ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) bacteria producing heat-stable toxin (STa) and/or heat-labile toxin (LT) are among top causes of children's diarrhea and travelers’ diarrhea. Currently no vaccines are available for ETEC associated diarrhea. A major challenge in developing ETEC vaccines is the inability to stimulate protective antibodies against the key STa toxin that is potently toxic and also poorly immunogenic. A recent study suggested toxoid fusion 3xSTaN12S-dmLT, which consists of a monomer LT toxoid (LTR192G/L211A) and three copies of STa toxoid STaN12S, may represent an optimal immunogen inducing neutralizing antibodies against STa toxin [IAI 2014, 82(5):1823-32]. In this study, we immunized mice with this fusion protein following a different parenteral route and using different adjuvants to further characterize immunogenicity of this toxoid fusion. Data from this study showed that 3xSTaN12S-dmLT toxoid fusion induced neutralizing anti-STa antibodies in the mice following subcutaneous immunization, as effectively as in the mice under intraperitoneal route. Data also indicated that double mutant LT (dmLT) can be an effective adjuvant for this toxoid fusion in mice subcutaneous immunization. Results from this study affirmed that toxoid fusion 3xSTaN12S-dmLT induces neutralizing antibodies against STa toxin, suggesting this toxoid fusion is potentially a promising immunogen for ETEC vaccine development.
Keywords: diarrhea, ETEC (enterotoxigenic E. coli), toxoid fusion, neutralizing antibodies, vaccine
Plain language summary
Toxoid fusion antigen 3xSTaN12S-dmLT of enterotoxigenic Escherichia coli is a desirable antigen for vaccines against children's diarrhea and travelers’ diarrhea.
INTRODUCTION
Escherichia coli bacteria that produce heat-labile toxin (LT) and/or heat-stable toxin (STa), known as enterotoxigenic E. coli (ETEC), are the leading cause of moderate-to-severe diarrhea to children in developing countries and to children and adults from developed countries traveling to ETEC endemic regions (Sanders et al. 2005; WHO 2006; Sack et al. 2007; Black et al. 2010; Kotloff et al. 2012). ETEC fimbrial or non-fimbrial adhesins and enterotoxins LT and STa are virulence determinants in diarrheal disease. Adhesins initiate adherence of ETEC bacteria to host cell receptors and promote bacteria colonization in small intestines. Enterotoxins enter host small intestinal epithelial cells and enzymatically disrupt homeostasis, resulting in elevation of intracellular cyclic AMP (by LT) and cyclic GMP (by STa) levels, fluid and electrolyte hypersecretion and watery diarrhea (Nataro and Kaper 1998; Svennerholm and Tobias 2008). Vaccination is regarded the most effective and the most practical preventive approach against ETEC diarrhea (Svennerholm 2011; Zhang 2012). Unfortunately, there are no vaccines available for ETEC-associated diarrhea.
Developing effective ETEC vaccines is proven difficult. One major challenge is the inability to identify safe antigens to induce protective antibodies against the key STa toxin (Svennerholm 2011; Zhang 2012). As potent enterotoxins, both native STa and LT cannot be used directly as vaccine components. However, unlike LT, of which non-toxic LT B subunit (LTB) or detoxified LT mutants can be used as safe antigens to elicit protective antibodies against LT, safe STa antigens to induce neutralizing antibodies against STa toxin had not been identified until very recently. In addition, the poorly immunogenic 19-amino acid STa toxin itself cannot induce host anti-STa antibody response. Recent studies demonstrated that STa with mutations of a single amino acid became less or not toxic (Zhang et al. 2010, 2013; Liu et al. 2011). These low or non-toxic STa molecules or toxoids were found safe STa antigens and also became immunogenic when they were genetically fused to a LT toxoid carrier, demonstrated by that derived STa-LT toxoid fusions induced antibodies neutralizing STa and LT toxins (Zhang et al. 2010, 2013; Liu et al. 2011). A more recent study revealed that LT-STa toxoid fusions possessing different STa toxoids exhibited anti-STa antigenicity variously. Among the LT-STa toxoid fusions we examined, 3xSTaN12S-dmLT, a peptide consisting of three STa toxoid STaN12S, one LT A subunit (LTA) and one (not five) LT B subunit (LTB), induced stronger neutralizing anti-STa antibodies in the intraperitoneally (IP) immunized mice (Ruan et al. 2014). This suggests that 3xSTaN12S-dmLT may represent an optimal toxoid fusion for inducing neutralizing anti-STa antibodies and potentially an immunogen for ETEC vaccines.
