High plasma fibroblast growth factor 23 (FGF23) levels in patients with CKD have been associated with adverse cardiorenal outcomes in most studies, thereby providing a rationale for FGF23-lowering strategies. Potassium intake influences kidney phosphate handling and reduces FGF23 in individuals with normal kidney function1; whether this also applies to patients with CKD is unclear. In this study, we describe the effects of short-term KCl supplementation on mineral parameters in patients with stage 3b-4 CKD.
Data were analyzed from 125 participants who completed a single-arm 2-week intervention with 40 mmol/d KCl during the run-in phase of an ongoing randomized placebo-controlled trial with ≥75% adherence to supplements (92% of the total population). The primary aim of the trial is to address the long-term effects of KCl or potassium citrate versus placebo on kidney function.2 The study was approved by the Erasmus Medical Center Medical Ethics Committee (MEC-2017-226) and registered at ClinicalTrials.gov (NCT03253172). We calculated that 106 participants would provide 80% power to detect an effect of 10 relative unit (RU)/ml in C-terminal FGF23 (cFGF23),1 with a two-sided significance of 0.05. Plasma intact FGF23 (iFGF23, BiomedicaGruppe), cFGF23 (Kainos), α-Klotho (ImmunoBiological), C-reactive protein (CRP), IL-6 (Roche), and mineral parameters were measured at baseline and after 2 weeks.
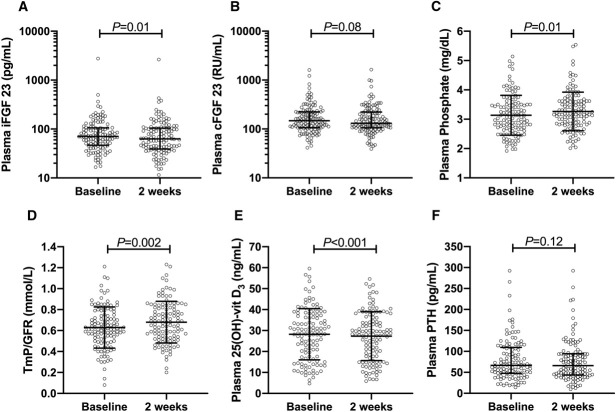
The mean age was 66±11 years, 72% were male, the mean body mass index was 28.2±4.5 kg/m2, and the mean eGFR was 32±8 ml/min per 1.73 m2, while 51% used vitamin D supplementation and 2% used phosphate binders. After KCl supplementation, both 24-hour urinary potassium excretion (2.8±0.9–4.1±1.1 g/d, P<0.001) and plasma potassium increased (4.2±0.5–4.7±0.6 mEq/L, P<0.001). The 24-hour urinary chloride excretion also increased (5.2±1.9–6.4±2.4 g/d, P<0.001), whereas plasma chloride did not change (104±3–105±4 mEq/L, P=0.07). iFGF23 decreased (70.5 [46.6–105.6] to 62.9 [39.1–104.6] pg/ml, P=0.007), but cFGF23 did not (147.1 [105.9–223.8] to 130.3 [105.8–221.3] RU/ml, P=0.08, Figure 1). Furthermore, iFGF23:cFGF23 ratio decreased (0.53 [0.31–0.72] to 0.47 [0.30–0.72], P=0.02). Plasma phosphate (3.1±0.7–3.3±0.65 mg/dl, P=0.01) and tubular maximum reabsorption of phosphate per GFR (TmP/GFR; 0.63±0.20–0.68±0.20 mmol/L, P=0.002) increased, while 24-hour urinary phosphate excretion (78 [56–93] to 76 [58–92] mg/d, P=0.23) and 24-hour urinary sodium excretion did not change (152±60–150±69 mmol/d, P=0.73). Plasma 25(OH)-vitamin D decreased (28.8 [17.6–37.3] to 28.0 [17.6–36.1] ng/ml, P<0.001), and fractional calcium excretion increased (0.49% [0.29%–0.84%] to 0.55% [0.31%–0.89%], P=0.03). No significant changes were observed in parathyroid hormone (PTH; 67.0 [48.0–109.4] to 66.0 [43.4–94.3] pg/ml, P=0.12), α-Klotho (410 [477–564] to 424 [488–579] pg/ml, P=0.23), CRP (2.1 [1.2–4.4] to 2.3 [1.0–4.8] mg/L, P=0.72), or IL-6 (2.88 [1.53–4.91] to 2.54 [1.50–4.77] pg/ml, P=0.35).
