Abstract
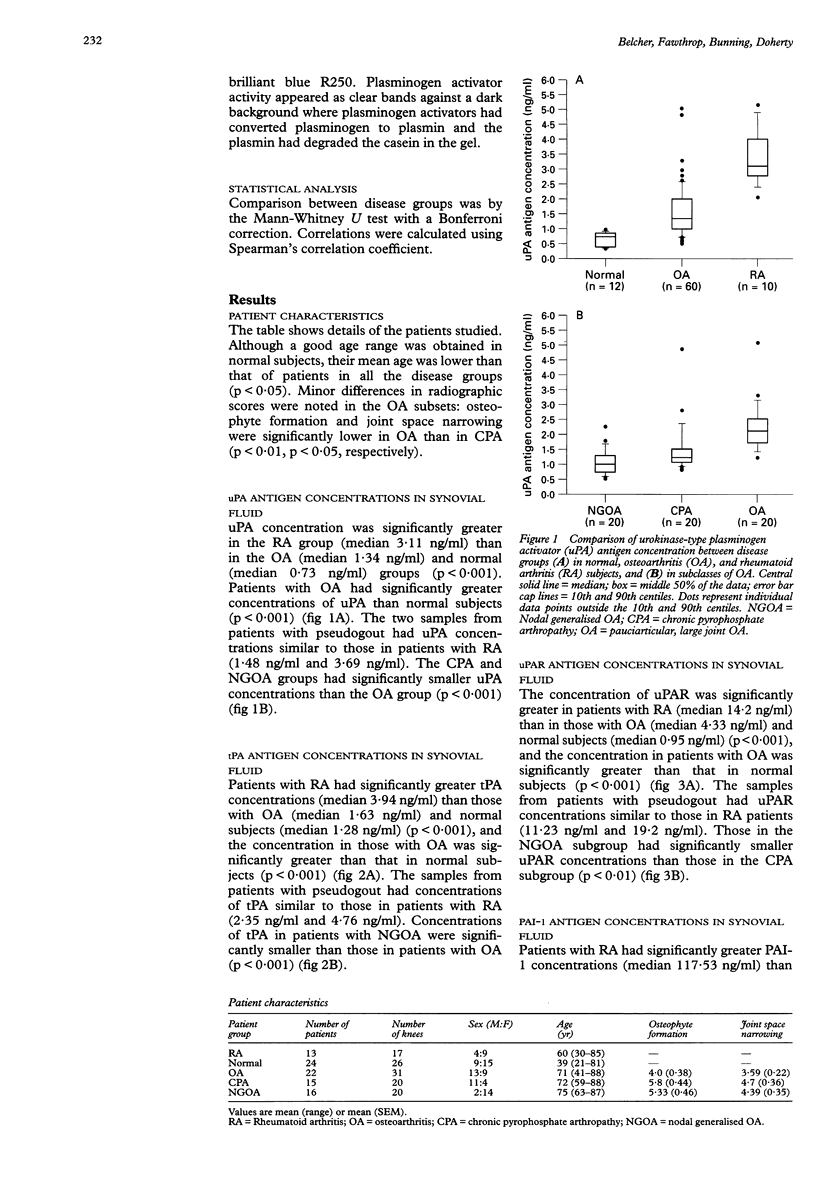
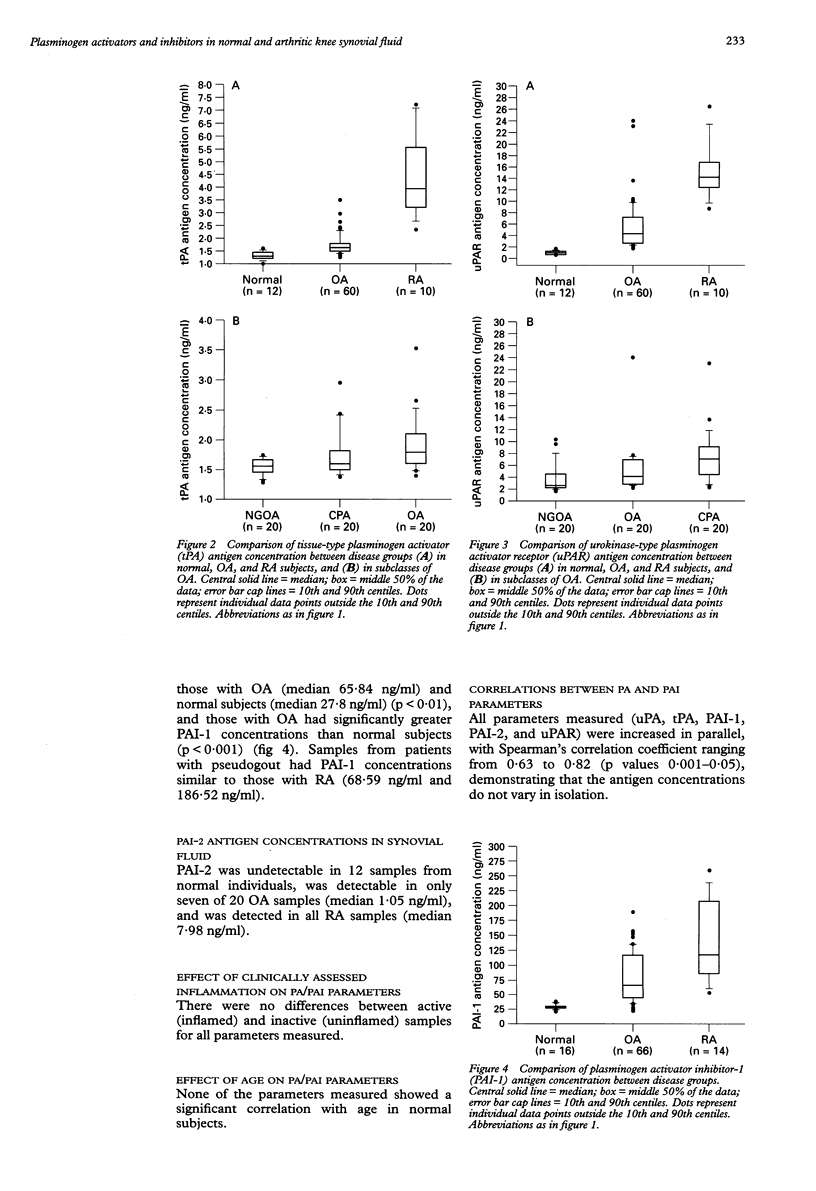
OBJECTIVES: To establish baseline concentrations of plasminogen activators and their inhibitors in normal knee synovial fluids, and to compare them with well characterised osteoarthritis (OA) and rheumatoid arthritis (RA) knee fluids. METHODS: A total of 26 normal subjects, 71 patients with OA, and 17 patients with RA underwent knee aspiration. Patients with OA were subclassified according to presence of nodal generalised OA (NGOA) and synovial fluid calcium pyrophosphate crystals. Clinical assessment of inflammation (graded 0-6) was undertaken in OA and RA patients. Plasminogen activator (PA), plasminogen activator inhibitor (PAI), and urokinase-type PA receptor (uPAR) antigen concentrations were determined by enzyme linked immunosorbent assay. The species of PAs present were determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. RESULTS: Concentrations of all antigens (uPA, tissue-type PA (tPA), uPAR, and PAI-1), were significantly greater in RA than OA; those in OA were significantly greater than normal. The concentrations showed no direct association with clinically assessed inflammation of the knee. In normal fluids, no associations with age were observed. Antigen concentrations (uPA, tPA, and uPAR) in NGOA differed from those in other subclasses of OA, but the species of PA present did not appear to vary between disease groups. The predominant PA appeared to have identity with uPA. CONCLUSION: Because of the greater concentrations of these antigens in OA compared with normal fluids, OA cannot be used as a surrogate normal control in studies of the PA/PAI system. Alteration of the PA/PAI system was confirmed in RA and OA knee fluids, with greater changes evident in RA. The finding of different concentrations of PA antigens in NGOA compared with other OA fluids further supports a different pathogenic mechanism in this subset.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993 Feb;15(2):105–111. doi: 10.1002/bies.950150206. [DOI] [PubMed] [Google Scholar]
