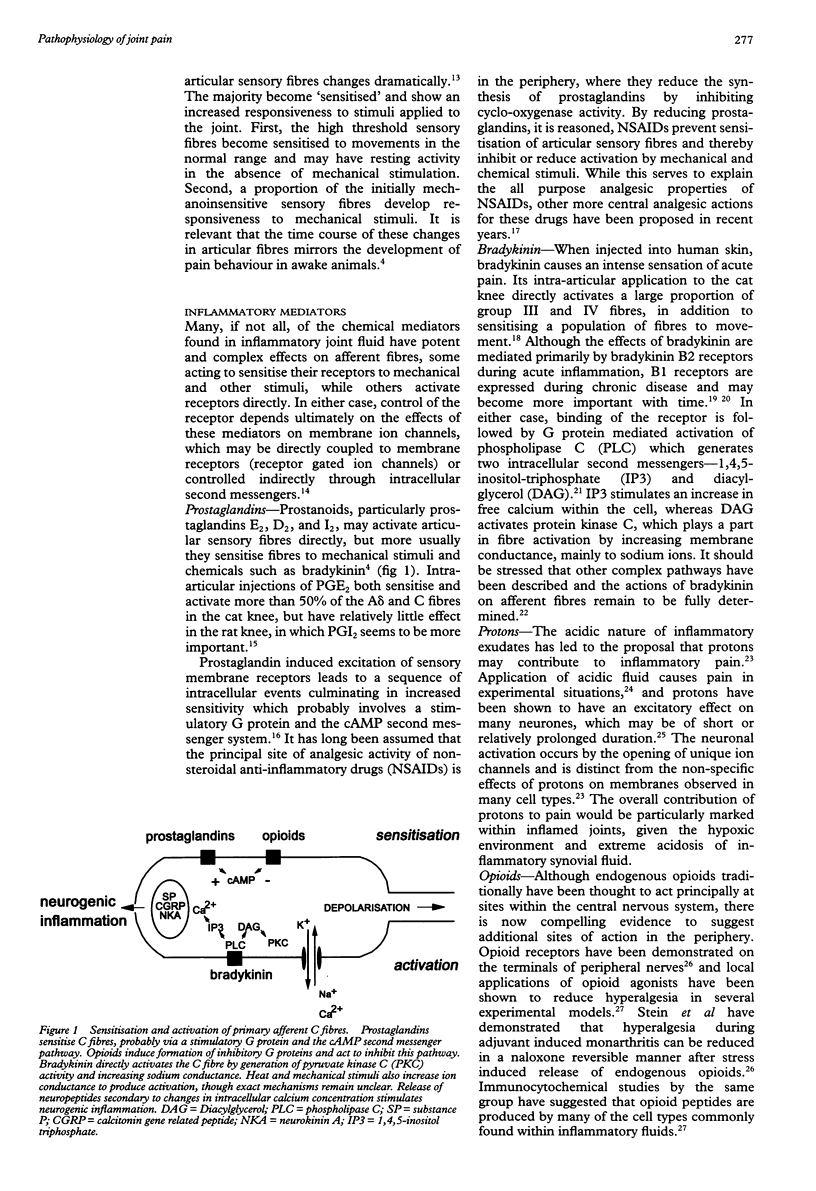
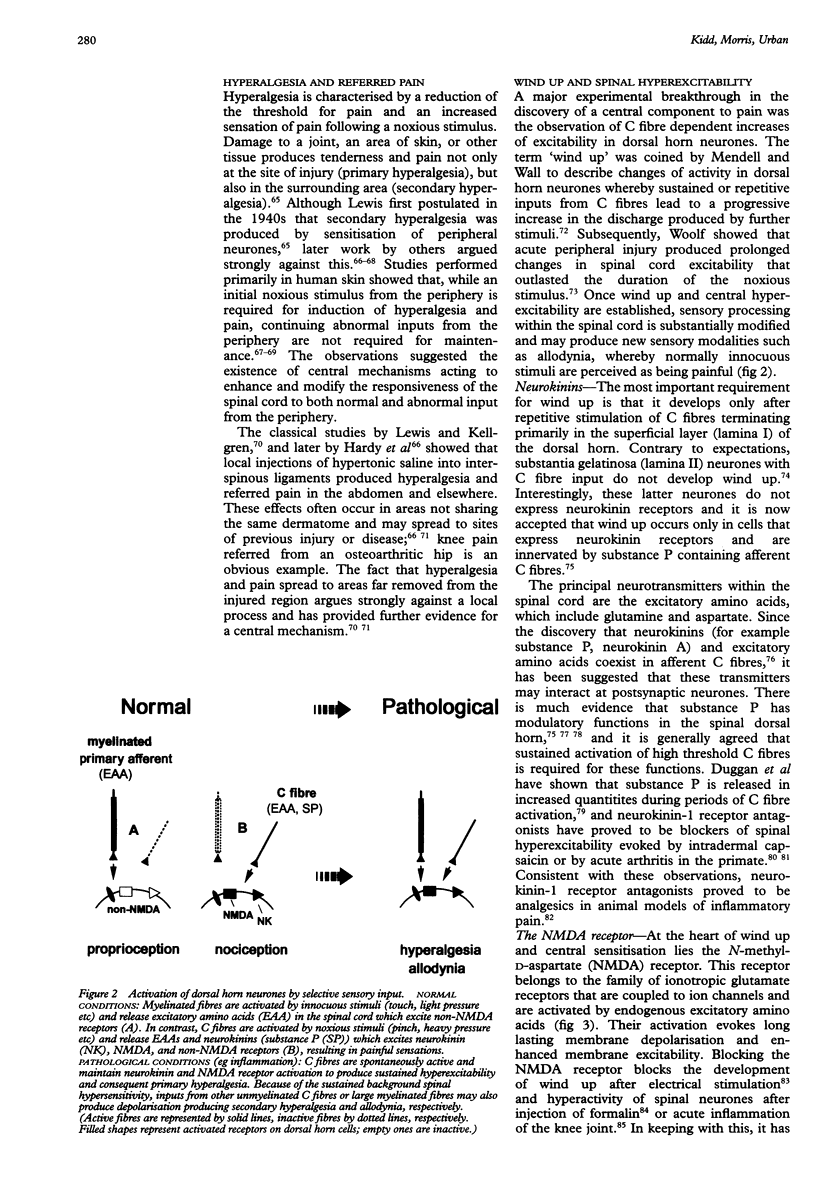
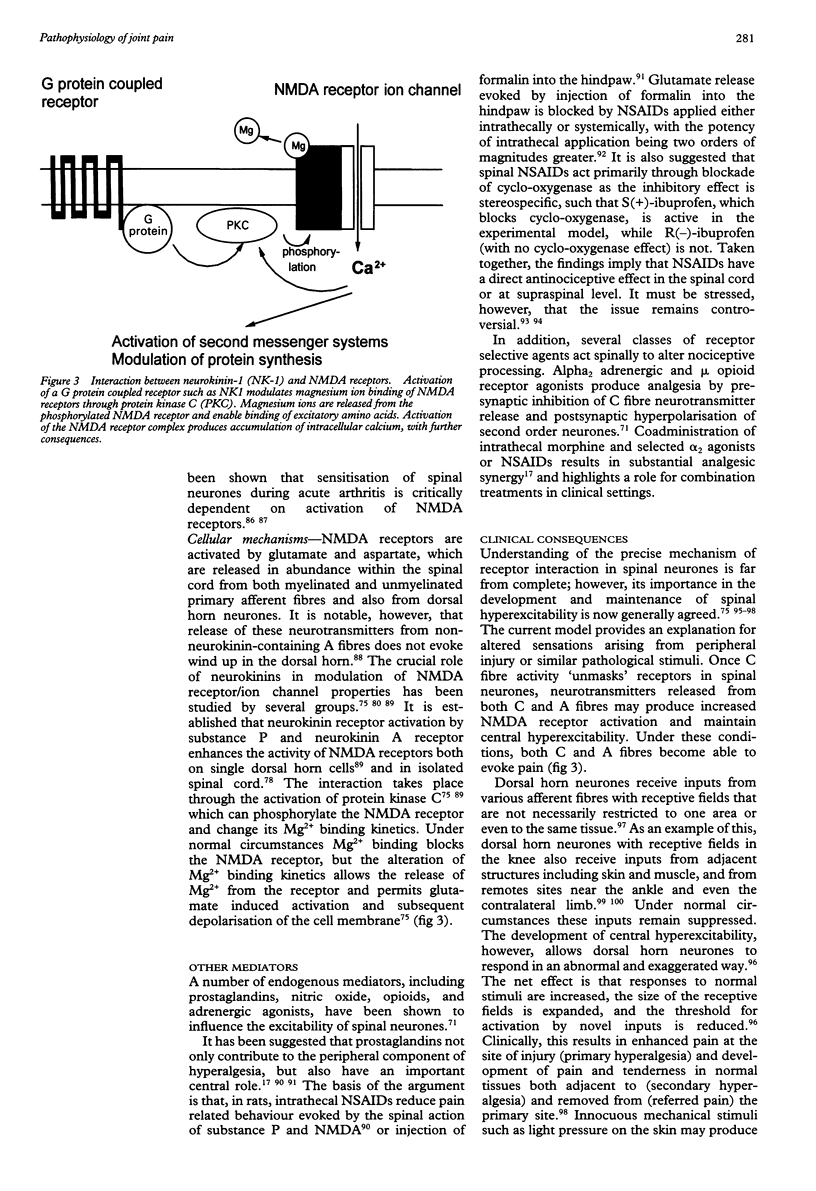
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Tuveri M. A., Carcassi U., Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992 Mar;35(3):351–355. doi: 10.1002/art.1780350315. [DOI] [PubMed] [Google Scholar]
- Baumann T. K., Simone D. A., Shain C. N., LaMotte R. H. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991 Jul;66(1):212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901 Feb 28;26(3-4):173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. J. The role of the sympathetic nervous system in painful peripheral neuropathy. Pain. 1991 Jun;45(3):221–223. doi: 10.1016/0304-3959(91)90045-Y. [DOI] [PubMed] [Google Scholar]
- Bevan S., Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994 Dec;17(12):509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Birrell G. J., McQueen D. S., Iggo A., Coleman R. A., Grubb B. D. PGI2-induced activation and sensitization of articular mechanonociceptors. Neurosci Lett. 1991 Mar 11;124(1):5–8. doi: 10.1016/0304-3940(91)90809-8. [DOI] [PubMed] [Google Scholar]
- Brune K., Beck W. S., Geisslinger G., Menzel-Soglowek S., Peskar B. M., Peskar B. A. Aspirin-like drugs may block pain independently of prostaglandin synthesis inhibition. Experientia. 1991 Mar 15;47(3):257–261. doi: 10.1007/BF01958153. [DOI] [PubMed] [Google Scholar]
- Chapman V., Dickenson A. H. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rat. Eur J Pharmacol. 1992 Sep 4;219(3):427–433. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Katz J., Vaccarino A. L., Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993 Mar;52(3):259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992 Sep;12(9):3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert F. C., Donnerer J., Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983 Apr 18;32(16):1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Cruwys S. C., Garrett N. E., Kidd B. L. Sensory denervation with capsaicin attenuates inflammation and nociception in arthritic rats. Neurosci Lett. 1995 Jul 7;193(3):205–207. doi: 10.1016/0304-3940(95)11704-z. [DOI] [PubMed] [Google Scholar]
- Cruwys S. C., Garrett N. E., Perkins M. N., Blake D. R., Kidd B. L. The role of bradykinin B1 receptors in the maintenance of intra-articular plasma extravasation in chronic antigen-induced arthritis. Br J Pharmacol. 1994 Nov;113(3):940–944. doi: 10.1111/j.1476-5381.1994.tb17083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. J., Perkins M. N. Induction of B1 receptors in vivo in a model of persistent inflammatory mechanical hyperalgesia in the rat. Neuropharmacology. 1994 Jan;33(1):127–133. doi: 10.1016/0028-3908(94)90107-4. [DOI] [PubMed] [Google Scholar]
