Key Points
Patients with obesity did not have any larger hematocrit drop after kidney biopsy compared with those without obesity.
Patients with obesity had fewer glomeruli sampled from kidney biopsy compared with those without obesity.
For patients with obesity, kidney biopsy is a safe procedure but may have lower diagnostic adequacy.
Keywords: obesity, kidney biopsy, pathology
Introduction
Patients with obesity are at higher risk of developing kidney disease.1 Although often assumed to be due to metabolic comorbidities or obesity-related glomerulopathy, recent studies indicate that kidney disease among patients with obesity is more heterogeneous and a kidney biopsy can often lead to changes in management.2 However, obesity is considered a relative contraindication to percutaneous kidney biopsy because of the technical challenges.3,4 While complication rates after kidney biopsy in the general population in both acute kidney disease and CKD have been well-examined,5–7 there is no study directly comparing complication rates in patients with obesity with those without obesity. To better support clinical decision making when caring for patients with obesity and kidney disease, further data are needed on the actual risk of biopsy in these patients. Moreover, it is also possible that diagnostic yield is lower in obese individuals because of poor visualization of the kidneys. We therefore sought to examine postbiopsy procedure-related outcomes of safety and adequacy in patients with obesity.
Methods
In two biopsy-based cohorts at Yale University and Johns Hopkins University that enroll patients undergoing clinically indicated kidney biopsies, we tested the independent association of class II or higher obesity (defined as body mass index [BMI]≥35 kg/m2) with the safety outcome of drop in hematocrit after a kidney biopsy in linear regression analyses controlling for prebiopsy risk factors for bleeding including platelet count, international normalized ratio (INR), BUN, and biopsy needle gauge. We also tested the adequacy outcome of the number of glomeruli available for diagnosis. Detailed methods are presented in the supplement. Patients included in the biopsy cohorts provided informed consent, and the study was approved by the Institutional Review Boards of Yale University and Johns Hopkins University.
Results
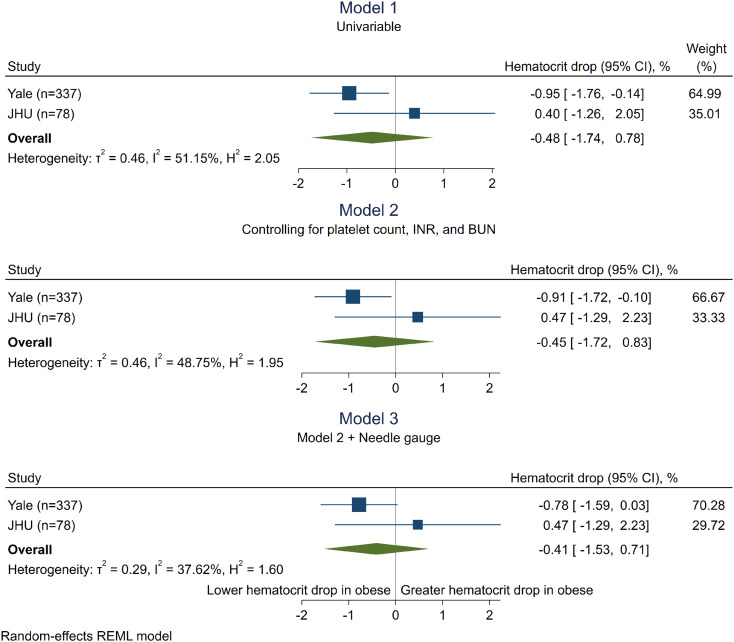
Of the 337 patients at Yale, 76 (23%) had BMI ≥35 kg/m2 and 261 (77%) had BMI<35 kg/m2. Participants with obesity were more likely to be female and have hypertension (Table 1). However, there were no significant differences in prebiopsy risk factors for bleeding including hematocrit, INR, platelet count, or use of anticoagulant medications between the two groups. Participants with obesity were more likely to be biopsied using a narrower 18-gauge needle rather than 16-gauge needle (48 [66%] versus 113 [45%], P=0.002), required computerized tomography rather than ultrasound guidance for biopsy (35 [47%] versus 75 [29%], P=0.004), and had longer procedure times (65 versus 58 minutes, P=0.04). Use of 18-G needles was associated with lesser drop in hematocrit compared with 16G needles (−2.3 [interquartile range {IQR} −1.0 to −4.1] versus −3.0 [IQR −1.4 to −4.7], P = 0.01; data not shown), and computed tomography (CT)–guided biopsies had longer procedure times than ultrasound-guided (69 [58–84] versus 52 [36–70], P = 0.0001). Participants with obesity experienced a lesser absolute drop in hematocrit from prebiopsy to postbiopsy in both univariable analysis (−2.1 [95% confidence interval {95% CI} −0.7 to −3.8] versus −3.0 [95% CI−1.4 to −4.6]%, P = 0.02; Figure 