Abstract
Significance Statement
Standard blood markers of kidney function undergo renal clearance and are thus inversely correlated with estimated glomerular filtration rate (eGFR). Recent work has shown that blood levels of the podocyte-derived protein testican-2 are positively correlated with eGFR among individuals with relatively normal kidney function. The current study considers blood testican-2 levels among three cohorts of >8,000 individuals in total, including many with established kidney disease. Testican-2 levels are positively correlated with eGFR across the full range of kidney health, with higher levels associated with lower risk of incident end stage kidney disease (ESKD), even after adjusting for baseline eGFR, proteinuria, and other kidney disease risk factors. This study highlights a positive association between testican-2 and kidney health and prognosis.
Background
Testican-2 was recently identified as a podocyte-derived protein that is released into circulation by the kidneys and is positively correlated with eGFR and eGFR slope. However, whether higher testican-2 levels are associated with lower risk of ESKD is unknown.
Methods
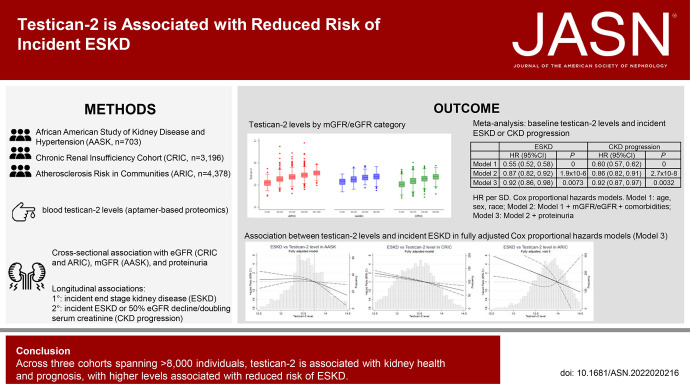
Aptamer-based proteomics assessed blood testican-2 levels among participants in the African American Study of Kidney Disease and Hypertension (AASK, n=703), the Chronic Renal Insufficiency Cohort (CRIC) study (n=3196), and the Atherosclerosis Risk in Communities (ARIC) study (n=4378). We compared baseline characteristics by testican-2 tertile and used Cox proportional hazards models to study the association of testican-2 with incident ESKD.
Results
Higher testican-2 levels were associated with higher measured GFR (mGFR) in AASK, higher eGFR in the CRIC and ARIC studies, and lower albuminuria in all cohorts. Baseline testican-2 levels were significantly associated with incident ESKD in Cox proportional hazards models adjusted for age, sex, and race (model 1) and model 1+mGFR or eGFR+comorbidities (model 2). In model 3 (model 2+proteinuria), the associations between testican-2 (per SD increase) and incident ESKD were AASK (hazard ratio [HR]=0.84 [0.72 to 0.98], P=0.023), CRIC (HR=0.95 [0.89 to 1.02], P=0.14), ARIC (HR=0.54 [0.36 to 0.83], P=0.0044), and meta-analysis (HR=0.92 [0.86 to 0.98], P=0.0073).
Conclusions
Across three cohorts spanning >8000 individuals, testican-2 is associated with kidney health and prognosis, with higher levels associated with reduced risk of ESKD.
CKD is a worldwide health problem.1 Standard blood markers of CKD, including creatinine and cystatin C, undergo renal clearance and are thus inversely correlated with eGFR.2 These markers are nonspecific, are late indicators of kidney damage, and are not functional participants in the disease process. The identification of a blood molecule associated with risk of disease progression, but not subject to glomerular filtration, has the potential to improve CKD diagnosis and to elucidate new biology.3
In addition to playing a fundamental role in clearance, the kidney also synthesizes and releases molecules such as erythropoietin and active vitamin D into circulation.4 Using an aptamer-based proteomic approach, we recently characterized renal arteriovenous gradients for >1300 proteins.5 Although most of the proteins that changed were significantly decreased from artery to vein, several were found to increase, the most significant of which was testican-2. Furthermore, we showed that blood testican-2 levels were positively correlated with eGFR cross-sectionally and that higher testican-2 levels at baseline were associated with the slower rate of a subsequent eGFR decline in two cohorts spanning >2600 individuals. Finally, the glomerular expression of testican-2 in human kidneys was demonstrated by microscopy while single-cell RNA sequencing showed expression of the cognate gene (SPOCK2) exclusively in podocytes, highlighting testican-2 as a potential marker of kidney biosynthetic function.5
However, because our prior study examined population-based cohorts with normal eGFR at baseline, we were unable to assess the association between testican-2 and progression of CKD, including development of ESKD. To address this gap, we now examine the association between blood testican-2 levels and CKD outcomes in the African American Study of Kidney Disease and Hypertension (AASK), which directly combines measured GFR (mGFR) using iothalamate with high kidney event rates, and in the Chronic Renal Insufficiency Cohort (CRIC) study and Atherosclerosis Risk in Communities (ARIC) study. Importantly, in all these studies, testican-2 was measured using the same aptamer-based platform used in our published work, providing analytical continuity across analyses. Taken together, these studies advance the hypothesis that kidney-derived testican-2 provides insight into kidney health across the spectrum of CKD severity.
Methods
Study Populations
The study population consisted of participants with blood samples available for proteomic profiling in AASK, the CRIC study, and the ARIC study. Study protocols were approved by the Institutional Review Board of each participating center.
