Keywords: transplantation, transplant outcomes, randomized controlled trials, infliximab
Abstract
Significance Statement
Peritransplant TNF blockade with infliximab should not be used in recipients of deceased-donor kidney transplants due to lack of efficacy and an increased incidence of BK virus infection, according to results of a randomized controlled clinical trial. Our results underscore the need for properly controlled and powered trials to avoid falsely accepting unproven therapeutics and reporting incorrect low adverse event rates derived from small, uncontrolled experiments.
Background
Ischemia-reperfusion (IR) of a kidney transplant (KTx) upregulates TNF α production that amplifies allograft inflammation and may negatively affect transplant outcomes.
Methods
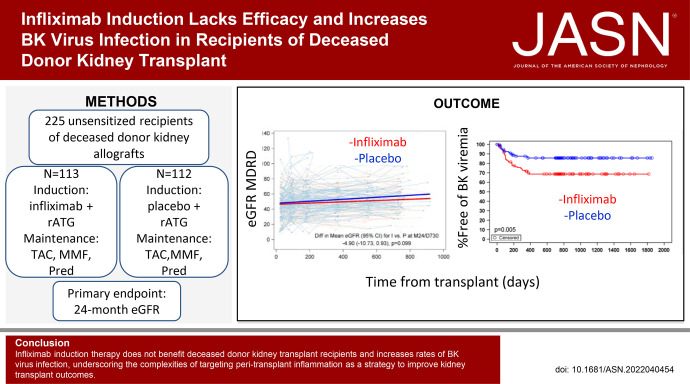
We tested the effects of blocking TNF peri-KTx via a randomized, double-blind, placebo-controlled, 15-center, phase 2 clinical trial. A total of 225 primary transplant recipients of deceased-donor kidneys (KTx; 38.2% Black/African American, 44% White) were randomized to receive intravenous infliximab (IFX) 3 mg/kg or saline placebo (PLBO) initiated before kidney reperfusion. All patients received rabbit anti-thymocyte globulin induction and maintenance immunosuppression (IS) with tacrolimus, mycophenolate mofetil, and prednisone. The primary end point was the difference between groups in mean 24-month eGFR.
Results
There was no difference in the primary end point of 24-month eGFR between IFX (52.45 ml/min per 1.73 m2; 95% CI, 48.38 to 56.52) versus PLBO (57.35 ml/min per 1.73 m2; 95% CI, 53.18 to 61.52; P=0.1). There were no significant differences between groups in rates of delayed graft function, biopsy-proven acute rejection (BPAR), development of de novo donor-specific antibodies, or graft loss/death. Immunosuppression did not differ, and day 7 post-KTx plasma analyses showed approximately ten-fold lower TNF (P<0.001) in IFX versus PLBO. BK viremia requiring IS change occurred more frequently in IFX (28.9%) versus PLBO (13.4%; P=0.004), with a strong trend toward higher rates of BKV nephropathy in IFX (13.3%) versus PLBO (4.9%; P=0.06).
Conclusions
IFX induction therapy does not benefit recipients of kidney transplants from deceased donors on this IS regimen. Because the intervention unexpectedly increased rates of BK virus infections, our findings underscore the complexities of targeting peritransplant inflammation as a strategy to improve KTx outcomes.
Clinical Trial registry name and registration number: clinicaltrials.gov (NCT02495077).
Despite impressive reduction in rates of acute kidney graft rejection, the widespread use of calcineurin inhibitors and accompanying immunosuppressive medication, including induction therapy with rabbit anti-thymocyte globulin (rATG), has had only a modest effect on prolonging transplant survival, particularly in recipients of deceased-donor organs. Five-year survival for deceased-donor kidneys is 74.4%—remarkably and significantly shorter than the 85.6% for living-donor kidneys over the same time period (https://www.UNOS.org). Moreover, despite trends showing continued improvements in long-term allograft survival, the half-life of deceased-donor allografts in the current era is only 11.7 years, which is also significantly shorter than the 19.2-year half-life for recipients of living-donor kidneys.1
Increasing evidence implicates inflammatory events related to ischemia-reperfusion (IR) injury occurring immediately after transplantation of deceased-donor organs as important factors driving the shortened short- and long-term survival in comparison to living-donor kidneys.2,3 Delayed graft function (DGF), the most significant consequence of post-transplant IR injury, results in ARF manifested by post-transplantation oliguria and requires dialytic support. DGF is associated with increased allograft immunogenicity, elevated risk of acute rejection episodes, and decreased long-term survival.2–4 On average, 30% of deceased-donor kidney transplant recipients develop DGF.2–4 Prolonged ischemic times are associated with an increased incidence of DGF,2,4,5 and longer cold ischemic storage times for kidney grafts are themselves associated with an increased incidence of acute rejection episodes and the development of graft fibrosis and arteriopathy.6–9 Development of DGF strongly correlates with increased acute rejection, the subsequent development of chronic allograft nephropathy or interstitial fibrosis and tubular atrophy, worse long-term kidney function, and poorer kidney graft survival.5,10 A meta-analysis found that patients who experienced DGF had a higher risk of acute rejection and worse long-term renal function.5 In addition, DGF is associated with prolonged hospitalization, higher transplantation costs, and adverse effects on the rehabilitation of transplant recipients.11,12 Thus, DGF is both an outcome after kidney transplantation and a predictor of long-term graft function.
Kidney IR-induced inflammation (reviewed in Schröppel et al.2 and Siedlecki et al.3) is complex, and a major initiator is the production of reactive oxygen species (ROS) that activate the vascular endothelium of the tissue to express adhesion molecules, produce complement components,13–15 and release a variety of proinflammatory cytokines2,3,16 and chemokines. The released proinflammatory molecules are hypothesized to provide an immunogenic, proinflammatory environment that results in DGF and potentially facilitates the induction and function of adaptive immune responses, ultimately resulting in graft failure.
Among the mediators implicated in the pathogenesis of DGF is TNF, a pleiotropic cytokine produced by multiple cell types, including monocytes, macrophages,17 T cells,18 and endothelial cells.19 TNF was originally identified by its ability to induce necrosis of implanted tumors in vivo (hence its name),20 but subsequent studies linking TNF’s proinflammatory function to multiple inflammatory diseases has resulted in the development of effective TNF-neutralization therapies currently in use for rheumatoid arthritis21,22 and inflammatory bowel disease.23 TNF ligates multiple receptors, including TNF receptor (TNFR) I and II. Expression levels of TNFR I/II vary on immune and nonimmune cells in the kidney, and the receptors mediate distinct, although in some contexts overlapping, functions.24
In transplantation, preclinical murine studies performed by multiple groups showed that blocking TNF binding to its receptors dampens production of other inflammatory molecules, attenuates tissue injury, and prolongs heterotopic heart transplant survival.25 Basic26 and translational clinical studies in human islet transplant recipients have revealed that peritransplant TNF blockade limits islet death and improves glycemic control.27,28 As a consequence, etanercept is routinely used for induction therapy by clinical centers performing islet transplants in humans.28 Together, these observations suggest that peritransplant TNF blockade could dampen the vicious cycle of IR-induced inflammation after kidney transplantation and consequently reduce DGF and, potentially, late allograft injury and loss.
To test this hypothesis, we performed a randomized, placebo-controlled, double-blind trial in which recipients of first, deceased-donor kidney transplants received peritransplant induction therapy with the anti-TNF monoclonal antibody infliximab (IFX) versus placebo (PLBO; normal saline), followed by rATG induction and tacrolimus (TAC)-based triple maintenance immunosuppression.
Methods
Study Development and Oversight
Initiated in 2016, this phase 2, multicenter, randomized, double-blind, placebo-controlled two-arm study of a target of 300 recipients of primary deceased-donor kidney transplants randomized enrolled patients 1:1 (150 per arm) to receive intraoperative IFX versus PLBO. The study was performed under an Investigational New Drug approved by the US Food and Drug Administration (FDA). All patients in both study arms were treated with rATG followed by TAC, a mycophenolic acid derivative (either mycophenolate mofetil [MMF] or enteric-coated mycophenolic acid [MPA]), and glucocorticoids.
We conducted this study as part of the National Institute of Allergy and Infectious Diseases (NIAID)-sponsored Clinical Trials in Organ Transplantation (CTOT) program, protocol CTOT-19, registered at clinicaltrials.gov (NCT02495077). We enrolled patients at 14 of the 15 North American transplant centers that participated in the trial. Each site participated under the auspices of its Institutional Review Board. An independent, NIAID-appointed Data Safety Monitoring Board (DSMB) was responsible for protocol review and approval and for periodic safety review.
Study Patients
Candidates for deceased-donor kidney transplants who were >18 years of age at enrollment. Age and self-identified race/ethnicity and sex data were collected for enrolled patients and donors to meet the requirements of the National Institutes of Health. Recipients were crossmatch negative or had a peak panel reactive antibody (PRA) of 0%, had a negative PPD or QuantiFERON test for latent tuberculosis (TB), and received kidney transplants from deceased brain-dead donors or donors after cardiac death with Kidney Donor Profile Indices (KDPI) ranging from ≥20 to <95. Exclusion criteria included recipients of living-donor transplants, presence of other transplanted organs, previous history of invasive fungal infections, active TB, any chest x-ray evidence of latent TB or fungal infection, current or former residents of regions in the United States endemic for coccidiomycosis, and candidates with any condition or characteristic that in the opinion of the local primary investigator made the participant unlikely to complete the study. Detailed inclusion and exclusion criteria are available on pages 39–41 of the CTOT-19 protocol in the Supplementary Material. Clinical data were collected from each site using standardized electronic case report forms.