- Brommer E. J., Dooijewaard G., Dijkmans B. A., Breedveld F. C. Plasminogen activators in synovial fluid and plasma from patients with arthritis. Ann Rheum Dis. 1992 Aug;51(8):965–968. doi: 10.1136/ard.51.8.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning R. A., Crawford A., Richardson H. J., Opdenakker G., Van Damme J., Russell R. G. Interleukin 1 preferentially stimulates the production of tissue-type plasminogen activator by human articular chondrocytes. Biochim Biophys Acta. 1987 Jun 22;924(3):473–482. doi: 10.1016/0304-4165(87)90163-2. [DOI] [PubMed] [Google Scholar]
- Collier S., Ghosh P. The role of plasminogen in interleukin-1 mediated cartilage degradation. J Rheumatol. 1988 Jul;15(7):1129–1137. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Del Rosso M., Fibbi G., Magnelli L., Pucci M., Dini G., Grappone C., Caldini R., Serni U., Colombo F., Borella F. Modulation of urokinase receptors on human synovial cells and osteoarthritic chondrocytes by diacetylrhein. Int J Tissue React. 1990;12(2):91–100. [PubMed] [Google Scholar]
- Doherty M., Richards N., Hornby J., Powell R. Relation between synovial fluid C3 degradation products and local joint inflammation in rheumatoid arthritis, osteoarthritis, and crystal associated arthropathy. Ann Rheum Dis. 1988 Mar;47(3):190–197. doi: 10.1136/ard.47.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn S., Gilbert R., Ojakian G., Schwimmer R., Quigley J. P. The extracellular matrix of normal chick embryo fibroblasts: its effect on transformed chick fibroblasts and its proteolytic degradation by the transformants. J Cell Biol. 1985 Nov;101(5 Pt 1):1790–1798. doi: 10.1083/jcb.101.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Cheung D., Filonzi E. L., Piccoli D. S., Wojta J., Gallichio M., McGrath K., Last K. Independent regulation of plasminogen activator inhibitor 2 and plasminogen activator inhibitor 1 in human synovial fibroblasts. Arthritis Rheum. 1992 Dec;35(12):1526–1534. doi: 10.1002/art.1780351217. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Rehemtulla A. Plasminogen activators and their inhibitors: regulators of extracellular proteolysis and cell function. Comp Biochem Physiol B. 1988;90(4):691–708. doi: 10.1016/0305-0491(88)90323-9. [DOI] [PubMed] [Google Scholar]
- Heiple J. M., Ossowski L. Human neutrophil plasminogen activator is localized in specific granules and is translocated to the cell surface by exocytosis. J Exp Med. 1986 Sep 1;164(3):826–840. doi: 10.1084/jem.164.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Tanaka S., Matsuo O. Plasminogen activator in synovial fluid from patients with rheumatoid arthritis. J Rheumatol. 1987 Jun;14(3):439–445. [PubMed] [Google Scholar]
