Abstract
MicroRNAs (miRNAs) are responsible for regulating gene expression post-transcriptionally. Are involved in several biological processes, such as wound healing. Understanding the miRNAs involved in this process is fundamental for the development of new therapies. So, due to the need to understand the role of these molecules, we aimed systematically review the literature in order to identify which miRNAs are involved in the wound healing and determine, through bioinformatics analysis, which signaling pathways are associated with these miRNAs. An electronic search was performed in the following databases: National Library of Medicine National Institutes of Health (PubMed), Science Direct, Scifinder, Scopus and Web of Science, using the descriptors: “(microRNA [MeSH])” and “(skin [MeSH])” and “(wound healing [MeSH])”. After the search, two independent and previously calibrated reviewers selected the articles that analyzed the expression pattern of miRNAs in wound healing in in vivo studies, using the software Zotero bibliography manager. Following, bioinformatic analysis was performed using the software DIANA Tools, mirPath v.3 and the data was interpreted. The bioinformatics analysis revealed that on the day 1 there were 13 union pathways, eight of which were statistically significant. Still on the day 1, among the miRNAs that had a decrease in their expression, 12 of 17 union pathways found were statistically significant. On the day 5, among the miRNAs with an increase in expression, 16 union pathways were found, 12 of which were statistically significant. Finally, among the miRNAs with decreased expression, 11 of 15 union pathways found were statistically significant. Although it has been found substantial heterogeneity in the studies, with this systematic review, it was possible to study the panorama of miRNAs that may be altered in the wound healing. The present review summarizes existing evidence of miRNAs associated to wound healing, and these findings can contribute to new therapeutic approaches.
1. Introduction
Wound healing is a biological process extremely complex and able to recover the barrier function of the skin. It requires the synchronization of several cell types beyond the interaction between cytokines and growth factors. It is composed of four different phases: hemostasis, inflammation, proliferation, and remodeling. Each one of these phases is important so that the process can occur properly. However, during the proliferative phase occurs a cascade of events of major importance for the process as a whole, such as re-epithelization, angiogenesis, and wound closure [1].
The events associated to wound healing can be affected by a variety of agents, changing the wound bed environment. Excessive wound healing–hypertrophic scar and keloid–and chronic wound are some of the sequels of impaired wound healing [2, 3]. Indeed, this process is highly complex and consists of several steps, being more susceptible to a fault. Since wound healing does not occur normally, a chronic wound may be the most recurrent consequence [4]. Nowadays, chronic non-healing wounds can affect millions of patients each year, resulting in higher morbidity and mortality of these patients [5]. Furthermore, the issue related to chronic wounds is not limited only to ulcers but is also associated with other challenges to be overcome, such as infectious and ischemic wounds. It is also known the treatment of chronic non-healing wounds remains a stretch goal on the subject of complexity and prevalence [6]. Considering this, it is clear the need for a therapeutic approach able of overcoming the problem of the complexity of this wound type in terms of treatment.
At the same time, several studies have reported miRNA as a potential therapeutic target for various diseases or conditions, from wound healing to cancer [7–9]. MiRNAs are small non-coding RNA molecules of approximately 22 nucleotides and are responsible for the regulation of gene expression at the post-transcriptional level [10, 11]. They are involved in an assorted of biological processes, from embryonic development to main cell functions, such as proliferation, differentiation, and apoptosis. The miRNA biogenesis and mechanism of action are known only about two decades ago and its molecular mechanism and is not yet totally elucidated [12, 13]. The miRNA research is clearly expanding, mainly in the last five years, because increasingly more studies have elucidated some points of miRNA molecular mechanisms. Moreover, as the miRNAs are important regulators of gene expression, may be promising targets for the development of biomarkers and can help in the development of a therapy [14].
Although there are still some questions about miRNA to be clarified, it is currently known that it is a key part of the entire epigenetic machinery acting as an important regulator of gene expression [15]. The epigenetic plays an important role in all the processes that occur in living organisms and can be used to explain several features of diseases and biological events, such as their pathway or late onset and end [16]. However, miRNAs are not only a part of the epigenetic machinery but are also can modify epigenetically by DNA methylation and histone modification just like another protein-coding gene [17, 18]. These changes in the miRNA expression pattern occur also during the wound healing, and as reported several miRNAs can be found decreased, including the members of the miR-200 family [19], or increased like miR-31, miR-33 and miR-196, among others [20–23]. Also was reported that certain miRNAs, such as miR-21, can mitigate possible aging-associated wound healing failures [24].
Therefore, comprehension of the miRNAs role in wound healing, as well as where and how it acts, can be applied in identifying the pathways involved in the process in order to bring new possibilities of molecular target therapies to the defects that can occur during wound healing. Further, considering the lack of studies about the panorama of miRNAs and their signaling pathways, mainly related to wound healing, we aimed to systematically review the available literature for the purpose of identifying which miRNAs are associated with the wound healing phases and their associated pathways using bioinformatics analysis.
2. Material and methods
2.1 Registration and review questions
The current systematic review is reported in accordance with the Preferred Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Due to the study design’s nature, the protocol was registered in the Open Science Framework and is available at the following link (https://osf.io/xp4tf/).
The review questions were: (1) Which miRNAs have been related to the wound healing process? (2) In which pathways do these miRNAs act?
2.2 Inclusion and exclusion criteria
The inclusion criterion for the articles was in vivo animal studies that analyzed the miRNA expression patterns in the wound healing process. The choice to use only in vivo animal studies is due to the similarity found in the methodology of these studies, reducing possible confounding variables. Only studies published in English were included. The exclusion criteria were congress summary, book section, literature reviews, hypothesis articles, opinion articles, methodological approaches, commentaries, previews, expert opinions, letters, news, patents, studies unrelated to miRNA and wound healing, and studies with confounding factors. Besides that, articles that were not written in English and not fully available were also excluded.
2.3 Search strategy
The electronic strategy was carried out without initial date restriction up to and including May 2020 in the following databases: PubMed, Science Direct, Scifinder, Scopus, and Web of Science. In this case, the Google Scholar database was not used due to the fact that it presents low precision in recently established themes, such as miRNAs [25, 26]. The search strategy was conducted using the following terms: “(MicroRNAs) AND (Skin) AND (Wound Healing)”. No language was applied in the search.
