Vascular access is a fundamental cornerstone of hemodialysis, and the arteriovenous fistula (AVF) is the preferred access for long-term KRT whenever possible. However, the creation and maturation of AVFs are often challenging, and primary failure rates remain high. After AVF surgery, 5%–20% of fistulae fail because of early thrombosis, and 20%–50% fail to mature, requiring assisted maturation by interventional procedures. Thus, the rate of unassisted maturation ranges between 30% and 70% for multiple and complex reasons.
The definition of AVF maturation is challenging. AVFs should allow using two needles, have adequate blood flow for the specific patient, and have minimal recirculation. The 2006 Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines addressed the issue of AVF maturation with the “rule of sixes,” which states that mature AVFs should achieve a blood flow of at least 600 ml/min, a diameter of at least 6 mm, and a depth of 6 mm or less from the surface of the skin. However, the 2019 update to the KDOQI guidelines did not confirm that statement, suggesting that AVF maturation should be based on clinical judgment. In 2018, the Hemodialysis Fistula Maturation study defined clinical maturation as AVF use for 75% of treatments over 4 weeks with adequate blood flow and clearance.1
AVF maturation needs a favorable pattern of vascular remodeling, characterized by an increase in blood vessel diameter and wall thickness. By contrast, nonmaturation is often related to predominant intimal hyperplasia and insufficient vessel diameter expansion. Unassisted AVF maturation was found to be significantly associated with AVF blood flow (odds ratio [OR], 1.30), presence of stenosis (OR, 0.45), AVF depth (OR, 0.88), and the interaction of AVF location with depth (OR, 0.50).2 Robbin et al.1 also reported that AVF blood flow, diameter, and depth predicted unassisted AVF clinical maturation. However, clinical maturation was influenced by a complex interaction of different combinations of vein diameter, blood flow, and vein depth.
Clinical factors have also been associated with AVF maturation, including age, sex, diabetes, prothrombotic disorders, hypertension, and peripheral vascular disease, but a robust causal association is lacking. On the other hand, AVF blood flow determinants after surgery are paramount to better understanding and predicting AVF maturation. Farrington et al.3 importantly underscored that preoperative vascular diameters demonstrated a linear association with AVF maturation with no clear threshold values. However, contrary to the expectations, the most relevant predictor of AVF maturation was preoperative arterial diameter, not venous diameter. Moreover, unassisted AVF maturation was linked to systolic blood pressure and left ventricular ejection fraction. These factors highlight the importance of adequate blood flow through AVF anastomosis. The increasingly common choice of creating AVFs with the larger arteries of the upper arm is in line with these findings. However, in the upper arm, better unassisted maturation rates may be linked to a higher number of complications, such as hand ischemia and high-output heart failure.
Are these hemodynamic factors linked to shear forces generated by the AVF? In this issue of CJASN, He et al.4 described how wall shear stress (WSS) and oscillatory shear index (OSI) influence the expansion of the AVF lumen. Using magnetic resonance imaging, the authors derived the postoperative WSS by computational fluid dynamic simulations and quantified the changes in the AVF lumen. Mean and max WSS positively correlated with the lumen area expansion at an early maturation stage—6 weeks—and after 6 months. Thus, high WSS is associated with vessel lumen expansion. On the other hand, high OSI, an index of variation in the blood flow direction, was significantly associated with reduced lumen expansion. Low OSI values indicate unidirectional flow, whereas high values are an index of flow with no predominant direction or turbulence.
The study by He et al.4 adds substantial evidence of a cause-and-effect relationship between disturbed flow and AVF maturation failure, showing that higher WSS correlates with a higher lumen expansion. By contrast, turbulent flow, indicated by high OSI levels, impairs maturation, as predicted by Remuzzi et al.5
Although the mean WSS is now an established predictor of AVF maturation, using magnetic resonance imaging to assess the WSS, the lumen expansion is not practical and cost-effective in the clinical setting. Pulsed wave Doppler ultrasound examination, which could also assess WSS, loses accuracy in the measurements of nonlaminar, complex flows.6 Ultrasound assessment of preoperative arterial diameter and the absence of intimal atherosclerosis, which will be linked to better WSS in the access vessels after AVF surgery, is the best practical approach.
Interestingly, the correlation between WSS, OSI, and AVF lumen expansion was weaker among diabetic patients, confirming the clinical observation of a higher risk of AVF failure in diabetic patients and possibly reflecting the pivotal role of endothelial dysfunction in AVF maturation.7 Indeed, shear stress levels and endothelium are strictly linked. Endothelial cell activation and proliferation, followed by the release of inflammatory and procoagulant substances, has been associated with neointimal hyperplasia and vascular constriction that, finally, results in a reduction of shear stress and blood flow.8 Consistent with the findings of He et al.,4 low WSS was associated with cephalic arch stenosis in upper-arm AVFs.9
He et al.4 outline some significant limitations of their study, which rely on several theoretical assumptions for constructing their model. Most importantly, they limited their analysis to AVF remodeling rather than evaluating AVF clinical maturation. However, they should be commended for clarifying the relationship between WSS, OSI, local hemodynamics, and AVF remodeling in the clinical setting, overcoming previous experimental studies.