- Kirchheimer J. C., Nong Y. H., Remold H. G. IFN-gamma, tumor necrosis factor-alpha, and urokinase regulate the expression of urokinase receptors on human monocytes. J Immunol. 1988 Dec 15;141(12):4229–4234. [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G., Wanivenhaus A., Binder B. R. Increased proteolytic activity on the surface of monocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1430–1433. doi: 10.1002/art.1780341114. [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Harpel P. C., Nachman R. L. Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J Clin Invest. 1987 Oct;80(4):1082–1089. doi: 10.1172/JCI113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof E. K., Tran-Thang C., Ransijn A., Bachmann F. Demonstration of a fast-acting inhibitor of plasminogen activators in human plasma. Blood. 1984 Oct;64(4):907–913. [PubMed] [Google Scholar]
- Kummer J. A., Abbink J. J., de Boer J. P., Roem D., Nieuwenhuys E. J., Kamp A. M., Swaak T. J., Hack C. E. Analysis of intraarticular fibrinolytic pathways in patients with inflammatory and noninflammatory joint diseases. Arthritis Rheum. 1992 Aug;35(8):884–893. doi: 10.1002/art.1780350806. [DOI] [PubMed] [Google Scholar]
- LACK C. H., ROGERS H. J. Action of plasmin on cartilage. Nature. 1958 Oct 4;182(4640):948–949. doi: 10.1038/182948a0. [DOI] [PubMed] [Google Scholar]
- Laroche M., Arlet P., Ader J. L., Durand D., Arlet J., Mazieres B. Phosphate diabetes associated with bone metastases of oat cell lung cancer. J Rheumatol. 1991 Jan;18(1):106–109. [PubMed] [Google Scholar]
- Leizer T., Clarris B. J., Ash P. E., van Damme J., Saklatvala J., Hamilton J. A. Interleukin-1 beta and interleukin-1 alpha stimulate the plasminogen activator activity and prostaglandin E2 levels of human synovial cells. Arthritis Rheum. 1987 May;30(5):562–566. doi: 10.1002/art.1780300511. [DOI] [PubMed] [Google Scholar]
- Levin E. G. Latent tissue plasminogen activator produced by human endothelial cells in culture: evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6804–6808. doi: 10.1073/pnas.80.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenhayn K., Heilmann H. H., Regling G., Haupt R. Die Plasminogenaktivator-Aktivität der Synovialflüssigkeit als Indikator für Aktivierungsphänomene bei degenerativen Gelenkerkrankungen. Z Rheumatol. 1989 Sep-Oct;48(5):246–253. [PubMed] [Google Scholar]