2.4 Study selection
The search results have been exported to the Zotero bibliography manager. Initially, duplicate records were excluded. Titles, abstracts, and study methodologies were screened based on the inclusion and exclusion criteria by two blinded and independent reviewers (MLA and RGS). All records were compared and in case of disagreement, a consensus was reached by discussion. When consensus was not achieved, a different reviewer decided if the article should be included (RGL).
2.5 Data extraction
Data were extracted and tabulated independently by two reviewers (MLA and RGS) in an Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA) to be submitted for descriptive analysis. Cases of disagreement were handled as described above.
2.6 Bioinformatics analysis
After extracting the data, the miRNAs that showed different expression patterns (higher or lower expression) and were repeated on days 1 and 5 during the wound healing process were separately inserted, using the software DIANA Toll mirPath v.3. The inclusion criterion for bioinformatics analysis were the miRNAs that were described more than one time at different times analyzed in the selected studies, and the exclusion criterion was the miRNAs described just one time in the selected studies beyond the miRNAs do not index in mirPath v.3. So, the miRNAs that were not recognized by the software were disregarded in this analyze.
The analyses were performed in real-time using the Kyoto Encyclopedia of Genes and Genoma (KEGG) selecting the murine specie to investigate the miRNAs. The interactions dataset chosen for all miRNAs was TarBase v7.0 and in the advanced statistics options, False Discovery Rate (FDR) correction was chosen. The Fisher’s Exact Test was used with a p-value threshold of 0.05. After inserting all miRNAs into the software, the pathway associated were observed and tabulated, specifying its p-value and its relationship to the wound healing process.
2.7 Quality assessment
To assess the quality of the studies included in this systematic review, the Review Manager 5.4.1 software was used. The checklist was composed of three domains: wound healing assay, miRNA analysis, and the presence of a control group (uninjured skin). Two independent researchers (MLA and RGS) assessed the quality of the studies based on criteria previously established. In cases of disagreement were discussed until a consensus was reached, and when a consensus was not obtained, a third reviewer participated in the discussion (RGL).
3. Results
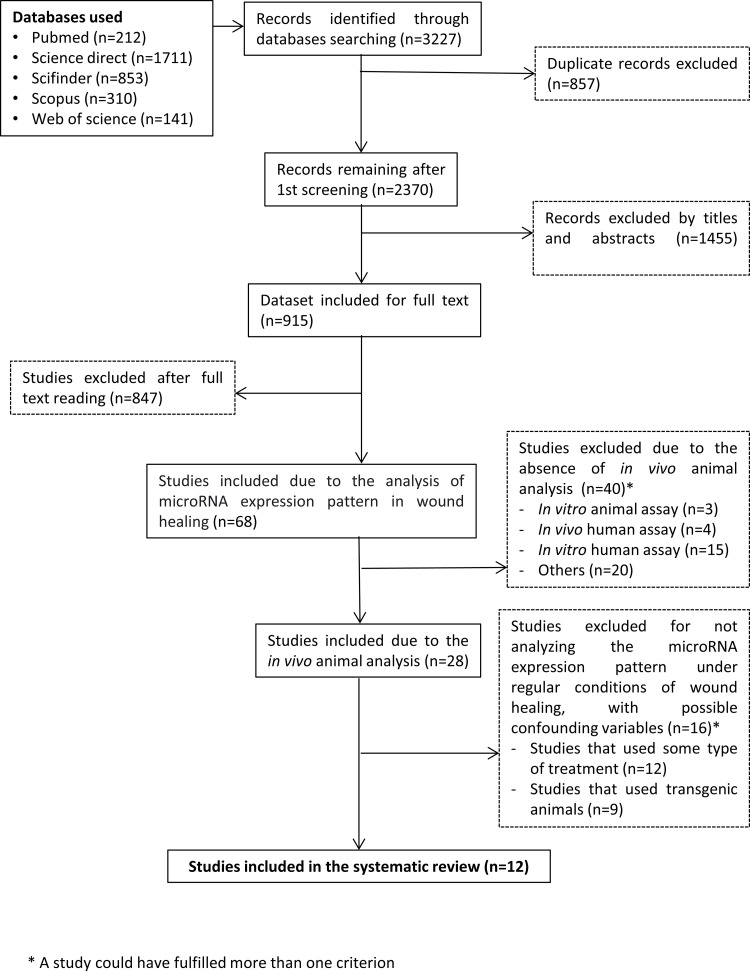
The initial search amounted to 3,227 records. After the removal of duplicates, a total of 2,370 articles remained for the title and abstract screening. So, based on the title and abstract, 915 articles remained for full-text reading screening. After reading the full text, were excluded 847 articles, remaining 68 articles satisfy the inclusion criteria. These of 68 articles, 40 were excluded for not performing the analysis in vivo animal. A total of 28 articles remained, and 16 of these ended up being excluded for showing possible confounding variables, such as studies that used some type of treatment or transgenic animal. In other words, studies did not analyze wound healing under regular conditions. Finally, remained 12 articles that present viable data to analyze, as shown in the PRISMA flowchart for the study selection process (Fig 1).
Fig 1. Flow diagram of study selection.
Flow diagram of the study selection process according to PRISMA Statement. * A Study could have fulfilled more than one criterion.
Among the 12 selected studies, it is clear that the country of publication of the most recurrent articles is the USA, totaling 6 of the 12 articles, followed by China with 4 of these publications. Regarding the year of publication, the oldest article date from 2012, demonstrating how recent the miRNAs studies, especially in relation to wound healing, is recent. These results are also described and illustrated in the figure below (Fig 2).
Fig 2. Correlation between publication countries and years of selected articles.
Correlation between publication countries and years of selected articles.
Table 1 refers to the main methodologies used in the 12 selected articles. Concerning the animal model used, the choice was unanimous. Absolutely all articles used mice as an animal model, there were a few studies that used rats; however, these ended up being excluded due to eligibility criteria. The most frequent strains were C57BL/6, C57BL/6J, and Balb-C. Interestingly, the SKH-1 strain, which is hairless mice in the skin, was reported in only one article. Almost half of the studies specified the gender of their animals–female (n = 3) and male (n = 4)–the other half did not specify. Most animals were between 8–10 weeks old. The more frequent methodology applied for the induction of the lesion was full-thickness wound by biopsy punch in 100% of the articles. The recurrent induction site of the lesion was on the dorsal skin, and the lesions were sized between 3–6 mm.
