Abstract
Antimicrobial resistance (AMR) is a burgeoning challenge of global priority, warranting immediate action to prevent the explosion of multidrug-resistant (MDR) pathogens. Indiscriminate antimicrobial use is the most important driver for AMR. AMR has led to depletion of the antibiotic pipeline and developing new antibiotics is extremely challenging due to technical and financial issues and also resistance emerges as soon any new antibiotic is introduced. At present, preserving the power of existing antibiotics by prudent use and curtailing spread of pathogens by infection prevention and control (biosecurity) in both humans and animals are the best available options to defer AMR crisis. Meanwhile, to reduce dependence on antibiotics, other alternatives such as vaccines, antibodies, pattern recognition receptors, probiotics, bacteriophages, peptides, phytochemicals, metals, and antimicrobial enzymes are being explored. This review provides an overview of various promising, potential and under investigative strategies as alternatives to antibiotics.
Keywords: Antibiotics, antimicrobial resistance, antibiotic alternatives, bacteriophages, multidrug resistance, probiotics, vaccine
Introduction
Antimicrobial resistance (AMR) is a burgeoning challenge of global priority1. It is estimated that by 2050, approximately 10 million deaths will occur annually with a cumulative loss of US$ 100 trillion to the global economy, if concrete actions are not taken to slow down the current trend of AMR progression1. Antibiotic use is the most important driver for AMR, and more than 50 per cent of antibiotic use is unjustified with irresponsible use occurring in humans, veterinary practice and animal husbandry for therapeutic and non-therapeutic indications (growth promotion)1,2. Antimicrobials are used indiscriminately as a substitute for asepsis, cleanliness and infection prevention and control (IPC) across community, hospitals and farms, leading to survival, selection and spread of multidrug-resistant (MDR) pathogens. The magnitude and extent of antibiotic abuse are alike in both human and animals1,2. Antibiotics, antibiotic residues and antibiotic-resistant pathogens/genes from humans and animals enter the environment through excreta/sewage and get incorporated in soil and water sources ultimately risking the ecosystem.
A holistic, multidimensional, multisectoral, multidisciplinary ‘one-health’ approach has been advocated globally to combat AMR across human–animal–environment interface3. The possible solution to combat this escalating threat of AMR is to reduce unjustified antibiotic use or develop new effective antibiotics2,4. Preventing infections is the most promising and long-term sustainable solution; several initiatives have been taken by the Government of India in the last decade to tackle the problem of AMR such as Swachh Bharat Abhiyan (2014) and Kayakalp Award Scheme (2015) which helped in raising awareness about sanitation and hygiene in the community and hospitals, but the impact of these initiatives on reduction in antimicrobial use yet needs to be consolidated5,6. Developing new antibiotics is a challenging task in view of various hurdles from discovering a new molecule to several commercial and regulatory issues7. Current pipeline for developing new antibiotics has only 27 drug candidates targeting the WHO priority pathogens, and majority are the modifications of previously known classes with already existing cross-resistance4. Discovery of new antibiotics is technically demanding and financially non-viable as it requires considerable time, effort and expenses with poor returns on investment4,5. Reducing and rationalizing antibiotic use are the next best sustainable solutions to address this crisis2.
There is an urgent need to explore innovative alternatives to antibiotics acting differently by preventing infections, reducing the emergence of resistance by targeting different mechanisms of action (MOAs) or increasing the effectiveness of existing antibiotics7–9. Use of these alternatives for community-acquired infections would ultimately reduce the dependency on antibiotics9,10.
Alternative to antibiotics refers to products such as vaccines, antibodies, pattern recognition receptors (PRRs), probiotics, bacteriophages, peptides, phytochemicals, metals and antimicrobial enzymes7,8. The available literature on alternatives to antibiotics is scattered in terms of different focus areas, different development stages and restricted to either human or animal use6,8,9. This review is aimed to provide a holistic overview of various promising, potential or under-investigation alternatives to antibiotics with their MOA, current status, challenges associated in commercialization and future scope. The Table gives an overview of status of the uses of alternatives and the Figure summarizes the major mechanism of action of antibiotic alternatives.
Table.
Uses of alternatives to antibiotics for health promotion, prophylactic and therapeutic purposes in humans, animals and food preservation*
| Substitute | Human use | Animal use | Food | Major strength | Major limitations | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Promotion$ | Prophylactic | Therapeutic | Promotion | Prophylactic | Therapeutic | Preservation/disinfection | |||
| Vaccines | X |

|
X | X |

|
X | X | Specific, efficient immune response with memory | Reversion to virulence, no/limited cross-protection, parenteral route |
| Polyclonal antibody | X |

|

|
X |

|

|
X | Immediate response for fatal infections | Supply chain issues |
| Monoclonal antibody | X |

|

|
X | ? | ? | X | -do- | Need for diagnosis/pathogen escape |
| Pattern recognition receptors | X |

|
X | X | ? | X | X | Increase immunogenicity of antigens | - |
| Antimicrobial peptides# | X | ? |

|

|

|

|

|
Broad-spectrum, target MDR pathogens | Resistance, toxicity |
| Probiotics |

|

|

|

|

|

|
? | Safe, multiple mechanisms of action | Standardization of dose, strain |
| Bacteriophages and lysins | X |

|

|
X |

|

|

|
High specificity, ease of administration | Integration in host genome, unstable |
| Phytochemicals |

|

|
? |

|

|

|
? | Immunomodulation | Poorly standardized products/toxicity |
| Antimicrobial enzymes |

|
? | ? |

|

|
? |

|
Specificity and strong catalytic activity | Denaturation, loss of activity |
| Heavy metals |

|

|
? |

|

|

|

|
Target multiple cellular processes | Metal toxicity, resistance |
| Nanomaterials | ? | ? | ? | ? | ? | ? |