Patient Randomization
Patients were randomized 1:1 using a fixed block size, and randomization was stratified by site. The randomization schedule was computer generated by the data coordinating center, and randomizations were performed using a web-based randomization system and dispensed by the unblinded research pharmacist at each clinical site. Study patients and other site clinical personnel, including the treating physician, remained blinded to treatment assignments throughout a patient’s participation.
Study Protocol–Defined Immunosuppression
All participating centers agreed to harmonize immunosuppression protocols before initiation of this study and adopted a standard of care. Randomized patients received a single dose of IFX (Remicade) 3 mg/kg (the FDA-approved dose for treatment of rheumatoid arthritis), or an equivalent volume of PLBO, as an intravenous infusion initiated in the operating room before reperfusion and administered over at least 4 hours. One of 113 patients randomized to IFX and two of 112 randomized to PLBO received <100% of the target dose. All patients received rATG initiated on the day of transplant, dosed daily with the intention of achieving a total dose of 4.5–6 mg/kg as tolerated. All patients received TAC, MMF/MPA, and prednisone. MMF/MPA was initiated no later than 48 hours after transplant with a target MMF dose equivalent of 2000 mg/day as tolerated. TAC was initiated no later than 48 hours after transplant with an initial total target dose of 0.1 mg/kg per day divided into twice-daily dosing or an equivalent dose of a once-daily formulation. Dosing was adjusted to target trough levels of 8–12 ng/ml at months 1–3 and then 5–8 ng/ml until study closure. Prednisone was administered perioperatively per center standard of practice and then tapered gradually to no less than 5 mg/day or 10 mg every other day by 3 months after transplant.
Cytomegalovirus Prophylaxis
Cytomegalovirus (CMV) prophylaxis was standardized across institutions as mandated by the study protocol. Seronegative recipients of seronegative donor organs did not receive CMV prophylaxis; CMV– recipients of CMV+ donor organs and CMV+ recipients (regardless of donor serology) received oral valganciclovir daily, with the dose adjusted per renal function for 6 months and 3 months. Additional details available in pages 59–60 of the study protocol.
BK Virus Monitoring
We collected PCR testing at 1, 3, 6, and 12 months after transplant. Outside of those four time points, all positive results were recorded until a negative result was obtained. Three negatives were then recorded after the initial negative result to document resolution. Management of BK viremia was determined by study site standard of care.
End Points
The primary end point approved by the FDA for this phase 2 trial was the difference between the mean 24-month eGFR (on the basis of the modified modification of diet in renal disease [MDRD] equation29,30) in the IFX (derived from 507 creatinine values from 111 patients) versus PLBO (derived from 479 creatinine values from 109 patients) groups (see Statistical Analyses section). All of these creatinine measurements were performed using dedicated samples analyzed at a core laboratory (Cleveland Clinic, EP, core laboratory director). We additionally performed post hoc analyses in which we utilized available locally collected creatinine values when a core laboratory value was not available, noting a correlation coefficient of 0.97 when both central and local creatinine values were present. Secondary efficacy end points included (see protocol pages 36–37) eGFR comparisons using alternate formulas (including race-independent measures31,32) and ΔeGFR at 24 months among other post-transplant time points, the proportion of patients in each group with death and/or graft failure within 2 years, DGF (need for dialysis within the initial 7 days after transplant), biopsy-proven acute rejection, and biopsy-proven antibody mediated rejection (see protocol pages 22–27 for definitions).
Prespecified safety end points included the proportion of patients in each group with mycobacterial or fungal infections, any infection requiring hospitalization or resulting in death, CMV or BK viremia requiring a change in immunosuppression, malignancy, and impaired wound healing at the transplant incision site. We also reported incidence of BK virus nephropathy (diagnosed centrally or at local labs) in each study arm.
Sample Size and Power Calculations
Sample size was initially determined using a two-sided, two-sample t test at an α level of 0.05, assuming a SD of 25 ml/min and a 15% dropout rate, to provide 90% power to detect a difference in eGFR at 24 months of at least 10 ml/min per 1.73 m2. We used this approach because of a lack of accessible software at the time of protocol submission for calculating power using the prespecified generalized linear mixed model, noting that this approach conservatively overestimated the sample size required.
In 2019, after 3 years of enrollment, we recognized that we would not reach our target enrollment and complete 2 full years of follow-up for all patients. We reassessed our original power calculation with an updated estimate of the SD on the basis of available eGFRs (blinded to treatment group) and decided with DSMB approval that we had >90% power to detect an 8 ml/min per 1.73 m2 difference in eGFR with a total of 220–230 randomized patients. The decision was made to halt enrollment with 225 patients transplanted and randomized.
At the completion of the study (post hoc), we recreated our sample size calculations using a mixed model approach with newly available GLIMMPSE software (https://glimmpse.samplesizeshop.org/). This software does not allow for dropouts or varying number of observations at the visits. Therefore, we calculated the power on the basis of having 75% of the final enrollment number of 225 participants and the same number of eGFR measurements. This analysis showed that we would have 97% power to detect an 8 ml/min per 1.73 m2 difference between the two treatment groups and 99% power to detect a 10 ml/min per 1.73 m2 difference.
Study Monitoring, Surveillance Biopsies, and Follow-Up
Protocol-directed kidney allograft biopsies were performed pre-implantation and at three and 24 months after transplant. These biopsies, submitted to the core laboratory, were read by a single kidney transplant pathologist (I.W.G.), and Banff 2013 scores33 were reported. Readings were performed in batches (not in “real time”), and readings were not provided to the clinical sites. SV40 immunostaining for identification of polyomavirus nephropathy was performed if pathologic findings were suggestive and/or BK viremia was present and was routinely performed on all 24-month post-transplant protocol biopsies.
Sixty-one percent of protocol-directed biopsies were also read by local pathologists, and these local reads were used to guide clinical care. Biopsies were also performed at the discretion of the local investigator for clinically indicated reasons. All of these “for-cause” biopsies were read by the local pathologists, and these readings guided clinical care. Due to logistical limitations, in only a subset of these cases (46%) were adequate unstained slides or paraffin-embedded cores sent to the core laboratory for a central reading.
Adverse Events
Clinical safety was monitored through routine physical examinations and laboratory assessments. At each study visit, an assessment was made for adverse events, serious adverse events, infections, rejections, death, post-transplant lymphoproliferative disorder, and hospitalizations. Study personnel reported all events using a designated electronic case report form, with interpretation and intervention as determined by the clinical site primary investigator.
Effect of the Coronavirus Disease 2019 Pandemic
The coronavirus disease 2019 pandemic halted clinical research at all centers participating in the trial, beginning in March 2020. Sites reopened research activities from early 2021, mostly with virtual visits, and did not fully reopen with in-person visits until the fall of 2021. These dates coincided with the 24-month visit in 66 enrolled patients, limiting collection of 24-month samples, including eGFR and protocol-directed biopsies, despite widening the visit windows through a protocol amendment. Of the 225 randomized and transplanted patients, 184 reached the 24-month time point: 110 (58 IFX, 52 PLBO) who had a core laboratory eGFR available from the 24-month visit; 31 who had their final visit conducted via telephone due to the pandemic; 18 (12 after March 1, 2020) for whom the final visit did not take place; two who experienced graft loss before the 24-month visit; two for whom the 24-month core laboratory creatinine sample was lost; and 21 (10 IFX, 11 PLBO) for whom a 24-month core laboratory eGFR was not collected at the time of their final visit.
Laboratory Studies
Monitoring: Assessment of Humoral Immunity
Donor-specific antibody (DSA) screening was performed using FlowPRA beads representing HLA-A, B, C, DR, DQ, and DP antigens (One Lambda, Canoga Park, CA). If positive, determination of HLA antibody specificities was performed using Flow-PRA single antigen Class I and II beads (One Lambda). HLA antibody specificities were confirmed using LABScreen single antigen beads. A DSA was deemed present if the mean fluorescence intensity (MFI) was ≥1400, with the caveat that when the antigen/allele in question belonged to a cross-reactive group (commonly referred to as CREG) or shared epitope(s), an MFI of >300 was considered positive.34
Plasma Cytokines
Plasma samples collected on days 7 and 30 after transplant obtained from the enrolled patients were assayed for inflammatory markers/cytokines IL-1β, IL-6, IL-8, and TNF using a MESO QuickPlex SQ120 using the V-PLEX Human Proinflammatory Panel II kit (Meso Scale Diagnostics, Rockville, MD) as per the manufacturer’s instructions.
Statistical Analyses
Statistical analyses were performed using the intent-to-treat (ITT) population (i.e., all transplanted and randomized patients who received at least a portion of the IFX/PLBO infusion). Continuous variables are summarized with means and SD, and categorical variables with counts and percentages.