- De Biasi S., Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. Nerve pathophysiology and mechanisms of pain in causalgia. J Auton Nerv Syst. 1983 Mar-Apr;7(3-4):371–384. doi: 10.1016/0165-1838(83)90090-5. [DOI] [PubMed] [Google Scholar]
- Donaldson L. F., Harmar A. J., McQueen D. S., Seckl J. R. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res Mol Brain Res. 1992 Nov;16(1-2):143–149. doi: 10.1016/0169-328x(92)90204-o. [DOI] [PubMed] [Google Scholar]
- Donnerer J., Schuligoi R., Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992 Aug;49(3):693–698. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- Dougherty P. M., Palecek J., Palecková V., Willis W. D. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994 Oct;72(4):1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993 Mar;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hendry I. A., Morton C. R., Hutchison W. D., Zhao Z. Q. Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res. 1988 Jun 7;451(1-2):261–273. doi: 10.1016/0006-8993(88)90771-8. [DOI] [PubMed] [Google Scholar]
- Ferrell W. R., Russell N. J. Extravasation in the knee induced by antidromic stimulation of articular C fibre afferents of the anaesthetized cat. J Physiol. 1986 Oct;379:407–416. doi: 10.1113/jphysiol.1986.sp016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C. Peptides and neurogenic inflammation. Br Med Bull. 1987 Apr;43(2):386–400. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- Freeman M. A., Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat. 1967 Jun;101(Pt 3):505–532. [PMC free article] [PubMed] [Google Scholar]
- Garrett N. E., Kidd B. L., Cruwys S. C., Tomlinson D. R. Changes in preprotachykinin mRNA expression and substance P levels in dorsal root ganglia of monoarthritic rats: comparison with changes in synovial substance P levels. Brain Res. 1995 Mar 27;675(1-2):203–207. doi: 10.1016/0006-8993(95)00066-y. [DOI] [PubMed] [Google Scholar]
- Grigg P., Schaible H. G., Schmidt R. F. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J Neurophysiol. 1986 Apr;55(4):635–643. doi: 10.1152/jn.1986.55.4.635. [DOI] [PubMed] [Google Scholar]
- Grubb B. D., Stiller R. U., Schaible H. G. Dynamic changes in the receptive field properties of spinal cord neurons with ankle input in rats with chronic unilateral inflammation in the ankle region. Exp Brain Res. 1993;92(3):441–452. doi: 10.1007/BF00229032. [DOI] [PubMed] [Google Scholar]
