RA. Macrophages undergo the phenomenon of macrophage polarization under the action of different stimulating factors, that is, M1 and M2 macrophages. During the occurrence and development of RA, the dynamic balance between M1 and M2 macrophages is disrupted by a variety of factors, causing an increase in M1 type pro-inflammatory macrophages and aggravate the inflammatory reaction.26 Therefore, the effective intervention of macrophage transformation to M1 type and restoration of dynamic balance between M1/M2 macrophages can promote the regression of inflammation.
Macrophages in synovial tissue mainly come from peripheral monocytes. It has been shown that monocytes can be stimulated by GM-CSF or M-CSF to convert into macrophages with stronger phagocytic ability. Macrophages can differentiate into different phenotypes and present different functions under different microenvironment signals, which is called macrophage plasticity.31,32 Th1 derived cytokines (such as IFN-γ, IL-1β) and LPS can induce M1 macrophages through classical activation pathway, presenting a pro-inflammatory phenotype, which can secrete a large number of pro-inflammatory factors, such as TNF-α, IL-1, IL-6, etc., playing the role of pro-inflammatory and antigen presentation. Conversely, Th2 derived cytokines, such as IL-4, IL-13, etc. can induce M2 macrophages through alternative activation pathway, showing anti-inflammatory effects. Inflammatory phenotype mainly involves high expression of anti-inflammatory factors, such as IL-10, IL-13, Il-14, and so on, which play the role of immune regulators, thereby promoting wound healing and tissue repair.33,34
Our results revealed that SKN relieved symptoms of inflammation in the joints in CIA mice. SKN inhibited M1 macrophage polarization but did not affect M2 macrophage polarization in the joints of CIA mice.
Meanwhile, SKN inhibited M1 polarization induced by LPS + IFN-γ, but did not affect M2 polarization induced by IL-4 + IL-13.
In conclusion, these findings suggest that SKN alleviated CIA through inhibiting M1 macrophage polarization, and therefore, it can be potentially deployed as a new drug for RA treatment.
5. ACKNOWLEDGEMENTS
The authors thank prof. Lin Na and Liu Chunfang, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, China, for guiding the experiment and reviewing the manuscript.
Contributor Information
Yu SHI, Email: shiyu@pkuszh.com.
Qingwen WANG, Email: wqw_sw@163.com.
REFERENCES
- [1]. Andújar I, Ríos JL, Giner RM, Recio MC. . Pharmacological properties of shikonin - a review of literature since 2002. Planta Med 2013; 79: 1685-97. [DOI] [PubMed] [Google Scholar]
- [2]. Wang F, Yao X, Zhang Y, Tang J.. Synthesis, biological function and evaluation of shikonin in cancer therapy. Fitoterapia 2019; 134: 329-39. [DOI] [PubMed] [Google Scholar]
- [3]. Dai Q, Fang J, Zhang FS.. Dual role of shikonin in early and late stages of collagen type II arthritis. Mol Biol Rep 2009; 36: 1597-604. [DOI] [PubMed] [Google Scholar]
- [4]. Lee CC, Wang CN, Lai YT, et al. Shikonin inhibits maturation of bone marrow-derived dendritic cells and suppresses allergic airway inflammation in a murine model of asthma. Br J Pharmacol 2010; 161: 1496-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Kim YO, Hong SJ, Yim SV.. The efficacy of shikonin on cartilage protection in a mouse model of rheumatoid arthritis. Korean J Physiol Pharmacol 2010; 14: 199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Tardito S, Martinelli G, Soldano S, et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev 2019; 18: 102397. [DOI] [PubMed] [Google Scholar]
- [7]. Yuan DP, Gu L, Long J, et al. Shikonin reduces endometriosis by inhibiting RANTES secretion and mononuclear macrophage chemotaxis. Exp Ther Med 2014; 7: 685-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Koike A, Shibano M, Mori H, Kohama K, Fujimori K, Amano F.. Simultaneous addition of shikonin and its derivatives with lipopolysaccharide induces rapid macrophage death. Pharm Bull 2016; 39: 969-76. [DOI] [PubMed] [Google Scholar]
- [9]. Remmers EF, Joe B, Griffiths MM, et al. Modulation of multiple experimental arthritis models by collagen-induced arthritis quantitative trait loci isolated in congenic rat lines: different effects of non-major histocompatibility complex quantitative trait loci in males and females. Arthritis Rheum 2002; 46: 2225-34. [DOI] [PubMed] [Google Scholar]