To verify anti-STa antigenicity of this 3xSTaN12S-dmLT toxoid fusion, we carried out mouse subcutaneous (SC) immunization and also mouse IP immunization with different adjuvant, and examined the antigen for induction of anti-STa antibody response. In addition, we evaluated antibodies derived from the SC and IP immunized mice for in vitro neutralization activity against STa toxin to assess potential of this toxoid fusion in ETEC vaccine development.
MATERIALS AND METHODS
Antigens and adjuvants used in mouse SC and IP immunization
Toxoid fusion 3xSTaN12S-dmLT was purified from recombinant Escherichia coli strain 9331 (Ruan et al. 2014) as the immunogen. This fusion protein has three copies of STa toxoid STaN12S fused to a monomeric LT mutant (one LTA subunit fused to one LTB subunit) as a single peptide. Montanide ISA51 (kindly provided by SEPPIC, Fairfield, NJ, USA), Freund's incomplete adjuvant (FIA; Sigma, ST. Louis, MO, USA) and holotoxin-structured double mutant LT (dmLT; provided by Walter Reed Army Institute of Research, Silver Spring, MD, USA) were used as adjuvants in mouse SC and IP immunization (Table 1).
Table 1.
Doses of immunogen 3xSTaN12S-dmLT and adjuvants used in mouse SC and IP immunization studies.
| Routes Doses | SC | IP |
|---|---|---|
| Antigen | 3xSTaN12S-dmLT (40 μg in 50 μl) | 3xSTaN12S-dmLT (200 μg in 200 μl) |
| (1) No adjuvant | (1) FIA (200 μl) | |
| Adjuvant | (2) ISA51 (50 μl) | (2) dmLT (0.2 μg in 200 μl) |
| (3) dmLT (2 μg in 50 μl) | (3) dmLT (2 μg in 200 μl) |
Mouse SC or IP immunization
A total of seven groups of 8-week-old female BALB/c mice (Charles River Laboratories International, Inc., Wilmington, MA, USA) were included in immunization studies: three SC immunization groups, three IP immunization groups and one negative control group.
For SC immunization, five mice in a group were each administered with 40 μg 3xSTaN12S-dmLT toxoid fusion protein without adjuvant, with adjuvant ISA51 (SEPPIC) or with 2 μg dmLT adjuvant. For IP immunization, a group of five mice was administered with 200 μg toxoid fusion protein (in 200 μl) and 200 μl FIA, a group of six mice was administered with 200 μg toxoid fusion protein and 0.2 μg dmLT adjuvant (in 200 μl) and a third group of six mice was administered with 200 μg toxoid fusion protein and 2 μg dmLT adjuvant (in 200 μl). Each immunized mouse received two boosters with the same route and the same dose of the primary, at the interval of 2 weeks. Five mice in the control group received no IP or SC administration. Mouse serum samples were collected before the primary administration and 10 days after the final booster and stored at −80°C.
Mouse immunization complied with the Public Health Service Policy on Humane Care and Use of Laboratory Animals (Revision 2015; http://grants.nih.gov/grants/olaw/references/phspol.htm). All mouse studies were approved by the Kansas State University IACUC and supervised by a university staff veterinarian.
Mouse serum IgG antibody titration
Mouse serum anti-STa and anti-LT IgG antibody responses were measured in ELISAs as previously described (Zhang et al. 2010, 2013; Ruan et al. 2014).
Mouse serum antibody neutralization assays
Anti-STa and anti-LT antibody neutralization activities were examined from mouse serum samples pooled from each immunization group or the control group (in triplicates) using T-84 cells and a cAMP EIA kit or a cGMP EIA kit (Enzo Life, Farmingdale, NY, USA), as described previously (Zhang et al. 2010, 2013; Liu et al. 2011; Ruan et al. 2014).
