Abstract
Background
Increasing antimicrobial resistance makes treating uncomplicated urinary tract infections (uUTIs) difficult. We compared whether adverse short-term outcomes among US female patients were more common when initial antimicrobial therapy did not cover the causative uropathogen.
Methods
This retrospective cohort study used data from female outpatients aged ≥12 years, with a positive urine culture and dispensing of an oral antibiotic ±1 day from index culture. Isolate susceptibility to the antimicrobial initially dispensed, patient age, and history of antimicrobial exposure, resistance, and all-cause hospitalization within 12 months of index culture were evaluated for associations with adverse outcomes during 28-day follow up. Outcomes assessed were new antimicrobial dispensing, all-cause hospitalization, and all-cause outpatient emergency department/clinic visits.
Results
Of 2366 uUTIs, 1908 (80.6%) were caused by isolates susceptible and 458 (19.4%) by isolates not susceptible (intermediate/resistant) to initial antimicrobial treatment. Within 28 days, patients with episodes caused by not susceptible isolates were 60% more likely to receive a new antimicrobial versus episodes with susceptible isolates (29.0% vs 18.1%; 95% confidence interval, 1.3–2.1; P < .0001). Other variables associated with new antibiotic dispenses within 28 days were older age, prior antimicrobial exposure, or prior nitrofurantoin-not-susceptible uropathogens (P < .05). Older age, prior antimicrobial-resistant urine isolates, and prior hospitalization were associated with all-cause hospitalization (P < .05). Prior fluoroquinolone-not-susceptible isolates or oral antibiotic dispensing within 12 months of index culture were associated with subsequent all-cause outpatient visits (P < .05).
Conclusions
New antimicrobial dispensing within the 28-day follow-up period was associated with uUTIs where the uropathogen was not susceptible to initial antimicrobial treatment. Older age and prior antimicrobial exposure, resistance, and hospitalization also identified patients at risk of adverse outcomes.
Keywords: antimicrobial resistance, health outcomes, hospitalization, treatment failure, uncomplicated UTI
Increasing antimicrobial resistance (AMR) makes treating uncomplicated urinary tract infections (uUTIs) difficult. Considering risk factors associated with AMR and common adverse short-term health outcomes related to treatment failure may improve patient-specific approaches in treating uUTIs.
Urinary tract infections (UTIs) are among the most common bacterial infections worldwide, affecting 150 million people each year [1]. UTIs are particularly common in women, with 50% experiencing at least 1 UTI in their lifetime [2]. Uncomplicated UTIs (uUTIs) are those that occur in women who do not present with urological or functional abnormalities of the urinary tract and do not have complicating comorbidities [3]. Escherichia coli (E coli) is the predominant causative uropathogen of community-acquired uUTIs, causing approximately 75% of cases [1, 4]. Treatment for uUTIs is generally empiric, without the aid of urine culture or antimicrobial susceptibility testing at the time of prescribing [5, 6]. A large proportion of total outpatient antimicrobial use comprises antibiotics prescribed to treat uUTIs [7]. Antimicrobial resistance (AMR) is a growing concern and increases the likelihood of patients receiving antibiotic treatments to which their uropathogens are not susceptible. As a result, patients are subjected to extended antimicrobial exposure and a delay in receiving effective therapy, which may in turn increase the risk of adverse treatment outcomes. This study evaluated the association between inadequate empiric therapy (where the uropathogen was not susceptible to the initial treatment) and 28-day short-term health outcomes among female patients with uUTI.
METHODS
Study Design and Patients
This was a retrospective cohort study of outpatient data from urine cultures collected at 9 facilities participating in the Becton, Dickinson and Company (BD) Insights Research Database (Franklin Lakes, NJ) and spanning all major regions of the United States (East Central, Middle Atlantic, West Central, and Pacific). Urine cultures were collected between January 1, 2015 and December 31, 2019 from female outpatients ≥ 12 years of age, who were not hospitalized within 24 hours of culture collection. The primary objective was to evaluate whether uUTI caused by a uropathogen not covered by initial antimicrobial therapy was associated with adverse 28-day health outcomes. An index uUTI episode was defined as a positive noncontaminated urine culture from an outpatient setting, followed by dispensing of antibiotics. The same patient could have more than 1 uUTI event included, as long as the events were at least 30 days apart. Dispensing was defined as the prescription and collection of an antibiotic. A positive index culture was defined as containing at least 1 uropathogen (Staphylococcus saprophyticus or Enterobacterales [eg, E coli]). Uropathogens were evaluated as susceptible or not susceptible (intermediate or resistant) to the antimicrobial initially dispensed. Antimicrobials had to be administered orally and received 1 day before or up to 1 day after index urine culture. The antimicrobials included were nitrofurantoin (NFT); trimethoprim-sulfamethoxazole (SXT); cephalexin; ciprofloxacin; levofloxacin; amoxicillin-clavulanate; amoxicillin; cefdinir; cefpodoxime; and fosfomycin. Patients were also required to have at least 12 months of baseline data and 28 days of follow-up data available for assessment. Patients were excluded if they received an intravenous (IV) or intramuscular antibiotic for the index UTI episode, to eliminate potential cases of complicated UTI. Exclusions were also made if there was evidence of the following complicating comorbidities at baseline within 6 months of index culture: uncontrolled diabetes mellitus (defined as a hemoglobin A1C result >8.0%); immunosuppression (defined as dispensing of immunosuppressive therapies such as systemic corticosteroids or anti-neoplastic agents); pregnancy; or severe renal impairment (defined as an estimated creatinine clearance <30 mL/min). Data collected from the 12-month baseline period included patient age, prior all-cause hospitalization, prior antimicrobial exposure (fluoroquinolone [FQ] or non-FQ), and prior urine culture status (positive/negative, the presence of isolates not susceptible to the predefined antibiotics [NFT, FQ, SXT, extended-spectrum beta-lactamase producing [ESBL+ ]). The model was adjusted to account for any significant differences (P < .05) in these baseline variables.