|
Improved pharmacokinetics | Toxicity |
| CRISPR/Cas | ? | ? | ? | ? | ? | ? | ? | Re-sensitization of bacteria to antibiotics | Delivery option, target mutation |
| Predatory bacteria | ? | ? | ? | ? | ? | ? | ? | Potential for resistance rare | Risk of gut microbiome alteration |
*Efficacy varies with host species and reasons of use;  Promising strategies (evidence of efficacy in systemic review, meta-analysis or review of authoritative organizations (WHO, FAO), commercially available;
Promising strategies (evidence of efficacy in systemic review, meta-analysis or review of authoritative organizations (WHO, FAO), commercially available;  Potential strategies (scientific evidence of usefulness available but not sufficient to justify large scale commercial use/market approvals); $Efficacy in direct health promotion not by prevention of infections; #Many AMPs such as colistin banned for animal use to save them for human use; ? Investigational strategies (approaches in pre-clinical/clinical research); X No efficacy. CRISPR/Cas, clustered regularly interspaced short palindromic repeats/CRISPR-associated; AMPs, associated molecular patterns
Potential strategies (scientific evidence of usefulness available but not sufficient to justify large scale commercial use/market approvals); $Efficacy in direct health promotion not by prevention of infections; #Many AMPs such as colistin banned for animal use to save them for human use; ? Investigational strategies (approaches in pre-clinical/clinical research); X No efficacy. CRISPR/Cas, clustered regularly interspaced short palindromic repeats/CRISPR-associated; AMPs, associated molecular patterns
Figure.
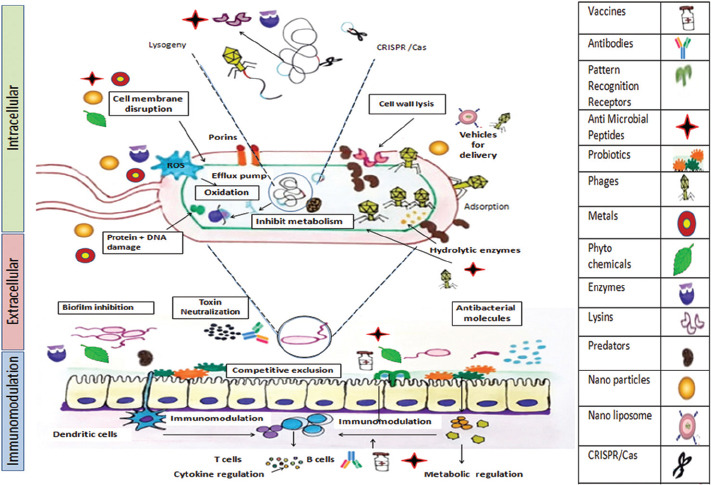
Major mechanisms of action of antibiotic alternatives wherein alternatives to antibiotics (shown as pictograms in the right panel) bring anti-microbial effect by intracellular action (bacterial cell damage), extracellular action (neutralization of microbe derived products/toxins), production of antibacterial molecules, enhancing mucosal barrier, competitive exclusion by adherence to the mucosa/epithelium, biofilm inhibition and immunomodulation (metabolic regulation and inducing protective cytokines). The antipathogenic action as discussed in this review is shown by the combination of pictograms with the mechanism of action as shown in boxes. CRISPR /Cas: Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated.
Immunomodulation
Modulating immune response has proved to be a promising approach in various autoimmune, inflammatory, anticancer therapies, with considerable potential in reducing the incidence of infections12. Immune response to any foreign antigen is brought by a complex-interdependent interaction of innate and adaptive immunity ultimately mediated by effectors such as cytokines, chemokines, inflammatory cells and their products12. Pathogenic microbes downregulate the innate immunity, thereby allowing pathogen survival and multiplication within host12. Immunomodulators have the potential to counteract this downregulation and induce protective immunity13. Immune modulators enhance the efficacy of antibiotics, augment host-specific immunity or limit pathogen-specific toxic effects13–15. A variety of biological or synthetic substances (such as cytokines, interleukins, chemokines, synthetic cytosine phosphate-guanosine, oligodeoxynucleotides, glucans, granulocyte colony-stimulating factor, interferons, imiquimod and cellular membrane fractions) can modulate immune system to boost immunity and indirectly fight infections12. The immunomodulators with potential to reduce antibiotic use are briefly discussed below.
Vaccines: Vaccines generate a highly specific and efficient immune response with rapid and robust response in case of re-infection13,15. These prevent the establishment of infection or reduce the disease severity, if infection sets in13–16. Vaccines also provide herd immunity and protect unvaccinated individuals from infections15. Antibiotics are often misused for viral illness due to diagnostic uncertainty. Further, antibiotics are often prescribed to prevent secondary bacterial infections in these patients8,17.
Applications: Introduction of vaccines for diphtheria, pertussis, tetanus, meningococcal meningitis, tuberculosis, typhoid fever, pneumococcus and Haemophilus influenzae in human beings; vaccines for parvovirus, rabies, distemper and viral hepatitis in pets and vaccines for necrotic enteritis, coccidiosis, infectious bronchitis, Escherichia coli, rotavirus, pink eye and brucellosis in livestock have met with considerable success in reducing the antibiotic prescriptions8,12,13,16. In addition, vaccines bring indirect health benefits and growth promotion by preventing diseases, thereby reducing the need of antibiotics8.
Challenges: Advancement in vaccinology has led to licensing of many novel vaccines in the last 40 yr, but most of these target viruses with a paucity of vaccines for bacterial pathogens16,17. There is urgency to develop vaccines for pathogens in which AMR is of critical concern such as E. coli resistant to third-generation cephalosporin and fluoroquinolone; Klebsiella pneumoniae resistant to carbapenem; methicillin-resistant Staphylococcus aureus (MRSA); penicillin-resistant S. pneumoniae and fluoroquinolones-resistant non-typhoidal Salmonella and Shigella and Neisseria gonorrhoeae, with reduced susceptibility to third-generation cephalosporins18,19. Development of vaccines against Gram-negative infections can make a difference for diseases, such as burns and severe injuries, which need prolonged use of antibiotics16,17. Large-scale coverage for vaccine-preventable diseases is also an issue depending on the type of vaccine used as there are safety concerns (reversion to virulence) and difficulty in cold chain maintenance with live vaccines, parenteral route of administration with killed vaccines, suboptimum immunogenicity with subunit vaccines and risk of integration in host genome with DNA vaccines16,17.
Future prospects: It is an arduous task to develop vaccines against a myriad of pathogens infecting humans and animals due to limited understanding of host–pathogen interactions, suboptimal challenge models and long, complex research in developing vaccines along with regulatory hurdles in licensing17 However, there is some hope as some vaccines against AMR pathogens such as Clostridium difficile, S. aureus, M. tuberculosis, carbapenem-resistant and extraintestinal E. coli have entered clinical trials17.
Polyclonal/monoclonal antibodies: Antibodies are produced by the host immune system in response to any foreign antigen to rapidly eliminate it by multiple mechanisms, such as preventing adhesion, neutralization, complement fixation and opsonization by phagocytes and antibody-dependent cellular toxicity20.
Polyclonal antibodies: Passive immunization by transfer of polyclonal antibodies is a time-tested tool for prophylaxis and treatment of several human and animal diseases. These antibodies are derived from pooled plasma/serum of convalescent patients, immune people or animals20.
Applications: Antibodies are used for prophylaxis and treatment of acute viral infections [hepatitis, measles, varicella, vaccinia, cytomegalovirus (CMV), etc.] and bacterial infections (tetanus, anthrax, botulism, diphtheria, bacteraemia due to S. aureus, etc.) in humans20. There are some licensed polyclonal antibodies for passive immunization in livestock for Arcanobacterium pyogenes, clostridioides infections, tetanus, West Nile virus, septicaemia, Rhodococcus equi infections, etc21.
Challenges: Polyclonal antibodies have limitations with regard to standardization of quality and quantity of antibodies as these are pooled from multiple animals or convalescent patients and there is a risk of cross-reactions and non-specific interaction within the antibody pool20.
Monoclonal antibodies (mAbs): mAbs are being investigated for their prophylactic or therapeutic potential against bacterial infections as stand alone therapy, along with antibiotics, or as adjuvant because of high specificity and low risk of development of resistance in acute infections22. These hold promise in emerging rare/fatal diseases and immunocompromised states where passive infusion might bring immediate protection by neutralizing the foreign antigens22,23. Many mAbs are approved for the treatment of cancers, autoimmune diseases and chronic diseases, but much success has not been achieved for infectious diseases22.