AASK was a 2×3 randomized trial of BP lowering and antihypertensive medication in African American participants with hypertension-attributed CKD.6 Exclusion criteria included mGFR <20 or >65 ml/min per 1.73 m2, urine protein-to-creatinine ratio (PCR) >2.5 g/g, diabetes mellitus, and known glomerular or autosomal dominant polycystic kidney disease. Randomization occurred between February 1995 and September 1998. After trial completion on September 30, 2001, participants without kidney failure were invited to participate in a cohort phase. For this study, a subset of 703 participants who had serum samples at baseline available for proteomic profiling were included. The CRIC study is a prospective cohort of 3939 participants with mild-to-moderate CKD. Eligibility was determined by eGFR: 20–70 ml/min per 1.73 m2 for participants aged 21–44 years, 20–60 ml/min per 1.73 m2 for participants aged 45–64 years, and 20–50 ml/min per 1.73 m2 for participants aged 65–74 years.7,8 Participants were excluded for polycystic kidney disease, multiple myeloma, active glomerulonephritis, a previous dialysis requirement, recent immunotherapy for kidney disease and/or vasculitis, New York Heart Association class III or IV heart failure, and cirrhosis. Recruitment was completed between 2003 and 2008, and follow-up is ongoing. For this study, a subset of 3249 participants with available plasma proteomic data at the year 1 visit were included. The ARIC study is a prospective cohort study of 15,792 individuals between 45 and 64 years of age, from four communities in the United States.9 Visit 1 was carried out between 1987 and 1989, and follow-up is ongoing. For this study, we included 4378 participants without ESKD at visit 5 (between 2011 and 2013) who had serum proteomic data.
Proteomic Profiling and Measurement of Serum Testican-2
Frozen serum samples from the baseline visit in AASK, plasma samples from the year 1 visit in the CRIC study, and plasma samples from visit 5 in the ARIC study were sent for proteomic profiling using the single-stranded DNA aptamer-based SOMAscan proteomics platform, as previously described.5 Relative concentrations of proteins or protein complexes were quantified using the V4.1 platform in AASK in 2021, the V4 platform for CRIC in 2019, and the V4 platform for ARIC in 2018. Quality control was run separately for each study, with blind duplicate samples and standard SomaLogic reference standards run on each plate. The coefficient of variation for testican-2 across blind duplicates was 4.88% in AASK, 4.56% in CRIC, and 5.22% in ARIC. Testican-2 levels, reported in relative fluorescence units, were log-transformed (base 2) because of skewed distribution, and values outside of five SDs on the log-2 scale were winsorized.
Outcomes
The primary study outcome was incident ESKD. We also examined a composite outcome of CKD progression defined as incident ESKD and/or doubling of serum creatinine concentration (equivalent to approximately 57% decline in GFR) in AASK, or incident ESKD and/or decline in eGFR by ≥50% in the CRIC study and ARIC study. In sensitivity analyses, the composite CKD progression definition of ESKD and/or decline in eGFR by ≥50% was also applied in AASK. Death was treated as a censoring event. In AASK, GFR was measured by the urinary clearance of 125I Iothalamate. In CRIC and ARIC, eGFRcr-cys (stated as eGFR throughout the article unless otherwise specified) was calculated from serum creatinine and serum cystatin C concentrations using the 2012 Chronic Kidney Disease Epidemiology Collaboration equation.10 In sensitivity analyses, eGFR was also calculated from cystatin C alone (eGFRcys). In AASK and CRIC, ESKD was ascertained per protocol throughout the study periods. In ARIC, ESKD was ascertained through linkage to the United States Renal Data System (USRDS) in December 2018.11 As per USRDS policy, any table cell with fewer than 11 individuals is shown as <11 to maintain study participant anonymity; for statistical analyses, the actual number in each cell was used.
Covariates
For each cohort, covariates were assessed at the same visit as proteomic profiling. In AASK, these included age, sex, history of heart disease, history of diabetes mellitus, total and HDL cholesterol, systolic blood pressure, body mass index (BMI), mGFR, and urine PCR. In CRIC and AASK, we also assessed self-reported race and used eGFR instead of mGFR. In CRIC, proteinuria was also assessed by PCR, whereas in ARIC, it was assessed by urine albumin-to-creatinine ratio (ACR). PCR was converted to ACR using the following equation: ACR=exp(0.3072×log(min(PCR/50,1)) + 1.5793×log(max(min(PCR/500,1), 0.1))+1.1266×log(−max(PCR/500, 1))+5.3920) if missing(PCR).12 In all cohorts, history of heart disease was self-reported. In CRIC, diabetes was defined as self-reported use of insulin or oral hypoglycemic medications, fasting blood glucose ≥126 mg/dl or a nonfasting level ≥200 mg/dl, or an HbA1c ≥6.5%. In ARIC, diabetes was defined as current use of a diabetes medication, fasting blood glucose ≥126 mg/dl or a nonfasting level ≥200 mg/dl, or self-report of a physician's diagnosis of diabetes.
Statistical Analyses
We used descriptive statistics, including means and proportions, to compare baseline characteristics by serum testican-2 tertile. Formal testing was performed using t-test or Wilcoxon rank-sum test for continuous variables and chi-squared for categorical variables. Cox proportional hazards models were constructed to study the associations of testican-2 with (1) incident ESKD and (2) CKD progression, defined as incident ESKD and/or doubling serum Cr (AASK) or incident ESKD and/or ≥50% eGFR decline (CRIC and ARIC). We adjusted for the covariates using three different models. Model 1 was adjusted for age, sex, and race (the latter in CRIC and ARIC only). Model 2 was additionally adjusted for baseline GFR (mGFR in AASK, eGFR in CRIC and ARIC), heart disease, smoking, systolic blood pressure, BMI, and diabetes (the latter in CRIC and ARIC only). Model 3 was additionally adjusted for either log PCR in AASK and CRIC or log ACR in AASK. In sensitivity analyses, models were adjusted for eGFRcys instead of eGFRcr-cys. To evaluate for nonlinear relationships, we also investigated the fully adjusted association between testican-2 and ESKD modeling testican-2 (IDI) with cubic splines. Hazard ratios (HRs) were also estimated within subgroups stratified by GFR and albuminuria, and interactions were evaluated by including product terms of testican-2 among the covariates. Improvement in model performance was evaluated by estimating the change in Harrell's C-statistic, the net reclassification index (NRI), and the integrated discrimination index for model 3 compared to model 3 with testican-2 or the Kidney Failure Risk Equation compared to the Kidney Failure Risk Equation with testican-2.13 Finally, we performed a fixed effect meta-analysis of the association of testican-2 with incident ESKD and CKD progression across AASK, the CRIC study, and the ARIC study using the “meta” package in R (version 4.0.3). A P value <0.05 was considered significant.
