Abstract
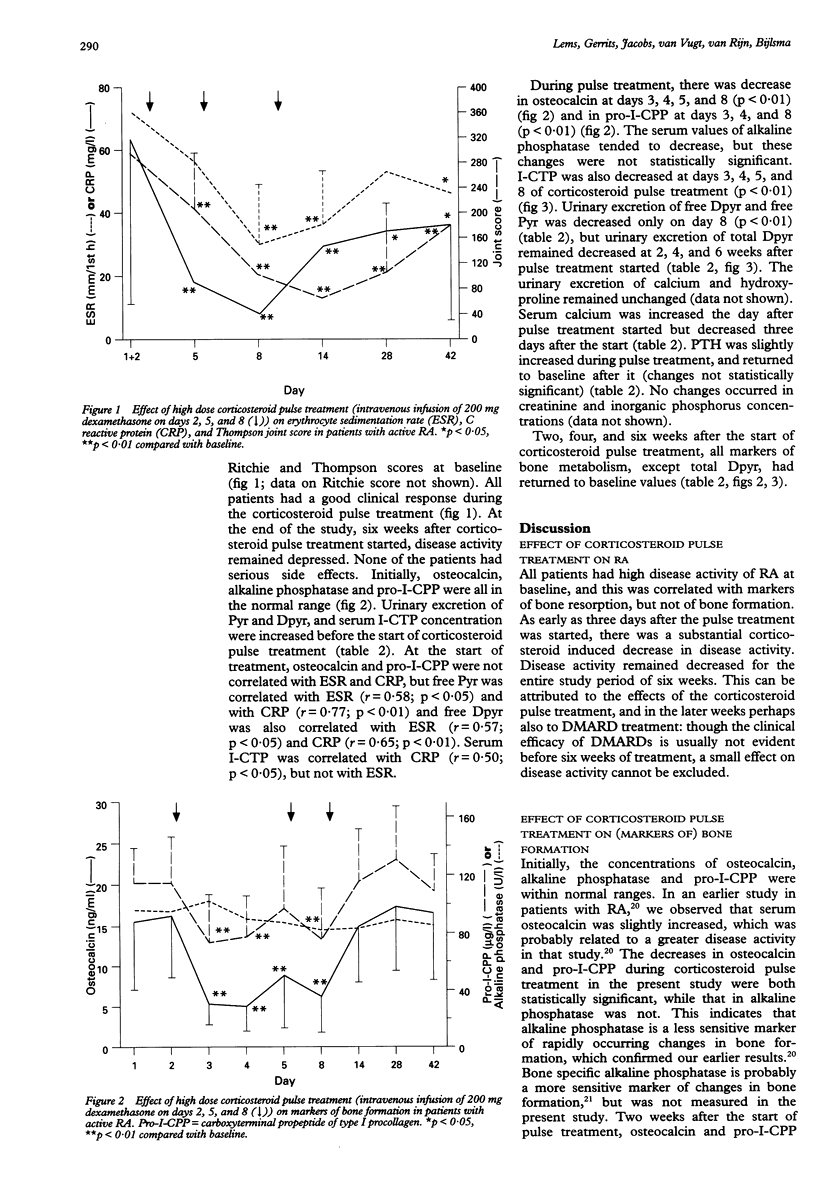
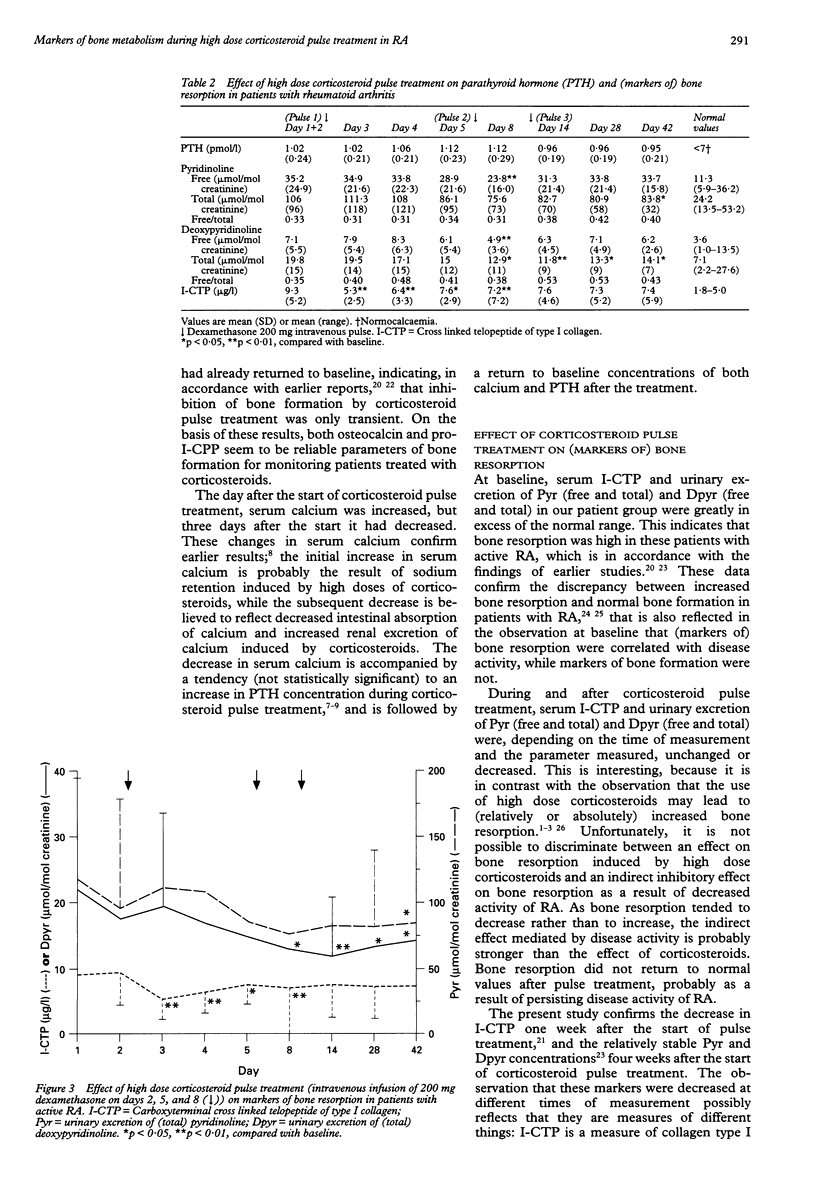
OBJECTIVE: To examine the effect of high dose corticosteroid pulse treatment (three times 200 mg dexamethasone intravenously in eight days) on calcium and bone metabolism in 17 consecutive patients with active rheumatoid arthritis (RA). METHODS: Bone formation was quantified by measurement of serum alkaline phosphatase, osteocalcin, and carboxyterminal propeptide of type I procollagen (pro-I-CPP) concentrations. Bone resorption was measured by urinary excretion of calcium, hydroxyproline, (free and total) deoxypyridinoline (Dpyr), (free and total) pyridinoline (Pyr), and serum concentrations of the carboxyterminal cross linked telopeptide of type I collagen (I-CTP). Disease activity of RA was measured by erythrocyte sedimentation rate, C reactive protein, and Ritchie and Thompson joint scores. RESULTS: Disease activity was initially high, and decreased during corticosteroid pulse treatment and the following five weeks. Osteocalcin, alkaline phosphatase, and pro-I-CPP concentrations were initially within normal limits, while I-CTP, Dpyr, and Pyr were increased. Osteocalcin and pro-I-CPP concentrations decreased (p < 0.01) during corticosteroid pulse treatment, but rapidly returned to baseline after the treatment. No changes were observed in alkaline phosphatase and urinary excretion of calcium and hydroxyproline. Bone resorption measured by serum I-CTP and urinary excretion of Pyr and Dpyr was unchanged or decreased (p < 0.05-0.01), depending on the time of measurement and the parameter measured. CONCLUSIONS: In these patients with active RA, bone resorption was increased, while bone formation was within normal limits. During high dose corticosteroid pulse treatment, bone formation was only transiently decreased, while markers of bone resorption were unchanged or decreased. Because corticosteroid pulse treatment has only a short term negative effect on bone formation, and because it probably reduces bone resorption, at least partly as a result of the decreased disease activity, the effect of corticosteroid pulse treatment on bone may be assumed to be relatively mild.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi J. D., Bensen W. G., Hodsman A. B. Corticosteroid-induced osteoporosis. Semin Arthritis Rheum. 1993 Jun;22(6):375–384. doi: 10.1016/s0049-0172(05)80029-0. [DOI] [PubMed] [Google Scholar]