For analysis of the primary end point (eGFR at 24 months), a repeated-measures mixed model with random effects for the intercept and collection day of the eGFR serum sample was used, enabling all collected eGFR data (not simply those collected at 24 months) to contribute to the analysis.35
Treatment group comparisons for secondary clinical and safety categorical end points were performed using chi-squared, Fisher’s exact, or Cochran–Mantel–Haenszel tests, and continuous end points were compared using t tests, Wilcoxon tests, or repeated-measures mixed-model analysis. Time-to-event analyses were performed using Kaplan–Meier plots and log-rank tests. All statistical analyses were two-tailed tests, using α=0.05 as the level of statistical significance and performed using SAS v9.4 (SAS Institute, Inc., Cary, NC) and GraphPad Prism v5.04 (GraphPad Software, San Diego, CA). The analytic plan was approved by the FDA as part of our Investigational New Drug application.
Results
Study Cohort Characteristics
We enrolled 242 patients over 3 years (Figure 1), and we randomized 225 transplanted patients to receive a single dose of intraoperative IFX (n=113) or PLBO (n=112). We chose IFX over other anti-TNF agents because of its relatively short half-life (7.7–9.2 days) and its availability as an intravenous preparation for administration during the transplant procedure, with the goal of inhibiting peritransplant inflammation but without prolonged additional immunosuppression over standard of care. As detailed in the Methods section, we ceased enrollment before reaching our study goal of 300 with DSMB approval due to decreasing enrollment rates.
Figure 1.
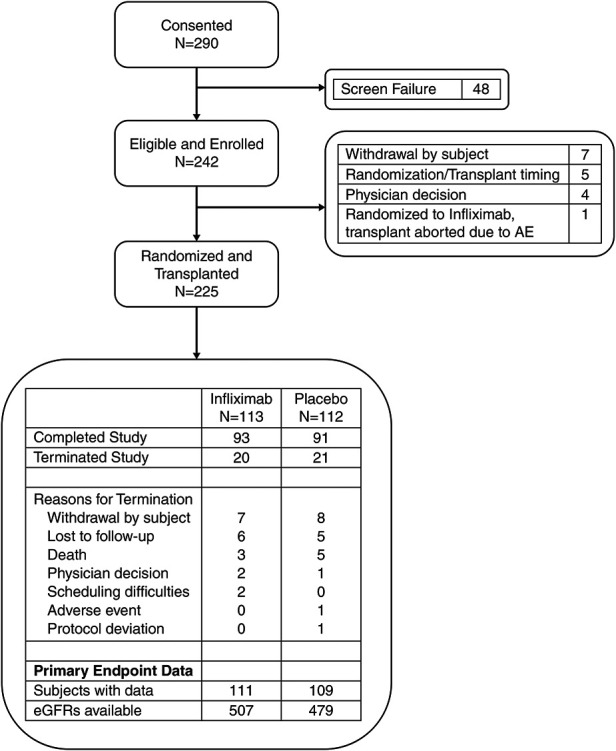
Two hundred twenty-five subjects were evaluable for the primary end point. Flow of all patients who provided consent through the study. Reasons for termination before and after transplant are provided. For randomized and transplanted patients, disposition is presented, along with a summary of data available for primary end point analysis. AE, adverse event.
Baseline characteristics of the enrolled patients (Supplemental Table 1, Table 1) showed that 60% of the recipients were men with a median age of 55 years. The cohort was 38% Black or African American, 13% Hispanic/Latino, and 44% White (mean percentages in United States from 2016 to 2019 were 26.7% Black or African American, 18.7% Hispanic/Latino, and 46% White; https://www.unos.org). Approximately 92% of the cohort had been on dialysis for a mean of 70.7 months before transplantation. The average number of HLA mismatches between donor and recipient for the cohort was 4.3, and this did not differ between groups (Table 1). Fifty-six IFX and 51 PLBO patients had 0% PRA. Of the patients with >0% PRA, median pretransplant PRA was 71% in IFX and 75.5% in PLBO (range 1%–100% for each group). Six enrolled patients (three in each arm) had low-level DSA detected retrospectively by the core HLA laboratory (Supplemental Table 2). Median donor age was 42 years, median donor graft KDPI was 52 (range 20–93), mean cold ischemic time was 1004 minutes (SD=16.7 hours), and 35% of the allografts underwent pump perfusion. Of 113 patients in the IFX arm, 93 completed the study, of 112 patients in the PLBO arm, 91 completed the study (Figure 1).
Table 1.
Baseline characteristics of the ITT population
| Characteristic | Infliximab | Placebo | Total |
|---|---|---|---|
| (n=113) | (n=112) | (N=225) | |
| Age, yr at transplant | |||
| Mean±SD | 53.2±10.7 | 53.6±10.69 | 53.4±10.67 |
| Median | 54 | 55 | 55 |
| Min, max | 29, 72 | 27, 73 | 27, 73 |
| Women, n (%) | 42 (37.2) | 48 (42.9) | 90 (40) |
| Race, n (%) | |||
| American Indian or Alaskan Native | 1 (0.9) | 1 (0.9) | 2 (0.9) |
| Asian | 7 (6.2) | 6 (5.4) | 13 (5.8) |
| Black or African American | 40 (35.4) | 46 (41.1) | 86 (38.2) |
| Native Hawaiian or Other Pacific Islander | 2 (1.8) | 2 (1.8) | 4 (1.8) |
| White | 51 (45.1) | 48 (42.9) | 99 (44) |
| Unknown or not reported | 12 (10.6) | 9 (8) | 21 (9.3) |
| EBV IgG+, n (%) | 113 (100) | 112 (100) | 225 (100) |
| Donor and Recipient CMV IgG, n (%) | |||
| D+, R+ | 39 (34.5) | 46 (41.1) | 85 (37.8) |
| D+, R– | 25 (22.1) | 19 (17) | 44 (19.6) |
| D–, R+ | 32 (28.3) | 28 (25) | 60 (26.7) |
| D–, R– | 16 (14.2) | 17 (15.2) | 33 (14.7) |
| D indeterminate/not done, R+ | 1 (0.9) | 2 (1.8) | 3 (1.3) |
| Pump perfusion used, n (%) | |||
| Yes | 41 (36.3) | 38 (33.9) | 79 (35.1) |
| No | 59 (52.2) | 58 (51.8) | 117 (52) |
| Unknown | 13 (11.5) | 16 (14.3) | 29 (12.9) |
| HLA (A, B, DR) mismatch | |||
| n | 91 | 88 | 179 |
| Mean±SD | 4.5±1.35 | 4.1±1.53 | 4.3±1.45 |
| Median | 5 | 4 | 5 |
| Min, max | 0, 6 | 0, 6 | 0, 6 |
| Donor age (yr) | |||
| Mean±SD | 41.6±12.87 | 39.0±15.92 | 40.3±14.49 |
| Median | 43 | 41.5 | 42 |
| Min, max | <1, 69 | 4.0, 69 | <1, 69 |
| Donor sex, women, n (%) | 58 (51.3) | 43 (38.4) | 101 (44.9) |
| Donor race, n (%) | |||
| American Indian or Alaskan Native | 0 | 2 (1.8) | 2 (0.9) |
| Asian | 2 (1.8) | 9 (8) | 11 (4.9) |
| Black or African American | 13 (11.5) | 22 (19.6) | 35 (15.6) |
| Native Hawaiian or Other Pacific Islander | 0 | 1 (0.9) | 1 (0.4) |
| White | 79 (69.9) | 65 (58) | 144 (64) |
| Unknown or Not Reported | 19 (16.8) | 13 (11.6) | 32 (14.2) |
| Donation after cardiac death, n (%) | 28 (24.8) | 25 (22.3) | 53 (23.6) |
| Donor KDPI | |||
| Mean±SD | 52.85±20.2 | 54.29±19.71 | 53.56±19.93 |
| Median | 53 | 49.5 | 52 |
| Min, max | 20, 93 | 21, 93 | 20, 93 |
Min, minimum; max, maximum; EBV, Epstein–Barr virus.
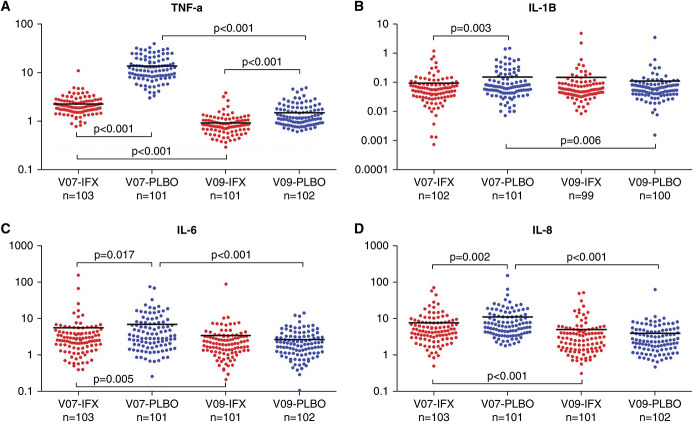
IFX Reduced Post-Transplant Plasma TNF among Other Inflammation Markers
To assess the effectiveness of a single dose of IFX (median half-life 7.7–9.2 days) on inhibiting post-transplant inflammation, we quantified plasma levels of TNF, IL-1β, IL-6, and IL-8 (Figure 2) on days 7 and 30 after transplant. These assays showed significantly lower levels of plasma TNF in the IFX group compared with the PLBO group on day 7 (P<0.001), with persistently lower levels on day 30 in the IFX arm (P<0.001), together demonstrating that the single intraoperative dose markedly reduced plasma TNF levels. Interestingly, we also observed significantly lower day 7 post-transplant serum IL-1β, IL-6, and IL-8 in the IFX group, suggesting that intraoperative TNF blockade broadly reduced post-transplant inflammatory markers. Plasma levels of these other cytokine markers on day 30 did not differ between groups.