Table 1. Correlation of the main methodological approaches found in the selected articles.
| Author, Year | Animal conditions | Wound procedures | |||||
|---|---|---|---|---|---|---|---|
| Animal | Strain | Sex | Age | Injury induction site | Injury type | Injury size | |
|
Aunin et al., 2017 |
Mice |
- |
- |
8 weeks | Back skin | Full-thickness wound by biopsy punch | 3 mm |
| 2 years | Back skin | Full-thickness wound by biopsy punch | 3 mm | ||||
| Chan et al., 2012a | Mice | C57BL/6 | Male | 8–10 weeks | Dorsal skin | 2 or 4 full-thickness excisional wounds by biopsy punch | 6 mm |
|
Chan et al., 2012b |
Mice | C57BL/6 | Male | - | Dorsal skin | 2 full-thickness wound | 8x16 mm |
| Mice | C57BL/6 | Male | - | Dorsal skin | 2 or 4 full-thickness excisional wounds by biopsy punch | 6 mm | |
|
Chen et al., 2019 |
Mice | Balb/c | Female | 8–10 weeks | Dorsal skin | 2 full-thickness incisional wounds using a pair of scissors | 10 mm |
| 2 full-thickness excisional wounds by biopsy punch | 5 mm | ||||||
| Anterior of the hard palate | 3 incisional wounds using a scalpel blade | 50 mm | |||||
| Etich et al., 2017 | Mice | C57BL/6N | - | 8–10 weeks | Back skin | Full-thickness wounds by biopsy punch | 6 mm |
| Jin and Chung, 2018 | Mice | SKH-1 | Female | 8 weeks | Dorsal skin | 2 full-thickness wounds by biopsy punch | 3.5 mm |
|
Shi et al., 2018 |
Mice |
- |
- |
7–8 weeks |
Dorsal skin |
Full-thickness wounds by biopsy punch |
6 mm |
| K14-Cre | |||||||
|
Simões et al., 2019 |
Mice |
Balb/c |
Female |
8–10 weeks |
Dorsal skin | Full-thickness wounds by biopsy punch | 5mm |
| Hard palate from maxilla | Full-thickness with a pair of forceps | - | |||||
| Dorsal skin | 2 full-thickness excisional wounds by biopsy punch | 5 mm | |||||
|
van Solingen et al., 2014 |
Mice |
B6.Cg-Mirn155tm1.1Rsky/J | - | 10–12 weeks | - | 2 full-thickness by biopsy punch | 6 mm |
| C57BL | - | 10–12 weeks | - | 2 full-thickness by biopsy punch | 6 mm | ||
| Wang et al., 2012 | Mice | C57BL | Male | Adult | Back | Full-thickness skin excision | 10 mm |
|
Wang et al., 2019 |
Mice | C57BL (H-2b) | Male | Adult | Back | 2 full-thickness by biopsy punch | 6 mm |
| B6.Cg-Mirn155tm1.1Rsky/J | Male | 6–8 weeks | Back | 2 full-thickness by biopsy punch | 6 mm | ||
|
Zhao et al., 2020 |
Mice |
C57BL/6J | - | 8–12 weeks | Back | 2 circular full-thickness excisional wounds | 6 mm |
| C57BL/6J | - | 8–12 weeks | Back | 2 circular full-thickness excisional wounds | 6 mm | ||
| - | Male and female | - | Back | 2 circular full-thickness excisional wounds | 6 mm | ||
Main findings regarding the methodological approaches of the studies include animal and strain, injury induction site, injury size, and wound procedure.
As shown in S1 Table, the main techniques used to analyze the expression of miRNA were RT-qPCR, reported by most articles, and microarray. Some studies combined the two methodologies. The total amount of miRNA that had its expression analyzed varied according to the days of analysis. Among the most recurrent analysis times that were related to the wound healing phases, three days stood out: day 1, day 3, and day 5. However, the relationship between the down and up expression on day 3 was verified by only one of the selected articles (Aunin et al., 2017). The other articles found alteration of the expression in only one of these two patterns and not concurrently (Chan et al., 2012 (a), Chan et al., 2012 (b) and Zao et al., 2020 found alterations in just decreased expression. While, Shi et al., 2018 and Wang et al, 2019 found alterations in just increased expression). So the bioinformatics analysis was performed according to the data obtained on days 1 and 5.
On day 1, a total of 273 miRNAs were analyzed, and just two of these were not affected, in other words, do not have alteration in their expression pattern. Among these that showed some alteration in expression pattern, 153 miRNAs had an increase in their expression, while 118 miRNAs, decreased. On day 5, the time of analysis with the highest amount of analyzed miRNA, the not affected ones totaled 5 miRNAs, while those with increased and decreased expression were 192 and 130, respectively. At last, considering these two days of analysis of the 12 selected articles, a total of 600 miRNAs had their expression pattern analyzed during the wound healing process. The most frequently found miRNAs on days 1 and 5 that were analyzed by the bioinformatic are described in Table 3.
Table 3. Statistically significant union pathways refer to the miRNAs with a decreased expression on day 1.