Figure 1.
Effects of 2-week potassium chloride supplementation on mineral parameters in patients with stage 3b-4 CKD. Effects of 2-week supplementation of 40 mmol/d KCl on (A) iFGF23, (B) cFGF23 (both FGF23 values are log-transformed depicted), (C) plasma phosphate, (D) TmP/GFR, (E) 25-OH-vitamin D, and (F) PTH in 125 patients with grade 3b–4 CKD. Depicted are unadjusted means and SD or median and interquartile range for iFGF23, cFGF23, and PTH.
Changes in FGF23 levels were positively correlated with changes in plasma phosphate (iFGF23: Spearman ρ=0.36, P<0.001; cFGF23: ρ=0.37, P<0.001) and TmP/GFR (iFGF23: ρ=0.29, P<0.001; cFGF23: ρ=0.29, P<0.001). In addition, changes in FGF23 were inversely correlated with changes in plasma chloride (iFGF23: ρ=−0.30, P<0.001; cFGF23: ρ=−0.22, P=0.01) and eGFR (iFGF23: ρ=−0.19, P=0.04; cFGF23: ρ=−0.37, P<0.001).
This study shows that a modest increase in potassium intake may lead to a small but significant reduction in iFGF23 levels in patients with advanced CKD, in line with our prior study in individuals with normal kidney function.1 Mechanistically, increased potassium intake inhibits sodium chloride cotransporter (NCC) expression in the distal tubule. Interestingly, osteoblasts, the main FGF23-producing cells, also express NCC,3 and NCC blockade reduced FGF23 transcription in murine osteoblasts and attenuated plasma FGF23 levels in patients with CKD.4 Moreover, reversing low intracellular chloride concentration may also reduce NCC phosphorylation.5 Thus, the KCl-associated reduction in iFGF23 might be mediated by increased osteocyte NCC expression or phosphorylation.
The decrease in FGF23 levels likely led to an increase in TmP/GFR and, in turn, to a temporarily decrease in urinary phosphate excretion and increased plasma phosphate levels, independent of PTH. Urinary phosphate excretion eventually returned to baseline levels because of unchanged phosphate intake. Although the positive, and not inverse, correlation between change in FGF23 and phosphate may seem counterintuitive, on a group level, KCl led to a decrease in iFGF23 and an increase in phosphate levels. Plasma vitamin D decreased, presumably as a consequence of the increase in plasma phosphate. The iFGF23:cFGF23 ratio decreased, suggesting either decreased FGF23 transcription or slightly increased cleavage of iFGF23. It is unlikely that inflammation mediated the FGF23-lowering effect of KCl as CRP and IL-6 levels did not change.
In this uncontrolled study, we could not account for dietary intake, plasma 1,25-dihydroxy vitamin D, or diurnal variation in phosphate homeostasis. Furthermore, we could not discriminate whether potassium, chloride, or both contributed to the reduction in plasma iFGF23. In the ongoing trial, participants are randomized to KCl, potassium citrate, or placebo for 2 years,2 allowing for more specific analyses.
In conclusion, the 2-week supplementation of 40 mmol/d KCl led to lower iFGF23, but not lower cFGF23 levels, while plasma phosphate levels increased. Further research should address whether long-term KCl provides a sustainable reduction in FGF23 levels, and whether this translates into improved clinical outcomes.