- Adinoff A. D., Hollister J. R. Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med. 1983 Aug 4;309(5):265–268. doi: 10.1056/NEJM198308043090502. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Astbury C., Bird H. A., McLaren A. M., Robins S. P. Urinary excretion of pyridinium crosslinks of collagen correlated with joint damage in arthritis. Br J Rheumatol. 1994 Jan;33(1):11–15. doi: 10.1093/rheumatology/33.1.11. [DOI] [PubMed] [Google Scholar]
- Bijlsma J. W., Duursma S. A., Huber-Bruning O. Bone metabolism during methylprednisolone pulse therapy in rheumatoid arthritis. Ann Rheum Dis. 1986 Sep;45(9):757–760. doi: 10.1136/ard.45.9.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell A., Russell R. G., Eastell R. Factors affecting the assay of urinary 3-hydroxy pyridinium crosslinks of collagen as markers of bone resorption. Eur J Clin Invest. 1993 Jun;23(6):341–349. doi: 10.1111/j.1365-2362.1993.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Cosman F., Nieves J., Herbert J., Shen V., Lindsay R. High-dose glucocorticoids in multiple sclerosis patients exert direct effects on the kidney and skeleton. J Bone Miner Res. 1994 Jul;9(7):1097–1105. doi: 10.1002/jbmr.5650090718. [DOI] [PubMed] [Google Scholar]
- Delmas P. D. Biochemical markers of bone turnover. J Bone Miner Res. 1993 Dec;8 (Suppl 2):S549–S555. doi: 10.1002/jbmr.5650081323. [DOI] [PubMed] [Google Scholar]
- Delmas P. D., Gineyts E., Bertholin A., Garnero P., Marchand F. Immunoassay of pyridinoline crosslink excretion in normal adults and in Paget's disease. J Bone Miner Res. 1993 May;8(5):643–648. doi: 10.1002/jbmr.5650080516. [DOI] [PubMed] [Google Scholar]
- Dykman T. R., Gluck O. S., Murphy W. A., Hahn T. J., Hahn B. H. Evaluation of factors associated with glucocorticoid-induced osteopenia in patients with rheumatic diseases. Arthritis Rheum. 1985 Apr;28(4):361–368. doi: 10.1002/art.1780280402. [DOI] [PubMed] [Google Scholar]
- Eggelmeijer F., Papapoulos S. E., Westedt M. L., Van Paassen H. C., Dijkmans B. A., Breedveld F. C. Bone metabolism in rheumatoid arthritis; relation to disease activity. Br J Rheumatol. 1993 May;32(5):387–391. doi: 10.1093/rheumatology/32.5.387. [DOI] [PubMed] [Google Scholar]