Applications: mAbs showed reduced mortality in Ebola virus outbreak but did not get approval in view of safety and efficacy concerns24. Palivizumab was the first-approved mAb for respiratory syncytial virus infection in high-risk infants22. Subsequently, bezlotoxumab and obiltoxaximab were approved to reduce the recurrence of C. difficile infection (CDI) and prevention of inhalational anthrax, respectively22. Obiltoxaximab was approved on compassionate grounds only based on animal studies in view of high mortality due to anthrax22. Itolizumab was recently approved for emergency use for the treatment of moderate-to-severe complications of SARS-CoV-225. mAbs are being extensively explored for prophylactic and therapeutic use for animals, but currently, there is no approved product for commercial use26.
Challenges: Economic viability due to high production cost of mAb is a concern coupled with poor return on investment due to efficacy against single disease target/antigenic site. Moreover, gathering adequate data for rare, emerging/fatal diseases for conducting randomized clinical trials is a strenuous task because of unpredictability of outbreaks, unspecified epidemiology, difficulty in inclusion of patients and high fatality rates22. There is also risk of neutralization escape if mutation occurs in the targeted single antigenic site22.
Future prospects: Research for finding effective human mAb has gained impetus for nosocomial bacterial pathogens; S. aureus, Enterococcus faecium, K. pneumoniae, Pseudomonas aeruginosa, Enterobacter species, Acinetobacter baumannii, and C. difficile22,26. There is a considerable possibility of mAbs cocktails, multivalent antibody and multiple variable region constructs of different specificities to reach market in the near future22,26.
Targeting pattern recognition receptors (PRRs): PRRs such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain proteins and nucleotide-binding domain leucine-rich repeat-containing receptors are important components of innate immune system27. These are expressed either on the cell surface or in the endosomal compartment of a wide variety of cells such as spleen, peripheral blood leucocytes, in lungs and gut epithelium27. Microbial cell surfaces and damaged cells have a defined molecular signature called pathogen-associated molecular patterns, which are recognized by PRRs27. After recognition, PRRs get activated and induce the expression of various pro-inflammatory, anti-infective molecules with activation of T and B lymphocytes, macrophages and dendritic cells to boost immune response and combat infections27. Thus, TLR agonists are promising candidates to boost the adaptive immune response to eliminate pathogens and attenuate pro-inflammatory immune response and as adjuvant to increase immunogenicity of vaccines27.
Applications: Currently, only one TLR4 agonist (monophosphoryl lipid A) is licensed for use as an adjuvant in hepatitis B virus (HBV) and human papillomavirus vaccine27.
Future prospects: Several TLR agonists with potential of limiting chronic inflammation and tissue damage caused by viral pathogens [HCV (ANA773, PF-487861), HIV (GS-620), influenza (VAX102), CMV (CBLB502) and melioidosis (CRX527)] are in preclinical and phase 1 clinical trial27. In addition, several TLR agonists alone or in combinations (CpG DNA, Pam3CSK4) are being evaluated as adjuvant in vaccines27.
Host defence peptides (HDPs) [antimicrobial peptides (AMPs), defensins]: Host defence peptides (HDPs) are components of innate immune defence, which are produced constitutively or induced in response to any foreign insult by almost all species from prokaryotes, insects, plants to higher animals28. Antimicrobial peptides (AMPs) are positively charged amphipathic small peptides (approximately 12-50 amino acids), which are produced in abundance at all sites exposed to pathogens such as skin and mucosal epithelium28,29.
Mechanism of action (MOA): AMPs act by bactericidal and/or modulating host immune response against a broad range of pathogens (bacteria, fungus, virus and MDR pathogens) and have potential to inhibit/eradicate biofilms14,29. Bactericidal action is brought mainly by two mechanisms: (i) alteration of cell membrane permeability leading to spore formation with leakage of cell contents or blockage of membrane respiration and cell death, and (ii) by intracellular damage to mitochondria and other organelles, DNA fragmentation and inhibition of macromolecules synthesis10,29. Immunomodulation is brought by balancing of pro- and anti-inflammatory immune response, with stimulation of adaptive immunity29,30. These molecules bring local non-inflammatory resolution of infections by suppression of pro-inflammatory cellular response29.
Applications: AMPs are selectively toxic to bacterial cells as these interact with negatively charged cell membranes sparingly uncharged or neutral eukaryotic cell membranes10,14. Many AMPs such as bacitracin, dalbavancin, daptomycin, enfuvirtide, oritavancin, teicoplanin, telaprevir, telavancin, vancomycin, polymyxin B and colistin are highly efficacious last resort anti-infectives for serious/life-threatening infections by MDR pathogens as topical, oral or systemic preparations11,28,30,31. Despite limited research on animal use, AMPs gained extensive popularity for growth promotion and therapeutic purposes in food animals, poultry and aqua-culture, thereby resulting in imposition of bans for their use (particularly, colistin and vancomycin) in animals in several countries, to preserve these antibiotics for human use32. AMPs are also used commercially in the food industry (spheniscin, protamine, magainins, nisin, bacteriocins, etc.) as preservatives to prevent the growth of food-spoilage organisms, to increase shelf life of dairy, meat, fish, etc33.
Challenges: Development of resistance to AMPs is a major challenge as bacteria evade these molecules by increasing their net positive charge, thus not allowing AMP interaction with cell membranes, by producing proteases for peptide degradation or activating efflux pumps34. Besides, there are issues due to instability, degradation by endogenous proteases, short half-life, toxicity, high costs and unreliable pharmacokinetics34.
Future prospects: Many synthetic long-lasting AMP analogues and short peptides are being evaluated to avoid peptide degradation and to reduce production costs14,35. Unique delivery mechanisms to deliver AMPs inside bacterial cells such as liposome encapsulation are being explored to enhance stability and reduce toxicity35. In addition, various inducers of natural AMPs (butyrate, histone deacetylase inhibitors, vitamin D3, etc.) are in different phases of clinical trials for local/systemic AMR pathogens and inflammatory disorders35. These inducers offer some distinct advantages such as lowered potential for emergence of AMR, requirement for fewer doses and thus reduced cost and toxicity to host cells35.
Pro-, pre-, post- and syn-biotics: Gut microbiome consists of a variety of commensal organisms (>1000 types) which promote human and animal health by energy metabolism, digestion and immune functions and by regulating gut–brain axis36,37. Probiotics refer to live microorganisms when administered in adequate amounts bring health benefit to host38. These include organisms such as Lactobacillus, Bacillus, Bifidobacterium, Saccharomyces boulardii, non-pathogenic strains of E. coli, Bacillus, Clostridioides, Veillonella, Peptostreptococcus and certain Enterococci36–38. Probiotics have anti-pathogenic, anti-inflammatory, antidiabetic, anticancer, anti-allergic, anti-obesity and angiogenic properties along with modulation of gut–brain axis8,37.
Prebiotics, probiotics and post biotics act by similar mechanisms. Prebiotics are naturally occurring non-digestible products such as fibres, natural sugars, vegetables and fruits, which act as food for commensal bacteria and stimulate their growth. Post biotics refer to metabolic by-products of probiotics such as bacteriocins, ethanol, organic acids, diacetyl, acetaldehydes and hydrogen peroxide that bring biologic activity in the host37. Synbiotics are a combination of prebiotics and probiotics in definite proportion37.
MOA: These agents bring anti-pathogenic effect by several ways: (i) interfering with pathogen attachment and entry into gut mucosa by enhancing mucosal barrier, (ii) producing antibacterial substances such as bacteriocins and organic acids, (iii) destruction of toxins produced, (iv) restoration of gut dysbiosis, and (v) bringing in immunomodulation by inducing protective cytokines (IL-10, TGF-beta) and suppressing pro-inflammatory cytokines (TNF)8,36,37.