- Grönblad M., Konttinen Y. T., Korkala O., Liesi P., Hukkanen M., Polak J. M. Neuropeptides in synovium of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol. 1988 Dec;15(12):1807–1810. [PubMed] [Google Scholar]
- HARDY J. D., WOLFF H. G., GOODELL H. Experimental evidence on the nature of cutaneous hyperalgesia. J Clin Invest. 1950 Jan;29(1):115–140. doi: 10.1172/JCI102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J. E., Sullivan A. F., Dickenson A. H. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990 Jun 4;518(1-2):218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Helme R. D., Littlejohn G. O., Weinstein C. Neurogenic flare responses in chronic rheumatic pain syndromes. Clin Exp Neurol. 1987;23:91–94. [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Hu S. J., Zhu J. Sympathetic facilitation of sustained discharges of polymodal nociceptors. Pain. 1989 Jul;38(1):85–90. doi: 10.1016/0304-3959(89)90077-8. [DOI] [PubMed] [Google Scholar]
- Jolliffe V. A., Anand P., Kidd B. L. Assessment of cutaneous sensory and autonomic axon reflexes in rheumatoid arthritis. Ann Rheum Dis. 1995 Apr;54(4):251–255. doi: 10.1136/ard.54.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka R., Schaible H. G., Schmidt R. F. Activation of fine articular afferent units by bradykinin. Brain Res. 1985 Feb 18;327(1-2):81–90. doi: 10.1016/0006-8993(85)91501-x. [DOI] [PubMed] [Google Scholar]
- Kessler W., Kirchhoff C., Reeh P. W., Handwerker H. O. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance P. Exp Brain Res. 1992;91(3):467–476. doi: 10.1007/BF00227842. [DOI] [PubMed] [Google Scholar]
- Kidd B. L., Cruwys S. C., Garrett N. E., Mapp P. I., Jolliffe V. A., Blake D. R. Neurogenic influences on contralateral responses during experimental rat monoarthritis. Brain Res. 1995 Aug 7;688(1-2):72–76. doi: 10.1016/0006-8993(95)00512-o. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Lundberg L. E., Torebjörk H. E. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992 Mar;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Effects of interactions of naturally-occurring neuropeptides on blood flow in the rat knee joint. Br J Pharmacol. 1993 Mar;108(3):694–699. doi: 10.1111/j.1476-5381.1993.tb12863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Inhibition of carrageenan induced inflammation in the rat knee joint by substance P antagonist. Ann Rheum Dis. 1989 Nov;48(11):928–932. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford L. A., Schmidt R. F. Afferent and efferent axons in the medial and posterior articular nerves of the cat. Anat Rec. 1983 May;206(1):71–78. doi: 10.1002/ar.1092060109. [DOI] [PubMed] [Google Scholar]
