Abstract
We report our long-term results of apico-aortic conduit implantation in patients with isolated idiopathic hypertrophic subaortic stenosis. Between December 1977 and July 1983, apico-aortic prosthetic-valved conduits were implanted in 4 such patients (age range, 24–65 years) who had severe left ventricular hypertrophy and small left ventricular chambers. In this procedure, the distal end of the conduit was anastomosed to the ascending aorta in 3 patients and to the upper abdominal aorta in 1. Postoperative echocardiography showed relief of the left ventricle–aortic gradient and enlargement of the left ventricular chamber in all cases. One patient died of perioperative wound infection. One patient died of unnatural causes 13 years after the initial operation; in his case, the conduit was known to be occluded. Two patients are alive 15 and 19 years, respectively, after the initial operation.
Three instances of conduit obstruction due to bioprosthetic calcification were observed. Despite the high incidence of reoperation due to conduit valve failure, apico-aortic conduit implantation has produced good hemodynamic outcome and has improved the quality of life in patients who have idiopathic hypertrophic subaortic stenosis and anatomic features unsuitable for Morrow's operation. Improvements in bioprostheses and in apical implantation techniques may allow a revival of apico-aortic conduit implantation in selected patients with idiopathic hypertrophic subaortic stenosis.
Key words: Aorta, abdominal/surgery; bioprosthesis; cardiomyopathy, hypertrophic/surgery; coronary disease/surgery; follow-up studies; heart valve prosthesis; heart ventricle/physiopathology
Idiopathic hypertrophic subaortic stenosis (IHSS) includes a wide range of anatomic lesions, from simple subaortic stenosis caused by a hypertrophic ventricular septum to severe left ventricular hypertrophy accompanied by a very small left ventricular chamber. 1 Although many surgical 2 and percutaneous techniques 3–5 have been proposed, the treatment for IHSS remains controversial. Localized subaortic septal hypertrophy with a significant subaortic gradient is best treated with Morrow's technique. 1 However, diffuse septal thickening with severe left ventricular hypertrophy and a very small left ventricle is not amenable to septal resection. 6,7
Cardiac transplantation has been performed in patients with IHSS on some occasions, but the need for immunosuppression and the shortage of donors are limiting this option to patients with end-stage heart failure. 8,9 Cooley and colleagues, 10 in 1975, proposed the implantation of a composite conduit (apical left ventricle-to-abdominal aorta) for 1-stage treatment of complex left ventricular outflow obstructions. In their initial series of 23 patients, 1 patient with IHSS underwent apico-aortic conduit implantation. 6
Beginning in December 1977, we implanted apico-aortic prosthetic-valved conduits in 4 patients who had IHSS with severe left ventricular hypertrophy. 11 One postoperative death, along with 3 cases of conduit thrombosis due to bioprosthetic degeneration, led us to abandon the program. Recently, however, we reviewed our long-term results after noting the 19-year survival of 1 of these patients.
Patients and Methods
From December 1977 through July 1983, implantation of an apico-aortic bioprosthetic-valved conduit was performed at our institution in 12 patients with complex left ventricular outflow obstruction, 4 of whom had isolated IHSS. The indication for apico-aortic conduit implantation rather than septal resection was the presence of IHSS with diffuse unresectable septal thickening and a small left ventricular chamber. Clinical case reports and follow-up of the 4 patients who had IHSS are presented herein.
Patient 1
Patient 1 was a 49-year-old man with a history of smoking and poorly controlled hypertension. He was admitted to our institution in November 1977 with unstable angina and dyspnea on minimal exertion (New York Heart Association [NYHA] functional class III). Echocardiography showed a small left ventricular cavity with a hypertrophic septum obstructing the left ventricular outflow tract. No mitral insufficiency was observed (Table I). Cardiac catheterization showed subaortic obstruction caused by asymmetric septal hypertrophy with a 120-mmHg pressure gradient between the left ventricular apex and the ascending aorta. Coronary angiography showed severe (75%) stenosis of the right coronary artery.