Applications: Probiotics meet health promotive, preventive and therapeutic uses. These improve intestinal health and enhance immune response and prevent super infections by organisms such as C. difficile and AMR pathogens by maintaining microbiota composition in patients treated with antibiotics8,36–39. These are also being used for prevention of certain diseases (travellers’ diarrhoea, antibiotic-associated diarrhoea, vaginitis, sepsis, atopic dermatitis); treatment of acute (CDIs, diarrhoea, constipation) and chronic diseases (irritable bowel disease/syndrome, hepatic encephalopathy) and preventing side effects after chemotherapy or standard antibiotic therapies40,41.
Faecal microbiota transplant (FMT), wherein stool containing commensal microorganism, is introduced in the gut, has shown promise as the second-line therapy for recurrent CDI not responsive to standard therapies and clears colonization by many MDR pathogens and to prevent neonatal sepsis42. FMT offers decreased risks to the host and increased ease of administration with reduced production costs42.
Several probiotics, prebiotics (fructo-oligosaccharides, malto-oligosaccharides, short chain fructo-oligosaccharides) and synbiotics are being commercially used to improve productivity and health of food animals43. By increasing feed conversion efficiency, these bring growth promotion and reduction in mortality in farm animals43.
Challenges: Choosing the correct probiotic(s) product is challenging as their efficacy is strain-dependent, disease-specific and dose-dependent44. Probiotics act by different MOAs on different pathogens, and same probiotic strain or mixture of strains may be effective for one disease and yet not effective for other disease subtypes, for example, Lactobacillus rhamnosus GG, is effective for preventing paediatric antibiotic-associated diarrhoea but is not effective for disease subtypes such as Crohn’s disease, CDIs, nosocomial infections or traveller’s diarrhoea44. Standardizing the dose required is highly intricate as attaining the optimum number of viable cells for effective gut colonization depends on manufacturing processes, quality control, interaction among different bacterial species administered together, acid and bile tolerance44. Moreover, like antibiotics, there is a risk of horizontal transfer of AMR-resistant genes from probiotic strains to other co-infected pathogens and vice versa40.
Future prospects: Probiotics hold a promising future to reduce antibiotic use, but over-the-counter availability of a large number of probiotic combinations of doubtful efficacy along with irrational usage and undefined doses may be problematic39,43.
Phage therapy: Bacteriophages are viruses that infect bacterial cells and can be genetically engineered for a range of antibacterial activities by several mechanism of action as mentioned below36,45.
MOA: Lytic phages enter inside bacterial cell by binding to variety of receptors and multiply therein using host cell machinery and bring out cell lysis, while lysogenic phages integrate with bacterial genome and replicate passively without producing virions but can switch to lytic cycle, thereby killing host cell45. Lysogenic phages/phagemids (plasmids carrying bacteriophage genes) are genetically engineered to express certain proteins, enzymes, AMPs or toxins to disrupt the normal metabolic processes in bacteria and remove virulence or resistance genes. Bacteriophages encode a variety of peptidoglycan hydrolases such as endolysins and holins to digest bacterial cell wall for phage entry inside cell or release before cell lysis45.
Applications: Bacteriophages are highly specific and replicate only in target bacteria without disrupting normal microbiome46. They offer favourable safety profiles, tolerability and ease of administration and are cheaper than antibiotics47. These have been extensively evaluated for safety and therapeutic efficacy in treatment of several bacterial infections in humans and animals, following topical or oral administration48. Bacteriophages have shown efficacy in burns, wound infection, diabetic foot ulcer and also for treatment of systemic infections by several pathogens48. Their potential for synergistic activity with antibiotics and for treatment of MRSA, MDR P. aeruginosa, A. baumanii and K. pneumoniae has met with considerable success48. These have also shown promising results for disease prevention and treatment in food animals6.
Some phage cocktails were initially licensed in a few countries only for clinical, animal and environment use in view of equivocal efficacy but are slowly gaining recognition in other countries as well45. Some phage preparations are available for selected indications for topical use in chronically infected cutaneous wounds/diabetic ulcers refractory to systemic antibiotic treatment, for the treatment of purulent infections, for oral use in diarrhoea and dysentery, etc45. Phage therapy is currently approved in the United States, only for emergency treatment under compassionate grounds (‘off-license’ approval) for terminally ill patients in the absence of any effective antibiotic or alternative treatment49,50. A recent compendium of case series for compassionate use has revealed phage therapy to be safe and clinically efficacious therapy, resulting in eradication of pathogens50.
Bacteriophages have also been approved for prophylaxis and treatment of infections due to Salmonella (PLSV-1™) and Clostridium perfringens (INT-401™) in poultry in the USA51. Fixed phage mixtures are commercially available for biocontrol of food-borne pathogens such as E. coli Salmonella serotypes and Listeria monocytogenes, Shigella and for surface disinfection in various parts of the world including USA51,52. Lysins are most evaluated hydrolases used extensively in food industry for preventing food spoilage of cheese, meat and fresh cut fruit and inhibiting biofilm formation36.
Challenges: High phage specificity, difficulty in characterization and standardization of phage dosage limit their therapeutic usefulness. Phage preparations are unstable, and resistance emerges easily by modifications of phage binding targets in bacterial cells, by integration of antibiotic resistance genes by lysogenization of phage DNA into bacterial genome (transduction)47. Tetz and Tetz53 have highlighted a contradictory picture, in which phages themselves act as pathogens by killing the normal microbiome, by interacting with host cells, proteins and thus have proposed that their abundance may also be a target for therapeutic intervention This aspect needs to be explored further.
Future prospects: U.S. Food and Drug Administration (FDA) has accepted a new drug application for an intravenously administered bacteriophage-based therapy54. Phages are being researched to act as a vehicle to deliver bacterial biofilm/capsule-degrading enzymes, proteins to repress the DNA repair mechanism, antibiotics, photosensitizing agents, etc. directly inside bacterial cells, thereby avoiding binding to targets amenable to mediate resistance45. These can also act as vehicles for delivery of novel RNA-guided endonucleases to selectively cut, destroy the DNA sequences encoding for virulence and AMR in MDR Gram-negative pathogens [clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated (CAS) system discussed later]10,55. Phage cocktails promise a propitious future to target mixed bacterial populations based on signals of safety and therapeutic efficacy45.
Phytochemicals: Phytochemicals (phytobiotics, phytogenics, herbal medicines or functional foods) are natural bioactive compounds such as carotenoids, curcumin, organosulphur compounds, phytosterols and flavonoids that are derived from a variety of plants, fruits, vegetables, legumes, whole grains, etc. Besides antioxidant, anticancer, immune stimulant, anti-inflammatory, provitamin, enzymatic, anti-stress and hormone regulation properties, these are also being evaluated for antimicrobial (antibacterial, antifungal, antiviral, antiparasitic) activity56,57.