- LeVasseur S. A., Gibson S. J., Helme R. D. The measurement of capsaicin-sensitive sensory nerve fiber function in elderly patients with pain. Pain. 1990 Apr;41(1):19–25. doi: 10.1016/0304-3959(90)91104-Q. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Basbaum A. I., Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci. 1985 May;5(5):1380–1386. doi: 10.1523/JNEUROSCI.05-05-01380.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Roizen M. F., Helms C., Basbaum A. I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986 Dec;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Fields H. L., Basbaum A. I. Peptides and the primary afferent nociceptor. J Neurosci. 1993 Jun;13(6):2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G. R., Mendell L. M. Nerve growth factor and nociception. Trends Neurosci. 1993 Sep;16(9):353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Harmar A. J. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989 Jan 26;337(6205):362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Lynn B. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. Pain. 1990 Apr;41(1):61–69. doi: 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- MENDELL L. M., WALL P. D. RESPONSES OF SINGLE DORSAL CORD CELLS TO PERIPHERAL CUTANEOUS UNMYELINATED FIBRES. Nature. 1965 Apr 3;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992 Oct;263(1):136–146. [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. J Neurosci. 1995 Apr;15(4):2768–2776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992 Aug 28;257(5074):1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993 Aug;79(2):270–281. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- Mapp P. I., Kidd B. L., Gibson S. J., Terry J. M., Revell P. A., Ibrahim N. B., Blake D. R., Polak J. M. Substance P-, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37(1):143–153. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Koltzenburg M. Novel classes of nociceptors: beyond Sherrington. Trends Neurosci. 1990 Jun;13(6):199–201. doi: 10.1016/0166-2236(90)90159-8. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Lewin G. R., Wall P. D. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993 Aug;3(4):602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Neugebauer V., Lücke T., Schaible H. G. Differential effects of N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists on the responses of rat spinal neurons with joint input. Neurosci Lett. 1993 May 28;155(1):29–32. doi: 10.1016/0304-3940(93)90666-9. [DOI] [PubMed] [Google Scholar]