| Pathway | p-value | Target genes |
|---|---|---|
| Prion diseases | 6.72018e-13 | 7 |
| Hippo signaling pathway | 4.282252e-09 | 1 |
| Proteoglycans in cancer | 2.17414e-07 | 59 |
| Caffeine metabolism | 1.547323e-05 | 2 |
| Renal cell carcinoma | 9.472664e-05 | 17 |
| Steroid biosynthesis | 0.0001140205 | 5 |
| Lysine degradation | 0.00122778 | 18 |
| Adherens junctions | 0.001282294 | 20 |
| TGF-beta signaling pathway | 0.00177966 | 13 |
| FoxO signaling pathway | 0.003806427 | 47 |
| Endocytosis | 0.004723869 | 55 |
| N-Glycan biosynthesis | 0.0115209 | 15 |
| Drug metabolism–other enzymes | 0.01724572 | 6 |
| Protein processing in endoplasmatic reticulum | 0.0386971 | 55 |
| Cicardian entrainment | 0.04087073 | 1 |
| Morphine addiction | 0.04087073 | 1 |
| Pancreatic cancer | 0.04101645 | 23 |
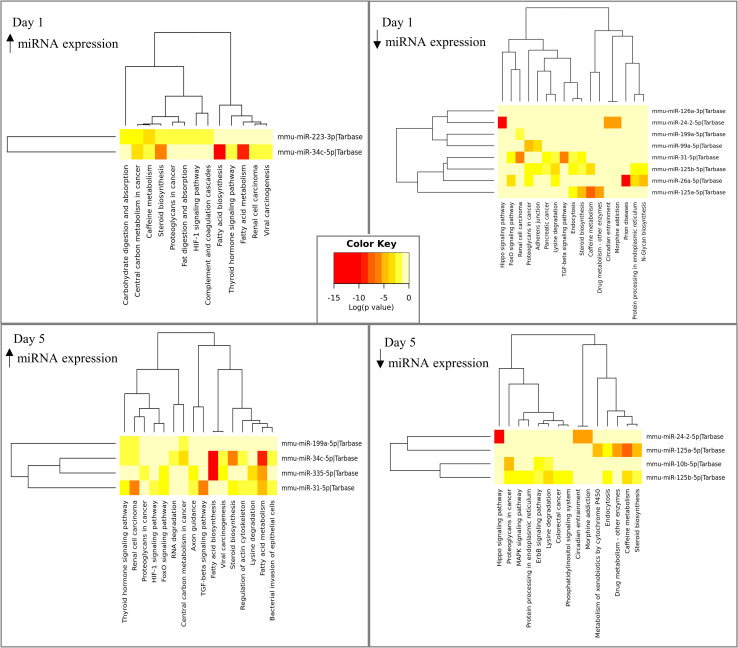
The bioinformatics analysis discloses that on the day 1, among the miRNAs that had their expression increased, there were three intersection pathways, but none with a statistically significant difference (p ≤ 0.05) and 13 union pathways, eight of which were statistically significant. In the miRNAs that had a decrease in their expression, 17 union pathways were found, 12 of which were statistically significant, and no one intersection pathway was found. On the day 5, in the miRNAs with an increase in expression, 16 union pathways were found, 12 of which were statistically significant, and also no one intersection pathway was found. Among the miRNAs with decreased expression, 15 union pathways were found, 11 of which were statistically significant, and no one intersection pathway was found (Tables 2–5), and signaling pathways related to the wound healing process are highlighted in bold.
Table 2. Statistically significant union pathways refer to the miRNAs with an increased expression on day 1.
| Pathway | p-value | Target genes |
|---|---|---|
| Fatty acid biosynthesis | <1e-325 | 2 |
| Fatty acid metabolism | 6.165574e-09 | 4 |
| Steroid biosynthesis | 6.921681e-08 | 3 |
| Central carbon metabolism in cancer | 2.532531e-05 | 9 |
| Caffeine metabolism | 2.639553e-05 | 2 |
| Proteoglycans in cancer | 0.009315449 | 8 |
| Carbohydrate digestion and absorption | 0.01156247 | 4 |
| Fat digestion and absorption | 0.01410009 | 2 |
| Complement and coagulation cascades | 0.04045149 | 7 |
| Thyroid hormone and signaling pathway | 0.04320222 | 11 |
| HIF-1 signaling pathway | 0.04440003 | 7 |
| Renal cell carcinoma | 0.04873515 | 7 |
| Viral carcinogenesis | 0.0496533 | 11 |
Table 5. Statistically significant union pathways refer to the miRNAs with a decreased expression on day 5.
| Pathway | p-value | Target genes |
|---|---|---|
| Hippo signaling pathway | 2.384482e-11 | 1 |
| Caffeine metabolism | 8.713539e-08 | 2 |
| Proteoglycans in cancer | 7.888269e-06 | 32 |
| Drug metabolism–other enzymes | 0.0001989493 | 6 |
| Steroid biosynthesis | 0.0003114483 | 2 |
| Cicardian entrainment | 0.0006915614 | 1 |
| Morphine addiction | 0.0006915614 | 1 |
| Lysine degradation | 0.001772916 | 12 |
| Metabolism of xenobiotics by cytochrome P450 | 0.00374839 | 4 |
| Endocytosis | 0.005002083 | 34 |
| ErbB signaling pathway | 0.008312138 | 18 |
| MAPK signaling pathway | 0.01457244 | 39 |
| Phosphatidylinositol signaling system | 0.01930813 | 16 |
| Colorectal cancer | 0.02517332 | 14 |
| Protein processing in the endoplasmic reticulum | 0.04973187 | 29 |
Correlation of binding pathways observed in miRNAs with increased and decreased expression and their respective target genes.
Table 4. Statistically significant union pathways refer to the miRNAs with an increased expression on day 5.
| Pathway | p-value | Target genes |
|---|---|---|
| Fatty acid biosynthesis | <1e-325 | 2 |
| Fatty acid metabolism | 4.440892e-16 | 11 |
| Renal cell carcinoma | 1.630683e-08 | 23 |
| Steroid biosynthesis | 3.491662e-07 | 5 |
| TGF-beta signaling pathway | 0.0001216682 | 13 |
| Lysine degradation | 0.0003168722 | 10 |
| Thyroid hormone signaling pathway | 0.0005841115 | 25 |
| FoxO signaling pathway | 0.0007019681 | 31 |
| Central carbon metabolism in cancer | 0.003639735 | 6 |
| Proteoglycans in cancer | 0.01120219 | 19 |
| HIF-1 signaling pathway | 0.01131124 | 17 |
| Viral carcinogenesis | 0.01320044 | 26 |
| Bacterial invasion of epithelial cells | 0.0176033 | 17 |
| RNA degradation | 0.0310473 | 10 |
| Regulation of actin cytoskeleton | 0.03361088 | 37 |
| Axon guidance | 0.04726244 | 25 |
The miRNAs and their associated pathways also are illustrated in Fig 3. In the Venn diagrams (Fig 4) it is possible to observe the signaling pathways that overlap on both days (1 and 5), as well as those that occur exclusively on either day 1 or day 5.
Fig 3. Analysis of the miRNAs expression.
Heatmap of KEGG pathways referring to increased or decreased expression of miRNAs at different times of analysis.