Statistical analysis
Mouse antibody titration and antibody neutralization data were analyzed with SAS program (SAS Institute, Cary, NC, USA) and presented in means ± standard deviations. Different treatment groups were pairwise compared using two-tailed distribution and two-sample equal or unequal variance. Student's t-test P value of <0.05 refers statistically significant difference.
RESULTS
Mice SC immunized with toxoid fusion 3xSTaN12S-dmLT developed antibodies specific to STa and LT toxins
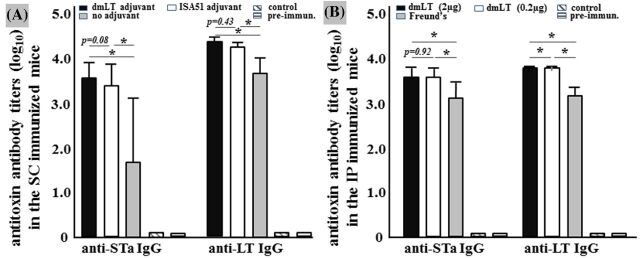
Mice developed antibody responses specific to STa and LT following SC administration of 3xSTaN12S-dmLT (Fig. 1A). Mice SC administered with the toxoid fusion and adjuvant dmLT and mice SC with the toxoid fusion and adjuvant ISA51 had similar anti-STa IgG titers (3.62 ± 0.42 and 3.42 ± 0.59; in log10) and anti-LT IgG titers (4.40 ± 0.15 and 4.30 ± 0.09) detected in their serum samples. No IgG antibody responses to STa or LT were detected from the control mouse serum samples or the serum samples collected prior to immunization (Fig. 1A).
Figure 1.
Mouse serum anti-STa and anti-LT IgG antibody titers (in log10) detected from the immunized groups and the control group.(A) Anti-STa and anti-LT IgG antibody titers from the serum samples of mice SC immunized with toxoid fusion 3xSTaN12S-dmLT without adjuvant, or with dmLT (2 μg) or Montanide ISA51 as the adjuvant.(B) Anti-STa and anti-LT IgG antibody titers from the serum samples of mice IP immunized with toxoid fusion 3xSTaN12S-dmLT with 0.2 μg dmLT, 2 μg dmLT or FIA as the adjuvant. Serum samples of the control mice and serum samples collected from mice prior to immunization were included for anti-STa and anti-LT IgG antibody titration. P values were calculated from Student's t-test. Boxes and bars represented means and standard deviations of antibody titers, respectively.
Serum IgG antibody titers specific to STa and LT in the mice administered with the fusion antigen alone (without adjuvant) were detected at 1.74 ± 1.66 and 3.76 ± 0.43 (log10), which were significantly lower than those from the mice administered with the toxoid fusion and dmLT adjuvant (P < 0.01) or ISA51 adjuvant (P < 0.01).
Mice IP administered with 3xSTaN12S-dmLT developed antibodies specific to STa and LT
Mice developed anti-STa and anti-LT antibody responses following IP administration of 3xSTaN12S-dmLT (Fig. 1B). Anti-STa IgG titers were 3.62 ± 0.21, 3.61 ± 0.18 and 3.12 ± 0.36 (log10) in the serum of the IP-administered groups using 2 μg dmLT, 0.2 μg dmLT or FIA as the adjuvant. Anti-LT IgG antibody titers were 3.8 ± 0.02, 3.73 ± 0.02 and 3.16 ± 0.24 in the serum of the three administered groups with adjuvant 2 μg dmLT, 0.2 μg dmLT or FIA, respectively. The STa and LT IgG titers in the two immunization groups with dmLT adjuvant were significantly greater than those in the immunization group with FIA adjuvant (P < 0.01, P < 0.01). No anti-STa or anti-LT IgG titers were detected from the serum samples of the control mice or the pre-immunization serum samples (Fig. 1B).
Anti-STa IgG antibody titers detected from the serum samples of the two IP administered groups using dmLT adjuvant were not different (P = 0.92), but IgG titers specific to LT in the group using 2 μg dmLT adjuvant were greater than those in the group using 0.2 μg dmLT adjuvant (P < 0.01).