Results
Study Population Characteristics
The study population with testican-2 measurements consisted of 703, 3196, and 4378 participants from AASK, the CRIC study, and the ARIC study, respectively (Table 1). Compared with other study subjects, AASK participants had the lowest mean age (54.5 years), the lowest proportion of women (38.6%), the highest prevalence of heart disease (50.9%), and the highest mean systolic blood pressure at baseline (151.1 mmHg). AASK was comprised of all Black participants, whereas 41.4% of CRIC study participants and 18.8% of ARIC study participants were Black. In addition, no participants in AASK had diabetes, whereas 48.7% of CRIC study participants and 30.0% of ARIC study participants had diabetes. AASK and CRIC study participants had more kidney function impairment at baseline than ARIC participants, with a mean mGFR of 45.7 ml/min per 1.73 m2 in AASK, mean eGFR of 42.8 ml/min per 1.73 m2 in CRIC, and mean eGFR of 65.0 ml/min per 1.73 m2 in ARIC. For the primary outcome of incident ESKD, the mean follow-up was 7.49 years in AASK, 9.04 years in the CRIC study, and 5.23 years in the ARIC study. The number of cases of incident ESKD was 210 (29.9%), 1028 (32.2%), and 31 (0.7%) in AASK, the CRIC study, and the ARIC study, respectively.
Table 1.
Baseline characteristics and outcomes by testican-2 tertile in AASK, CRIC, and ARIC
| AASK | CRIC | ARIC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total (n=703) | Tertile 1 | Tertile 2 | Tertile 3 | P | Total (n=3196) | Tertile 1 | Tertile 2 | Tertile 3 | P | Total (n=4378) | Tertile 1 | Tertile 2 | Tertile 3 | P |
| Age, yr | 54.5±10.7 | 53.2±11.0 | 53.9±11.1 | 54.4±16.9 | 0.48 | 59.1±10.7 | 57.9±11.3 | 59.8±10.7 | 59.5±9.8 | <0.001 | 75.7±5.2 | 76.4±5.3 | 75.6±5.2 | 75.2±4.9 | <0.001 |
| Black, % | 1322 (41.4) | 451 (42.3) | 423 (39.7) | 448 (42.1) | 0.91 | 823 (18.8) | 320 (21.9) | 260 (17.8) | 243 (16.7) | <0.001 | |||||
| Woman, % | 38.6 | 40.4 | 38.9 | 36.3 | 0.73 | 44.6 | 46.0 | 43.0 | 44.7 | 0.56 | 54.9 | 55 | 57 | 53 | 0.20 |
| Heart disease, % | 50.9 | 45.5 | 54.3 | 53.0 | 0.059 | 35.3 | 36.1 | 36.1 | 33.7 | 0.25 | 18.5 | 22 | 16 | 18 | 0.008 |
| Diabetes, % | — | — | — | — | — | 48.7 | 55.6 | 50.5 | 40.0 | <0.001 | 30.0 | 37 | 28 | 25 | <0.001 |
| TC, mg/dl | 211.9±45.7 | 210.8±47.2 | 213.4±43.7 | 211.6±46.2 | 0.53 | 181.6±43.4 | 179.8±43.9 | 180.9±43.9 | 184.1±42.4 | 0.026 | 179.4±41.6 | 177.4±43.2 | 181.2±41.1 | 179.6±40.4 | 0.16 |
| HDL, mg/dl | 48.3±16.0 | 47.0±16.1 | 47.5±15.4 | 50.3±16.3 | 0.77 | 48.6±15.8 | 46.0±15.0 | 48.1±15.2 | 51.6±16.5 | <0.001 | 51.4±14.0 | 49.2±13.3 | 51.8±13.7 | 53.1±14.4 | <0.001 |
| SBP, mm Hg | 151.1±24.9 | 149.9±22.6 | 150.3±26.0 | 153.2±25.8 | 0.84 | 126.8±21.6 | 130.3±22.5 | 126.2±21.4 | 124.0±20.5 | <0.001 | 130.5±18.1 | 131.8±18.9 | 130.4±17.9 | 129.1±17.5 | <0.001 |
| BMI, kg/m2 | 30.5±6.4 | 30.8±6.7 | 30.5±6.4 | 30.4±6.2 | 0.57 | 32.1±7.7 | 32.7±8.6 | 32.0±7.4 | 31.7±7.0 | 0.004 | 28.8±5.6 | 29.2±6.0 | 28.8±5.7 | 28.2±5.1 | <0.001 |
| eGFR,a ml/min per 1.73 m2 | 45.7±13.0 | 39.6±12.8 | 46.2±12.4 | 51.3±11.1 | <0.001 | 42.8±15.9 | 33.0±13.5 | 43.6±13.9 | 51.6±14.6 | <0.001 | 65.0±17.8 | 55.0±17.6 | 66.7±15.2 | 73.1±15.5 | <0.001 |
| PCR, g/g | 0.33±0.52 | 0.49±0.62 | 0.31±0.50 | 0.18±0.37 | <0.001 | 0.79±1.77 | 1.40±2.53 | 0.61±1.34 | 0.40±0.93 | <0.001 | |||||
| ACR, g/g | 0.05±0.28 | 0.09±0.44 | 0.03±0.14 | 0.04±0.17 | <0.001 | ||||||||||
| CKD progression, n (%) | 274 (39.0) | 125 (53.2) | 91 (38.9) | 58 (24.8) | 0.002 | 1222 (38.4) | 594 (55.7) | 385 (36.1) | 243 (22.8) | <0.001 | 69 (1.4) | 43 (2.7) | 15 (1.0) | 11 (0.8) | <0.001 |
| Follow-up time, yr | 7.02 (3.45) | 6.86 (4.98) | 5.22 (1.24) | ||||||||||||
| ESKD incident, n (%) | 210 (29.9) | 110 (46.8) | 70 (29.9) | 30 (12.8) | <0.001 | 1028 (32.2) | 552 (51.8) | 302 (28.3) | 174 (16.3) | <0.001 | 31 (0.7) | 27 (1.8) | <11 | <11 | <0.001 |
| Follow-up time, yr | 7.49 (3.36) | 9.04 (4.98) | 5.23 (1.24) | ||||||||||||
Values are mean±SD or percentage. For proteinuria, values are mean PCR±SD for AASK and CRIC; and mean ACR±SD for ARIC. AASK, American Study of Kidney Disease and Hypertension; ARIC, Atherosclerosis Risk in Communities; CRIC, Chronic Renal Insufficiency Cohort; TC, total cholesterol; SBP, systolic BP; BMI, body mass index; PCR, urine protein/creatinine ratio; ACR, urine albumin/creatinine ratio.