- [10]. Prado C, Ugalde V, González H, et al. STAT3 activation in combination with NF-KappaB inhibition induces tolerogenic dendritic cells with high therapeutic potential to attenuate. doi: 10.1155/2019/1982570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. He LH, Qin QX, He J, et al. ErMiao San inhibits angiogenesis in rheumatoid arthritis by suppressing JAK/STAT signaling path-ways. Evid Based Complement Alternat Med 2020; 2020: 1-12. [Google Scholar]
- [12]. He L, Liu C, Sun C, et al. Wu-Tou Decoction inhibits angiogenesis in experimental arthritis by targeting VEGFR2 signaling pathway. Rejuvenation Res 2018; 21: 442-55. [DOI] [PubMed] [Google Scholar]
- [13]. Liu CF, Kong XY, Li XB, et al. Wen Luo Yin inhibits angiogenesis in collagen-induced arthritis rat model and in vitro. J Ethnopharmacol 2013; 149: 478-89. [DOI] [PubMed] [Google Scholar]
- [14]. Liu CF, He LH, Wang JX, et al. Anti-angiogenic effect of shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J Ethnopharmacol 2020; 260: 113039. [DOI] [PubMed] [Google Scholar]
- [15]. He LH, Luan HJ, He J, et al. Shikonin attenuates rheumatoid arthritis by targeting SOCS1/JAK/STAT signaling pathway of fibroblast like synoviocytes. Chin Med 2021; 16: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Ying W, Cheruku PS, Bazer FW, Safe SH, Zhou B. . Investigation of macrophage polarization using bone marrow derived macrophages. J Vis Exp 2013; 50323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Burke B, Giannoudis A, Corke KP, et al. Hypoxia-induced gene ex-pression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol 2003; 163: 1233-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Viatte S, Plant D, Raychaudhuri S.. Genetics and epigenetics of rheumatoid arthritis. Nature reviews. Rheumatol 2013; 9: 141-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. McInnes IB, Schett G.. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 365: 2205-19. [DOI] [PubMed] [Google Scholar]
- [20]. Alvaro-Gracia JM, Zvaifler NJ, Firestein GS.. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granu-locyte macro-phage colony-stimulating factor production by rh-eumatoid arthritis synoviocytes. J Clin Invest 1990; 86: 1790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Athanasou NA, Quinn J, Heryet A, Puddle B, Woods CG, McGee JO.. The immunohistology of synovial lining cells in normal and inflamed synovium. J Pathol 1988; 155: 133-42. [DOI] [PubMed] [Google Scholar]
- [22]. Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. . The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum 2010; 62: 647-57. [DOI] [PubMed] [Google Scholar]
- [23]. Danning CL, Illei GG, Hitchon C, Greer MR, Boumpas DT, McInnes IB.. Macrophage-derived cytokine and nuclear factor kappaB p65 expression in synovial membrane and skin of patients with psoriatic arthritis. Arthritis Rheum 2000; 43: 1244-56. [DOI] [PubMed] [Google Scholar]
- [24]. Brennan FM, McInnes IB.. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008; 118: 3537-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Feldmann M, Brennan FM, Maini RN.. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 1996; 14: 397-440. [DOI] [PubMed] [Google Scholar]
- [26]. Kishimoto T.. Interleukin-6: from basic science to medicine-40 years in immunology. Annu Rev Immunol 2005; 23: 1-21. [DOI] [PubMed] [Google Scholar]
- [27]. Choy EH, Panayi GS.. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001; 344: 907-16. [DOI] [PubMed] [Google Scholar]
- [28]. Bresnihan B, Gerlag DM, Rooney T, et al. Synovial macrophages as a biomarker of response to therapeutic intervention in rheumatoid arthritis: standardization and consistency across centers. J Rheumatol 2007; 34: 620-2. [PubMed] [Google Scholar]
- [29]. Mulherin D, Fitzgerald O, Bresnihan B.. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 1996; 39: 115-24. [DOI] [PubMed] [Google Scholar]
- [30]. Haringman JJ, Gerlag DM, Zwinderman AH, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in pa-tients with rheumatoid arthritis. Ann Rheum Dis 2005; 64: 834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Gordon S.. The macrophage: past, present and future. Eur J Immunol 2007; 37 Suppl 1: S9-17. [DOI] [PubMed] [Google Scholar]
- [32]. Falconer J, Murphy AN, Young SP, et al. Review: synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis Rheum 2018; 70: 984-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Mantovani A, Sica A, Sozzani S, AllavenaP, Vecchi A, Locati M.. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677-86. [DOI] [PubMed] [Google Scholar]
- [34]. Hamilton JA.. Colony-stimulating factors in inflammation and autoimmunity. Nature reviews. Immunology 2008; 8: 533-44. [DOI] [PubMed] [Google Scholar]