Serum samples of the mice SC or IP administered with 3xSTaN12S-dmLT neutralized STa toxin
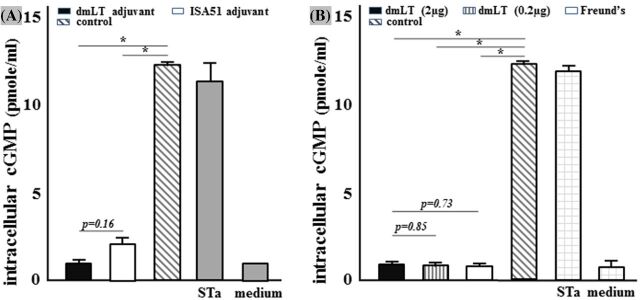
Serum samples pooled from mice following SC or IP immunization neutralized STa toxin in vitro (Fig. 2). Intracellular cGMP levels of the T-84 cells treated with 2 ng STa and 30 μl pooled serum from the SC immunization group using dmLT and ISA51 adjuvant were 1.06 ± 0.34 and 2.12 ± 0.59 pmole ml−1, respectively (Fig. 2A).
Figure 2.
Mouse serum antibodies against STa toxin in vitro antibody neutralization assay with T-84 cells and EIA cGMP kit. Pooled serum samples (30 μl) from each immunization group or the control group were used to incubate STa (2 ng). The serum/toxin mixture samples were added to T-84 cells. After 1 h incubation, intracellular cGMP levels (pmole ml−1) in T-84 cells were measured.(A) Intracellular cGMP levels in T-84 cells incubated with STa toxin and pooled serum from the SC immunized group using 2 μg dmLT or ISA51 adjuvant, or from the control group. (B)Intracellular cGMP levels in T-84 cells incubated with STa toxin and pooled serum from the IP immunized groups using 2 μg dmLT, 0.2 μg dmLT or FIA as the adjuvant, or from the control group. Baseline cGMP levels (T-84 cells incubated with cell culture medium) and cGMP levels in T-84 cells incubated with 2 ng STa (as positive reference) were included. P values were calculated with Student's t-test. Boxes and bars represented means and standard deviations of cGMP levels, respectively.
Intracellular cGMP levels of the T-84 cells treated with 2 ng STa and 30 μl pooled serum from the IP immunization groups using 2 μg dmLT adjuvant, 0.2 μg dmLT adjuvant and FIA were 0.81 ± 0.16, 0.79 ± 0.06 and 0.77 ± 0.05 pmole ml−1, respectively (Fig. 2B).
Baseline intracellular cGMP levels of T-84 cells, in which cells were treated with culture medium only, were 0.67 ± 0.37 pmole ml−1. In contrast, the cGMP levels in the T-84 cells treated with STa alone and STa with the control mouse serum were 11.7 ± 0.47 and 12.3 ± 0.06 pmole ml−1, respectively.
Serum samples of the SC or IP administrated mice neutralized CT
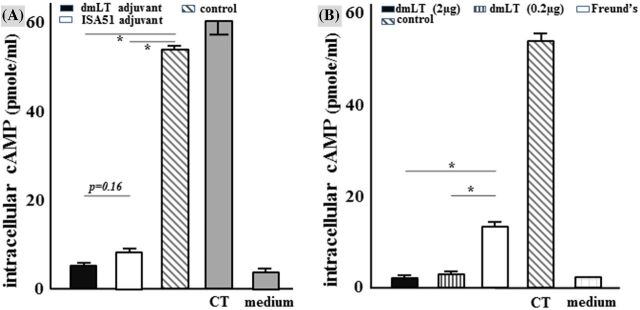
Intracellular cAMP levels of the T-84 cells that were treated with 10 ng CT and 30 μl pooled serum sample of the mice SC administered with the toxoid fusion and dmLT adjuvant or ISA51 adjuvant were 5.42 ± 0.08 and 7.54 ± 0.46 pmole ml−1, respectively (Fig. 3A). Intracellular cAMP concentrations of the T-84 cells that were treated with 10 ng CT and 30 μl pooled serum from the mice IP administered groups using 2 μg dmLT adjuvant, 0.2 μg dmLT adjuvant or FIA were 2.3 ± 0.37, 2.7 ± 0.06 and 13.3 ± 1.2 pmole ml−1, respectively (Fig. 3B).