In AASK, GFR was directly measured.
Testican-2 Levels and Kidney Health at Baseline
As shown in Table 1, higher testican-2 levels were associated with better kidney health at baseline in all three cohorts. In AASK, mean mGFR in the lowest, middle, and highest tertiles of testican-2 was 39.6, 46.2, and 51.3 ml/min per 1.73 m2; in the CRIC study and ARIC study, mean eGFR by testican-2 tertile was 33.0, 43.6, and 51.6 ml/min per 1.73 m2 and 55.0, 66.7, and 73.1 ml/min per 1.73 m2, respectively (P<0.001 for all cohorts). Box and whisker plots depicting the correlation between testican-2 and GFR in each study cohort are shown in Figure 1. In AASK and the CRIC study, mean PCR by the lowest, middle, and highest tertiles of testican-2 was 0.49, 0.31, and 0.18 g/g and 1.40, 0.61, and 0.40 g/g, respectively; in the ARIC study, mean ACR by testican-2 tertile was 0.09, 0.03, and 0.04 g/g, respectively (P<0.001 for all cohorts). Box and whisker plots depicting the correlation between testican-2 and proteinuria in each study cohort are shown in Figure 2. In both the CRIC study and ARIC study, individuals in the highest tertile of testican-2 had lower prevalence of diabetes, lower HDL cholesterol levels, lower systolic blood pressure, and lower BMI than individuals in the lowest tertile of testican-2 (P=0.003 for BMI in CRIC, otherwise P<0.001 for all other traits). In the ARIC study, Black participants comprised 21.9%, 17.8%, and 16.7% of study participants in the lowest, middle, and highest tertiles of testican-2 (P<0.001); no trend for testican-2 by race was observed in the CRIC study (P=0.90).
Figure 1.
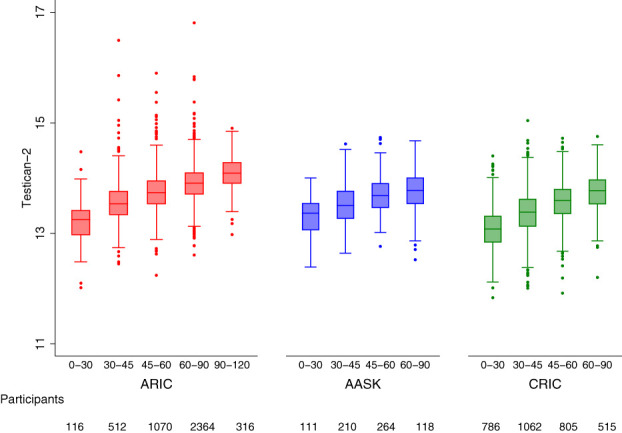
Testican-2 levels and GFR. Box and whisker plots showing the cross-sectional relationship between baseline eGFR (mGFR in AASK) and testican-2 levels in the ARIC study (left), AASK (middle), and the CRIC study (right). The x-axes show eGFR or mGFR categories in ml/min per 1.73 m2.
Figure 2.
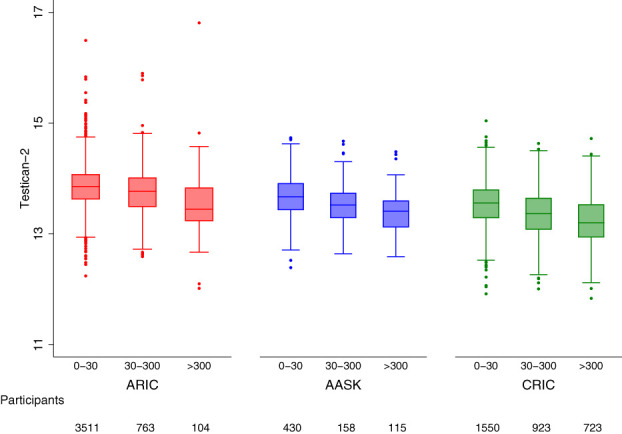
Testican-2 levels and proteinuria. Box and whisker plots showing the cross-sectional relationship between baseline proteinuria and testican-2 levels in the ARIC study (left), AASK (middle), and the CRIC study (right). The x-axes show proteinuria categories as albumin-to-creatinine ratios in mg/g.
Baseline Testican-2 Levels and Incident ESKD
In AASK, the numbers of cases of incident ESKD in the lowest, middle, and highest tertiles of testican-2 were 110, 70, and 30, respectively; in the CRIC study and ARIC study, the numbers of incident ESKD cases by testican-2 tertile were 552, 302, and 174 and 27, <11, and <11, respectively (P<0.001 for all cohorts, Table 1). The results were similar for the composite outcome of CKD progression, with lower rates of CKD progression for higher tertiles of testican-2 in all three cohorts.