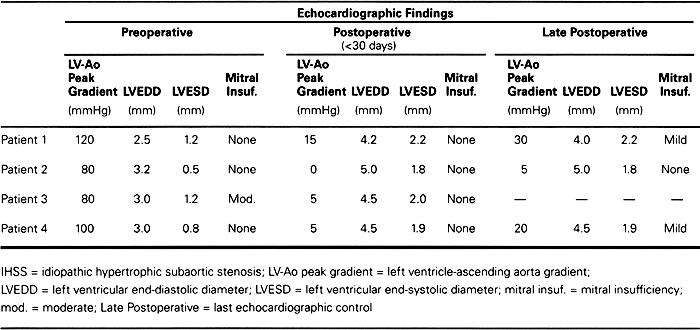
Table I. Echocardiographic Evaluation of 4 Patients Undergoing Apico-Aortic Conduit Implantation for IHSS
The patient underwent apico-aortic conduit implantation. A long median incision (median sternotomy and median laparotomy) was performed, and hypothermic (26 °C) cardiopulmonary bypass was initiated. The heart was arrested with crystalloid cardioplegia. An 18-mm rigid apical connector (Medtronic Inc.; Irvine, Calif), was implanted into the apex of the left ventricle and attached to the distal end of the conduit, which bore a 20-mm Hancock porcine valve (Medtronic Inc.; Irvine, Calif). The distal end of the conduit had previously been implanted end-to-side into the upper abdominal aorta with use of a large biting clamp. Coronary artery bypass was performed on the right coronary artery with a reversed saphenous vein.
The postoperative course was uneventful, and the patient was discharged from the hospital on the 12th postoperative day. Postoperative echocardiography and cardiac catheterization performed 1 month later showed a 15-mmHg gradient between the left ventricle and the aorta.
The patient was asymptomatic for 7 years, at which time he began experiencing dyspnea on minimal exertion and was readmitted to the hospital. Echocardiography showed conduit thrombosis, and the patient underwent reoperation. Through a median laparotomy, the conduit was isolated (clamped on both sides), and the middle portion containing the porcine valve was resected. A new composite conduit with another 20-mm Hancock porcine valve was implanted while the patient's circulation was supported by femoro-femoral bypass. The resected bioprosthesis was heavily calcified, and the conduit was thrombosed.
The patient's postoperative course was uneventful, and he was discharged 7 days postoperatively. Postoperative echocardiography did not show any gradient, and the patient was asymptomatic for another 6 years. He was then readmitted to our hospital with unstable angina and dyspnea at rest. Echocardiography showed a thrombosed conduit and a 100-mmHg gradient between the left ventricular apex and the ascending aorta. The patient refused any further surgery and was discharged with medical therapy alone.
Six months later, the patient took an overdose of verapamil (1600 mg), and thus died 13 years after the initial operation.
Patient 2
Patient 2 was a 24-year-old woman with a history of chest pain and dyspnea on moderate exertion. She was admitted to our hospital in October 1979 for further evaluation. She was experiencing vertigo and had recently sustained a syncopal attack. Echocardiography showed an 80-mmHg gradient between the left ventricle and the aorta, along with septal asymmetric hypertrophy (Table I). Cardiac catheterization confirmed the echocardiographic findings. The coronary arteries were normal.
The patient underwent apico-aortic conduit implantation with a prosthetic valve through a median sternotomy. An 18-mm rigid apical connector was implanted in the apex of the left ventricle. The distal end of the conduit was anastomosed end-to-side to the ascending aorta. The valve-bearing segment (with a 20-mm Hancock porcine bioprosthesis) was placed in the left pleural space through an incision in the pericardium anterior to the phrenic nerve.
The patient's postoperative course was uneventful, and she was discharged on the 8th postoperative day. Postoperative echocardiography (Table I) showed no gradient between the left ventricle and the aorta. Since the operation, the patient has been asymptomatic in NYHA functional class I without drug therapy for 19 years. She has had 2 pregnancies and delivered 2 normal babies. When we last saw her in December 1998, the patient did not have any notable gradient between the left ventricle and the aorta on echocardiogram, and the bioprosthesis showed no sign of degeneration or malfunction (Table I).