1, model 1; Table 1) and lesser drop in hematocrit relative to prebiopsy level (−6.1 [95% CI−2.6 to −12.0] versus −9.5 [95% CI−4.7 to −15.0]%, P = 0.02). Obesity was associated with a lesser drop in hematocrit in linear regression analysis controlling for prebiopsy INR, platelet count, and BUN (−0.92 [95% CI−1.73 to −0.11]%; Figure 1, model 2), but not after further controlling for needle gauge (−0.80 [95% CI−1.60 to 0.01]%; Figure 1, model 3). There were no significant differences between obese and nonobese participants in the presence of hematoma, need for transfusions, need for interventions, pain, escalation in care, or death after biopsy (Table 1). In the JHU biopsy cohort (N=78, 12 with obesity), where all biopsies were performed using 18-gauge needles, obese participants had similar hematocrit drop as nonobese (0.40 [95% CI−1.26 to 2.05]%; Figure 1, model 1, second row). Meta-analysis of the two cohorts found no association between obesity with hematocrit drop (−0.48 [95% CI−1.74 to 0.78]%, Figure 1, all models, third row). Sensitivity analyses using two alternate definitions of obesity as obesity class I or higher (BMI>30 kg/m2) and obesity class III or higher (BMI>40 kg/m2) showed similarly null association of obesity with hematocrit drop. Similar results were noted after additionally controlling for anticoagulant and antiplatelet medication use. Biopsy samples from participants with obesity in the Yale cohort had fewer glomeruli sampled (8 [IQR 5.0–13.5] versus 11 [IQR 7.0–16.0] glomeruli, P=0.003) and showed a trend toward being more likely to be deemed insufficient for diagnosis by pathologists' report (3.9% of patients with “inadequate” biopsies versus 0.8%, P=0.08). The association of obesity with fewer glomeruli sampled remained significant after controlling for needle gauge and procedure time (−2.5, [95% CI−4.4 to −0.5]).
Table 1.
Demographics, patient characteristics, and prebiopsy risk factors from Yale biopsy study
| Characteristics | BMI≥35 kg/m2 (N=76) | BMI<35 kg/m2 (N=261) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, years | 53.7 (43.4, 63.8) | 58.3 (46.3, 67.2) | 0.07 |
| BMI, kg/m2 | 39.2 (36.6, 44.1) | 27.2 (23.9, 30.1) | <0.001* |
| Female | 46 (60.5%) | 106 (40.8%) | 0.002* |
| Diabetes | 31 (41.3%) | 87 (33.5%) | 0.21 |
| Hypertension | 64 (84.2%) | 182 (69.7%) | 0.01* |
| AKI | 55 (72.4%) | 201 (77.0%) | 0.40 |
| CKD | 51 (75.0%) | 162 (64.0%) | 0.09 |
| Creatinine at biopsy, mg/dl | 2.3 (1.6, 4.7) | 2.8 (1.6, 4.2) | 0.58 |
| Prebiopsy risk factors | |||
| Hematocrit, % | 31.7 (27.1, 37.0) | 31.2 (27.0, 36.0) | 0.86 |
| Platelet count, ×109/L | 234 (178, 296) | 223 (169, 283) | 0.29 |
| INR | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.2) | 0.16 |
| BUN, mg/dl | 33.0 (24.0, 55.0) | 38.0 (27.0, 60.0) | 0.29 |
| AC medication | 19 (25%) | 69 (26%) | 0.80 |
| Antiplatelet medication | 4 (6%) | 17 (7%) | 0.79 |
| CT-guided biopsy (versus ultrasound-guided) | 35 (47%) | 75 (29%) | 0.004* |
| Performed by fellow (versus attending) | 30 (39%) | 118 (47%) | 0.26 |
| 18-gauge needle used (versus 16-gauge) | 48 (66%) | 113 (45%) | 0.002* |
| Procedure time (minutes) | 65 (52, 78) | 58 (41, 75) | 0.04* |
| Postbiopsy complication | |||
| Absolute drop in hematocrit, % | −2.1 (−0.7, −3.8) | −3.0 (−1.4, −4.6) | 0.02* |
| Relative drop in hematocrit, % of prebiopsy hematocrit | −6.1 (−2.6, −12.0) | −9.5 (−4.7, −15.0) | 0.02* |
| Hematoma detected after biopsy | 6 (7.9%) | 22 (8.5%) | 1.00 |
| Received transfusion as result of biopsy | 2 (2.6%) | 11 (4.2%) | 0.74 |
| Required intervention due to bleeding after biopsy | 0 (0.0%) | 4 (1.5%) | 0.58 |