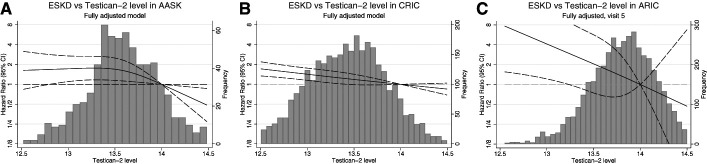
In age-, sex-, and race-adjusted Cox proportional hazards models, baseline testican-2 levels (per SD) were strongly associated with reduced incident ESKD in all three cohorts (Table 2). These associations were attenuated but remained significant after further adjustment for baseline mGFR or eGFR and comorbidities. In fully adjusted Cox proportional hazards models that additionally adjusted for proteinuria, baseline testican-2 levels remained significantly associated with reduced incident ESKD in AASK (HR=0.84 [0.72 to 0.98], P=0.023) and the ARIC study (HR=0.54 [0.36 to 0.83], P=0.044) and demonstrated a trend for association in CRIC (HR=0.95 [0.89 to 1.02], P=0.14) (Table 2). We observed a potential interaction in CRIC whereby testican-2 was significantly associated with incident CKD among participants with urine PCR <0.1 g/g, but not among participants with urine PCR >0.1 g/g; however, no interaction was noted by proteinuria category in AASK or ARIC or by eGFR category in any cohort (Supplementary Table 1). For all three cohorts, cubic spline graphs of the association between baseline testican-2 levels and incident ESKD in fully adjusted Cox proportional hazards models are shown in Figure 3. Baseline testican-2 levels were also significantly associated with the composite CKD progression outcome in fully adjusted Cox proportional hazards models in the CRIC study (HR=0.94 [0.88 to 1.00], P=0.048) and the ARIC study (HR=0.75 [0.57 to 0.99], P=0.039), with a similar trend in AASK (HR=0.88 [0.77 to 1.01], P=0.064) (Table 2). The results for both incident ESKD and CKD progression were similar in all three cohorts when eGFR was calculated using cystatin C only (Supplementary Table 2). In meta-analysis of all three cohorts (Table 3), baseline testican-2 levels were significantly associated with reduction in both incident ESKD (HR=0.92 [0.86 to 0.98], P=0.0073) and the composite CKD progression outcome (HR=0.92 [0.87 to 0.97], P=0.0032) in fully adjusted Cox proportional hazards models. The results were unchanged when CKD progression was defined as incident ESKD and/or ≥50% decline in eGFR in AASK (Supplementary Tables 3 and 4). Furthermore, baseline testican-2 levels remained significantly associated with a reduction in incident ESKD and the composite CKD progression outcome in a meta-analysis of CRIC and AASK data only (Supplementary Table 5). When added to the fully adjusted model or to the Kidney Failure Risk equation, testican-2 did not improve the C-statistic, IDI, or NRI for incident ESKD (not shown).
Table 2.
Association of testican-2 and incident ESKD and CKD progression in AASK, CRIC, and ARIC
| ESKD | CKD Progression | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| AASK | ||||
| Model 1 | 0.54 (0.47 to 0.63) | 1.3 × 10−17 | 0.63 (0.55 to 0.71) | 5.2 × 10−14 |
| Model 2 | 0.76 (0.65 to 0.89) | 4.6 × 10−4 | 0.80 (0.70 to 0.92) | 0.0012 |
| Model 3 | 0.84 (0.72 to 0.98) | 0.023 | 0.88 (0.77 to 1.01) | 0.064 |
| CRIC | ||||
| Model 1 | 0.56 (0.53 to 0.59) | 4.9 × 10−99 | 0.60 (0.57 to 0.63) | 3.1 × 10−92 |
| Model 2 | 0.89 (0.84 to 0.95) | 8.0 × 10−4 | 0.88 (0.83 to 0.93) | 1.4 × 10−5 |
| Model 3 | 0.95 (0.89 to 1.02) | 0.14 | 0.94 (0.88 to 1.00) | 0.048 |
| ARIC | ||||
| Model 1 | 0.23 (0.17 to 0.30) | 8.8 × 10−23 | 0.45 (0.36 to 0.56) | 1.5 × 10−12 |
| Model 2 | 0.58 (0.38 to 0.89) | 0.012 | 0.76 (0.58 to 1.00) | 0.051 |
| Model 3 | 0.54 (0.36 to 0.83) | 0.0044 | 0.75 (0.57 to 0.98) | 0.038 |
HRs are per SD. Covariates: model 1. AASK: adjusted for age and sex; CRIC/ARIC: adjusted for age, sex, and race. Model 2: AASK: model 1+mGFR, heart disease, smoking, systolic BP, and BMI; CRIC/ARIC: model 1+eGFR, heart disease, diabetes, smoking, systolic BP, anti–hypertensive medications, and BMI. Model 3. AASK/CRIC: model 2+Log (PCR); ARIC: model 2+Log (ACR). HR, hazard ratio; CI, confidence interval; AASK, American Study of Kidney Disease and Hypertension; CRIC, Chronic Renal Insufficiency Cohort; ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; PCR, urine protein-to-creatinine ratio; ACR, urine albumin-to-creatinine ratio.
Figure 3.
Testican-2 levels and risk of incident ESKD. Cubic spline plots showing the association between baseline testican-2 levels and incident ESKD in fully adjusted Cox proportional hazards models in AASK (A), the CRIC study (B), and the ARIC study (C). The left y-axes denote the HR for incident ESKD at each testican-2 level and correspond to the cubic spine plots. The right y-axes denote the frequency (number of study participants) at each testican-2 level and correspond to the histograms.
Table 3.
Meta-analysis of testican-2 and incident ESKD and CKD progression in AASK, CRIC, and ARIC
| ESKD | CKD Progression | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1 | 0.55 (0.52 to 0.58) | 0 | 0.60 (0.57 to 0.62) | 0 |
| Model 2 | 0.87 (0.82 to 0.92) | 1.9 × 10−6 | 0.86 (0.82 to 0.91) | 2.7 × 10−8 |
| Model 3 | 0.92 (0.86 to 0.98) | 0.0073 | 0.92 (0.87 to 0.97) | 0.0032 |
HRs are per SD. Covariates: model 1. AASK: adjusted for age and sex; CRIC/ARIC: adjusted for age, sex, and race. Model 2: AASK: model 1+mGFR, heart disease, smoking, systolic BP, and BMI; CRIC/ARIC: model 1+eGFR, heart disease, diabetes, smoking, systolic BP, anti–hypertensive medications, and BMI. Model 3. AASK/CRIC: model 2+Log (PCR); ARIC: model 2+Log (ACR). HR, hazard ratio; AASK, American Study of Kidney Disease and Hypertension; CRIC, Chronic Renal Insufficiency Cohort; ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; PCR, urine protein-to-creatinine ratio; ACR, urine albumin-to-creatinine ratio.