Patient 3
Patient 3 was a 65-year-old man who was admitted to our hospital in December 1981 with chest pain and dyspnea on moderate exertion. Echocardiography showed muscular subaortic stenosis, with systolic anterior movement of the mitral valve and moderate mitral insufficiency. Cardiac catheterization showed normal coronary arteries, moderate mitral insufficiency, and subaortic obstruction by the interventricular septum, causing an 80-mmHg gradient between the apical portion of the left ventricle and the ascending aorta. The patient underwent apico-aortic prosthetic-valved conduit implantation. A 22-mm rigid apical connector was implanted. The distal part of the conduit, containing a 23-mm Hancock porcine valve, was anastomosed to the ascending aorta just a few centimeters below the aortic arch.
The initial postoperative course was uneventful. Postoperative echocardiography showed a 5-mmHg gradient between the left ventricle and the aorta and mild mitral insufficiency (Table I). On the 8th postoperative day, wound dehiscence necessitated reoperation. Sternal rewiring was performed, and intraoperative cultures showed no bacterial growth. However, the patient could not be weaned from the ventilator. On the 7th day after reoperation, he became febrile with leukocytosis. Blood cultures grew Pseudomonas aeruginosa. Despite aggressive antibiotic therapy, the patient died on the 30th postoperative day of multiorgan failure due to sepsis.
Patient 4
Patient 4 was a 49-year-old man who was admitted to the hospital in June 1983 with palpitations and shortness of breath on moderate exertion. Echocardiography showed hypertrophic subaortic stenosis with a 100-mmHg gradient between the left ventricle and the aorta. No mitral insufficiency was present. Cardiac catheterization confirmed the gradient between the apex of the left ventricle and the aorta. The coronary arteries were normal.
The patient underwent implantation of a bioprosthetic-valved apico-aortic conduit. A 22-mm rigid connector was implanted in the left ventricular apex. The distal segment of a conduit containing a 22-mm Carpentier-Edwards porcine valve (Baxter-Edwards Inc.; Irvine, Calif) was anastomosed to the ascending aorta.
The patient's postoperative course was complicated by gastrointestinal bleeding secondary to a gastric ulcer. Despite medical treatment with cimetidine, the bleeding continued and gastric resection was necessary.
The patient recovered well after the 2nd surgery and was discharged from the hospital on the 15th postoperative day. Postoperative echocardiography showed a larger ventricular diameter, a reduced gradient between the left ventricle and the aorta (Table I), and a patent conduit.
Three years later, the patient was readmitted to the hospital with dyspnea at rest. Echocardiography showed a gradient of 100 mmHg between the left ventricle and the aorta and no flow across the conduit. Reoperation was performed through a left anterolateral thoracotomy on a beating heart with no extracorporeal circulatory support. The conduit was resected above and below the porcine valve, and a conduit with a Carpentier-Edwards 22-mm porcine valve was implanted. The explanted bioprosthesis was heavily calcified.
The postoperative course was uneventful and the patient was discharged from the hospital on the 7th postoperative day. Postoperative echocardiography showed a gradient between the left ventricle and the aorta of 30 mmHg. The most recent echocardiographic examination was performed in July 1998, 15 years after the 1st operation. The results showed a 20-mmHg gradient between the left ventricle and the aorta and a gradient across the conduit bioprosthesis of 15 mmHg, which is considered mild. No mitral insufficiency was seen. The patient was in NYHA functional class II.
Discussion
The idea that another left ventricular outflow could be created in order to relieve complex left intraventricular obstruction is not new. In 1910, Alexis Carrel 12 described his experimental work in which bypasses were established between the left ventricle and the thoracic aorta. In 1955, Sarnoff, 6 using a Hufnagel valve in a Lucite tube, was able to direct the entire cardiac output from the left ventricle to the thoracic aorta (in animals), permanently occluding the ascending aorta. In 1963, Templeton 6 implanted Sarnoff's prosthesis in 5 patients. One patient survived more than 10 years postoperatively.
In 1975, Bernhard and coworkers reported a reoperation in which a conduit was implanted between the left ventricle and the thoracic aorta. 6 That same year, Cooley and colleagues 10 published a case report describing their technique based on the same concept. The surgical approach was a long median incision; the distal end of the conduit was implanted in the abdominal aorta. Cooley and Norman's initial series 6 using apical left ventricular-abdominal aortic composite conduits comprised 23 patients. The operative mortality rate was 13% and the rate of late deaths was 8.7%. The main indication for use of an apico-aortic conduit was congenital aortic stenosis or complex multiple obstructions of the left ventricular outflow tract. An apico-aortic conduit was used in only 1 patient with IHSS in their series. After that report, further experience in apico-aortic conduit implantation was gained in both pediatric and adult patients. 13,14 Dembitsky and Weldon 15 implanted the distal end of the conduit into the ascending aorta to restore blood flow and to reduce the risk of a steal effect on coronary perfusion.