Acknowledgments
We thank all patients who participated in this study and nephrologists who helped with the inclusion of patients. We thank research nurses M. Cadogan, E. Kreukniet, M. Boer-Verschragen, B. Nome, and J. Sierra. We thank Biomedica, Vienna, Austria, for providing the iFGF23 kits.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Disclosures
S.J.L. Bakker reports employment with University Medical Center Groningen; research funding from Astellas Pharma and Chiesi; and advisory or leadership roles for Dutch Health Council and Scientific Board of the Dutch Kidney Foundation. M.H. de Borst reports employment with University Medical Center Groningen, The Netherlands; consultancy agreements with Astellas, AstraZeneca, Kyowa Kirin, Pharmacosmos, Sanofi Genzyme, and Vifor Pharma; research funding from Sanofi Genzyme and Vifor Pharma; and serving as an Associate Editor of Nephrology Dialysis Transplantation. M. Gritter reports employment with Erasmus MC Rotterdam. E.J. Hoorn reports employment with Erasmus Medical Center; research funding from Aurinia; honoraria from UpToDate; serving on the Editorial Boards of American Journal of Physiology—Renal Physiology, Journal of Nephrology, and Journal of the American Society of Nephrology; and serving as a Board Member of ERA Working Group on Inherited Kidney Diseases and a Board Member of Dutch Federation of Nephrology. J.I. Rotmans reports employment with LUMC; research funding from Dutch Kidney Foundation and Dutch Ministry of Science; advisory or leadership role for Advisory Board Neokidney; and serving as Chair of Thematic Working Group Vascular Tissue Engineering at TERMIS, president-elect of Vascular Access Society, and a member of the guideline committee Dutch Society of Nephrology. J.J. van Zanden reports employment with Certe. L. Vogt reports employment with Amsterdam University Medical Centers; consultancy agreements with AstraZeneca, Bayer BV Netherlands, Carlsbad, CA, ISIS Pharmaceuticals, Inc, and Vifor Pharma; research funding from Dutch Kidney Foundation and Health Holland; and serving as an Associate Editor of BMC Nephrology. R.D. Wouda reports employment with Amsterdam University Medical Centers, location AMC. S.M.H. Yeung reports employment with University Medical Center Groningen.
Funding
This study was supported by the Dutch Kidney Foundation (grants CP16.01 and 21OK+013).
Author Contributions
M.H. de Borst, E.J. Hoorn, and S.M.H. Yeung conceptualized the study; M.H. de Borst and S.M.H. Yeung were responsible for formal analysis; S.M.H. Yeung was responsible for investigation; M.H. de Borst and S.M.H. Yeung were responsible for methodology; M. Gritter was responsible for data curation and visualization; M.H. de Borst, E.J. Hoorn, and J.J. van Zanden were responsible for resources; M.H. de Borst provided supervision; S.M.H. Yeung wrote the original draft; and S.J.L. Bakker, M.H. de Borst, M. Gritter, E.J. Hoorn, J.I. Rotmans, J.J. van Zanden, L. Vogt, and R.D. Wouda reviewed and edited the manuscript.
References
- 1.Humalda JK Yeung SMH Geleijnse JM, et al. Effects of potassium or sodium supplementation on mineral homeostasis: a controlled dietary intervention study. J Clin Endocrinol Metab. 2020;105(9):e3246-e3256. doi: 10.1210/clinem/dgaa359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritter M Vogt L Yeung SMH, et al. Rationale and design of a randomized placebo-controlled clinical trial assessing the renoprotective effects of potassium supplementation in chronic kidney disease. Nephron. 2018;140(1):48-57. doi: 10.1159/000490261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dvorak MM De Joussineau C Carter DH, et al. Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone. J Am Soc Nephrol. 2007;18(9):2509-2516. doi: 10.1681/ASN.2007030348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasco RFV, Takayama L, Pereira RMR, Moyses RMA, Elias RM. Effects of diuretics furosemide and hydrochlorothiazide on CKD-MBD: a prospective randomized study. Bone Rep. 2021;14:100746. doi: 10.1016/j.bonr.2021.100746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terker AS Zhang C McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21(1):39-50. doi: 10.1016/j.cmet.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]