Figure 2.
IFX reduces serum levels of TNF among other proinflammatory cytokines. Plasma levels (pg/ml) of (A) TNF, (B) IL-1β, (C) IL-6, and (D) IL-8 are presented at study visits 7 (day 7 after transplant or discharge visit, whichever occurred first) and visit 9 (day 30/month 1 after transplant) for the IFX (red dots) and PLBO (blue dots) groups. Horizontal black lines within each group represent the mean cytokine level at the indicated visit. P values for between-group comparisons result from a two-sample t test; P values for within-group comparisons between visits 7 and 9 result from a paired t test.
Primary eGFR End Point Analysis
Assessment of the protocol-defined (prespecified) primary end point for the ITT cohort using core laboratory analyzed serum creatinine values (Figure 3) estimated the mean eGFR at 24 months (calculated using the MDRD equation) to be 52.45 (95% confidence interval [CI], 48.38 to 56.52) in the IFX arm (507 measurements from 111 patients) versus 57.35 (95% CI, 53.18 to 61.52) in the PLBO arm (479 measurements from 109 patients), with a difference in eGFR for IFX versus PLBO at 24 months of –4.9 (95% CI, –10.73 to 0.93; P=0.1). Recalculating the 24-month eGFR using the CKD-EPI equation or the race-independent CKD-EPI 2021 equation also showed no significant differences between arms: –4.79 (95% CI, –11.09 to 1.5; P=0.14) and –4.15 (95% CI, –10.62 to 2.32; P=0.21), respectively (Supplemental Table 3).
Figure 3.
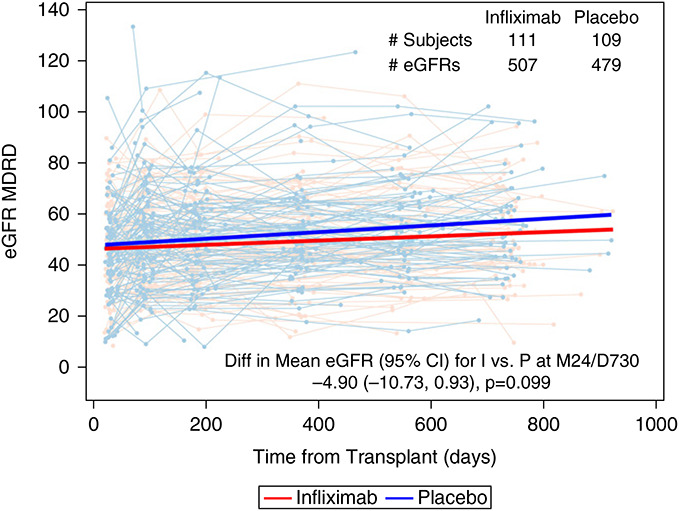
The 24-month eGFR did not differ between study arms. eGFR values are plotted over time for each patients in the IFX (red) and PLBO (blue) groups. A longitudinal repeated-measures mixed model with random effects for the intercept and day of eGFR sample collection estimated the month 24 treatment group difference to be –4.9 ml/min (95% CI, –10.73 to 0.93; P=0.1). The bold red and blue lines represent the treatment group-specific predicted lines resulting from the mixed model. I, infliximab; P, placebo; M24/D730, month 24/day 730.
In additional post hoc analyses, we supplemented the primary model with available locally obtained serum creatinine values when a central serum creatinine was unavailable. This resulted in a mean eGFR at 24 months (MDRD) of 52.22 (95% CI, 48.26 to 56.17) in the IFX arm (635 measurements from 112 patients) versus 55.7 (95% CI, 51.68 to 59.73) in the PLBO arm (592 measurements from 110 patients), with a difference in eGFR for IFX versus PLBO at 24 months of –3.49 (95% CI, –9.13 to 2.16; P=0.23; Supplemental Table 3), validating the conclusion of no difference between the study arms.
Comparison of the same end point (using only core laboratory creatinine values) within subgroups of African American patients, men, women, and patients >60 years of age showed no significant differences between study arms (Supplemental Table 3). Additional protocol-specified secondary analyses included differences in eGFR between study arms at 24 months and from 3 to 24 months, 6 to 24 months, and from the time of post-transplant creatinine nadir to 24 months, each calculated by MDRD. These analyses showed no significant differences (Supplemental Table 3) but were notably underpowered.
Importantly, average administered rATG doses (IFX 428.6±123.43 mg versus PLBO 416.2±128.86 mg; P=0.46), serum TAC levels and average SD (a measure of adherence36; Supplemental Figure 1, A and B), and MPA and prednisone doses (Supplemental Table 4) did not differ between study arms. MMF levels were significantly lower in the IFX group during the first year (Supplemental Table 4). None of the patients in either arm underwent steroid withdrawal. During the course of the study, four IFX and two PLBO patients were switched from TAC to either sirolimus/everolimus (one IFX and two PLBO) or belatacept (three IFX).
Other Secondary Efficacy End Point Analyses
DGF (Table 2) occurred in 31% of patients in the IFX arm and 36% in the PLBO arm (P=0.45). The incidence of primary nonfunction, the severity of DGF as defined by duration of DGF (in days), or number of dialysis treatments within the first 8 weeks did not differ between groups (Table 2). Moreover, we did not observe differences between groups in various prespecified definitions of slow graft function (Table 2). As the cohort included recipients of donation after circulatory death (DCD) kidneys, and evidence indicates that IR injury preferentially promotes inflammation after transplantation of brain-dead versus DCD donors,37,38 in a post hoc analysis, we reanalyzed the data, excluding recipients of DCD donors. This analysis revealed a numerically lower rate of DGF in the IFX (23.5%) versus PLBO (34.5%) groups, but the difference did not meet statistical significance (P=0.11). Reanalysis of the primary eGFR end point, excluding recipients of DCD kidneys, showed a nonsignificant difference in eGFR between groups (–5.01; 95% CI, –11.79 to 1.76; P=0.15).
Table 2.
Summary of secondary efficacy end points
| Efficacy End Point | Infliximab | Placebo | P Valuea | Total |
|---|---|---|---|---|
| (n=113) | (n=112) | (N=225) | ||
| DGF, n (%) | 35 (31) | 40 (35.7) | 0.45 | 75 (33.3) |
| Duration of DGF (days), n | 33 | 39 | 72 | |
| Mean±SD | 13.27±13.89 | 15.74±38.37 | 0.71 | 14.61±29.6 |
| Median | 10 | 6 | 7 | |
| Min, max | 1, 57 | 1, 232 | 1, 232 | |
| n=109 | n=107 | n=216 | ||
| Primary nonfunction, n (%) | 3 (2.8) | 1 (0.9) | 0.62 | 4 (1.9) |
| Number of dialysis sessions in first 8 wk after transplant | 111 | 109 | 220 | |
| Mean±SD | 0.14±1 | 0.26±2.32 | 0.61 | 0.2±1.77 |
| Median | 0 | 0 | 0 | |
| Min, max | 0, 10 | 0, 24 | 0, 24 | |
| Slow graft function end points, n | 78 | 72 | 150 | |
| Number of patients assessedb | ||||
| Serum creatinine >3 mg/dl at day 5, n (%) | 37 (47.4) | 30 (42.9) | 0.58 | 67 (45.3) |
| Serum CRR on day 2, n | 77 | 70 | 147 | |
| Mean±SD | 24.28±22.6 | 20.97±16.64 | 0.31 | 22.7±19.99 |
| Median | 26.81 | 22.37 | 23.89 | |
| Min, max | −24.2, 66.96 | −18.87, 54.14 | −24.2, 66.96 | |
| n=77 | n=70 | n=147 | ||
| Serum CRR <30% on day 2, n (%) | 44 (57.1) | 48 (68.6) | 0.15 | 92 (62.6) |
| Serum CRR on day 5, n | 78 | 69 | 147 | |
| Mean±SD | 47.06±28.86 | 43.37±25.79 | 0.42 | 45.33±27.43 |
| Median | 52.41 | 49.07 | 51.17 | |
| Min, max | −43.44, 88.03 | −20.23, 83.5 | −43.44, 88.03 | |
| n=78 | n=69 | n=147 | ||
| Serum CRR <70% on day 5, n (%) | 58 (74.4) | 61 (88.4) | 0.03 | 119 (81) |
CRR, creatinine reduction ratio.
P value results from a chi-squared or Fisher’s exact test for categorical variables or t test for continuous variables.