Fig 4.
(A). Analysis of significant union pathways overlapping, and non-overlapping. Venn diagram representing the signaling pathways overlapping, and non-overlapping refer to the miRNAs with an increased expression on days 1 and 5. (B). Analysis of significant union pathways overlapping, and non-overlapping. Venn diagram representing the signaling pathways overlapping, and non-overlapping refer to the miRNAs with a decreased expression on days 1 and 5.
Based on the quality assessment of the 12 studies, the three pre-established domains proved to be adequate (Fig 5). The requirement to perform the miRNA analysis in addition to the presence of a control group were the domains that presented insufficient or absent definitions according to pre-established domains.
Fig 5. Analysis of risk of bias of the selected studies.
Risk of bias of each included study in the domains: wound healing assay, miRNA analysis, and presence of a control group.
4. Discussion
In this systematic review, based on the results obtained, can be observed a panorama of altered miRNAs during the wound healing process and their pathways associated (Table 2).
Recent studies have often reported the importance of the role of miRNA in several cellular processes, including wound healing. These studies claim that during the process the miRNA expression pattern can alternate according to the day, suggesting its regulatory function in these cases [27–29]. In our results, it was possible to observe these patterns on different days of wound healing. on each of the days analyzed, essentially all the analyzed miRNAs suffered some alteration in their expression pattern. The miRNAs found with some alterations were quite varied, considering all analysis times.
On day 1, among the miRNAs with an increase in their expression (mmu-miR-223-3p and mmu-miR-34c-5p), already it is possible to observe the correlation with wound healing. Evidence had shown that mmu-miR-223-3p can ameliorates vascular endothelial lesions through the IL6ST and STAT3 signaling pathways [23, 30]. Furthermore, members of the miR-223 family have been associated as important regulators in the inflammatory process that occurs during early phases of wound healing, which justifies its increase in expression on day 1, when the inflammatory process of tissue repair occurs. Also, the miR-223 family is effective in increasing the activation of neutrophils after episodes of bacterial infections, and consequently, improving the course of the healing process [31, 32]. The miR-34 family also seems to be related to wound healing; it has been reported that several family members are up-regulated in epidermal keratinocytes during wound healing. In addition, it being able to improve the inflammatory process occurring during healing due to the release of inflammatory cytokines [33, 34].
The miR-31 family is also closely related to the wound healing process, these miRNAs can regulate keratinocytes proliferation, differentiation, and migration through the regulation of the signaling pathways NF-κB, RAS/MAPK, Notch, and cytokines [35, 36]. The miR-31-5p was found with expression decreased on day 1 and increased on day 5. Already described for its important role in cell migration, the miR-199a-5p was found decreased on both days [37], but also there is evidence that miR-199a-5p can, in a negative way, regulate the angiogenic responses through responses by directly targeting ETS proto-oncogene 1 and transcription factor (ETS-1) [38]. The first miRNAs associated with inflammatory response were miR-132 and miR-125b, the expression was induced in a monocytic cell line treated with lipopolysaccharide [39–41]. However, the miR-125a-5p and miR-125b-5p were found with a decreased expression on both days. The angiogenesis also is regulated by the miRNAs. For instance, some studies already related the downregulation of miR-199a-5p expression in the dermis and endothelial tissue during the wound healing process. Also, by targeting an angiogenesis-related transcription factor and its mediator, the miR-199a-5p can, negatively, regulate the angiogenic response of dermal microvascular endothelial cells in humans. In mice with homozygous deletions in the ETS-1 gene, was related to an impaired of angiogenesis, insufficient formation of granulation tissue, and compromised wound closure [41–43]. The miRNA-199a-5p was found with an expression decreased on day 1, but not on day 5.
In our bioinformatics analysis, we investigated the associated pathways highlighting the statistically significant and related to wound healing. Among them, the thyroid hormone signaling pathway (p = 0.04) was identified. This pathway can be related to several biological processes by regulating gene expression. The thyroid hormone, for instance, already was described as one of the most potent stimulators of growth and metabolic rate, it can induce the angiogenesis through the increase of bGFG mRNA expression via the integrin αvβ3/PKD/HDAC5 signaling pathway [44, 45]. In a culture of human keratinocytes, the exogenous thyroid human stimulated the expression of keratin genes, these genes are responsible for 30% of the protein of the epidermis, and there is clear evidence about the relation to keratin genes and wound healing specific phases [46]. Another representative pathway associated with miRNAs with an increased expression is the Hypoxia Inducible Factor-1 (HFI-1) signaling pathway (p = 0.04). Several studies already related the role of HIF-1 in wound healing, contributing to cell migration and division under hypoxic conditions, beyond the growth factor release and extracellular matrix [47, 48].
The forkhead box O (FoxO) signaling pathway was also found among the miRNAs with an increased expression (p = 0.0007) targeting more than 30 genes. The FoxO family is constituted of transcription factors responsible for regulating gene expression in several cellular events and biological processes, such as apoptosis, cell-cycle control, oxidative stress resistance and wound healing stimulation [49]. Also, the members of this family are recruited for keratinocyte mobilization and migration due to their ability to regulate, positively, transforming growth factor-beta (TGF-β1) expression. The TGF-β1 exerts effects on wound healing through immune modulation, cell proliferation, migration and differentiation regulation, and extracellular matrix production [50]. The TGF-β1 signaling pathway was also found associated to wound healing (p = 0.0001). There are three isoforms (TGF-β1, TGF-β2 and TGF-β3) and all of these appears to exert effects on wound healing through the SMAD pathway, mainly. The TGF-β1 is more frequently related to scarless wound healing formation, whereas the TGF-β3 already was observed in fibrotic scarring [51]. These findings corroborate the TGF-β1 decrease described in chronic non-healing wounds [52]. In general, the TGF-β family can play a role he wound healing through inflammation regulation, fibroblast proliferation, angiogenesis simulation, and deposition and remodeling of the extracellular matrix [53].