Discussion
In a study sample enriched for individuals with CKD and at high risk for CKD, we find that increased blood testican-2 levels are associated with better kidney health, including cross-sectional associations with higher GFR and lower proteinuria, and in meta-analysis, reduced risk of incident ESKD even after adjusting for established risk factors. Furthermore, heterogeneity in baseline kidney function, age, race, sex, geography, and comorbidities across the examined cohorts supports the robustness and generalizability of the observed associations.
Encoded by the SPOCK2 gene, testican-2 is a 424 amino acid-secreted glycoprotein originally cloned in neuronal cells.14–16 On the basis of gene and protein expression databases, testican-2 is widely expressed; for example, the Human Protein Atlas demonstrates strong immunohistochemical staining in brain, adrenal, lung, gastrointestinal, kidney, testes, and lymphoid tissue.17 Testican-2 has been characterized as a component of the extracellular matrix, but little is known about its biologic function(s).14–16 In glioma cells in culture, testican-2 has been found to promote cell migration on collagen by enhancing membrane-type 1 matrix metalloproteinase (MT1-MMP) activity.18 In the lung, overexpression of Spock2 in mice has been found to alter lung alveolar development while inhaled testican-2 in adult mice protects lung epithelial cells against influenza virus infection.19,20 In the kidney, single-cell RNA sequencing data demonstrate SPOCK2 gene expression exclusively in podocytes, with immunofluorescence and electron microscopy demonstrating testican-2 secretion into the glomerular basement membrane. Furthermore, testican-2 increases glomerular endothelial cell tube formation and motility and increases MMP-2 and MMP-9 activity in vitro, raising the possibility that its secretion has a functional role within the glomerulus.5
Two recent proteomics studies have highlighted an association between testican-2 and kidney function. As noted, one study found that testican-2 had the most significant augmentation from aorta to renal vein among all measured proteins in 22 individuals (mean increase of approximately 40%), consistent with net kidney release of the protein. In turn, testican-2 was found to have the strongest positive correlation with eGFR of all measured proteins in the Jackson Heart Study (n=1928), with a replication of this association in the Framingham Heart Study (n=1621). Furthermore, higher testican-2 levels were associated with the lower rate of a subsequent eGFR decline in both cohorts in models adjusted for baseline eGFR, albuminuria, and other CKD risk factors.5 In the second study, testican-2 was found to have the strongest positive association with eGFR among approximately 1100 measured proteins in the KORA study (Cooperative Health Research in the Region of Augsburg), with a replication of the association in the INTERVAL study, the Nord-Trøndelag Health study, and the Qatar Metabolomics Study on Diabetes (total n=2882 across the four studies). Using Mendelian randomization analyses, this study also supports a positive causal effect of eGFR on testican-2 levels (no statistically significant effect of testican-2 levels on eGFR was identified).21
Compared to these previous analyses, where mean eGFR was ≥80 ml/min per 1.72 m2 in all cohorts, this study examines testican-2 levels among individuals with substantially worse kidney function. More specifically, nearly all participants in AASK and the CRIC study had CKD at the time of testican-2 assay, with the mean mGFR and eGFR of 45.7 and 42.6 ml/min per 1.73 m2, respectively, whereas mean eGFR was 65.0 ml/min per 1.73 m2 in the ARIC study. Thus, our study extends the literature on testican-2 to individuals with established CKD or at high risk for CKD. We note that although the association between testican-2 and both incident ESKD and the secondary composite CKD progression outcome was significant in meta-analysis, only a trend for association for incident ESKD was observed in CRIC and for CKD progression in AASK, and testican-2 did not improve the C-statistic, IDI, or NRI when added to the fully adjusted model. In particular, adjustment for baseline mGFR or eGFR markedly attenuated the association between testican-2 and outcomes. This is not surprising given the very strong cross-sectional correlation between testican-2 and GFR. However, we emphasize that this correlation is positive, in contrast to other blood markers of CKD such as creatinine and cystatin C that undergo renal clearance and are thus inversely correlated with GFR.
Because testican-2 is expressed exclusively by podocytes in the kidney, adjustment for GFR has the potential to obscure physiologically or biologically informative associations. For example, testican-2 levels may serve as a general proxy for nephron mass. Low nephron number, for example, because of low birth weight, is well-established as a risk factor for CKD later in life.22 Noninvasive methods for estimating the number of glomeruli (and podocytes) by measuring urine podocyte mRNA have been developed,23 and a comparison of how these measures correlate with blood testican-2 levels would be of interest. In addition to reporting on podocyte number, testican-2 levels may provide specific insight into podocyte health; assessment of testican-2 in individuals with primary glomerular diseases is required to explore this possibility. Finally, testican-2 levels may play an active functional role in supporting kidney health. Our findings motivate future mechanistic studies to explore the biologic actions of testican-2 in the glomerulus.
This study has several key strengths. Differences in assay selection and performance can introduce challenges in analyzing protein associations across distinct studies. However, in all the studies of testican-2 and kidney function to date, testican-2 has been measured using the SOMAscan aptamer-based platform, ensuring a high degree of analytical consistency across analyses. In addition, the specificity of the aptamer for testican-2 has been corroborated by Genome-Wide Association Study, whereby variants in the cognate gene SPOCK2 have been shown to be significantly associated with blood levels of testican-2.5,24 Finally, our study spans >8000 individuals from three independent cohorts, with approximately 1200 that reached the primary outcome of ESKD. Our study also has limitations. The aptamer-based measurement of testican-2 does not provide absolute quantitation. Furthermore, this assay has not been rigorously validated for analysis of urine, which is lacking in the studies of testican-2 to date. Finally, because tissue samples are not available in AASK, the CRIC study, or the ARIC study, we are unable to associate testican-2 levels with specific CKD etiologies or histopathologic lesions.
