Dear Editor,
A previous study showed that expression of the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome, an innate immunity pathway, was up-regulated in cancer patients infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and possibly associated with the severity of the coronavirus disease 2019 (Covid-19).1 It has been shown that SARS-CoV-2 infection led to the activation of the NLRP3 inflammasome, and suggested that inflammasomes could be used as markers of the disease severity.2 Indeed, it has been observed that SARS-CoV-2 replication and infection in lung human macrophages is a critical driver of the Covid-19 disease.3
Innate immunity is the first line of defense in response to pathogens, which acts locally and also leads the stimulation of adaptive immunity through at least with Interleukin-1β (IL-1β), one of the major secreted cytokine of the NLRP3 inflammasome.4
The aim of this study was to assess and compare the level of IL-1β secretion in monocyte-like cells infected with six different SARS-CoV-2 variants of concern and possibly observed a variant-dependent pattern of inflammasome activation.
Six SARS-CoV-2 variants (historical (B.1, D614G), Alpha, Beta, Gamma, Delta and Omicron BA.1) were isolated from COVID-19 molecular proven hospitalized patients by inoculation of Vero or Vero-TRMPSS2 cells. THP-1 monocyte-like, that are the most commonly used cell line for the study of inflammasome activation, were cultured with RPMI-hepes 10% FBS-0.05 mM 2-mercaptoethanol. A total of 5 × 104 of THP-1 cells was plated per well in 96-wells plate and differentiated in macrophages-like cells with 10 nM of PMA for 24 h.5
The SARS-CoV-2 infection of the differentiated THP-1 was first confirmed by RT-qPCR. The relative viral load (VL) was assessed from CT values (ORF1ab target gene) obtained by the TaqPath COVID-19 RT-PCR assay (ThermoFisher, Waltham, USA) and by linear regression in log10 copies/ml with a standard curve realized from a SARS-CoV-2 positive nasopharyngeal sample quantified by Droplet-Digital PCR (Bio-Rad). Cells were infected with the historical SARS-CoV-2 strain at an MOI of 0.1 and relative VL were measured at 4 and 24 h post infection in the supernatant.
Differenciated-THP-1 were first primed with LPS 1 µg/ml for 2 h and infected with different SARS-CoV-2 variants at an MOI of 0.1. IL-1β was measured by the Lumit Human IL-1β Immunoassay in the supernatant after 24 h of infection. Results come from multiple independent experiments.
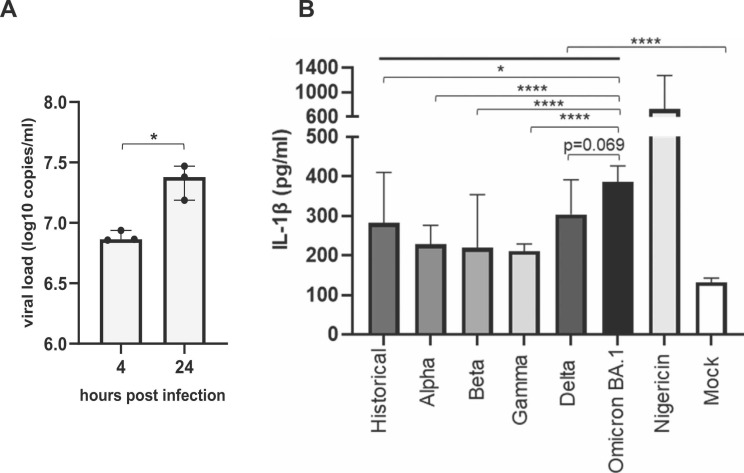
We first observed an increase of the VL between 4 h and 24 h post infection which showed that the THP-1 cells are permissive to the historical SARS-CoV-2 strain ( Fig. 1A).
Fig. 1.
SARS-CoV-2 variants-dependent IL-1β secretion in macrophage-like THP-1 cells. A) Supernatant relative viral load of differentiated-THP-1 cells infected by the SARS-CoV-2 historical strain after 4 and 24 h post infection at an MOI of 0.1 indicative of a permissive infection. B) Histograms represent IL-1β quantification in supernatants of non- (mock) or SARS-CoV-2 infected cells. Nigericin is used as positive control for NLRP-3 activation in differentiated-THP-1 cells. *p < 0.05; ****p < 0.0001. Data shown result from multiple independent experiments.