Among the signaling pathways associated with the miRNAs with decreased expression and related to the wound healing process, was found the steroid biosynthesis pathway (p = 0.0001). This signaling pathway can be related to wound healing in many ways; glucocorticoids (GC) are able to inhibit wound healing through their membranous glucocorticoid receptor. This receptor via activation of the Wnt-like 6 PLC/PKC signaling cascade interferes with the keratinocytes migration and, consequently, on wound closure [54]. The Wnt signaling pathway already was, frequently, related to several aspects of skin development and physiology. Also, in cases of Wnt pathway reduction, the regenerative capacities and abilities are impaired. This pathway regulates the β-catenin activation, and this process appears to be one of the several inflammatory responses to injury [54, 55]. Although the Wnt signaling pathway in the inflammatory response is not yet very understood, some evidence, through the observation of gene Wnt5 increased expression in patients with severe sepsis, suggests β-catenin-independent Wnt signaling may be a proinflammatory stimulus, a key event for the wound healing process [56].
Cell adhesion, mediated by adherens junctions, is crucial and closely related to the wound healing process. The adherens junction signaling pathway (p = 0. 001) plays an important role in cell plasticity, providing both cell-cell adhesion and fast cell-cell contact remodeling during several biological processes, such as wound healing [57]. The adherens junctions are also a key target of endocytosis during wound healing; the endocytosis signaling pathway (p = 0.004), also found in miRNAs with decreased expression, provides the endocytic remodeling of adherens junctions. This cell adhesion is required to control the actin assembly on the wound edge increasing the speed of wound closure [58]. There is some evidence suggesting that most cell-cell adhesion proteins can be modified by N-Glycans, increasing the probability of the occurrence of defects in the formation of the protective barrier and cell differentiation and adhesion [59]. The N-Glycans biosynthesis signaling pathway (p = 0.01), and these glycans are clearly involved in processes responsible for regulating the terminal differentiation products in keratinocytes [60].
In addition, were identified two signaling pathways among the miRNAs with decreased expression involving the protein kinase. The ErbB (epidermal growth factor receptor) signaling pathway (p = 0.08) is a family of receptor tyrosine kinases (RTKs) responsible for binding extracellular growth factor to intracellular pathways, to regulate some biological responses–cell proliferation, differentiation, and motility [61]. These growth factors, acting via RTKs, are able to control different cell types in skin wound healing, particularly macrophages and neutrophils. For this reason, the aberrant expression of the growth factors or their receptors is related to difficult wound healing [62]. Cell proliferation is also regulated for the mitogen-activated protein kinase (MAPK) signaling pathway (p = 0.01). Through more than 18 target genes, the activation of the MAPK pathway, mainly the extracellular signal-regulated kinase (ERK), is the most important regulator of several cell types’ migration. The ERK/MAPK pathway can be activated by skin damage and this activation has a strong effect on keratinocyte migration [63–66].
Ultimately, this systematic review has limitations that need to be highlighted. Different strains of mice were used to observe the miRNA pattern expression during wound healing, although there are certain strains most frequently used, their differences must be considered so that the results are properly interpreted. In different strains, beside the miRNA pattern expression, the signaling pathways also can be altered [67, 68]. Another variable that can induce confusion and doubts is the analysis time. Wound healing is a complex and extensive process, in other words, does not occur in a fixed time, but in three or four phases taking to days to months [1, 3]. Thus, it is necessary to define the most adequate analysis time to obtain the most reliable data possible, according to the studied species and the biological processes that the objective is to study. Nonetheless, in some cases, such heterogeneity may be important, for example, to identify new pathways that would not be related otherwise.
Hence, the results found in this systematic review and bioinformatics analysis contribute to the identification of miRNAs altered during the wound healing process, as well as the associated signaling pathways. Furthermore, they can be used as a study tool for the next works that aim to relate miRNAs to wound healing, in the search for new potential biomarker targets, whether for diagnosis or therapy.
5. Conclusion
In conclusion, the results of our systematic review demonstrated that some miRNAs are altered during the wound healing process. The bioinformatics analysis revealed that on day 1, among the miRNAs with increased and decreased expression, there are 20 union pathways that were statistically significant. And on day 5, we can observe a similar amount, totalizing 23 union pathways statistically significant. Most miRNAs identified in our study play a role in wound healing through regulating, mainly, cell proliferation and differentiation, by several signaling pathways. It is worth noting the limitation we found in the selected studies regarding their significant heterogeneity, which can be explained by the differences in the target populations—in this case, the wound healing is observed in different strains of mice -, and timing of outcome measurements. In this sense, even though there is a diversity in the studies found, this heterogeneity can be important in order to identify new pathways that would probably not be considered in another manner, as in studies with low heterogeneity. But even then, the results we present help to better understand the complex network of miRNAs, as well as their role in the healing of wounds. With this systematic review, it was possible to study the panorama of miRNAs that may be altered in the wound healing, understanding which miRNAs and its respective signaling pathways may be involved in the wound healing process. The present review summarizes existing evidence of miRNAs associated to wound healing, and these findings can contribute to new therapeutic approaches.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99: 665–706. doi: 10.1152/physrev.00067.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu S, Shukla V. Complications of wound healing. In: Measurements in wound healing. Springer. 2012: 109–144. [Google Scholar]