Outcomes
Outcomes evaluated in the 28-day follow-up period were the dispensing of a new antimicrobial (of those listed above) suggesting empiric treatment failure, all-cause hospitalization, and all-cause outpatient emergency department (ED) or clinic visits.
Statistical Analysis
Descriptive statistics were used to report the n (%) of isolated organisms, antimicrobials dispensed, and summary statistics of study outcomes. The covariates considered in modeling analysis were patient age, prior AMR, and baseline uUTI episode demographics (see a full list in Supplementary Tables 1 and 2). In the univariate analysis, either the χ2 test or Fisher's exact test methods were used to explore (1) the bivariate associations between each study outcome and antimicrobial susceptibility status of isolates (susceptible/not susceptible) as well as (2) each potential covariate. A candidate variable with a bivariate P value of .25 or less was considered in the multiple regression modeling phase. Generalized linear mixed models were used for multivariable modeling to (1) evaluate the effect of infection with not-susceptible isolates on outcomes and (2) to identify other covariates. The optimal models were selected based on goodness-of-fit statistics (Akaike's information criterion and Bayesian information criterion). Three models were developed, one for each outcome of interest, with the primary objective of evaluating the association with outcomes of isolates being not susceptible to the initial antibiotic received. To address potential multicollinearity, we examined pairwise Spearman correlations among candidate covariates. Some effects that were deemed clinically important, such as prior FQ-not-susceptible uropathogens and prior infection with ESBL+ uropathogens, were included in the final models regardless of statistical significance (P values) if the univariate assessment for those effects were statistically significant. Analyses were conducted using Statistical Analysis System (SAS) V9.4 (SAS Institute, Cary, NC). Results were reported using odds ratio (OR) and 95% confidence intervals (CIs).
Patient Consent Statement
This study was approved by the New England Institutional Review Board and Human Subjects Research Committee in Wellesley, Massachusetts and exempted from consent due to the use of a limited retrospective dataset for the purposes of an epidemiological study. The study was conducted in compliance with the standards set by the Health Insurance Portability and Accountability Act.
RESULTS
Overall, 2366 index uUTI episodes with positive urine cultures from 2087 female patients were analyzed. The mean age (range) of patients included was 48 (12–90) years. Of the episodes analyzed, 1908 (80.6%) had isolates that were susceptible and 458 (19.4%) had isolates that were not susceptible to the initial antimicrobial prescribed. E. coli was the most common uropathogen, isolated in 1858 (78.5%) episodes, followed by Klebsiella pneumoniae, which was found in 267 (11.3%) episodes (Table 1). The most common antimicrobials initially dispensed were NFT (34.3% episodes), SXT (22.5%), cephalexin (20.4%), and FQs (ciprofloxacin and levofloxacin; 18.4%) (Table 2). Approximately 20% of patients who were initially dispensed these agents required a new antimicrobial within 28 days of dispensing: 18.1% of patients whose isolates were susceptible to the initial antimicrobial prescribed and 29.0% of those whose isolate was not susceptible. New antimicrobials were as follows: NFT, 21.0%; SXT, 19.4%; cephalexin, 19.7%; ciprofloxacin, 17.9%; and levofloxacin, 19.0%. There was a greater rate of new antimicrobial dispensing when cefpodoxime and amoxicillin were initially dispensed (25.0% and 31.7%, respectively) (Table 2). Variables collected at baseline included whether E coli was present, age, prior ESBL+ isolates, prior isolates not susceptible to antimicrobials (FQs, NFT, or SXT), prior positive urine culture, prior negative urine culture, prior oral antibiotics, and prior hospitalization. The distribution of these baseline variables was assessed, in addition to bivariate correlation with isolate susceptibility to antimicrobial dispensed at index (Table 3). The model was then adjusted to account for any significant differences in baseline variables between patients with isolates susceptible versus not susceptible to the antimicrobial dispensed at index visit. There were significant differences between the groups for the following baseline variables: E coli presence (P < .0010), age (P = .0010), prior ESBL+ isolates (P = .0014), prior FQ-not-susceptible isolates (P = .0002), prior SXT-not-susceptible isolates (P = .0003), prior NFT-not-susceptible isolates (P = .0245), prior negative urine culture (P = .0410), and prior oral antimicrobial use (P < .0001).