Figure 3.
Mouse serum antibodies against CT toxin in vitro antibody neutralization assays with T-84 cells and EIA cAMP kit. Pooled serum samples (30 μl) from each immunization group or the control group were used to incubate CT (10 ng). The serum/toxin mixture samples were added to T-84 cells. After 3 h incubation, intracellular cAMP levels (pmole ml−1) in T-84 cells were measured. (A)Intracellular cAMP levels in T-84 cells incubated with CT toxin and pooled serum from the SC immunized groups using 2 μg dmLT or ISA51 adjuvant or the control group. (B)Intracellular cAMP levels in T-84 cells incubated with CT toxin and pooled serum from the IP immunized groups using 2 μg dmLT, 0.2 μg dmLT or FIA as the adjuvant or the control group. Baseline cAMP levels (T-84 cells incubated with cell culture medium) and cAMP levels in T-84 cells incubated with 10 ng CT (as positive reference) were included as controls. P values were calculated with Student's t-test. Boxes and bars represented means and standard deviations of cAMP levels, respectively.
Intracellular cAMP levels of the T-84 cells treated with 10 ng CT and the control mouse serum were 53.7 ± 1.3 pmole ml−1. The baseline cAMP levels in T-84 cells that were cultured with cell medium were 2.5 ± 0.03 pmole ml−1.
DISCUSSION
Results from this study affirmed that toxoid fusion 3xSTaN12S-dmLT induces antibodies neutralizing STa toxin and CT toxin that is structurally and functionally homologous to LT. Mice SC or IP administered with 3xSTaN12S-dmLT, with different adjuvants, develop strong antibody responses specific to STa and LT. More importantly, induced mouse antibodies were shown to neutralize STa and CT. These results suggest that toxoid fusion 3xSTaN12S-dmLT is potentially a desirable antigen for ETEC vaccines. With a safe toxoid fusion antigen that induces neutralizing anti-STa antibodies, we potentially overcome a key challenge and are able to accelerate ETEC vaccine development.
Data from this study also suggest dmLT an effective adjuvant in SC immunization. ADP-ribosylating bacterial toxins including CT of Vibrio cholerae and LT of ETEC have been explored as vaccine adjuvants (Lycke and Holmgren 1986; Clements, Hartzog and Lyon 1988; Freytag and Clements 2005; Lycke 2005). Unfortunately, CT or LT potent toxicity results in adverse effects, limiting application as a safe adjuvant (Levine et al. 1984; Gagliardi and De Magistris 2003; Lewis et al. 2009). Detoxified CT and LT derivatives were produced as the second generation of adjuvants (Chong, Friberg and Clements 1998; Yamamoto et al. 2001; Lycke 2004). However, remaining enterotoxicity in some LT toxoids is linked to diarrhea following oral administration or Bell's palsy in volunteers following intranasal immunization (Kotloff et al. 2001; Gagliardi and De Magistris 2003; Mutsch et al. 2004; van Ginkel et al. 2005). Double-mutant holotoxin-structured LT toxoid (dmLT; LTR192G/L211A, one LTA subunit and five LTB subunits forming LT holotoxin), which had toxicity further reduced but retained LT adjuvanticity (Norton et al. 2012), was shown to positively immunoregulate antigen-specific mucosal immunity following oral, intragastric, sublingual and intranasal immunizations (Summerton et al. 2010; Leach et al. 2012; Holmgren et al. 2013; Martinez-Becerra et al. 2013; Sjokvist Ottsjo et al. 2013). However, dmLT adjuvanticity in parenteral immunizations has not been characterized, nor has its potential application as an adjuvant for ETEC subunit vaccines been explored.
Results from SC immunization study showed that dmLT was an effective adjuvant to immunoregulate toxoid fusion 3xSTaN12S-dmLT in inducing anti-STa antibodies. Anti-STa and anti-LT IgG titers in the serum of mice immunized with toxoid fusion and ISA51 adjuvant or dmLT adjuvant were significantly greater than the anti-STa (P < 0.01) and anti-LT (P < 0.01) IgG titers in the serum of the mice SC immunized with the toxoid fusion alone (without adjuvant). This suggests that dmLT is an adjuvant equally effective as ISA51 adjuvant in enhancing 3xSTaN12S-dmLT for induction of IgG antibodies to STa toxin.