We started our program of apico-aortic conduit implantation for complex left ventricular outflow obstructions in December 1977. 11 Early in our series, we performed an end-to-side anastomosis between the distal end of the conduit and the abdominal aorta in 1 patient. In the other patients, we anastomosed the outflow tract of the conduit to the ascending aorta in order to limit the invasiveness of both the approach and the procedure, as well as to enable an approach to the conduit through a small anterolateral thoracotomy in case of prosthetic malfunction. Regardless of the site of the distal anastomosis, we observed a high incidence of bioprosthetic failure necessitating reoperation. Therefore, we discontinued the program of apico-aortic conduit implantation in July 1983.
Recently, a follow-up of patients with isolated IHSS who had undergone apico-aortic conduit implantation showed a late survival beyond 10 years in 3 of the 4 patients. All 3 had originally had complex lesions unsuitable for operation by Morrow's technique or any other traditional technique. Efficacy of the apico-aortic valved conduit to relieve symptoms of left ventricular outflow obstruction was confirmed by a dramatic improvement in postoperative function. Survivors were asymptomatic and had an excellent quality of life. Symptoms returned only when conduit thrombosis occurred. Late postoperative echocardiographic studies showed low left ventricle-aorta gradient and nearly normal left ventricular diameters. 11 Finally, during more than 600 months of cumulative follow-up, none of our patients experienced any malignant ventricular arrhythmias.
When apico-aortic conduits are implanted, the mechanism of enlargement of the left ventricular cavity and reduction of hypertrophy is still unknown, although we know that improved left ventricular output is important. The rigid apical connector may help avoid systolic collapse and maintain an acceptable left ventricular diameter.
An alternative approach to septal myectomy was performed by Cooley's group in 1973 16—mitral valve replacement with a low-profile prosthesis—and was shown to relieve subaortic obstruction. Despite initial encouraging results, 17 the technique is currently limited to patients who have associated severe mitral insufficiency. Recent improvements 3–5 in invasive cardiology for the treatment of IHSS, such as ablation of the bundle of His followed by pacemaker implantation, and alcohol-induced septal branch occlusion, have been performed with high success rates and low operative mortality rates. However, these techniques are unsuitable for patients with diffuse septal thickening and severe left ventricular hypertrophy. Cardiac transplantation is the only therapeutic option in patients with IHSS when end-stage cardiac failure begins. Unfortunately, the shortage of donors and the incidence of sudden death of patients on the transplant waiting list limit the success of this approach. 8,9
Apico-aortic conduit implantation may still be a suitable surgical option in patients with severe left ventricular hypertrophy, a small left ventricle, and diffuse septal thickening. Furthermore, many other technologic advances, such as a new generation of bioprostheses treated with antimineralizing agents, 18 the use of collagen-impregnated conduits, 19 the use of a composite conduit with a bileaflet valve, and the incorporation of a Novacor left ventricular assist system (Baxter Healthcare Corporation; Deerfield, Ill) into the apico-aortic technique, 20 may further reduce the postoperative mortality and morbidity rates associated with apico-aortic conduit implantation. We conclude that the use of this technique is still worthy of consideration in selected patients.