Slow graft function end points were only assessed in patients who did not experience DGF.
There were 380 post-transplant allograft biopsies with either a central or local read obtained in the cohort over the course of the study. Of the 380 biopsies, 146 biopsies were read by the local pathologist and were not read by the core pathology laboratory. Of the biopsies read by the core laboratory (including the surveillance biopsies; Table 3), 10 (12.8%) patients in the IFX arm experienced borderline or higher acute T cell–mediated rejection (ACR; four Banff 1A or higher) rejections versus five (7%) patients (three Banff 1A or higher) in the PLBO arm (P=0.24), and a total of four episodes of suspicious or higher AMR (three in the IFX arm versus one in the PLBO arm; P=0.62) over the 24-month follow-up.
Table 3.
Summary of central and local biopsy collection and rejection events
| Rejection/Biopsy Characteristic | Central Read Only | Local Read Only | Central or Local Read |
|---|---|---|---|
| B=234 | B=309 | B=380 | |
| I=78 | I=83 | I=98 | |
| P=71 | P=73 | P=88 | |
| ACR 1A or higher, n (%) | |||
| Biopsiesa | 7 (3%) | 16 (5.2%) | 20 (5.3%) |
| Infliximab patientsb | 4 (5.1%) | 10 (12.05%) | 11 (11.2%) |
| Placebo patientsc | 3 (4.2%) | 5 (6.85%) | 7 (7.95%) |
| P valued | >0.1 | 0.27 | 0.45 |
| Borderline or higher ACR, n (%) | |||
| Biopsiesa | 19 (8.1%) | 64 (20.7%) | 77 (20.3%) |
| Infliximab patientsb | 10 (12.8%) | 24 (28.9%) | 29 (29.6%) |
| Placebo patientsc | 5 (7%) | 23 (31.5%) | 26 (29.5%) |
| P valued | 0.24 | 0.73 | 0.1 |
| Locally treated rejection | B=234, I=78, P=71 | ||
| Number of biopsiesa | n/a | 62 (15.9%) | n/a |
| Infliximab patientsb | 16 (16.2%) | ||
| Placebo patientsc | 24 (27%) | ||
| 0.07 | |||
B, biopsy; I, infliximab; P, placebo; n/a, not applicable.
Percentage of biopsies is calculated on the basis of the column total of B biopsies.
Percentage of Infliximab patients is calculated on the basis of the column total of I patients.
Percentage of Placebo patients is calculated on the basis of the column total of P patients.
P value results from a chi-square or Fisher’s exact test.
Using the local read only, we observed 24 (28.9%) IFX patients with borderline or higher ACR compared with 23 (31.5%) PLBO patients (P=0.73; Table 3). When considering rejection from either the central pathologist or local pathologist, 29.6% of IFX patients and 29.5% of PLBO patients experienced borderline or higher ACR (P=0.1; Table 3).
De novo DSA within 24 months after transplant occurred in nine (8%) patients (two class I, five class II, and two class I/II) in the IFX arm and in four (3.6%) patients in the PLBO arm (two class I and two class II; P=0.163; MFI listed in Supplemental Table 2). Twenty-four-month allograft biopsies were collected and analyzed for evidence of fibrosis (ci+ct scores) from 48 enrollees (26 IFX and 22 PLBO). In this small subset, we observed 73.1% of IFX patients versus 36.4% of PLBO patients with a ci+ct score of at least 2 at 24 months (P=0.01). No difference in change in ci+ct score from implantation to 24 months was observed. In a post hoc analysis, we compared median protein/creatinine ratios between study arms at 1, 3, 6, and 12 months after transplant (IFX: 0.14 [interquartile range (IQR) 0.09–0.25]; PLBO: 0.13 [IQR 0.07–0.27]), and at 24 months after transplant (IFX: 0.17 [IQR 0.1–0.42]; PLBO: 0.13 [IQR 0.07–0.3]); no significant differences were noted. Death or graft failure occurred in six (5.3%) IFX-treated versus eight (7.1%) PLBO-treated patients (P=0.57).
Safety End Points
All safety analyses were performed using all patients who received any IFX (n=114) or PLBO (n=112) infusion. The proportions of patients experiencing serious adverse events and nonserious adverse events did not differ between groups (Table 4). Infection requiring hospitalization or resulting in death occurred in 49 (43%) patients in the IFX arm versus 44 (39%) in the PLBO arm (P=0.57; Table 4). The incidences of mycobacterial or fungal infection, CMV viremia including cases requiring a change in immunosuppression, malignancy, and impaired wound healing did not differ between groups (Table 4).
Table 4.
Summary of safety end points for the safety population
| Safety End Point | Infliximab | Placebo | P Valueb | Total |
|---|---|---|---|---|
| (n=114a) | (n=112) | (N=226) | ||
| n (%) | n (%) | n (%) | ||
| SAE [# SAE] | 87 (76.3) [240] | 82 (73.2) [251] | 0.59 | 169 (74.8) [491] |
| Non-SAE [# non-SAE] | 44 (38.6) [77] | 41 (36.6) [81] | 0.76 | 85 (37.6) [158] |
| Any infection requiring hospitalization or resulting in death | 49 (43) | 44 (39.3) | 0.57 | 93 (41.2) |
| Any mycobacterial or fungal infection | 7 (6.1) | 7 (6.3) | 0.97 | 14 (6.2) |
| CMV viremia | 31 (27.2) | 22 (19.6) | 0.18 | 53 (23.5) |
| CMV viremia requiring change in immunosuppression or antiviral medication | 21 (18.4) | 13 (11.6) | 0.15 | 34 (15) |
| BK viremia | 52 (45.6) | 32 (28.6) | 0.008 | 84 (37.2) |
| BK viremia requiring change in immunosuppression or antiviral medication | 33 (28.9) | 15 (13.4) | 0.004 | 48 (21.2) |
| Any malignancy | 2 (1.8) | 1 (0.9) | >0.1 | 3 (1.3) |
| Type of malignancy | ||||
| Leukemia | 0 | 1 (100) | 1 (33.3) | |
| Ovarian neoplasm | 1 (50) | 0 | 1 (33.3) | |
| Prostate cancer | 1 (50) | 0 | 1 (33.3) | |
| Impaired wound healing at site of transplant incision | 9 (7.9) | 13 (11.6) | 0.35 | 22 (9.7) |
SAE, serious adverse event.
Includes one patient who received a partial infusion of infliximab in the operating room before the transplant being aborted; this patient is not included in the ITT population.
P value results from chi-squared or Fisher’s exact test.
We notably observed that the IFX-treated patients had a significantly higher rate of BK viremia overall and a significantly higher rate of BK viremia requiring a change in immunosuppression versus the PLBO group (29% versus 13%; P=0.004). Time to development of BK viremia was also shorter in the IFX arm (P=0.005; Figure 4). The frequency of BK virus nephropathy diagnosed either centrally or locally showed a strong trend toward higher rates in the IFX arm (13.3%) versus PLBO (4.9%; P=0.06; Supplemental Table 5). To assess whether IFX induction therapy improved eGFR in patients who did not develop BK disease, we reanalyzed the eGFR primary end point data excluding patients who developed BK viremia or BK nephropathy (Supplemental Table 5). These analyses showed no significant improvements in eGFR between groups.
Figure 4.
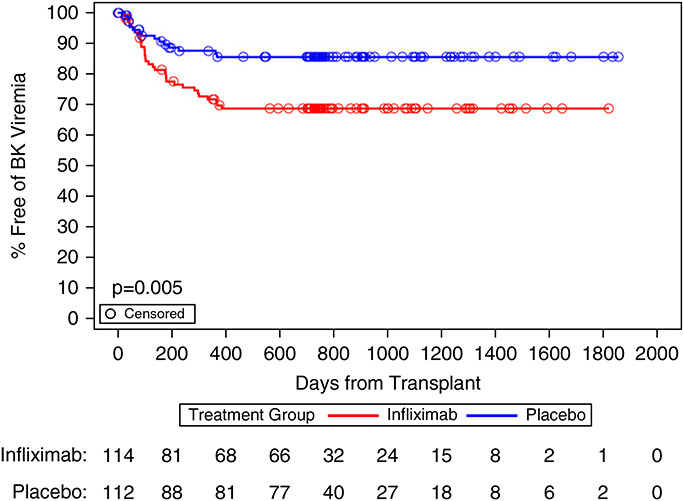
BK viremia occurred more frequently in the IFX-treated subjects. Number of patients at risk is presented at selected days after transplant and includes all patients who received IFX (n=114, red) or PLBO (n=112, blue) infusion. Patients are censored at day of last follow-up. P value results from log-rank test comparing the treatment groups. BK viremia defined as BK virus infection requiring a change in immunosuppression or antiviral therapy.