The enhanced antibody response to LT in SC administered mice using dmLT adjuvant can be resulted from adjuvant dmLT, since this dmLT adjuvant likely also plays a role as an antigen to induce anti-LT antibodies. Enhanced anti-LT antibody response nevertheless is desired for ETEC vaccines since LT is also a key virulence determinant in ETEC-associated children's diarrhea and travelers’ diarrhea.
Mice IP administered with the toxoid fusion and dmLT (2 or 0.2 μg) developed greater antibody responses to STa and LT compared to the mice IP administered with the same antigen but FIA adjuvant. Without a treatment group administered with the toxoid fusion only (without adjuvant), we were unable to characterize adjuvanticity of dmLT for toxoid fusion 3xSTaN12S-dmLT in the IP immunization study. However, serum anti-STa IgG antibody titers in IP immunized group using 2 or 0.2 μg dmLT were significantly greater than those in mice immunized using FIA adjuvant (P < 0.01, P < 0.01), suggesting that dmLT could immunoregulate this toxoid fusion equally or more effectively as FIA. Future studies to include a treatment immunized with the toxoid fusion alone and also treatments with various doses of dmLT will help to characterize dmLT adjuvanticity in IP immunization route.
In this study, we used LT as the coating antigen in the anti-LT antibody titration ELISA and CT in the anti-LT antibody neutralization assay. While the structurally homologous LT and CT used as ELISA coating antigens led to same outcomes in anti-LT antibody titration, we preferred CT for anti-LT antibody neutralization assay. CT was commercially available when this assay was developed. Also, we found that CT is more biologically effective in elevating cGMP levels in T-84 cells and that the assay is more reproducible when CT is used. As quality of commercial LT product gets improved, LT can be used in future studies for anti-LT antibody titration as well as in vitro antibody neutralization assay.
Antitoxin antibody neutralizing STa and CT was assessed in vitro in this study. In vivo studies using suckling infant mice can better characterize anti-STa antibody neutralization activity against STa toxin. However, animal challenge studies will be needed to assess protection of antibodies induced by this toxoid fusion. Although data from our preliminary study showed that this toxoid fusion induced neutralizing anti-STa antibodies in intramuscularly administered pigs and passive antibodies were shown to protect suckling piglets against STa+ ETEC challenge (data not shown), additional challenge studies using this pig model or other animal challenge models will be needed to further evaluate induced anti-STa antibodies for protection against STa+ ETEC diarrhea.
In conclusion, results from the current study revealed that mice SC or IP administered with toxoid fusion 3xSTaN12S-dmLT developed antibodies specific to STa and LT and the induced mouse antibodies neutralized STa and CT toxins. These results provided further evidence that toxoid fusion 3xSTaN12S-dmLT can induce neutralizing anti-STa (and anti-LT) antibodies and this toxoid fusion may potentially be a suitable toxin immunogen for ETEC subunit vaccine development.
ACKNOWLEDGEMENTS
The authors thank PATH for arranging dmLT, SEPPIC (Fairfield, NJ) for providing Montanide ISA 51, Dr. DC Robertson (Kansas State University) for STa-ovalbumin conjugates, and Dr. JD Clements (Tulane University) for purified STa toxin.
FUNDING
Financial support for this study was provided by () and .
Conflict of interest. None declared.