- 3.Wang PH, Huang BS, Horng HC, Yeh CC. Wound healing. J Chin Med Assoc. 2017;20: 1–8. [DOI] [PubMed] [Google Scholar]
- 4.Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34: 599–610. doi: 10.1007/s12325-017-0478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Z, Niu J, Cheng B. Prevalence of chronic skin wounds and their risk factors in an inpatient hospital setting in northern China. Adv Wound Care. 2020;33: 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Andersen C, Black J, de Leon J, Fife C, John CL, et al. Management of Chronic Wounds: Diagnosis, Preparation, Treatment, and Follow-up. Wounds. 2017;29: 19–36. [PubMed] [Google Scholar]
- 7.Christopher AF, Kaur RP, Kaur G, Kaur A, Gupta V, Bansal P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect Clin Res. 2016;7: 68. doi: 10.4103/2229-3485.179431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16: 203–222. doi: 10.1038/nrd.2016.246 [DOI] [PubMed] [Google Scholar]
- 9.Soliman AM, Das S, Abd Ghafar N, Teoh SL. Role of microRNA in proliferation phase of wound healing. Front genet. 2018;9: 38. doi: 10.3389/fgene.2018.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front genet. 2019;10: 478. doi: 10.3389/fgene.2019.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17: 1712. doi: 10.3390/ijms17101712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creugny A, Fender A, Pfeffer S. Regulation of primary micro RNA processing. FEBS Lett. 2018;592: 1980–1996. doi: 10.1002/1873-3468.13067 [DOI] [PubMed] [Google Scholar]
- 13.Macgregor-Das AM, Das S. A microRNA’s journey to the center of the mitochondria. Am J Physiol Heart Circ Physiol. 2018;315: 206–215. [DOI] [PubMed] [Google Scholar]
- 14.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141: 1202–1207. doi: 10.1016/j.jaci.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piletič K, Kunej T. MicroRNA epigenetic signatures in human disease. Arch Toxicol. 2016;90: 2405–2419. doi: 10.1007/s00204-016-1815-7 [DOI] [PubMed] [Google Scholar]
- 16.Yao Q, Chen Y, Zhou X. The roles of microRNAs in epigenetic regulation. Curr Opin Chem Biol. 2019;51: 11–17. doi: 10.1016/j.cbpa.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 17.Cui J, Zhou B, Ross SA, Zempleni J. Nutrition, microRNAs, and human health. Adv Nutr. 2017;8: 105–112. doi: 10.3945/an.116.013839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234: 5451–5465. doi: 10.1002/jcp.27486 [DOI] [PubMed] [Google Scholar]
- 19.Aunin E, Broadley D, Ahmed MI, Mardaryev AN, Botchkareva NV. Exploring a role for regulatory miRNAs in wound healing during ageing: involvement of miR-200c in wound repair. Sci Rep. 2017;7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan YC, Roy S, Huang Y, Khanna S, Sem CK. The microRNA miR-199a-5p down-regulation switches on wound angiogenesis by derepressing the v-ets erythroblastosis virus E26 oncogene homolog 1-matrix metalloproteinase-1 pathway. J Biol Chem. 2012;287: 41032–41043. doi: 10.1074/jbc.M112.413294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etich J, Bergmeier V, Pitzler L, Brachvogel B. Identification of a reference gene for the quantification of mRNA and miRNA expression during skin wound healing. Connect Tissue Res. 2017;58: 196–207. doi: 10.1080/03008207.2016.1210606 [DOI] [PubMed] [Google Scholar]
- 22.Shi J, Ma X, Su Y, Song Y, Tian Y, Yuan S, et al. MiR-31 mediates inflammatory signaling to promote re-epithelialization during skin wound healing. J Investig Dermatol. 2018; 138: 2253–2263. doi: 10.1016/j.jid.2018.03.1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CR, Zhu HF, Zhu Y. Knockout of microRNA-155 ameliorates the Th17/Th9 immune response and promotes wound healing. Curr Med Sci. 2019;39: 954–964. doi: 10.1007/s11596-019-2128-x [DOI] [PubMed] [Google Scholar]
- 24.Long S, Zhao N, Ge L, Wang G, Ran X, Wang J, et al. MiR‐21 ameliorates age‐associated skin wound healing defects in mice. J Genet Med. 2018;20: 3022. doi: 10.1002/jgm.3022 [DOI] [PubMed] [Google Scholar]
- 25.Boeker M, Vach W, Motschall E. Google Scholar as replacement for systematic literature searches: good relative recall and precision are not enough. BMC Med Res Methodol. 2013;13: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silveira RG, Ferrúa CP, do Amaral CC, Garcia TF, de Souza KB, Nedel, F. MicroRNAs expressed in neuronal differentiation and their associated pathways: systematic review and bioinformatics analysis. Brain Res Bull. 2020;157: 140–148. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Yang C, Wang X, yan Zhou L, Juan Lao G, Liu D, et al. MicroRNA-129 and-335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes. 2018;67: 1627–1638. doi: 10.2337/db17-1238 [DOI] [PubMed] [Google Scholar]
- 28.Jiang Z, Wei J, Yang W, Li W, Liu F, Yan X, et al. MicroRNA-26a inhibits wound healing through decreased keratinocytes migration by regulating ITGA5 through PI3K/AKT signaling pathway. Biosci Rep. 2020;40: BSR20201361. doi: 10.1042/BSR20201361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Zhang K, Liu R, Zhang H, Chen D, Yu S, et al. MicroRNA-21-3p accelerates diabetic wound healing in mice by downregulating SPRY1. Aging. 2020;12: 15436. doi: 10.18632/aging.103610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanoaga O, Braicu C, Chiroi P, Andreea N, Hajjar NA, Mărgărit S, et al. The Role of miR-155 in Nutrition: Modulating Cancer-Associated Inflammation. Nutrients. 2012;13(7): 2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolska-Gawron K, Bartosińska J, Rusek M, Kowal M, Raczkiewicz D, Krasowska D. Circulating miRNA-181b-5p, miRNA-223-3p, miRNA-210-3p, let 7i-5p, miRNA-21-5p and miRNA-29a-3p in patients with localized scleroderma as potential biomarkers. Sci Rep. 2020;10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Córdova-Rivas S, Fraire-Soto I, Mercado-Casas Torres A, Servín-González LS, Granados-López AJ, López-Hernández Y, et al. 5p and 3p strands of miR-34 family members have differential effects in cell proliferation, migration, and invasion in cervical cancer cells. Int J Mol Sci. 2019;20: 545. doi: 10.3390/ijms20030545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Li X, Li D, Ren X, Li Y, Herter EK, et al. MicroRNA-34 family enhances wound inflammation by targeting LGR4. J Investig Dermatol. 2020;140: 465–476. doi: 10.1016/j.jid.2019.07.694 [DOI] [PubMed] [Google Scholar]