In summary, higher blood testican-2 levels are associated with lower risk of ESKD among individuals with CKD. Future studies should simultaneously assess blood, urine, and kidney tissue in individuals with a range of kidney disease severity and etiology, including glomerular disease. Development of a targeted testican-2 assay that enables absolute quantitation, with reduced per-sample cost relative to discovery proteomics approaches, would facilitate this line of investigation. Furthermore, more work is required to investigate testican-2 biology and to determine if it is a causal mediator of kidney health.
ACKNOWLEDGMENTS
We thank the staff and participants of the AASK trial and cohort, CRIC study, and ARIC study for their important contributions.
The African American Study of Kidney Disease and Hypertension (AASK) was conducted by the AASK Investigators and supported by the NIDDK. This article was not prepared in collaboration with Investigators of the AASK study and does not necessarily reflect the opinions or views of the AASK study, the NIH, or the NIDDK. The AASK trial and cohort were supported by institutional grants from the NIH and NIDDK (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, DK 2818-02, DK057867, and DK048689), and the following pharmaceutical companies: King Pharmaceuticals, Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn. Funding for the CRIC study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902, and U24DK060990). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, and Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Some of the data reported in this study have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. The opinions presented in this paper do not necessarily reflect those of the National Institute of Diabetes Digestive and Kidney Diseases, the National Institutes of Health, the Department of Health and Human Services, or the government of the United States.
The CKD Biomarkers Consortium members are Alison Abraham, Amanda Anderson, Shawn Ballard, Joseph Bonventre, Clary Clish, Heather Collins, Steven Coca, Josef Coresh, Rajat Deo, Michelle Denburg, Ruth Dubin, Harold I. Feldman, Bart S. Ferket, Meredith Foster, Susan Furth, Peter Ganz, Daniel Gossett, Morgan Grams, Jason Greenberg, Orlando M. Gutierrez, Tom Hostetter, Lesley A. Inker, Joachim Ix, Paul L. Kimmel, Jon Klein, Andrew S. Levey, Joseph Massaro, Gearoid McMahon, Theodore Mifflin, Girish N. Nadkarni, Chirag Parikh, Vasan S. Ramachandran, Casey Rebholz, Eugene Rhee, Brad Rovin, M Sarnak, Venkata Sabbisetti, Jeffrey Schelling, Jesse Seegmiller, Michael G. Shlipak, Haochang Shou, Adriene Tin, Sushrut Waikar, Bradley Warady, Krista Whitehead, and Dawei Xie.
Published online ahead of print. Publication date available at www.jasn.org.
D.W. and L.Z. contributed equally to this work.
Disclosures
C.M. Ballantyne reports: Consultancy: 89Bio, Abbott Diagnostics, Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Denka Seiken, Esperion, Genentech, Gilead, Illumina, Matinas BioPharma Inc, Merck, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic; Research Funding: Abbott Diagnostic, Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, Novo Nordisk, Regeneron, and Roche Diagnostic; Advisory or Leadership Role: Arrowhead, Merck, and Pfizer. J. Coresh reports consultancy: Healthy.io, and SomaLogic; Ownership interest: Healthy.io; research funding: National Institutes of Health, National Kidney Foundation (NKF, which receives industry support); and advisory or leadership role: Healthy.io, and National Kidney Foundation. R. Deo reports Consultancy: Boehringer Ingelheim, Pfizer, Janssen Pharmaceuticals, Biotronik; Research Funding: iRhythm Technologies; Advisory or Leadership Role: Editorial Board of Circulation, Editorial Board of Heart Rhythm O2; and Other Interests or Relationships: Steering Committee for KDIGO. H.I. Feldman reports Consultancy: Kyowa Hakko Kirin Co, Ltd., National Kidney Foundation; Honoraria: InMed, Inc.; Advisory or Leadership Role: Steering Committee Chair of the Chronic Renal Insufficiency Cohort Study, Member of Advisory Board of the National Kidney Foundation, American Journal of Kidney Disease (AJKD) Editor in Chief; and Speakers Bureau: InMed, Inc. P. Ganz reports Consultancy: SomaLogic; and Advisory or Leadership Role: medical advisory board of SomaLogic (unpaid). M.E. Grams reports Advisory or Leadership Role: AJKD, CJASN, JASN Editorial Board, NKF Scientific Advisory Board, KDIGO Executive Committee, USRDS Scientific Advisory Board; and Other Interests or Relationships: Grant funding from NKF—which receives funding from multiple pharmaceutical companies, grant funding from the National Institutes of Health (NIH), payment from academic institutions for grand rounds, and payment from NephSAP. R.C. Hoogeveen reports Consultancy: Denka Seiken; and Research Funding: Denka Seiken. P.L. Kimmel reports Ownership Interest: CVS; Patents or Royalties: Elsevier, Royalties for coediting Chronic Renal Disease and Psychosocial Aspects of Chronic Kidney Disease; Advisory or Leadership Role: Unpaid member of Board of Directors of Academy of Medicine of Washington, DC; and Other Interests or Relationships: Co-Editor of Chronic Renal Disease Academic Press, Co-Editor of Psychosocial Aspects of Chronic Kidney Disease, and Academic Press Royalties. M.G. Shlipak reports Consultancy: Cricket Health, Intercept Pharmaceuticals; Ownership Interest: TAI Diagnostics; Research Funding: Bayer Pharmaceuticals; Honoraria: Bayer, AstraZeneca, Boehringer Ingelheim; and Advisory or Leadership Role: American Journal of Kidney Disease, Journal of the American Society of Nephrology, Circulation, and Board Member of Northern California Institute for Research and Education. R.S. Vasan reports Consultancy: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); and Honoraria: spouse has consulted for BioGen (with regard to an anti-Alzheimer drug). S.S. Waikar reports Consultancy: Wolters Kluwer, GSK, Ikena, Senda Biosciences, Strataca/3ive, BioMarin, Regeneron, Bain, CANbridge; Ownership Interest: Amazon, Apple, Southwest Airlines, Boeing, Walt Disney, Citigroup; Research Funding: Vertex, Pfizer, JNJ; and Other Interests or Relationships: expert witness for litigation related to laboratory testing (Davita), PPIs (Pfizer), and PFAO exposure (Dechert LLP). Z. Zheng reports Employer: Bayer, LLC, Brigham and Women's Hospital, and University of Pennsylvania; and Consultancy: Johnson & Johnson. L. Zhou reports Employer: Foundation Medicine, Inc.