MOA: Immunomodulation to boost host cell immunity is the most explored and validated mechanism of phytochemicals for health and growth promotion57. Different compounds exert antibacterial activity by different mechanisms, i.e. by damaging bacterial membranes, suppressing virulence factors, interacting with membrane proteins, leakage of ions, coagulation of the cell content, inhibiting enzymes/toxins or bacterial biofilm formation57. Some also modulate antibiotic resistance mechanisms in bacteria and can bring synergistic effect with antibiotics, thereby reducing the dose of antibiotics or making them more effective58–62.
Applications: Phytochemicals such as ashwagandha, curcumin and lycopenes are extensively used as nutraceuticals to bring a variety of health benefits in humans and livestock6,59. A large number of herbal drugs [Ocimun basilucum (basil), Caryophyllus aromaticus (clove), Achillea millefolium (yarrow), Rosmarinus officinalis (rosemary), Melissa offficinalis (lemon-balm), Punica granatum (pomegranate)]; phytochemicals (carvacrol, benzoic acid, cinnamic acid, eugenol and farnesol, etc.) and essential oils (cinnamon, oregano, thyme, Lemon grass, Sage, Caraway, Nutmeg, etc.) have shown good antimicrobial activity (for Gram-positive, Gram-negative and MDR pathogens) in vitro and are being used for their anti-inflammatory, prophylactic and therapeutic potential in infections without definitive evidence of efficacy and safety58,59,61–63. Topical application of combination of Aloe vera, C. longa and calcium hydroxide is currently used in sub-clinical mastitis in cattle because of broad-spectrum antimicrobial, anti-inflammatory and immunomodulatory activities64. Herbal medicines have shown potential in food units to control food spoilage by a variety of Gram-positive and -negative bacteria65.
Challenges: Although herbal medicines, phytochemicals are being used alone or with antibiotics for prophylactic and therapeutic purposes, there are no methodological clinical trials for their safety and efficacy. Like antibiotics, there are reports of insensitivity/tolerance/resistance to herbal medicines, phytochemicals as well65–67. Herbal medicines have been believed to be safe and non-toxic compared to allopathic medicines, but there is increasing number of reports of unacceptable health risks to consumers because of contamination with toxic levels of metals and carcinogens68.
Future prospects: There is a need for creation of comprehensive databases for natural product-based drug discovery69. An Indian database Indian Medicinal Plants Phytochemicals and Therapeutic uses has a subset of 60 potential druggable phytochemicals, and most of these are different from existing FDA-approved drugs69. There is hope that phytochemicals may emerge as a possible source of effective, cheap and safe antimicrobial agents.
Antimicrobial enzymes (enzybiotics): A wide variety of proteolytic (subtilin, lysostaphin, bacteriophage lysins), polysaccharide-degrading (lysozyme, amylase, dispersin B, alginate lyase), oxidative (myeloperoxidase, cellobiose dehydrogenase, horseradish peroxidase, quorum-quenching enzymes (acyl homoserine lactonase, acylase, etc.) are being explored for their antimicrobial action70. Several enzyme-based products are used in healthcare specifically for their anti-inflammatory properties (hyaluronidase), in food and biomedical industries71.
MOA: Enzymes bring bactericidal effect by degrading structural components of microorganisms; cell oxidation by inducing production of hydrogen peroxide, by catalytic reactions, by quenching quorum sensing, thereby preventing biofilm formation70.
Applications: Exogenously administered enzymes bring catalytic breakdown of food into smaller substances for better digestibility and nourishment. Enzymes have selective bactericidal action, non-toxic to normal flora with rare potential for emergence of AMR70,71. These have demonstrated good antimicrobial activity against Pseudomonas, Streptococcus, Bacillus and MRSA in vitro but have not shown conclusive evidence of efficacy and safety in clinical studies70. However, a combination of xylanases and beta-glucanases is commercially used for growth promotion in food animals and for prophylactic purposes for some diseases in chickens as feed enzymes71.
Challenges: Major disadvantage of enzymes is propensity to get denatured in extreme conditions during storage, transport, sterilization of enzyme-coated devices and cost-intensive purification processes.
Future prospects: Research to design different enzyme formulations to increase their stability, improve pharmacokinetics, administration by different routes, with decreased side effects and toxicity has gained impetus.
Heavy metals: Metals such as iron, cobalt, manganese, copper, molybdenum and zinc are useful for a variety of physiochemical and biological functions of the body in permissible amounts72. These are natural ingredients of many foods and used in a variety of formulations as micronutrients to bring health and growth promotion in humans and food animals72. Deficiency of these metals predisposes to infectious diseases by impairing immune defence mechanisms72. Metals such as copper and silver have been evaluated for a number of antimicrobial effects and have shown efficacy as monotherapy or with antibiotics (additive and/or synergistic effect) to inhibit a variety of Gram-positive and -negative bacteria73.
MOA: Metals bring bactericidal effect at very low concentration. These act by targeting multiple cellular processes of bacterial cells, such as damage to cell membranes, oxidative damage by generating free radicals, inhibiting essential enzymatic activities and breakdown of nucleic acids72,73. Metals also lead to deactivation, precipitation of bacterial cellular proteins, thereby cell death73.
Applications: Metals have been an integral component of a large number of Ayurvedic medicines (Indian Traditional Health System) for health-promoting, prophylactic and therapeutic uses but have recently gone in disrepute due to reports of metal toxicity72,73. Silver is used for health and growth promotion, for bactericidal activity in wound dressing and burns, as topical application72. Although metals are being evaluated for preventive and therapeutic antibacterial uses as topical and systemic preparations, there are not sufficient randomized controlled trials to assert efficacy and safety74. These are being explored as disinfectants on coatings of medical devices such as urinary catheters, intrauterine devices and hip prosthesis and have shown inconsistent efficacy to warrant large-scale use75. Metals have gained prominence for use as surface coatings in hospitals, food industry and water disinfection systems to prevent growth of pathogenic microorganisms75.
Challenges: Metals offer very low permissible limits and slight increase in exposure results in metal toxicity with interruptions of intracellular homeostasis due to free radical-induced damage to lipids, proteins, enzymes and DNA76. Studies have shown that some metals containing implantable medical devices predispose individuals to local or systemic inflammatory immune reactions77. Striking a fine balance between metal dose and toxicity is highly intricate. Furthermore, microbes develop resistance to metals by reducing uptake, activating efflux, extracellular/intracellular sequestration, metabolic bypass, repair of oxidized molecules, chemical modification, etc78. These also lead to cross-resistance to other metals (multimetal resistance) and antibiotics as the genes encoding for antibiotic resistance and heavy metals are genetically linked78.
Future prospects: Metal complexed antibiotics and metallic nanoparticles are being evaluated to improve the biocompatibility, bioavailability, synergistic effect and circumventing drug-resistant mechanisms. In addition, microbes capable of producing metal nanomaterials may hold promise in mitigating metal pollutants with simultaneous antibacterial properties79.
Nanomaterials: Nanostructured materials such as nanoparticles (NPs) and liposomal/polymer-based nano-drug carriers are attractive options to combat AMR as these can permeate cell membranes because of their ultra-small size80. NPs can be alloyed with metals such as silver, gold, aluminium and copper to bring bactericidal action and can also act as vehicles to deliver antibiotic/(s), antibiotics formulation with metals, immunomodulators/silencing agents safely inside host cells circumventing drug resistance mechanisms80–83. The slow release of antibiotics from encapsulated particles reduces the dose required and side effects along with favouring pharmacokinetics, therapeutic index and cost-effectiveness81. Silver NPs are being used as disinfectants in water filters, textiles, medical masks and food packaging industry with some success in animal husbandry and human use84.