- Neugebauer V., Lücke T., Schaible H. G. N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat's knee joint. J Neurophysiol. 1993 Oct;70(4):1365–1377. doi: 10.1152/jn.1993.70.4.1365. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Raja S. N., Meyer R. A., Campbell J. N. Peripheral mechanisms of somatic pain. Anesthesiology. 1988 Apr;68(4):571–590. doi: 10.1097/00000542-198804000-00016. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Bevan S., Dray A. Chemical activation of nociceptive peripheral neurones. Br Med Bull. 1991 Jul;47(3):534–548. doi: 10.1093/oxfordjournals.bmb.a072491. [DOI] [PubMed] [Google Scholar]
- Rusin K. I., Ryu P. D., Randic M. Modulation of excitatory amino acid responses in rat dorsal horn neurons by tachykinins. J Neurophysiol. 1992 Jul;68(1):265–286. doi: 10.1152/jn.1992.68.1.265. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Grubb B. D. Afferent and spinal mechanisms of joint pain. Pain. 1993 Oct;55(1):5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Responses of fine medial articular nerve afferents to passive movements of knee joints. J Neurophysiol. 1983 May;49(5):1118–1126. doi: 10.1152/jn.1983.49.5.1118. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988 Dec;60(6):2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F., Willis W. D. Convergent inputs from articular, cutaneous and muscle receptors onto ascending tract cells in the cat spinal cord. Exp Brain Res. 1987;66(3):479–488. doi: 10.1007/BF00270680. [DOI] [PubMed] [Google Scholar]
- Schaible Hans-Georg, Grubb Blair D., Neugebauer Volker, Oppmann Maria. The Effects of NMDA Antagonists on Neuronal Activity in Cat Spinal Cord Evoked by Acute Inflammation in the Knee Joint. Eur J Neurosci. 1991;3(10):981–991. doi: 10.1111/j.1460-9568.1991.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Sluka K. A., Jordan H. H., Westlund K. N. Reduction in joint swelling and hyperalgesia following post-treatment with a non-NMDA glutamate receptor antagonist. Pain. 1994 Oct;59(1):95–100. doi: 10.1016/0304-3959(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Sluka K. A., Lawand N. B., Westlund K. N. Joint inflammation is reduced by dorsal rhizotomy and not by sympathectomy or spinal cord transection. Ann Rheum Dis. 1994 May;53(5):309–314. doi: 10.1136/ard.53.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka K. A., Westlund K. N. Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain. 1993 Nov;55(2):217–225. doi: 10.1016/0304-3959(93)90150-N. [DOI] [PubMed] [Google Scholar]