We next compared IL-1β secretion induced by the 6 SARS-CoV-2 sublineages after infection of macrophages-like THP-1. We observed that THP-1 cells infected with SARS-CoV-2 variants presented a significantly higher IL-1β secretion than non-infected cells (Fig. 1B) and that some SARS-CoV-2 variants led to a stronger IL-1β secretion. We observed a significantly higher level of IL-1β cells infected with Omicron BA.1 sublineage compared to other tested variants, in particular. Indeed, Omicron BA.1 infected cells presented the higher IL-1β secretion (median 385.7 pg/ml IQR [302.6–426.3]) follows by the Delta and the historical variants (median 303.6 [266.3–391.9] and 281.9 [207.2–410], respectively). Alpha, Beta and Gamma variants presented the lowest IL-1β secretion (median 228.1 [192.5–276.4], 219.1 [185.1–354.2] and 211 [149.8–228.8]) (Fig. 1B).
Our results showed, as expected, that inflammasome activation is induced by all the 6 SARS-CoV-2 sublineages. However, a variation in the level of IL-1β secretion was observed between the variants. Hence, our results shown that Omicron BA.1 sublineage lead to a higher IL-1β secretion, which could possibly activate adaptative T cell immunity faster. Our results therefore suggested that Omicron BA.1 was more sensed by the innate immune cells or was less prone to counteract inflammasome activation than the other variants. This higher activation of the inflammasome pathway could lead to a viral neutralization with a better efficiency, and associated to the upper respiratory tract tropism of the Omicron BA.1, could possibly explain its less clinical virulence. Indeed, several studies have shown that Omicron BA.1 sublineage present a stronger tropism for the upper airway cells with a less efficient replication in the lung cells than others SARS-CoV-2 variants.6, 7 Even with a higher response of IL-1β secretion, Omicron infection probably not led to a cytokine storm in the lung, which is associated with a poor prognosis, due to its upper respiratory tract preferential localization.
Pyroptosis, the pore-forming inflammatory cell death pathway triggered by NRLP3 inflammasome was observed in SARS-CoV-2 infection and known to contribute to Covid-19 pathology.8 It would be interesting to further our study and explore if a variant-dependent modulation of this cell death pathway could be observed.
Taking together, these results suggest that the innate immune response and precisely, IL-1β secretion pathways were induced in a SARS-CoV-2 variant-dependent manner.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors acknowledged all the members of the Pitié-Salpêtrière, for their implication in the SARS-CoV-2 patient care. We thank the ANRS-MIE (Agence Nationale de Recherches sur le SIDA et les hépatites virales-Maladies Infectieuses Emergentes) (AC43, Medical Virology) and the Emergen Consortium for their support and funding.
References
- 1.Cui Haoran, Liu Jiaxin, Zhang Leiliang. The high expression of key components of inflammasome and pyroptosis might lead to severe COVID‐19 infection in cancer patients. J Infect. 2022;84(4):e19–e21. doi: 10.1016/j.jinf.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues Tamara S., de Sá Keyla S.G., Ishimoto Adriene Y., Amanda Becerra, Samuel Oliveira, Leticia Almeida, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218(3) doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sefik Esen, Qu Rihao, Junqueira Caroline, Kaffe Eleanna, Mirza Haris, Zhao Jun, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606(7914):585–593. doi: 10.1038/s41586-022-04802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Den Eeckhout Bram, Tavernier Jan, Gerlo Sarah. Interleukin-1 as innate mediator of T cell immunity. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.621931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. Elsevier Enhanced Reader〉; n.d. DOI: 〈10.1016/j.jim.2016.01.012. [DOI] [PubMed]
- 6.Nori Wassan, Ghani Zghair Muna Abdul. Omicron targets upper airways in pediatrics, elderly and unvaccinated population. World J Clin Cases. 2022;10(32):12062–12065. doi: 10.12998/wjcc.v10.i32.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng Bo, Abdullahi Adam, Ferreira Isabella A.T.M., Goonawardane Niluka, Saito Akatsuki, Kimura Izumi, et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706–714. doi: 10.1038/s41586-022-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittner Zsofia Agnes, Schrader Markus, George Shilpa Elizabeth, Amann Ralf. Pyroptosis and its role in SARS-CoV-2 infection. Cells. 2022;11(10):1717. doi: 10.3390/cells11101717. [DOI] [PMC free article] [PubMed] [Google Scholar]