MOA: NPs bring antibacterial action by several mechanisms: (i) triggering generation of reactive oxygen species thereby leading to oxidative damage, (ii) disruption of cell wall, (iii) inhibiting enzymes mediating AMR, (iv) inhibiting biofilm formation, quorum sensing, (v) protein deactivation, (vi) DNA damage, (vii) damaging bacterial efflux pump, and (viii) plasmid curing82,83.
Applications: Many metal-based NPs have been tested for efficacy and safety in vitro, in animals and clinical studies with mixed results84. However, silver NPs have been found safe and are commercially used as disinfectant coatings on wound dressings, polyurethane ventricular catheter, hand gels, intravenous catheters, cavity fillers, etc.84. In addition, these are used for surface disinfection in food industry85.
Challenges: It is difficult to calibrate dose and identify appropriate routes of administration because of the highly efficient cellular uptake of NPs across cells, tissues and organs as it has potential to attain toxic levels quickly84. Several studies have shown accumulation of NPs in liver, heart, lungs and spleen following intravenous or inhalation administration81.
Future prospects: Currently, many NP-based metal alloys, liposomal nanomer-carrying antibiotics and other novel compounds are being explored for effectiveness against MDR Gram-negative bacteria and inhibition of biofilm formation. NPs coating of implantable devices such as heart valves, dental implants and catheters have been studied to reduce seeding and growth of bacteria82. Some of these may prevent emergence of resistance in bacteria or revert antibiotic sensitivity.
Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas)-mediated gene disruption: CRISPR/Cas is an important component of the bacterial adaptive immune system. This is basically an array of short repeated sequences separated by spacers. Short repeats encode proteins (e.g. Cas 3/5/9) to bring functional activity (e.g. endonuclease activity to cut and remove the undesired sequences). Spacers are unique DNA sequences which are acquired from bacteriophages, plasmids or transposons and serve to counter any future attack with similar nucleic acids sequences. CRISPR array is transcribed and processed into short CRISPR RNAs that guide Cas nucleases to destroy target nucleic acids in case of any subsequent attack8,86.
MOA: CRISPR/Cas protein is employed for gene editing to remove AMR genes from bacteria, thereby reversing their sensitivity to antibiotics. A single guide RNA consisting of a crRNA sequence (specific to the DNA target encoding virulence factors/antibiotic resistance/foreign DNA) with a transfer crRNA sequence (that interacts with the Cas protein) is delivered by means of engineered bacteriophages/plasmids. crRNA sequence binds to the target DNA and Cas protein with DNA endonuclease activity brings cleavage in double stranded DNA followed by error prone DNA repair resulting in disruption of gene functions55,86,87.
Applications: The gene editing is being explored for precise removal of virulence and antimicrobial-resistant genes in S. aureus, E. coli, carbapenem-resistant E. coli and enterohemorrhagic E. coli with re-sensitization of bacteria to antibiotics55,86,87.
Challenges: Choosing effective delivery options in highly complex environmental populations, to evolution of resistance as a result of mutations in target sequences, loss of Cas activity and uncertainty due to legislative and social issues are some of the challenges87.
Future prospects: There is a ray of hope with the beginning of the first antibacterial clinical trial for treating urinary tract infections using phages to deliver CRISPR-Cas388.
Predatory bacteria: Bdellovibrio and like organisms, Micavibrio and others are bacteria found ubiquitously in soils and water and can eat up bacteria completely without spilling any of the bacterial contents in the extracellular milieu (unlike phages), thereby averting inflammatory immune response89,90.
MOA: These bacteria bring bactericidal action by releasing hydrolytic enzymes to digest the bacterial cell content either by entering inside or attaching to the cell surface. These attack a range of Gram-negative bacteria including Salmonella, E. coli and some AMR pathogens and may also break biofilms, thereby allowing antibiotics to act89,91.
Applications: These have demonstrated efficacy as bactericidal agents in experimental and ex vivo studies from periodontal, sub-gingival, ocular and respiratory samples91,92. Moreover, predators can restore gut dysbiosis and bring biological control by preying on MDR pathogens91. Emergence of resistance is unlikely because killing does not involve binding to any specific receptor or protein89.
Future prospects: These are in early stage of research and are associated with inherent risk of altering gut microbiota besides manufacturing and regulatory challenges89.
Conclusion
Vaccines appear to be the most promising alternatives to antibiotics for several viral and bacterial diseases and can substantially reduce pressure on antibiotics. While there is a need for developing new vaccines, the focus should be more on utilization and expanded coverage of existing vaccines. Probiotics offer an optimistic choice for the treatment of a variety of infective gastrointestinal disorders, particularly CDIs, and acute diarrhoeal diseases but need stringent quality control to standardize the dose and species used. Although AMPs are highly efficacious anti-infectives for treating serious, life-threatening infections mainly by MDR pathogens but have a high potential for developing resistance, hence should be reserved for only human use. Bacteriophages are also getting acceptance for the treatment of many bacterial diseases including MDR pathogens and life-threatening illnesses. Herbal medicines and phytochemicals have been used since antiquity for health promotion, prophylactic and therapeutic uses but need methodologically rigorous clinical evaluation for safety and efficacy to warrant large-scale prophylactic and therapeutic use. Others like PRR agonists, metals and NPs, CRISPR/Cas and predatory bacteria are in early stages of development and require intensive research before being used as antibiotic alternatives. Like with antibiotics, enforcement of regulations and monitoring systems would be critical to prevent abuse of these alternatives leading to safety concerns and risk of emergence of resistance to some of these strategies.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.O'Neill J. The review on antimicrobial resistance. Tackling drug resistant infections globally:A crisis for the health and wealth of nations. 2014. [accessed on February 2, 2021]. Available from: https://amr-review.org/sites/default/files/.pdf .
- 2.Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–87. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 3.White A, Hughes JM. Critical importance of a one health approach to antimicrobial resistance. Ecohealth. 2019;16:404–9. doi: 10.1007/s10393-019-01415-5. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2021 Antibacterial agents in clinical and preclinical development. [accessed on January 6, 2021]. Available from: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-gcp-irc/clinical-and-preclinical-database-infographics_2021.pdf?sfvrsn=78df3248_3 .
- 5.Ministry of Health and Family Welfare, Government of India. (2015). Guidelines for Implementation of “Kayakalp” Initiative. [accessed on March 29, 2019]. Available from: http://www.nhm.gov.in/images/pdf/infocus/Implementation_Guidebook_for_Kayakalp.pdf.%20 .
- 6.Swachh Bharat Mission. About SBM. [accessed on September 20, 2020]. Available from: https://swachhbharatmission.gov.in/SBMCMS/about-us.html .
- 7.The Pew Charitable Trusts. Tracking the global pipeline of antibiotics in development. 2020. [accessed on June 27, 2020]. Available from: https://www.pewtrusts.org .
- 8.The Pew Charitable Trusts. Alternatives to antibiotics in animal agriculture; 2017. Available from: https://www.pewtrusts.org/~/media/assets/2017/07/alternatives_to_anti biotics_in_animal_agriculture.pdf . accessed on August 9, 2020.
- 9.Allen H. Alternatives to antibiotics:Why and how? Natl Acad Med. 2017;7:1–4. [Google Scholar]