Funding
This work was supported by NIDDK grants U01DK106981, U01DK108809, R01DK108803, and R01DK124399.
Author Contributions
M.E. Grams, E.P. Rhee, and A. Surapaneni conceptualized the study; M.E. Grams, E.P. Rhee, and L. Zhou were responsible for data curation; M.E. Grams, E.P. Rhee, D. Wen, Z. Zheng, and L. Zhou were responsible for formal analysis; C.M. Ballantyne, J. Coresh, R. Deo, R.F. Dubin, H.I. Feldman, P. Ganz, M.E. Grams, R.C. Hoogeveen, P.L. Kimmel, E.P. Rhee, M.G. Shlipak, A. Surapaneni, R.S. Vasan, S.S. Waikar, D. Wen, L. Zhou, and Z. Zheng were responsible for investigation; M.E. Grams, E.P. Rhee, D. Wen, Z. Zheng, and L. Zhou were responsible for methodology; M.E. Grams, E.P. Rhee, D. Wen, and Z. Zheng were responsible for validation; M.E. Grams and E.P. Rhee were responsible for funding acquisition and provided supervision; and M.E. Grams, E.P. Rhee, and D. Wen wrote the original draft and reviewed and edited the manuscript.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Materials
This article contains the following supplemental material online at http://links.lww.com/JSN/D627.
Supplementary Table 1. Association of testican-2 and incident ESKD by eGFR and proteinuria category.
Supplementary Table 2. Association of testican-2 and incident ESKD and CKD progression in AASK, CRIC, and ARIC adjusting for eGFRcys.
Supplementary Table 3. Association of testican-2 and CKD progression in AASK as defined by incident ESKD or ≥50% eGFR decline.
Supplementary Table 4. Meta-analysis of testican-2 and CKD progression in AASK, CRIC, and ARIC using a uniform definition of incident ESKD or ≥50% eGFR decline.
Supplementary Table 5. Meta-analysis of testican-2 and incident ESKD and CKD progression in AASK and CRIC.
References
- 1.Center for Disease Control and Prevention. Chronic Kidney Disease Surveillance System—United States. http://www.cdc.gov/ckd. Accessed August 1, 2022 [Google Scholar]
- 2.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi: 10.1056/nejmoa1214234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubin RF, Rhee EP. Proteomics and metabolomics in kidney disease, including insights into etiology, treatment, and prevention. Clin J Am Soc Nephrol. 2020;15(3):404-411. doi: 10.2215/cjn.07420619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acharya V, Olivero J. The kidney as an endocrine organ. Methodist Debakey Cardiovasc J. 2018;14(4):305-307. doi: 10.14797/mdcj-14-4-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngo D, Wen D, Gao Y, et al. Circulating testican-2 is a podocyte-derived marker of kidney health. Proc Natl Acad Sci U S A. 2020;117(40):25026-25035. doi: 10.1073/pnas.2009606117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol. 2003;14(suppl 2):S154-S165. doi: 10.1097/01.asn.0000070080.21680.cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(suppl 2):S148-S153. doi: 10.1097/01.asn.0000070149.78399.ce [DOI] [PubMed] [Google Scholar]
- 8.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302-1311. doi: 10.2215/cjn.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk In Communities) study: JACC Focus Seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939-2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi: 10.1056/nejmoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Renal Data System. 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney DIseases; 2021 [Google Scholar]
- 12.Sumida K, Nadkarni GN, Grams ME, et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020;173(6):426-435. doi: 10.7326/m20-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559. doi: 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 14.FANTOM Consortium; RIKEN Genome Exploration Research Group Phase I & II Team, Furuno M, Kasukawa T, Adachi J, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420(6915):563-573. doi: 10.1038/nature01266 [DOI] [PubMed] [Google Scholar]
- 15.Mammalian Gene Collection Program Team, Feingold EA, Grouse LH, Derge JG, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99(26):16899-16903. doi: 10.1073/pnas.242603899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vannahme C, Schubel S, Herud M, et al. Molecular cloning of testican-2: defining a novel calcium-binding proteoglycan family expressed in brain. J Neurochem. 2002;73(1):12-20. doi: 10.1046/j.1471-4159.1999.0730012.x [DOI] [PubMed] [Google Scholar]
- 17.https://www.proteinatlas.org/ENSG00000107742-SPOCK2/tissue. Accessed August 1, 2022
- 18.Nakada M, Miyamori H, Yamashita J, Sato H. Testican 2 abrogates inhibition of membrane-type matrix metalloproteinases by other testican family proteins. Cancer Res. 2003;63(12):3364-3369 [PubMed] [Google Scholar]
- 19.Ahn N, Kim WJ, Kim N, Park HW, Lee SW, Yoo JY. The interferon-inducible proteoglycan Testican-2/SPOCK2 functions as a protective barrier against virus infection of lung epithelial cells. J Virol. 2019;93(20):e00662-19. doi: 10.1128/jvi.00662-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadchouel A, Franco-Montoya ML, Guerin S, et al. Overexpression of Spock2 in mice leads to altered lung alveolar development and worsens lesions induced by hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2020;319(1):L71–L81. doi: 10.1152/ajplung.00191.2019 [DOI] [PubMed] [Google Scholar]
- 21.Matías-García PR, Wilson R, Guo Q, et al. Plasma proteomics of renal function: a trans-ethnic meta-analysis and Mendelian randomization study. J Am Soc Nephrol. 2021;32(7):1747-1763. doi: 10.1681/asn.2020071070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21(6):898-910. doi: 10.1681/asn.2009121248 [DOI] [PubMed] [Google Scholar]
- 23.Wickman L, Afshinnia F, Wang SQ, et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol. 2013;24(12):2081-2095. doi: 10.1681/asn.2013020173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun BB, Maranville JC, Peters JE, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73-79. doi: 10.1038/s41586-018-0175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are available in this article.