- Martel-Pelletier J., Faure M. P., McCollum R., Mineau F., Cloutier J. M., Pelletier J. P. Plasmin, plasminogen activators and inhibitor in human osteoarthritic cartilage. J Rheumatol. 1991 Dec;18(12):1863–1871. [PubMed] [Google Scholar]
- Martel-Pelletier J., Zafarullah M., Kodama S., Pelletier J. P. In vitro effects of interleukin 1 on the synthesis of metalloproteases, TIMP, plasminogen activators and inhibitors in human articular cartilage. J Rheumatol Suppl. 1991 Feb;27:80–84. [PubMed] [Google Scholar]
- Mochan E., Keler T. Plasmin degradation of cartilage proteoglycan. Biochim Biophys Acta. 1984 Aug 21;800(3):312–315. doi: 10.1016/0304-4165(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Mochan E., Uhl J. Elevations in synovial fluid plasminogen activator in patients with rheumatoid arthritis. J Rheumatol. 1984 Apr;11(2):123–128. [PubMed] [Google Scholar]
- Nachman R. L., Hajjar K. A., Silverstein R. L., Dinarello C. A. Interleukin 1 induces endothelial cell synthesis of plasminogen activator inhibitor. J Exp Med. 1986 Jun 1;163(6):1595–1600. doi: 10.1084/jem.163.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Howell D. S., Ghandur-Mnaymneh L., Enis J. E., Woessner J. F., Jr Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983 Jan;26(1):63–68. doi: 10.1002/art.1780260110. [DOI] [PubMed] [Google Scholar]
- Roche P. C., Campeau J. D., Shaw S. T., Jr Comparative electrophoretic analysis of human and porcine plasminogen activators in SDS-polyacrylamide gels containing plasminogen and casein. Biochim Biophys Acta. 1983 May 30;745(1):82–89. doi: 10.1016/0167-4838(83)90172-3. [DOI] [PubMed] [Google Scholar]
- Rønne E., Pappot H., Grøndahl-Hansen J., Høyer-Hansen G., Plesner T., Hansen N. E., Danø K. The receptor for urokinase plasminogen activator is present in plasma from healthy donors and elevated in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1995 Mar;89(3):576–581. doi: 10.1111/j.1365-2141.1995.tb08366.x. [DOI] [PubMed] [Google Scholar]
- Saxne T., Lecander I., Geborek P. Plasminogen activators and plasminogen activator inhibitors in synovial fluid. Difference between inflammatory joint disorders and osteoarthritis. J Rheumatol. 1993 Jan;20(1):91–96. [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]