| Significant pain after biopsy | 3 (3.9%) | 19 (7.3%) | 0.43 |
| Required escalation of care after biopsy | 3 (3.9%) | 13 (5.0%) | 1.00 |
| Death within 30 daysa | 0 (0.0%) | 4 (1.5%) | 0.58 |
| Adequacy of kidney biopsy | |||
| Number of glomeruli sampled | 8.0 (5.0, 13.5) | 11.0 (7.0, 16.0) | 0.003* |
| Biopsy sample inadequate | 3 (3.9%) | 2 (0.8%) | 0.08* |
Data presented as median (25th percentile, 75th percentile) or n (%). Chi-square test or Fisher exact test for categorical and Wilcoxon rank-sum test for continuous variables. BMI, body mass index; INR, international normalized ratio; CT, computed tomography; AC medication, received anticoagulation medication 7 days before the biopsy.
No death resulted from biopsy-related complications.
*P<0.05.
Figure 1.
Association of class II obesity with postkidney biopsy hematocrit drop. Linear regression analysis showing association of class 2 obesity with hematocrit drop after kidney biopsy. Beta coefficient and 95% confidence intervals are shown. A negative coefficient indicates that there was lesser drop in hematocrit among those with obesity. Model 1 is univariable. Model 2 controls for platelet count, INR, and BUN. Model 3 additionally controls for needle gauge (16 versus 18). CI, confidence interval.
Discussion
Our results show that participants with obesity did not have more adverse safety outcomes after kidney biopsy. In fact, in the Yale cohort, class II obesity was associated with a lesser drop in hematocrit after biopsy, a finding that was robust even when controlling for prebiopsy risk factors for bleeding but dissipated after adjusting for needle gauge. Meanwhile, in the JHU cohort, where all biopsies were performed using 18-gauge needles, there was no significant difference in hematocrit drop. Thus, the lesser drop in hematocrit among obese participants in the Yale cohort may be due to more conservative technical approaches to the biopsy in this group, including use of smaller gauge needles. This suggests that obesity by itself should not be considered a barrier to kidney biopsy due to safety concerns. However, obese participants had fewer glomeruli sampled and showed a trend toward a greater likelihood of their biopsy being deemed inadequate for diagnosis. Obese participants also had longer procedure times perhaps reflecting the technical challenges of the procedure in obese patients and greater use of CT-guided biopsies in this population. The association of obesity with fewer glomeruli sampled persisted even after controlling for needle gauge and procedure time, indicating that technical challenges may explain the lower kidney biopsy adequacy in obese individuals. Our findings indicate that kidney biopsy procedure in obese patients does not pose a greater bleeding risk than in nonobese patients but may be associated with a greater likelihood of obtaining fewer glomeruli for establishing the histological diagnosis. These findings can help clinicians make informed decisions and guide potential biopsy-eligible patients with obesity on the safety and adequacy of biopsy. Strengths of our study include a large sample size and external validation. Limitations include selection bias, such as only selecting obese patients at low risk of complications for a biopsy; however, we did not notice any significant differences in known prebiopsy risk factors for biopsy-related bleeding. Our findings provide information to guide clinical decision making when considering kidney biopsy procedures for patients with obesity.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
These findings were presented at the American Society of Nephrology Kidney Week, 2022.