- Steen K. H., Reeh P. W., Anton F., Handwerker H. O. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992 Jan;12(1):86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Comisel K., Haimerl E., Yassouridis A., Lehrberger K., Herz A., Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991 Oct 17;325(16):1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- Stein C., Hassan A. H., Przewłocki R., Gramsch C., Peter K., Herz A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5935–5939. doi: 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993 Jan;76(1):182–191. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- Taiwo Y. O., Bjerknes L. K., Goetzl E. J., Levine J. D. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32(3):577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. W., Dray A., Urban L. Injury-induced plasticity of spinal reflex activity: NK1 neurokinin receptor activation and enhanced A- and C-fiber mediated responses in the rat spinal cord in vitro. J Neurosci. 1994 Jun;14(6):3672–3687. doi: 10.1523/JNEUROSCI.14-06-03672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. W., Gerber G., Sivilotti L. G., Woolf C. J. Long duration ventral root potentials in the neonatal rat spinal cord in vitro; the effects of ionotropic and metabotropic excitatory amino acid receptor antagonists. Brain Res. 1992 Nov 6;595(1):87–97. doi: 10.1016/0006-8993(92)91456-o. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Lundberg L. E., LaMotte R. H. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992 Mar;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban L., Naeem S., Patel I. A., Dray A. Tachykinin induced regulation of excitatory amino acid responses in the rat spinal cord in vitro. Neurosci Lett. 1994 Feb 28;168(1-2):185–188. doi: 10.1016/0304-3940(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol. 1974 Jun;43(3):580–593. doi: 10.1016/0014-4886(74)90197-6. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Winter J., James I. F., Rang H. P., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988 Sep;8(9):3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J. Generation of acute pain: central mechanisms. Br Med Bull. 1991 Jul;47(3):523–533. doi: 10.1093/oxfordjournals.bmb.a072490. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., King A. E. Subthreshold components of the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat lumbar spinal cord. J Neurophysiol. 1989 Oct;62(4):907–916. doi: 10.1152/jn.1989.62.4.907. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Safieh-Garabedian B., Ma Q. P., Crilly P., Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994 Sep;62(2):327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yaksh T. L. Stereospecific effects of a nonpeptidic NK1 selective antagonist, CP-96,345: antinociception in the absence of motor dysfunction. Life Sci. 1991;49(26):1955–1963. doi: 10.1016/0024-3205(91)90637-q. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]