Discussion
In this phase 2 study, IFX induction had no effect on eGFR, on any early end points hypothesized to be inflammation dependent, including DGF, or on the incidence of ACR, ABMR, or DSA, using protocol-defined, prespecified analyses or using post hoc analyses including various at-risk subsets. The study design, including the primary end point and analytic plan, was approved by the FDA. The study was carefully executed, double blind, multicenter, and placebo controlled, and was sufficiently powered to detect clinically relevant differences in eGFR between groups if they were present. Although the coronavirus disease 2019 pandemic negatively affected core laboratory sample collection for analysis of the primary eGFR end point, our statistical approach adequately accounted for the missing data. Post hoc supplementation of the analysis with locally measured serum creatinine values validated this negative result, strengthening the conclusion that there was no difference between study arms. The amount of rATG and prednisone administered to each patients, measured TAC levels, and SD of serum TAC levels did not differ between study arms. MMF levels were lower in the IFX arm during the first year, likely a reflection of the increased BK virus rates observed in this treatment. In support of this contention, reanalysis of the MMF levels excluding all patients with BK viremia or nephropathy showed MMF levels did not differ between groups at all time points (data not shown). Enrollee demographics were reasonably representative of deceased-donor recipients in North America. Graft characteristics including cold ischemia time were similar between groups, and the prevalence of DGF in the trial was within the expected range for a North American cohort of deceased-donor kidney transplant recipients. Analyses of day 7 post-transplant plasma inflammatory markers showed that IFX induction therapy markedly reduced serum TNF and significantly reduced all three of the other measured cytokine markers. Together, the results are consistent with a truly negative efficacy outcome. We acknowledge that this observed lack of efficacy formally applies only to the IS regimen tested in this trial (rATG induction followed by TAC, MMF/MPA, and prednisone).
Recent studies by others indicate that a composite of eGFR, DSA, proteinuria, and allograft histology (iBOX) can function as a strong predictor of late allograft failure,39,40 noting that eGFR, DSA, and urinary protein/creatinine ratio account for the vast majority of iBOX’s prognostic utility.40 We did not formally use iBOX as an end point in our study. Nonetheless, in our cohort, the documented absence of difference between study arms for eGFR, incidence of DSA, protein/creatinine ratio, and, for a subset of patients, graft interstitial fibrosis and tubular atrophy lower the likelihood that iBOX results differ between groups, again underscoring the lack of efficacy of IFX in our study.
This study is the largest and most comprehensive trial reported involving TNF blockade in human kidney transplant recipients. Beyond pilot analyses conveyed only in abstract form, the Berlin group performed a small trial to test the safety and efficacy of an immunosuppressive regimen that included alemtuzumab induction and day 2 post-transplant IFX to minimize immunosuppression.41 The results suggested that the combined therapy permitted long-term graft survival in five patients treated with maintenance TAC monotherapy. Although intriguing, the small size and study design were insufficient to determine efficacy of the IFX therapy within the entire study regimen. In a randomized trial testing the efficacy of ex vivo administration of etanercept during hypothermic machine perfusion of 100 kidneys, Diuwe and colleagues showed no effect on short- or long-term kidney function or survival.42
The absence of efficacy demonstrated for peritransplant TNF blockade in our study of kidney transplant recipients does not fully exclude a role for peritransplant inflammation as a driver of DGF. Our post hoc analysis in fact showed a trend for lower rates of DGF in our cohort when we excluded recipients of DCD kidneys from the analysis. As previous work by others showed (1) transplantation of deceased-donor kidneys from brain-dead donors undergoing prolonged cold ischemic storage is associated with potent proinflammatory cytokine production, whereas (2) transplantation of DCD kidneys exposed to prolonged warm ischemia induce a blunted proinflammatory response,37,38 the trend of IFX to limit DGF in recipients of brain-dead organs is consistent with known underlying mechanisms. Regardless, although multiple studies indicate that DGF in recipients of either kidneys from DCD or brain-dead donors can negatively affect long-term graft outcome, our findings show that any potential early post-transplant effects on reducing DGF rates do not translate into long-term effects on preventing development of cellular/antibody-mediated rejection and/or late graft dysfunction.
We note that TNF is only one of many inflammatory mediators released after transplantation of deceased-donor kidneys, and likely functions as a mediator of inflammation without triggering a self-sustaining cycle of post-transplant inflammation. In support, although we observed significant reductions in day 7 post-transplant plasma IL-6, IL-1β, and IL-8 in the IFX versus PLBO arms, these reductions were modest, and in contrast to the effects on serum TNF, the mean day 7 values were well above those obtained on day 30. Recent reports in the islet transplant literature have shown that the addition of IL-1β blockade to etanercept therapy further improves post-transplant islet survival over etanercept alone,43 consistent with the concept that multiple inflammatory mediators are involved. Results of an ongoing study of IL-6 receptor blockade in heart transplantation (clinicaltrials.gov NCT03644667) will be of interest in this regard as well.
Perhaps more important than the absence of documented efficacy, our study showed that IFX had a pronounced biologic effect that resulted in an unexpected adverse outcome: BK virus infections requiring changes in IS occurred more frequently and earlier in patients within the IFX arm. These differences were significant and occurred within the first post-transplant year, consistent with the timing of the IFX administration. We also observed a strong trend for higher rates of BK virus nephropathy in the IFX-treated patients, underscoring the clinical relevance of the observed effect. Interestingly, IFX did not affects rates of CMV infection in the study, suggesting that TNF is specifically required for protection against BK virus.
This finding expands upon a single case report44 describing IFX use being associated with a clinically relevant BK virus infection in transplantation. Independently, one previous analysis of an inflammatory bowel disease cohort showed significantly higher BK virus infections of native kidneys associated with long-term TNF blockade compared with controls.45 The fact that BK infections occurred in the absence of transplant-associated IS and in native kidneys in this inflammatory bowel disease cohort treated with anti-TNF supports the conclusion that TNF participates in preventing BK virus infection/reactivation. TNF’s established proinflammatory effects, its ability to activate46 NK cells that have a known protective function against BK infection,47,48 and reports that TNF contributes to T cell18 and natural killer cell cytotoxic killing49 of infected target cells suggest mechanistic links to account for our observations. Further supporting a mechanistic connection between TNF preventing BK infection, in vitro analyses of BK infection of proximal tubular cells and molecular analyses of BK infected kidneys show active BK virus infection is associated with lower TNF expression.50,51
In conclusion, IFX does not benefit deceased-donor kidney transplant recipients across the outcomes and follow-up period studied. As IFX induction increased rates of BK infection, our findings underscore the complexities of targeting peritransplant inflammatory effectors as a strategy to improve kidney transplant outcomes.
Supplementary Material
Acknowledgments