Disclosures
D.G. Moledina and C.R. Parikh are named coinventor on pending patent “Methods and Systems for Diagnosis of Acute Interstitial Nephritis.” C.R. Parikh serves as a member of the advisory board of and owning equity in RenalytixAI. F. Perry Wilson is the founder of Efference, LLC, a medical communications company. D.G. Moledina and C.R. Parikh are founders of Predict AIN, LLC, a diagnostics company. D.G. Moledina reports the following: Ownership Interest: Predict AIN, LLC; Research Funding: NIDDK K23DK117065, R01DK12681, R01128087, UH3DK114866, P30DK079310; Honoraria: National Kidney Foundation; Remedy health media; British medical journal; Patents or Royalties: D.G. Moledina is a coinventor of the pending patent application “Methods and Systems for Diagnosis of Acute Interstitial Nephritis”; and Advisory or Leadership Role: Kidney360, editorial board member. S. Menez reports the following: Research Funding: RenalytixAI. C. Parikh reports the following: Consultancy: Genfit Biopharmaceutical Company; Ownership Interest: Renaltix AI; Research Funding: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); National Heart, Lung and Blood Institute (NHLBI); and Advisory or Leadership Role: Genfit Biopharmaceutical Company; Renalytix. M.A. Perazella reports the following: Honoraria: UpToDate; and Advisory or Leadership Role: Kidney360 (Deputy Editor), AJKD, Journal of Ono-Nephrology (Co-Editor-in-Chief), Clinical Nephrology (AKI Series Editor), Clinical Journal of the American Society of Nephrology, Kidney International, and Kidney International Reports (Editorial Board). H. Thiessen-Philbrook reports the following: Other Interests or Relationships: Statistical Editor, Kidney360. J.M. Turner reports the following: Consultancy: BOA biomedical, Sequana Medical; and Research Funding: Sequana Medical, Bayer, Boehringer Ingelheim. F. Perry Wilson reports the following: Consultancy: Translational Catalyst, LLC; Ownership Interest: Owner of Efference, LLC; Research Funding: Boeringher-Ingelheim; Amgen; Vifor, Whoop; Advisory or Leadership Role: Editorial Board—American Journal of Kidney Disease; Editorial Board—Clinical Journal of the American Society of Nephrology; and Other Interests or Relationships: Medical columnist—Medscape. All remaining authors have nothing to disclose.
Funding
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases awards K23DK117065 (D.G. Moledina), R01DK128087 (D.G. Moledina), R01DK126815 (D.G. Moledina), and UH3DK114866 (C.R. Parikh, D.G. Moledina, F.P. Wilson) and the Yale O'Brien Center (P30DK079310).
Author Contributions
D.G. Moledina and L. Qian conceptualized the study; D. Hu, R.L. Luciano, H. Melchinger, S. Menez, H. Thiessen-Philbrook, J.M. Turner, C.R. Parikh, L. Qian, and J. Weinstein were responsible for data curation; D. Hu and L. Qian were responsible for formal analysis; L. Qian was responsible for investigation and visualization; D.G. Moledina and L. Qian were responsible for methodology; C.P. Corona Villalobos, S. Menez, D.G. Moledina, C.R. Parikh, M.A. Perazella, F. Perry Wilson, and H. Thiessen-Philbrook provided supervision; L. Qian wrote the original draft; and all authors reviewed and edited the manuscript.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
References
- 1.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73(1):19–33. doi: 10.1038/sj.ki.5002586 [DOI] [PubMed] [Google Scholar]
- 2.Choung HYG Bomback AS Stokes MB, et al. The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int. 2019;95(3):647–654. doi: 10.1016/j.kint.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 3.Luciano RL, Moeckel GW. Update on the native kidney biopsy: Core Curriculum 2019. Am J Kidney Dis. 2019;73(3):404–415. doi: 10.1053/j.ajkd.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 4.Bandari J, Fuller TW, Turner Іі RM, D'Agostino LA. Renal biopsy for medical renal disease: indications and contraindications. Can J Urol. 2016;23(1):8121–8126. [PubMed] [Google Scholar]
- 5.Poggio ED McClelland RL Blank KN, et al. Systematic review and meta-analysis of native kidney biopsy complications [published correction appears in Clin J Am Soc Nephrol. 2021;16(2):293]. Clin J Am Soc Nephrol. 2020;15(11):1595–1602. doi: 10.2215/CJN.04710420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corapi KM, Chen JL, Balk EM, Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60(1):62–73. doi: 10.1053/j.ajkd.2012.02.330 [DOI] [PubMed] [Google Scholar]
- 7.Moledina DG Luciano RL Kukova L, et al. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. 2018;13(11):1633–1640. doi: 10.2215/cjn.04910418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript and/or supporting information.