- 10.Ghosh C, Sarkar P, Issa R, Haldar J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019;27:323–38. doi: 10.1016/j.tim.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 11.van Dijk A, Hedegaard CJ, Haagsman HP, Heegaard PMH. The potential for immunoglobulins and host defense peptides (HDPs) to reduce the use of antibiotics in animal production. Vet Res. 2018;49:68. doi: 10.1186/s13567-018-0558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012;10:243–54. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Lillehoj H. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim Feed Sci Technol. 2019;250:41–50. [Google Scholar]
- 14.Jansen KU, Anderson AS. The role of vaccines in fighting antimicrobial resistance (AMR) Hum Vaccin Immunother. 2018;14:2142–9. doi: 10.1080/21645515.2018.1476814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia R. Vaccines as a tool to contain antimicrobial resistance. Indian J Med Microbiol. 2019;37:1–4. doi: 10.4103/ijmm.IJMM_19_223. [DOI] [PubMed] [Google Scholar]
- 16.Hoelzer K, Bielke L, Blake D, Cox E, Cutting S, Devriendt B, et al. Vaccines as alternatives to antibiotics for food producing animals. Part 1:Challenges and needs. Vet Res. 2018;49:70. doi: 10.1186/s13567-018-0560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vetter V, Denizer G, Friedland LR, Krishnan J, Shapiro M. Understanding modern-day vaccines:What you need to know. Ann Med. 2018;50:110–20. doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO publishes a list of bacteria for which new antibiotic are urgently needed. [accessed on August 9, 2020]. Available from: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed/
- 19.Centre of Disease Control. Antibiotic resistant threats in United States 2019. Atlanta, GA: U.S Department of Health and Human Services; 2019. [Google Scholar]
- 20.Sparrow E, Friede M, Sheikh M, Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull World Health Organ. 2017;95:235–7. doi: 10.2471/BLT.16.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedegaard CJ, Heegaard PM. Passive immunisation, an old idea revisited:Basic principles and application to modern animal production systems. Vet Immunol Immunopathol. 2016;174:50–63. doi: 10.1016/j.vetimm.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zurawski DV, McLendon MK. Monoclonal antibodies as an antibacterial approach against bacterial pathogens. Antibiotics (Basel) 2020;9:155. doi: 10.3390/antibiotics9040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motley MP, Fries BC. A new take on an old remedy:Generating antibodies against multidrug-resistant gram-negative bacteria in a post antibiotic world. mSphere. 2017;2:e00397–17. doi: 10.1128/mSphere.00397-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulangu S, Dodd LE, Davey RT, Jr, Tshiani Mbaya O, Proschan M, Mukadi D, et al. A Randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Press Trust of India. DCGI approves itolizumab for restricted emergency use in COVID-19 treatment. [accessed on August 12, 2020]. Available from: https://www.expresspharma.in/covid19-updates .
- 26.Bustamante-Córdova L, Melgoza-González EA, Hernández J. Recombinant antibodies in veterinary medicine:An update. Front Vet Sci. 2018;5:175. doi: 10.3389/fvets.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flórez-Álvarez L, Ruiz-Perez L, Taborda N, Hernandez JC. Toll-like receptors as a therapeutic target in cancer, infections and inflammatory diseases. Immunotherapy. 2020;12:311–22. doi: 10.2217/imt-2019-0096. [DOI] [PubMed] [Google Scholar]
- 28.Lei J, Sun L, Huang S, Zhu C, Li P, He J, et al. The antimicrobial peptides and their potential clinical applications. Am J Transl Res. 2019;11:3919–31. [PMC free article] [PubMed] [Google Scholar]
- 29.Raheem N, Straus SK. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front Microbiol. 2019;2866;10 doi: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides:Functions and clinical potential. Nat Rev Drug Discov. 2020;19:311–32. doi: 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- 31.Pfalzgraff A, Brandenburg K, Weindl G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front Pharmacol. 2018;9:281. doi: 10.3389/fphar.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centre for Science and Environment. Indian Govt's ban on Colistin is big win in fight against antimicrobial resistance, says CSE. [accessed on August 7, 2020]. Available form: https://www.fssai.gov.in/upload/media/FSSAI_News_Colistin_Thisday_31_07_2019.pdf .
- 33.Meng S, Xu H, Wang F. Research advances of antimicrobial peptides and applications in food industry and agriculture. Curr Protein Pept Sci. 2010;11:264–73. doi: 10.2174/138920310791233369. [DOI] [PubMed] [Google Scholar]
- 34.Joo HS, Fu CI, Otto M. Bacterial strategies of resistance to antimicrobial peptides. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150292. doi: 10.1098/rstb.2015.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta P, Das S. Mammalian antimicrobial peptides:Promising therapeutic targets against infection and chronic inflammation. Curr Top Med Chem. 2016;16:99–129. doi: 10.2174/1568026615666150703121819. [DOI] [PubMed] [Google Scholar]
- 36.Cheng G, Hao H, Xie S, Wang X, Dai M, Huang L, et al. Antibiotic alternatives:The substitution of antibiotics in animal husbandry? Front Microbiol. 2014;5:217. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.George Kerry R, Patra JK, Gouda S, Park Y, Shin HS, Das G. Benefaction of probiotics for human health:A review. J Food Drug Anal. 2018;26:927–39. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Food and Agriculture Organization, World Health Organization. Guidelines for the evaluation of probiotics in food London, Ontario, Canada. 2002. [accessed on February 8, 2021]. Available from: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf .
- 39.Ouwehand AC, Forssten S, Hibberd AA, Lyra A, Stahl B. Probiotic approach to prevent antibiotic resistance. Ann Med. 2016;48:246–55. doi: 10.3109/07853890.2016.1161232. [DOI] [PubMed] [Google Scholar]
- 40.Imperial IC, Ibana JA. Addressing the antibiotic resistance problem with probiotics:Reducing the risk of its double-edged sword effect. Front Microbiol. 2016;1983;7 doi: 10.3389/fmicb.2016.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58((Suppl 10)):S164–79. doi: 10.1002/jcph.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KO, Gluck M. Fecal microbiota transplantation:An update on clinical practice. Clin Endosc. 2019;52:137–43. doi: 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markowiak P, Śliżewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018;10:21. doi: 10.1186/s13099-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McFarland LV, Evans CT, Goldstein EJC. Strain-specificity and disease-specificity of probiotic efficacy:A systematic review and meta-analysis. Front Med (Lausanne) 2018;5:124. doi: 10.3389/fmed.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordillo Altamirano FL, Barr JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev. 2019;32:e00066–18. doi: 10.1128/CMR.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kakasis A, Panitsa G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int J Antimicrob Agents. 2019;53:16–21. doi: 10.1016/j.ijantimicag.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Principi N, Silvestri E, Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front Pharmacol. 2019;10:513. doi: 10.3389/fphar.2019.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres-Barceló C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg Microbes Infect. 2018;7:168. doi: 10.1038/s41426-018-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duplessis CA, Biswas B. A review of topical phage therapy for chronically infected wounds and preparations for a randomized adaptive clinical trial evaluating topical phage therapy in chronically infected diabetic foot ulcers. Antibiotics (Basel) 2020;9:377. doi: 10.3390/antibiotics9070377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duplessis CA, Stockelman M, Hamilton T, Merril G, Brownstein M, Bishp-Lilly K, et al. A case series of emergency investigational new drug applications for bacteriophages treating recalcitrant multi-drug resistant bacterial infections:Confirmed safety and a signal of efficacy. J Intensive Crit Care. 2019;5:377. [Google Scholar]
- 51.Żbikowska K, Michalczuk M, Dolka B. The use of bacteriophages in the poultry industry. Animals (Basel) 2020;10:872. doi: 10.3390/ani10050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhardwaj N, Sanjeev K, Bhardwa S, Deep A, Dahiya S, Kapoor S, et al. Lytic bacteriophages as biocontrol agents of foodborne pathogens. Asian J Anim Vet Adv. 2015;10:708–23. [Google Scholar]