REFERENCES
- Black RE Cousens S Johnson HL et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis Lancet 2010. 375 1969 87 [DOI] [PubMed] [Google Scholar]
- Chong C Friberg M Clements JD LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp Vaccine 1998. 16 732 40 [DOI] [PubMed] [Google Scholar]
- Clements JD Hartzog NM Lyon FL Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens Vaccine 1988. 6 269 77 [DOI] [PubMed] [Google Scholar]
- Freytag LC Clements JD Mucosal adjuvants Vaccine 2005. 23 1804 13 [DOI] [PubMed] [Google Scholar]
- Gagliardi MC De Magistris MT Maturation of human dendritic cells induced by the adjuvant cholera toxin: role of cAMP on chemokine receptor expression Vaccine 2003. 21 856 61 [DOI] [PubMed] [Google Scholar]
- Holmgren J Bourgeois L Carlin N et al. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant Vaccine 2013. 31 2457 64 [DOI] [PubMed] [Google Scholar]
- Kotloff KL Blackwelder WC Nasrin D et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study Clin Infect Dis 2012. 55 Suppl 4 S232 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL Sztein MB Wasserman SS et al. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection Infect Immun 2001. 69 3581 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach S Clements JD Kaim J et al. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens PLoS One 2012. 7 e51718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM Black RE Clements ML et al. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine Infect Immun 1984. 43 515 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ Huo Z Barnett S et al. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin PLoS One 2009. 4 e6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M Ruan X Zhang C et al. Heat-labile- and heat-stable-toxoid fusions (LTR(1)(9)(2)G-STaP(1)(3)F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies Infect Immun 2011. 79 4002 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N From toxin to adjuvant: the rational design of a vaccine adjuvant vector, CTA1-DD/ISCOM Cell Microbiol 2004. 6 23 32 [DOI] [PubMed] [Google Scholar]
- Lycke N From toxin to adjuvant: basic mechanisms for the control of mucosal IgA immunity and tolerance Immunol Lett 2005. 97 193 8 [DOI] [PubMed] [Google Scholar]
- Lycke N Holmgren J Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens Immunology 1986. 59 301 8 [PMC free article] [PubMed] [Google Scholar]
- Martinez-Becerra FJ Chen X Dickenson NE et al. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine Infect Immun 2013. 81 4470 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsch M Zhou W Rhodes P et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland New Engl J Med 2004. 350 896 903 [DOI] [PubMed] [Google Scholar]
- Nataro JP Kaper JB Diarrheagenic Escherichia coli Clin Microbiol Rev 1998. 11 142 201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton EB Lawson LB Mahdi Z et al. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens Infect Immun 2012. 80 2426 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X Robertson DC Nataro JP et al. the STa Toxoid Vaccine Consortium Group Characterization of heat-stable (STa) toxoids of enterotoxigneic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies Infect Immun 2014. 82 1823 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack DA Shimko J Torres O et al. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. Coli diarrhoea of travellers to Guatemala and Mexico Vaccine 2007. 25 4392 400 [DOI] [PubMed] [Google Scholar]
- Sanders JW Putnam SD Riddle MS et al. Military importance of diarrhea: lessons from the Middle East Curr Opin Gastroenterol 2005. 21 9 14 [PubMed] [Google Scholar]
- Sjokvist Ottsjo L Flach CF Clements J et al. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection Infect Immun 2013. 81 1532 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerton NA Welch RW Bondoc L et al. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin Vaccine 2010. 28 1404 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A-M From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development Indian J Med Res 2011. 133 188 96 [PMC free article] [PubMed] [Google Scholar]
- Svennerholm AM Tobias J Vaccines against enterotoxigenic Escherichia coli Expert Rev Vaccines 2008. 7 795 804 [DOI] [PubMed] [Google Scholar]
- van Ginkel FW Jackson RJ Yoshino N et al. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract Infect Immun 2005. 73 6892 902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries Wkly Epidemiol Rec 2006. 81 97 107 [PubMed] [Google Scholar]
- Yamamoto M McGhee JR Hagiwara Y et al. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant Scand J Immunol 2001. 53 211 7 [DOI] [PubMed] [Google Scholar]
- Zhang C Knudsen DE Liu M et al. Group STTVC Toxicity and immunogenicity of Enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTa(A14Q)-LT(S63K/R192G/L211A) in a murine model PLoS One 2013. 8 e77386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W Sack DA Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans Expert Rev Vaccines 2012. 11 677 84 [DOI] [PubMed] [Google Scholar]
- Zhang W Zhang C Francis DH et al. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies Infect Immun 2010. 78 316 25 [DOI] [PMC free article] [PubMed] [Google Scholar]