We thank the following individuals for their invaluable contributions to enrolling and following patients at each of the participating centers: Cleveland Clinic: C.C.F. Joshua, J. Augustine, and Jennifer Czerr (research coordinators); Emory University: Andrew B. Adams, Aneesh Mehta (co-investigators), and Donna Kang, Oluwakemi Oladipupo, April Roberson, Amanda Strudwick, and Francie Lasseter (study coordinators); Johns Hopkins Medical Center: Sami Alasfar, Darrin Ostrander, and Obi Ezennia (research coordinators); Mount Sinai Hospital: Sander Florman, Director Recanati Miller Transplant Institute, and Brandy Haydel (lead research coordinator); NIAID: Helena Diop (project manager); Rho, Inc.: Michele Cosgrove (data manager), Allison Ashley (clinical research associate); University of Alabama Birmingham: Gaurav Agarwal, Clifton Kew, and the following coordinators and research personnel: Tina Ayer, Elizabeth Harmon, Jennifer Newby, and Tina Parkhill; University of California at Los Angeles: Nakul Datta (research coordinator); University of California at San Francisco: Sarah Chen and Savanah Trewman (research coordinators); University Hospitals Cleveland Medical Center: Tricia Young (research coordinator); University of Maryland: Mariela Pinedo (research coordinator); University of Michigan Medical Center: Milagros D. Samaniego-Picota; Shared Health Manitoba: Denise Pochinco (laboratory manager); University of Wisconsin: Kristi Schneider (research coordinator); Washington University: Rowena Delos Santos, Anuja Java, Andrew Malone, Haris Murad, Massini Merzkani (co-investigators), Omar Alomar, and Devin Wall (research coordinators); Yale University Hospital: Ricarda Tomlin (research coordinator). We thank Nancy Bridges (Chief of the Transplant Branch, Division of Allergy, Immunology, and Transplantation, NIAID) for her leadership, guidance, and support of the CTOT consortia, and Gohar Mosoyan and Steven Coca (Renal Division, Icahn School of Medicine at Mount Sinai) for their assistance with the plasma cytokine assays. Finally, we express our thanks to all of the patients and families for their dedication and support of the trial.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Disclosures
T. Alhamad reports consultancy for CareDx, Mallinckrod, and Veloxis; research funding from Angion, CareDx, Europhines, and Natera; honoraria from CareDx, Sanofi, and Veloxis; an advisory or leadership role for CareDx, Europhines, and QSANT; and participation in a speakers’ bureau for CareDx, Sanofi, and Veloxis. D.C. Brennan reports consultancy for CareDx, Hansa, Medeor Therapeutics, Sanofi, and Vera Therapeutics; research funding from Amplyx (Vera Therapeutics), Allovir, CareDx, and Natera; honoraria from CareDx and Sanofi; and an advisory or leadership role for Transplantation and UpToDate (on editorial boards). J.S. Bromberg reports consultancy for Eurofins and Pfizer; research funding from Angion, Astellas, CareDx, Natera, Novartis, and Quark; and an advisory or leadership role for the National Institutes of Health and Transplantation. S. Bunnapradist reports consultancy for CareDx, Natera, Nephrosant, Takeda, Transplant Genomics, and Veloxis; research funding from Allovir, Astellas, CareDx, Merck, Natera, and Transplant Genomics; honoraria from Astellas, Allovir, CareDx, Merck, Natera, Nephrosant, Sanofi, Takeda, Transplant Genomics, and Veloxis; and participation in a speakers’ bureau for CareDx, Natera, Nephrosant, Sanofi, Takeda, Transplant Genomics, and Veloxis. S. Chandran reports consultancy for Bridge Bio Gene Therapy and Everest Clinical Research. R.L. Fairchild reports consultancy for Eurofins/Viracor; royalties from Eurofins/Viracor for a license monitoring urine RNA from kidney transplant patients for gene expression signature indicating T cell–mediated rejection; and an advisory or leadership role for Eurofins/Viracor (scientific advisory board). R. Formica reports consultancy for Mallinckrodt Pharmaceuticals, Sanofi, and Veloxis Pharmaceuticals; participation in a speakers’ bureau for Sanofi (nonbranded educational lectures); and other interests or relationships with OPTN (member of the board of directors). K. Kesler reports being an employee of Rho, Inc. S.J. Kim reports an advisory or leadership role for the Canadian Blood Services, Canadian Organ Replacement Register, Canadian Society of Transplantation, Eledon Pharmaceuticals (Data Monitoring Committee member for phase 1b trial; paid), and Health Canada. R.B. Mannon reports research funding from Transplant Genomics, Inc., Verici DX; honoraria from CSL Behring, Olaris Inc., and Vitaerris; patents or royalties for Eurofins; an advisory or leadership role for Verici Dx (steering committee) and the Vitaerris VKTX01 IMAGINE Trial (steering committee); and other interests or relationships with the American Society of Nephrology (grants committee, chair of the policy and advocacy committee), the Data and Safety Monitoring Board, the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health, the Scientific Registry of Transplant Recipients Review Committee (co-chair), TTS 2020 and 2022 (program committee), and Women in Transplantation (chair). M.C. Menon reports ownership interest in Renalytix AI; research funding from Natera Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases; and an advisory or leadership role for the American Society of Transplantation (scientific review committee), Clinical Transplantation (associate editor), JASN (editorial fellow of the transplantation section 2020), and the Journal of Clinical Medicine (editorial board). K.A. Newell reports consultancy for Care Dx, CSL Behring, Hansa, Immucor, Sangamo, Takeda, and Talaris Therapeutics. P. Nickerson reports consultancy for CSL Behring and Paladin Labs; research funding from the National Institutes of Health; and honoraria from Astellas and One Lambda. E.D. Poggio reports consultancy for CareDx, Transplant Genomics, and Verici; and honoraria from CareDx, Gador, Natera, Sanofi, and Scienzia. R. Sung reports research funding from Talaris Therapeutics and other interests or relationships with the American Society of Transplant Surgeons, Gift of Life Michigan, National Kidney Foundation of Michigan, and Organ Donation and Transplantation Alliance. R. Shapiro reports other interests or relationships with Clinical Transplantation (editor in chief). F. Vincenti reports research funding from Angion, Astellas, CSL Behring, Merck, Novartis, Pfizer, and Viela Bio; and honoraria from Veloxis. P.S. Heeger reports consultancy for Mallinckrodt Pharmaceuticals and Vertex; honoraria from Mallinckrodt Pharmaceuticals and Vertex; and an advisory or leadership role for Mallinckrodt Pharmaceuticals (paid scientific consultant) and Vertex (paid scientific consultant). All remaining authors have nothing to disclose.
Funding
This work was supported by National Institute of Allergy and Infectious Diseases of the National Institutes of Health awards U01AI063594 (P.S. Heeger) and UM2AI117870 (Rho, Inc.).
Author Contributions
T. Alhamad, B. Armstrong, D.C. Brennan, J. Bromberg, S. Bunnapradist, S. Chandran, R.L. Fairchild, D.P. Foley, R.N. Formica, Jr., I.W. Gibson, P.S. Heeger, D.E. Hricik, K.L. Kesler, J. Kim, R.B. Mannon, M.C. Menon, K. Newell, P. Nickerson, J. Odim, E.D. Poggio, R. Shapiro R.S. Sung, K. Tinckam, and F. Vincenti reviewed and edited the manuscript; T. Alhamad, D.C. Brennan, J. Bromberg, S. Bunnapradist, S. Chandran, R.L. Fairchild, D.P. Foley, R.N. Formica, Jr., I.W. Gibson, P.S. Heeger, D.E. Hricik, J. Kim, R.B. Mannon, M.C. Menon, K. Newell, P. Nickerson, E.D. Poggio, R. Shapiro, R.S. Sung, K. Tinckam, and F. Vincenti were responsible for the investigation; T. Alhamad, D.C. Brennan, J. Bromberg, S. Bunnapradist, D.P. Foley, R.N. Formica, Jr., P.S. Heeger, D.E. Hricik, K.L. Kesler, J. Kim, R.B. Mannon, M.C. Menon, K. Newell, P. Nickerson, J. Odim, R.S. Sung, K. Tinckam, and F. Vincenti were responsible for supervision; B. Armstrong, I.W. Gibson, P.S. Heeger, and K.L. Kesler were responsible for the formal analysis; B. Armstrong and K.L. Kesler were responsible for the software; B. Armstrong, P. Nickerson, and E.D. Poggio were responsible for data curation; R.L. Fairchild, I.W. Gibson, P.S. Heeger, and K.L. Kesler were responsible for resources; R.L. Fairchild and P.S. Heeger were responsible for the conceptualization; P.S. Heeger was responsible for funding acquisition and wrote the original draft of the manuscript; P.S. Heeger and K.L. Kesler were responsible for the methodology; and P.S. Heeger and J. Odim were responsible for project administration.
Data Sharing Statement
There are no data underlying this work.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/D622.
Supplemental Table 1. Additional baseline characteristics of the ITT population.
Supplemental Table 2. DSA specificities.
Supplemental Table 3. Additional analyses of eGFR.
Supplemental Table 4. Post-transplant immunosuppression.
Supplemental Table 5. Effects of BK nephropathy and BK viremia.
Supplemental Figure 1. Tacrolimus trough levels.