- 34.Childs DR, Murthy AS. Overview of wound healing and management. Surgical Clinics. 2017;97: 189–207. doi: 10.1016/j.suc.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Simões A, Chen Z, Zhao Y, Wu X, Dai Y, et al. Overexpression of the oral mucosa-specific microRNA-31 promotes skin wound closure. Int J Mol Sci. 2019;20: 3679. doi: 10.3390/ijms20153679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Gao Y, Yuan Y, Du R, Li P, Liu F, et al. MicroRNA-31 Can Positively Regulate the Proliferation, Differentiation and Migration of Keratinocytes. Biomed Hub. 2020;5(2): 1–12. doi: 10.1159/000508612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan S, Xu Z, Lou F, Zhang L, Ke F, et al. NF-κB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nat Commun. 2015;6(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gheinani AH, Burkhard FC, Rehrauer H, Fournier CA, Monastyrskaya K. MicroRNA MiR-199a-5p regulates smooth muscle cell proliferation and morphology by targeting WNT2 signaling pathway. J Biol Chem. 2015;290(11): 7067–7086. doi: 10.1074/jbc.M114.618694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. PNAS. 2006;103(33): 12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herter EK, Xu Landén N. Non-coding RNAs: new players in skin wound healing. Adv Wound Care. 2017;6(3):93–107. doi: 10.1089/wound.2016.0711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immun. 2007;179(8):5082–5089. [DOI] [PubMed] [Google Scholar]
- 42.Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146: 97–125. doi: 10.1016/j.addr.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan YC, Khanna S, Roy S, Sen CK. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem. 2011;286(3): 2047–2056. doi: 10.1074/jbc.M110.158790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Safer JD. Thyroid hormone and wound healing. J Thyroid Res. 2013;2013: 124538. doi: 10.1155/2013/124538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Zheng N, Shi YN, Yuan J, Li L. Thyroid hormone induced angiogenesis through the integrin αvβ3/protein kinase D/histone deacetylase 5 signaling pathway. J Mol Endocrinol. 2014;52(3): 245–254. [DOI] [PubMed] [Google Scholar]
- 46.Losner J, Courtemanche K, Whited JL. A cross-species analysis of systemic mediators of repair and complex tissue regeneration. Npj Regen Med. 2012;6(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong WX, Hu MS, Esquivel M, Liang GY, Rennert RC, McArdle A, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care. 2014;3(5): 390–399. doi: 10.1089/wound.2013.0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Liu X, Zhao F, Zhang Y, Wang Z. HIF1α overexpression enhances diabetic wound closure in high glucose and low oxygen conditions by promoting adipose-derived stem cell paracrine function and survival. Stem Cell Res Ther. 2020;11(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H. Understanding the perspectives of forkhead transcription factors in delayed wound healing. J Cell Commun Signal. 2019;13(2): 151–162. doi: 10.1007/s12079-018-0484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol. 2017;8: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burn Trauma. 2012;2(1): 18. [PMC free article] [PubMed] [Google Scholar]
- 52.Liarte S, Bernabé-García Á, Nicolás FJ. Role of TGF-β in skin chronic wounds: a keratinocyte perspective. Cells. 2020;9(2): 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang T, Wang XF, Wang ZC, Lou D, Fang QQ, Hu YY, et al. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020;129: 110287. [DOI] [PubMed] [Google Scholar]
- 54.Jozic I, Vukelic S, Stojadinovic O, Liang L, Ramirez HA, Pastar I, et al. Stress signals, mediated by membranous glucocorticoid receptor, activate PLC/PKC/GSK-3β/β-catenin pathway to inhibit wound closure. J Invest Dermatol. 2017;137(5): 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slominski AT, Zmijewski MA. Glucocorticoids inhibit wound healing: novel mechanism of action. J Invest Dermatol. 2017;137(5): 1012–1014. doi: 10.1016/j.jid.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ackers I, Malgor R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diab Vasc Dis Res. 2018;15(1): 3–13. doi: 10.1177/1479164117738442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi J, Barakat M, Chen D, Chen L. Bicellular tight junctions and wound healing. Int J Mol Sci. 2018;19(2): 3862. doi: 10.3390/ijms19123862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsubayashi Y, Coulson-Gilmer C, Millard TH. Endocytosis-dependent coordination of multiple actin regulators is required for wound healing. Int J Cell Biol. 2015;210(3): 419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dabelsteen S, Pallesen EM, Marinova IN, Nielsen MI, Adamopoulou M, Rømer TB, et al. Essential functions of glycans in human epithelia dissected by a CRISPR-Cas9-engineered human organotypic skin model. Dev Cell. 2020;54(5): 669–684. doi: 10.1016/j.devcel.2020.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin SP, Chung JH. Inhibition of N-glycosylation by tunicamycin attenuates cell–cell adhesion via impaired desmosome formation in normal human epidermal keratinocytes. Biosci Rep. 2018;38(6): BSR20171641. doi: 10.1042/BSR20171641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabbah DA, Hajjo R, Sweidan K. Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr Top Med Chem. 2020;20(10). doi: 10.2174/1568026620666200303123102 [DOI] [PubMed] [Google Scholar]
- 62.Kazanietz MG, Barrio-Real L, Casado-Medrano V, Baker MJ, Lopez-Haber C. The P-Rex1/Rac signaling pathway as a point of convergence for HER/ErbB receptor and GPCR responses. Small GTPases. 2018;94(2): 297–303. doi: 10.1080/21541248.2016.1221273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, Xu P, Wang X, Zhang M, Yan Y, Chen Y, et al. Activin B regulates adipose-derived mesenchymal stem cells to promote skin wound healing via activation of the MAPK signaling pathway. Int J Biochem. 2017;87: 69–76. doi: 10.1016/j.biocel.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 64.Lee S, Kim MS, Jung SJ, Kim D, Park HJ, Cho D. ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes. Sci Rep. 2018;8(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao N, Wang G, Long S, Hu M, Gao J, Ran X, et al. MicroRNA-34a deficiency leads to impaired wound closure by augmented inflammation in mice. Ann Transl Med. 2020;8(7). doi: 10.21037/atm.2020.03.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang T, Feng Y, Sun H, Zhang L, Hao L, Shi C, et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol. 2012;181(6):1911–1920. doi: 10.1016/j.ajpath.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 67.van Solingen C, Araldi E, Chamorro‐Jorganes A, Fernández‐Hernando C, Suárez Y. Improved repair of dermal wounds in mice lacking micro RNA‐155. (2014). J Cell Mol Med. 2014;18(6): 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bleul T, Zhuang X, Hildebrand A, Lange C, Böhringer D, Schlunck G, et al. Different innate immune responses in BALB/c and C57BL/6 strains following corneal transplantation. J Innate Immun. 2021;13(1): 49–59. doi: 10.1159/000509716 [DOI] [PMC free article] [PubMed] [Google Scholar]