- 53.Tetz G, Tetz V. Bacteriophages as new human viral pathogens. Microorganisms. 2018;6:54. doi: 10.3390/microorganisms6020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voelker R. FDA approves bacteriophage trial. JAMA. 2019;321:638. doi: 10.1001/jama.2019.0510. [DOI] [PubMed] [Google Scholar]
- 55.Gholizadeh P, Köse Ş, Dao S, Ganbarov K, Tanomand A, Dal T, et al. How CRISPR-Cas system could be used to combat antimicrobial resistance. Infect Drug Resist. 2020;13:1111–21. doi: 10.2147/IDR.S247271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sánchez E, Nabavi SF, et al. Phytochemicals for human disease:An update on plant-derived compounds antibacterial activity. Microbiol Res. 2017;196:44–68. doi: 10.1016/j.micres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Almodaifer S, Alsibaie N, Alhoumedan G, Alammari G, Kavita MS, Turki MA, et al. Role of phytochemicals in health and nutrition. BAOJ Nutr. 2017;3:028. [Google Scholar]
- 58.Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials –A review. Plants (Basel) 2017;6:16. doi: 10.3390/plants6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber GM, Michalczuk M, Huyghebaert G, Juin H, Kwakernaak C, Gracia MI. Effects of a blend of essential oil compounds and benzoic acid on performance of broiler chickens as revealed by a meta-analysis of 4 growth trials in various locations. Poult Sci. 2012;91:2820–8. doi: 10.3382/ps.2012-02243. [DOI] [PubMed] [Google Scholar]
- 60.Bhardwaj M, Singh BR, Sinha DK. Potential of herbal drug and antibiotic combination therapy:A new approach to treat multidrug resistant bacteria. Pharm Anal Acta. 2017;7:1–4. [Google Scholar]
- 61.Singh BR, Yadav A, Sinha DK, Vinodh Kumar OR. Potential of herbal antibacterials as an alternative to antibiotics for multiple drug resistant bacteria:An analysis. Res J Vet Sci. 2020;13:1–8. [Google Scholar]
- 62.Singh BR, Sinha DK, Or VK, Vadhana P, Bhardwaj M, Saraf A, et al. Antimicrobial activity of agarwood oil against Multiple-Drug-Resistant (MDR) microbes of clinical, food and environmental origin. Curr Drug Discov Technol. 2020;17:348–56. doi: 10.2174/1570163816666190125163536. [DOI] [PubMed] [Google Scholar]
- 63.White CM, Pasupuleti V, Roman YM, Li Y, Hernandez AV. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases:A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;146:104280. doi: 10.1016/j.phrs.2019.104280. [DOI] [PubMed] [Google Scholar]
- 64.Saidi R, Mimoune N, Baazizi R, Benaissa M, Khelef D, Kaidi R. A study of ethno-veterinary medicinal plants and in vitro antimicrobial activities against bovine mastitis isolated bacterial pathogens in Algeria. Bull Univ Agric Sci Vet Med Cluj Napoca. 2019;76:154. [Google Scholar]
- 65.Singh BR, Sinha DK, Vinodh Kumar OR. Effect of herbal antimicrobials on bacterial strains of foods of vegetable and animal origin. J Food Chem Nanotechnol. 2016;2:115–23. [Google Scholar]
- 66.Vadhana P, Singh BR, Bhardwaj M, Singh SV. Emergence of herbal antimicrobial drug resistance in clinical bacterial isolates. Pharm Anal Acta. 2015;6:434. [Google Scholar]
- 67.Singh BR, Vinodh Kumar OR, Sinha DK, Bhardwaj M, Vadhana P. Antimicrobial resistance profile of enteropathogens isolated from diarrhea patients:Herbal antimicrobials, a ray of hope. Ann Pharmacol Pharmaceut. 2017;2:1068–78. [Google Scholar]
- 68.Bode AM, Dong Z. Toxic phytochemicals and their potential risks for human cancer. Cancer Prev Res (Phila) 2015;8:1–8. doi: 10.1158/1940-6207.CAPR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohanraj K, Karthikeyan BS, Vivek-Ananth RP, Chand RPB, Aparna SR, Mangalapandi P, et al. IMPPAT:A curated database of Indian Medicinal Plants, Phytochemistry and therapeutics. Sci Rep. 2018;4329;8 doi: 10.1038/s41598-018-22631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thallinger B, Prasetyo EN, Nyanhongo GS, Guebitz GM. Antimicrobial enzymes:An emerging strategy to fight microbes and microbial biofilms. Biotechnol J. 2013;8:97–109. doi: 10.1002/biot.201200313. [DOI] [PubMed] [Google Scholar]
- 71.Hirose Y. Enzymes for human nutrition and health. In: Vogel A, May O, editors. Industrial enzyme applications. New Jersey: Wiley-VCH GmbH &Co; 2019. pp. 203–17. [Google Scholar]
- 72.Singh R, Bechan SB. In:Biomedical applications of metals. New York: Springer; 2018. Metal-based therapy in traditional and modern medicine systems; pp. 195–211. [Google Scholar]
- 73.Mittapally S, Taranum R, Parveen S. Metal ions as antibacterial agents. J Drug Del Ther. 2018;8:411–9. [Google Scholar]
- 74.Turner RJ. Metal-based antimicrobial strategies. Microb Biotechnol. 2017;10:1062–5. doi: 10.1111/1751-7915.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersen M, Flores-Mireles A. Urinary catheter coating modifications:The race against catheter-associated infections. Coatings. 2019;10:23. [Google Scholar]
- 76.Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM. Heavy metals and human health:Mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci. 2015;16:29592–630. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.U.S Food and Drug Adminsitration. Metals used in medical devices. 2019. [accessed on August 10, 2020]. Available form: https://www.fda.gov/medical-devices/products-and-medical-procedures/metals-used-med ical-devices .
- 78.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals:Mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11:371–84. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 79.Gupta P, Diwan B. Bacterial exopolysaccharide mediated heavy metal removal:A review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep (Amst) 2017;13:58–71. doi: 10.1016/j.btre.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, et al. Nano-strategies to fight multidrug resistant bacteria –“A battle of the titans”. Front Microbiol. 2018;1441;9 doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles:Present situation and prospects for the future. Int J Nanomedicine. 2017;12:1227–49. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hemeg HA. Nanomaterials for alternative antibacterial therapy. Int J Nanomedicine. 2017;12:8211–25. doi: 10.2147/IJN.S132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaweeteerawat C, Na Ubol P, Sangmuang S, Aueviriyavit S, Maniratanachote R. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J Toxicol Environ Health A. 2017;80:1276–89. doi: 10.1080/15287394.2017.1376727. [DOI] [PubMed] [Google Scholar]
- 84.Deshmukh SP, Patil SM, Mullani SB, Delekar SD. Silver nanoparticles as an effective disinfectant:A review. Mater Sci Eng C Mater Biol Appl. 2019;97:954–65. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh T, Shukla S, Kumar P, Wahla V, Bajpai V, Rather I. Application of nanotechnology in food science:Perception and overview. Front Microbiol. 2017;1501;8 doi: 10.3389/fmicb.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shabbir MA, Hao H, Shabbir MZ, Hussain HI, Iqbal Z, Ahmed S, et al. Survival and evolution of CRISPR-Cas system in prokaryotes and its applications. Front Immunol. 2016;7:375. doi: 10.3389/fimmu.2016.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pursey E, Sünderhauf D, Gaze WH, Westra ER, van Houte S. CRISPR-Cas antimicrobials:Challenges and future prospects. PLoS Pathog. 2018;14:e1006990. doi: 10.1371/journal.ppat.1006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hampton T. With first CRISPR trials, gene editing moves toward the clinic. JAMA. 2020;323:1537–9. doi: 10.1001/jama.2020.3438. [DOI] [PubMed] [Google Scholar]
- 89.Madhusoodanan J. Inner Workings:Probing predatory bacteria as an antibacterial remedy. Proc Natl Acad Sci U S A. 2019;116:22887–90. doi: 10.1073/pnas.1917513116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pérez J, Moraleda-Muñoz A, Marcos-Torres F, Muñoz-Dorado J. Bacterial predation:75 years and counting! Environ Microbiol. 2016;18:766–79. doi: 10.1111/1462-2920.13171. [DOI] [PubMed] [Google Scholar]
- 91.Kadouri DE, To K, Shanks RM, Doi Y. Predatory bacteria:A potential ally against multidrug-resistant Gram-negative pathogens. PLoS One. 2013;8:e63397. doi: 10.1371/journal.pone.0063397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loozen G, Boon N, Pauwels M, Slomka V, Rodrigues Herrero E, Quirynen M, et al. Effect of Bdellovibrio bacteriovorus HD100 on multispecies oral communities. Anaerobe. 2015;35:45–53. doi: 10.1016/j.anaerobe.2014.09.011. [DOI] [PubMed] [Google Scholar]