- Elomaa I., Virkkunen P., Risteli L., Risteli J. Serum concentration of the cross-linked carboxyterminal telopeptide of type I collagen (ICTP) is a useful prognostic indicator in multiple myeloma. Br J Cancer. 1992 Aug;66(2):337–341. doi: 10.1038/bjc.1992.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P., Gineyts E., Arbault P., Christiansen C., Delmas P. D. Different effects of bisphosphonate and estrogen therapy on free and peptide-bound bone cross-links excretion. J Bone Miner Res. 1995 Apr;10(4):641–649. doi: 10.1002/jbmr.5650100418. [DOI] [PubMed] [Google Scholar]
- Garnero P., Grimaux M., Demiaux B., Preaudat C., Seguin P., Delmas P. D. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J Bone Miner Res. 1992 Dec;7(12):1389–1398. doi: 10.1002/jbmr.5650071206. [DOI] [PubMed] [Google Scholar]
- Gerrits M. I., Thijssen J. H., van Rijn H. J. Determination of pyridinoline and deoxypyridinoline in urine, with special attention to retaining their stability. Clin Chem. 1995 Apr;41(4):571–574. [PubMed] [Google Scholar]
- Hall G. M., Spector T. D., Delmas P. D. Markers of bone metabolism in postmenopausal women with rheumatoid arthritis. Effects of corticosteroids and hormone replacement therapy. Arthritis Rheum. 1995 Jul;38(7):902–906. doi: 10.1002/art.1780380705. [DOI] [PubMed] [Google Scholar]
- Hofman D. M., Lems W. F., Witkamp T. D., Putte V. D., Bijlsma J. W. Demonstration of calf abnormalities by magnetic resonance imaging in polyarteritis nodosa. Clin Rheumatol. 1992 Sep;11(3):402–404. doi: 10.1007/BF02207202. [DOI] [PubMed] [Google Scholar]
- Kollerup G., Hansen M., Hørslev-Petersen K. Urinary hydroxypyridinium cross-links of collagen in rheumatoid arthritis. Relation to disease activity and effects of methylprednisolone. Br J Rheumatol. 1994 Sep;33(9):816–820. doi: 10.1093/rheumatology/33.9.816. [DOI] [PubMed] [Google Scholar]
- Lems W. F., Jacobs J. W., van den Brink H. R., van Rijn H. J., Bijlsma J. W. Transient decrease in osteocalcin and markers of type 1 collagen turnover during high-dose corticosteroid pulse therapy in rheumatoid arthritis. Br J Rheumatol. 1993 Sep;32(9):787–789. doi: 10.1093/rheumatology/32.9.787. [DOI] [PubMed] [Google Scholar]