Study Protocol
REFERENCES
- 1.Poggio ED, Augustine JJ, Arrigain S, Brennan DC, Schold JD: Long-term kidney transplant graft survival—Making progress when most needed. Am J Transplant 21: 2824–2832, 2021. 10.1111/ajt.16463 [DOI] [PubMed] [Google Scholar]
- 2.Schröppel B, Legendre C: Delayed kidney graft function: From mechanism to translation. Kidney Int 86: 251–258, 2014. 10.1038/ki.2014.18 [DOI] [PubMed] [Google Scholar]
- 3.Siedlecki A, Irish W, Brennan DC: Delayed graft function in the kidney transplant. Am J Transplant 11: 2279–2296, 2011. 10.1111/j.1600-6143.2011.03754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannon RB: Delayed graft function: The AKI of kidney transplantation. Nephron 140: 94–98, 2018. 10.1159/000491558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR: Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant 24: 1039–1047, 2009. 10.1093/ndt/gfn667 [DOI] [PubMed] [Google Scholar]
- 6.Giblin L, O’Kelly P, Little D, Hickey D, Donohue J, Walshe JJ, et al. : A comparison of long-term graft survival rates between the first and second donor kidney transplanted—The effect of a longer cold ischaemic time for the second kidney. Am J Transplant 5: 1071–1075, 2005. 10.1111/j.1600-6143.2005.00798.x [DOI] [PubMed] [Google Scholar]
- 7.Mikhalski D, Wissing KM, Ghisdal L, Broeders N, Touly M, Hoang AD, et al. : Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation 85[Suppl]: S3–S9, 2008. 10.1097/TP.0b013e318169c29e [DOI] [PubMed] [Google Scholar]
- 8.Salahudeen AK, Haider N, May W: Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int 65: 713–718, 2004. 10.1111/j.1523-1755.2004.00416.x [DOI] [PubMed] [Google Scholar]
- 9.van der Vliet JA, Warlé MC, Cheung CL, Teerenstra S, Hoitsma AJ: Influence of prolonged cold ischemia in renal transplantation. Clin Transplant 25: E612–E616, 2011. 10.1111/j.1399-0012.2011.01510.x [DOI] [PubMed] [Google Scholar]
- 10.Shoskes DA, Cecka JM: Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation 66: 1697–1701, 1998. 10.1097/00007890-199812270-00022 [DOI] [PubMed] [Google Scholar]
- 11.Buchanan PM, Schnitzler MA, Axelrod D, Salvalaggio PR, Lentine KL: The clinical and financial burden of early dialysis after deceased donor kidney transplantation. J Nephrol Ther 2012: 001, 2012. 10.4172/2161-0959.s4-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal JT, Danovitch GM, Wilkinson A, Ettenger RB: The high cost of delayed graft function in cadaveric renal transplantation. Transplantation 51: 1115–1118, 1991 [PubMed] [Google Scholar]
- 13.Farrar CA, Asgari E, Schwaeble WJ, Sacks SH: Which pathways trigger the role of complement in ischaemia/reperfusion injury? Front Immunol 3: 341, 2012. 10.3389/fimmu.2012.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrar CA, Tran D, Li K, Wu W, Peng Q, Schwaeble W, et al. : Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J Clin Invest 126: 1911–1925, 2016. 10.1172/JCI83000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Medof ME, Heeger PS, Sacks S: Graft-derived complement as a mediator of transplant injury. Curr Opin Immunol 19: 569–576, 2007. 10.1016/j.coi.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 16.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC: A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant 10: 2279–2286, 2010. 10.1111/j.1600-6143.2010.03179.x [DOI] [PubMed] [Google Scholar]
- 17.Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A: Secretion of TNF-alpha, IL-8 and nitric oxide by macrophages activated with polyanions, and involvement of interferon-gamma in the regulation of cytokine secretion. Cytokine 11: 571–578, 1999. 10.1006/cyto.1998.0472 [DOI] [PubMed] [Google Scholar]
- 18.Chun N, Ang RL, Chan M, Fairchild RL, Baldwin WM, 3rd, Horwitz JK, et al. : T cell-derived tumor necrosis factor induces cytotoxicity by activating RIPK1-dependent target cell death. JCI Insight 6: e148643, 2021. 10.1172/jci.insight.148643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranta V, Orpana A, Carpén O, Turpeinen U, Ylikorkala O, Viinikka L: Human vascular endothelial cells produce tumor necrosis factor-alpha in response to proinflammatory cytokine stimulation. Crit Care Med 27: 2184–2187, 1999. 10.1097/00003246-199910000-00019 [DOI] [PubMed] [Google Scholar]
- 20.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B: An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 72: 3666–3670, 1975. 10.1073/pnas.72.9.3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, et al. : Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 344: 1105–1110, 1994. 10.1016/s0140-6736(94)90628-9 [DOI] [PubMed] [Google Scholar]
- 22.Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Bijl H, et al. : Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 344: 1125–1127, 1994. 10.1016/s0140-6736(94)90632-7 [DOI] [PubMed] [Google Scholar]
- 23.Hanauer SB Feagan BG Lichtenstein GR Mayer LF Schreiber S Colombel JF et al. ; ACCENT I Study Group : Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 359: 1541–1549, 2002. 10.1016/S0140-6736(02)08512-4 [DOI] [PubMed] [Google Scholar]
- 24.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, et al. : TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J 19: 1637–1645, 2005. 10.1096/fj.05-3841com [DOI] [PubMed] [Google Scholar]
- 25.Ishii D, Schenk AD, Baba S, Fairchild RL: Role of TNFalpha in early chemokine production and leukocyte infiltration into heart allografts. Am J Transplant 10: 59–68, 2010. 10.1111/j.1600-6143.2009.02921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WH, Lee JW, Gao B, Jung MH: Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: Apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal 17: 1516–1532, 2005. 10.1016/j.cellsig.2005.03.020 [DOI] [PubMed] [Google Scholar]
- 27.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, et al. : Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 12: 1576–1583, 2012. 10.1111/j.1600-6143.2011.03977.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro AM, Pokrywczynska M, Ricordi C: Clinical pancreatic islet transplantation. Nat Rev Endocrinol 13: 268–277, 2017. 10.1038/nrendo.2016.178 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS Bosch JP Lewis JB Greene T Rogers N Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 30.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the modification of diet in renal disease and Cockcroft–Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005. 10.1681/ASN.2004060447 [DOI] [PubMed] [Google Scholar]
- 31.Diao JA, Inker LA, Levey AS, Tighiouart H, Powe NR, Manrai AK: In search of a better equation—Performance and equity in estimates of kidney function. N Engl J Med 384: 396–399, 2021. 10.1056/NEJMp2028243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inker LA Eneanya ND Coresh J Tighiouart H Wang D Sang Y et al. ; Chronic Kidney Disease Epidemiology Collaboration : New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 385: 1737–1749, 2021. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas M Sis B Racusen LC Solez K Glotz D Colvin RB et al. ; Banff Meeting Report Writing Committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions [published correction appears in Am J Transplant 15: 2784, 2015 10.1111/ajt.13517]. Am J Transplant 14: 272–283, 2014. 10.1111/ajt.12590 [DOI] [PubMed] [Google Scholar]
- 34.Tambur AR, Campbell P, Claas FH, Feng S, Gebel HM, Jackson AM, et al. : Sensitization in transplantation: Assessment of risk (STAR) 2017 Working Group meeting report. Am J Transplant 18: 1604–1614, 2018. 10.1111/ajt.14752 [DOI] [PubMed] [Google Scholar]
- 35.Leffondre K, Boucquemont J, Tripepi G, Stel VS, Heinze G, Dunkler D: Analysis of risk factors associated with renal function trajectory over time: A comparison of different statistical approaches. Nephrol Dial Transplant 30: 1237–1243, 2015. 10.1093/ndt/gfu320 [DOI] [PubMed] [Google Scholar]
- 36.Shemesh E, Fine RN: Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant 14: 940–943, 2010. 10.1111/j.1399-3046.2010.01396.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vries DK, Lindeman JH, Ringers J, Reinders ME, Rabelink TJ, Schaapherder AF: Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant 11: 1064–1070, 2011. 10.1111/j.1600-6143.2011.03466.x [DOI] [PubMed] [Google Scholar]
- 38.Stangl M, Zerkaulen T, Theodorakis J, Illner W, Schneeberger H, Land W, et al. : Influence of brain death on cytokine release in organ donors and renal transplants. Transplant Proc 33: 1284–1285, 2001. 10.1016/s0041-1345(00)02479-9 [DOI] [PubMed] [Google Scholar]
- 39.Aubert O, Divard G, Pascual J, Oppenheimer F, Sommerer C, Citterio F, et al. : Application of the iBox prognostication system as a surrogate endpoint in the TRANSFORM randomised controlled trial: Proof-of-concept study. BMJ Open 11: e052138, 2021. 10.1136/bmjopen-2021-052138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loupy A, Aubert O, Orandi BJ, Naesens M, Bouatou Y, Raynaud M, et al. : Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 366: l4923, 2019. 10.1136/bmj.l4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viklicky O, Hruba P, Tomiuk S, Schmitz S, Gerstmayer B, Sawitzki B, et al. : Sequential targeting of CD52 and TNF allows early minimization therapy in kidney transplantation: From a biomarker to targeting in a proof-of-concept trial. PLoS One 12: e0169624, 2017. 10.1371/journal.pone.0169624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diuwe P, Domagala P, Durlik M, Trzebicki J, Chmura A, Kwiatkowski A: The effect of the use of a TNF-alpha inhibitor in hypothermic machine perfusion on kidney function after transplantation. Contemp Clin Trials 59: 44–50, 2017. 10.1016/j.cct.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 43.Naziruddin B, Kanak MA, Chang CA, Takita M, Lawrence MC, Dennison AR, et al. : Improved outcomes of islet autotransplant after total pancreatectomy by combined blockade of IL-1β and TNFα. Am J Transplant 18: 2322–2329, 2018. 10.1111/ajt.14961 [DOI] [PubMed] [Google Scholar]
- 44.Lum EL, Bunnapradist S: BK viremia exacerbation with adalimumab coadministration. Transplant Direct 6: e557, 2020. 10.1097/TXD.0000000000001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flores V, Rodríguez-Sánchez B, Marín-Jiménez I, Bouza E, Menchén L, Muñoz P: Prospective study of BK virus infection in patients with inflammatory bowel disease. ScientificWorldJournal 2014: 970528, 2014. 10.1155/2014/970528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almishri W, Santodomingo-Garzon T, Le T, Stack D, Mody CH, Swain MG: TNFα augments cytokine-induced NK cell IFNγ production through TNFR2. J Innate Immun 8: 617–629, 2016. 10.1159/000448077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acott PD: Natural killer cell response to BK virus infection in polyoma virus-associated nephropathy of renal transplant recipients. Kidney Int 84: 233–235, 2013. 10.1038/ki.2013.148 [DOI] [PubMed] [Google Scholar]
- 48.Mishra R, Chen AT, Welsh RM, Szomolanyi-Tsuda E: NK cells and gammadelta T cells mediate resistance to polyomavirus-induced tumors. PLoS Pathog 6: e1000924, 2010. 10.1371/journal.ppat.1000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD: Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol 91: 299–309, 2012. 10.1189/jlb.0611308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahimi Z, Yaghobi R, Afshari A, Roozbeh J, Mokhtari MJ, Hosseini AM: The effect of BKV reactivation on cytokines behavior in kidney transplanted patients. BMC Nephrol 23: 20, 2022. 10.1186/s12882-021-02645-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ribeiro A, Merkle M, Motamedi N, Nitschko H, Köppel S, Wörnle M: BK virus infection activates the TNFα/TNF receptor system in polyomavirus-associated nephropathy. Mol Cell Biochem 411: 191–199, 2016. 10.1007/s11010-015-2581-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.