Footnotes
Address for reprints: Attilio Renzulli, MD, Via Aquila 144, 80143 Naples, Italy
References
- 1.Morrow AG. Hypertrophic subaortic stenosis. Operative methods utilized to relieve left ventricular outflow obstruction. J Thorac Cardiovasc Surg 1978;76:423–30. [PubMed]
- 2.Jeffery DL, Signorini W, Flemma RJ, Lepley D Jr, Mullen DC. Left ventricular myotomy. Physiologic approach to surgical therapy for IHSS. Chest 1981;80:550–6. [DOI] [PubMed]
- 3.Seggewiss H, Gleichmann U, Faber L, Fassbender D, Schmidt HK, Strick S. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol 1998;31:252–8. [DOI] [PubMed]
- 4.Fananapazir L, Cannon RO 3rd, Tripodi D, Panza JA. Impact of dual-chamber permanent pacing in patients with obstructive hypertrophic cardiomyopathy with symptoms refractory to verapamil and beta-adrenergic blocker therapy. Circulation 1992;85:2149–61. [DOI] [PubMed]
- 5.Nishimura RA, Danielson GK. Dual chamber pacing for hypertrophic obstructive cardiomyopathy: has its time come? Br Heart J 1993;70:301–3. [DOI] [PMC free article] [PubMed]
- 6.Cooley DA, Norman JC. Apical left ventricular abdominal aortic composite conduits for left ventricular outflow obstructions. In: Cohn LH. Modern Techniques in Surgery. New York: Futura, 1979:Vol 2:1–12. [PMC free article] [PubMed]
- 7.Norman JC, Nihill MR, Cooley DA. Valved apico-aortic composite conduits for left ventricular outflow tract obstructions. A 4 year experience with 27 patients. Am J Cardiol 1980;45:1265–71. [DOI] [PubMed]
- 8.Hosenpud JD, Novick RJ, Breen TJ, Keck B, Daily P. The Registry of the International Society for Heart and Lung Transplantation: Twelfth official report. 1995;14:805–15. [PubMed]
- 9.Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 1989;80:1675–80. [DOI] [PubMed]
- 10.Cooley DA, Norman JC, Mullins CE, Grace RR. Left ventricle to abdominal aorta conduit for relief of aortic stenosis. Bull Texas Heart Inst 1975;2:376–83. [PMC free article] [PubMed]
- 11.Cotrufo M, Nappi G, Scardone M, de Vivo F, Vosa C, de Luca L. Intermediate and late follow-up of the use of apico-aortic conduits in the surgical treatment of hypertrophic cardiomyopathy. Int J Cardiol 1986;12:35–43. [DOI] [PubMed]
- 12.Carrel A. Experimental surgery of the aorta and heart. Ann Surg 1910;52:83–95. [DOI] [PMC free article] [PubMed]
- 13.Ergin MA, Cooper R, Lacorte M, Golinko R, Griepp RB. Experience with left ventricular apicoaortic conduits for complicated left ventricular outflow obstruction in children and young adults. Ann Thorac Surg 1981;32:369–76. [DOI] [PubMed]
- 14.DiDonato RM, Danielson GK, McGoon DC, Driscoll DJ, Julsrud PR, Edwards WD. Left ventricle-aortic conduits in pediatric patients. J Thorac Cardiovasc Surg 1984;88:82–91. [PubMed]
- 15.Dembitsky WP, Weldon CS. Clinical experience with the use of a valve-bearing conduit to construct a second left ventricular outflow tract in cases of unresectable intraventricular obstruction. Ann Surg 1976;184:317–23. [DOI] [PMC free article] [PubMed]
- 16.Cooley DA, Wukasch DC, Leachman RD. Mitral valve replacement for idiopathic hypertrophic subaortic stenosis. Results in 27 patients. J Cardiovasc Surg (Torino) 1976; 17:380–7. [PubMed]
- 17.Cooley DA, Leachman RD, Wukasch DC. Diffuse muscular subaortic stenosis: surgical treatment. Am J Cardiol 1973;31:1–6. [DOI] [PubMed]
- 18.Flomenbaum MA, Shoen FJ. Effects of fixation back pressure and antimineralization treatment on the morphology of porcine aortic bioprosthetic valves. J Thorac Cardiovasc Surg 1993;105:154–64. [PubMed]
- 19.Quinones-Baldrich WJ, Moore WS, Ziomek S, Chvapil M. Development of a “leak-proof,” knitted Dacron vascular prosthesis. J Vasc Surg 1986;3:895–903. [DOI] [PubMed]
- 20.Portner PM, Oyer PE, Pennington DG, Baumgartner WA, Griffith BP, Frist WR, et al. Implantable electrical left ventricular assist system: bridge to transplantation and the future. Ann Thorac Surg 1989;47:142–50. [DOI] [PubMed]