- Lems W. F., Jahangier Z. N., Jacobs J. W., Bijlsma J. W. Vertebral fractures in patients with rheumatoid arthritis treated with corticosteroids. Clin Exp Rheumatol. 1995 May-Jun;13(3):293–297. [PubMed] [Google Scholar]
- Lukert B. P., Raisz L. G. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990 Mar 1;112(5):352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- MacDonald A. G., McHenry P., Robins S. P., Reid D. M. Relationship of urinary pyridinium crosslinks to disease extent and activity in osteoarthritis. Br J Rheumatol. 1994 Jan;33(1):16–19. doi: 10.1093/rheumatology/33.1.16. [DOI] [PubMed] [Google Scholar]
- Meunier P. J., Dempster D. W., Edouard C., Chapuy M. C., Arlot M., Charhon S. Bone histomorphometry in corticosteroid-induced osteoporosis and Cushing's syndrome. Adv Exp Med Biol. 1984;171:191–200. [PubMed] [Google Scholar]
- Risteli J., Elomaa I., Niemi S., Novamo A., Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clin Chem. 1993 Apr;39(4):635–640. [PubMed] [Google Scholar]
- Risteli L., Risteli J., Moniz C. Measuring collagen degradation. Eur J Clin Invest. 1993 Jun;23(6):339–340. doi: 10.1111/j.1365-2362.1993.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Sambrook P. N., Jones G. Corticosteroid osteoporosis. Br J Rheumatol. 1995 Jan;34(1):8–12. doi: 10.1093/rheumatology/34.1.8. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Kung V. T., Daniloff Y. N., Hesley R. P., Gomez B., Nielsen L. A., Rosen H. N., Zuk R. F. Immunoassay for urinary pyridinoline: the new marker of bone resorption. J Bone Miner Res. 1993 May;8(5):635–641. doi: 10.1002/jbmr.5650080515. [DOI] [PubMed] [Google Scholar]
- Spector T. D., James I. T., Hall G. M., Thompson P. W., Perrett D., Hart D. J. Increased levels of urinary collagen crosslinks in females with rheumatoid arthritis. Clin Rheumatol. 1993 Jun;12(2):240–244. doi: 10.1007/BF02231535. [DOI] [PubMed] [Google Scholar]
- Thompson P. W., Spector T. D., James I. T., Henderson E., Hart D. J. Urinary collagen crosslinks reflect the radiographic severity of knee osteoarthritis. Br J Rheumatol. 1992 Nov;31(11):759–761. doi: 10.1093/rheumatology/31.11.759. [DOI] [PubMed] [Google Scholar]
- Weusten B. L., Jacobs J. W., Bijlsma J. W. Corticosteroid pulse therapy in active rheumatoid arthritis. Semin Arthritis Rheum. 1993 Dec;23(3):183–192. doi: 10.1016/s0049-0172(05)80039-3. [DOI] [PubMed] [Google Scholar]