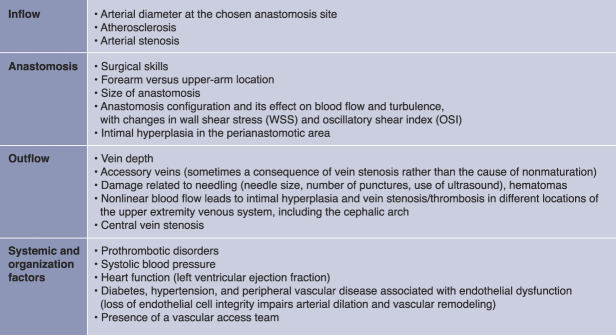
In summary, several systemic and local factors may affect AVF maturation related to its three main components: inflow, anastomosis, and outflow (Figure 1). More profound knowledge of hemodynamics effect on AVF clinical maturation was an unmet need in the vascular access field, and this study partially covered it. However, WSS and OSI alone can only partially capture the whole complexity of vascular changes in the AVF, which still needs to be broadly discovered.
Figure 1.
Pathophysiology of AVF (non)maturation. Inadequate AVF blood flow can be associated with problems of AVF inflow, anastomosis, and outflow, as well as systemic factors. AVF, arteriovenous fistula.
In the future, the implementation of artificial intelligence could provide elaborate algorithms capable of capturing the complexity of the interactions between hemodynamics, endothelial function, and uremic milieu in determining AVF maturation.
Acknowledgment
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the authors.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Association of Shear Stress with Subsequent Lumen Remodeling in Hemodialysis Arteriovenous Fistulas,” on pages 72–83.
Disclosures
M. Gallieni reports consultancy agreements with Vifor Pharma—advisory board for CKD-associated pruritus, BD—CEC committee for clinical studies in the vascular access devices area, and Sanofi Genzyme—Advisory Board, Fabry disease; honoraria as a speaker in meetings, from CME providers, at the national (Italian) and international level—sponsors of such meetings in the past 3 years have been: Amicus, Baxter, BD, Medtronic, Sanofi, and Vifor Pharma; serving as Editor-in-Chief of Journal of Vascular Access (SAGE Publishing); and serving as a member of the Board of Directors for Project for People (Italian NGO: www.projectforpeople.org/). The remaining author has nothing to disclose.
Funding
None.
Author Contributions
M. Gallieni and G. Sabiu equally contributed to the design and writing process of this editorial.
References
- 1.Robbin ML Greene T Allon M, et al. Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the Hemodialysis Fistula Maturation study. J Am Soc Nephrol. 2018;29(11):2735-2744. doi: 10.1681/ASN.2017111225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrington CA, Robbin ML, Lee T, Barker-Finkel J, Allon M. Postoperative ultrasound, unassisted maturation, and subsequent primary patency of arteriovenous fistulas. Clin J Am Soc Nephrol. 2018;13(9):1364-1372. doi: 10.2215/CJN.02230218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrington CA, Robbin ML, Lee T, Barker-Finkel J, Allon M. Early predictors of arteriovenous fistula maturation: a novel perspective on an enduring problem. J Am Soc Nephrol. 2020;31(7):1617-1627. doi: 10.1681/ASN.2019080848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y Shiu Y-T Imrey PB, et al. Association of shear stress with subsequent lumen remodeling in hemodialysis arteriovenous fistulas. Clin J Am Soc Nephrol. 2023;18(1):72-83. doi:10.2215/CJN.04630422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remuzzi A, Bozzetto M, Brambilla P. Is shear stress the key factor for AVF maturation? J Vasc Access. 2017;18(suppl 1):10-14. doi: 10.5301/jva.5000686 [DOI] [PubMed] [Google Scholar]
- 6.Du Y Goddi A Bortolotto C, et al. Wall shear stress measurements based on ultrasound vector flow imaging: theoretical studies and clinical examples. J Ultrasound Med. 2020;39(8):1649-1664. doi: 10.1002/jum.15253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Y Ye D Yang L, et al. A meta-analysis of the association between diabetic patients and AVF failure in dialysis. Ren Fail. 2018;40(1):379-383. doi: 10.1080/0886022X.2018.1456464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui MA, Ashraff S, Santos D, Carline T. An overview of AVF maturation and endothelial dysfunction in an advanced renal failure. Ren Replace Ther. 2017;3(1):42. doi: 10.1186/s41100-017-0123-x [DOI] [Google Scholar]
- 9.Hammes M, Cassel K, Boghosian M, Watson S, Funaki B, Coe F. A cohort study showing correspondence of low wall shear stress and cephalic arch stenosis in brachiocephalic arteriovenous fistula access. J Vasc Access. 2021;22(3):380-387. doi: 10.1177/1129729820942048 [DOI] [PubMed] [Google Scholar]