Table 1.
Frequency of Enterobacterales and Staphylococcus saprophyticus Isolates From Outpatient Urine Cultures With Rate of New Antimicrobial Dispensing at 28 Days
| Organism | Episodes, n (%) (N = 2366) |
Episodes Requiring New Antimicrobial at 28 Day, n (%) |
|---|---|---|
| Escherichia coli | 1858 (78.5) | 353 (19.0) |
| Klebsiella pneumoniae | 267 (11.3) | 69 (25.8) |
| Proteus mirabilis | 115 (4.9) | 31 (27.0) |
| Enterobacter aerogenes | 30 (1.3) | 7 (23.3) |
| Citrobacter freundii | 24 (1.0) | 4 (16.7) |
| Klebsiella oxytoca | 23 (1.0) | 6 (26.1) |
| S saprophyticus | 22 (0.9) | 4 (18.2) |
| Enterobacter cloacae | 20 (0.8) | 4 (20.0) |
| Morganella morganii | 4 (0.2) | 0 (0.0) |
| Serratia marcescens | 3 (0.1) | 1 (33.3) |
Table 2.
Frequency of Antimicrobials Dispensed for Index uUTI and Overall Efficacy Indicated by Rate of New Antimicrobial at 28 Days
| Initial Antimicrobial Dispensed | At Index Episode, n (%)a (N = 2366) |
Episodes Requiring Represcription at 28 Days, n (%) |
|---|---|---|
| Nitrofurantoin | 811 (34.3) | 170 (21.0) |
| Trimethoprim-sulfamethoxazole | 532 (22.5) | 103 (19.4) |
| Cephalexin | 482 (20.4) | 95 (19.7) |
| Ciprofloxacin | 335 (14.2) | 60 (17.9) |
| Levofloxacin | 100 (4.2) | 19 (19.0) |
| Amoxicillin-clavulanate | 60 (2.5) | 17 (28.3) |
| Amoxicillin | 41 (1.7) | 13 (31.7) |
| Cefdinir | 28 (1.2) | 6 (21.4) |
| Cefpodoxime | 12 (0.5) | 3 (25.0) |
| Fosfomycin | 1 (<0.1) | 0 (0.0) |
Abbreviations: uUTI, uncomplicated urinary tract infection.
Percentages do not sum to 100 as 36 patients received >1 antimicrobial.
Table 3.
Patient Baseline Variable Distribution and Bivariate Correlation With Isolate Susceptibility to Antimicrobial Dispensed at Index
| Baseline Variables | Study Cohort | Isolate Susceptibility to Initially Dispensed Antimicrobial | ||
|---|---|---|---|---|
| Susceptible | Not Susceptible | P Value | ||
| N (%) | n (%) | n (%) | ||
| Overall | 2366 (100.0) | 1908 (80.6) | 458 (19.4) | |
| Escherichia coli | … | … | … | <.0001 |
| No | 508 (21.5) | 370 (19.4) | 138 (30.1) | |
| Yes | 1858 (78.5) | 1538 (80.6) | 320 (69.9) | |
| Age Group, Years | … | … | … | .0010 |
| 12–17 | 100 (4.2) | 77 (4.0) | 23 (5.0) | |
| 18–24 | 379 (16.0) | 326 (17.1) | 53 (11.6) | |
| 25–50 | 851 (36.0) | 702 (36.8) | 149 (32.5) | |
| >50 | 1036 (43.8) | 803 (42.1) | 233 (50.9) | |
| Prior ESBL+ Isolates | … | … | … | .0014 |
| 0–90 days | 24 (1.0) | 15 (0.8) | 9 (2.0) | |
| 91–360 days | 34 (1.4) | 21 (1.1) | 13 (2.8) | |
| No prior AMR | 2308 (97.5) | 1872 (98.1) | 436 (95.2) | |
| Prior FQ-NS Isolates | … | … | … | .0002 |
| 0–90 days | 65 (2.7) | 40 (2.1) | 25 (5.5) | |
| 91–360 days | 91 (3.8) | 70 (3.7) | 21 (4.6) | |
| No prior AMR | 2210 (93.4) | 1798 (94.2) | 412 (90.0) | |
| Prior SXT-NS Isolates | … | … | … | .0003 |
| 0–90 days | 79 (3.3) | 53 (2.8) | 26 (5.7) | |
| 91–360 days | 100 (4.2) | 71 (3.7) | 29 (6.3) | |
| No prior AMR | 2187 (92.4) | 1784 (93.5) | 403 (88.0) | |
| Prior NFT-NS Isolates | … | … | … | .0245 |
| 0–90 days | 65 (2.7) | 48 (2.5) | 17 (3.7) | |
| 91–360 days | 95 (4.0) | 68 (3.6) | 27 (5.9) | |
| No prior AMR | 2206 (93.2) | 1792 (93.9) | 414 (90.4) | |
| Prior Positive Urine Culture | … | … | … | .0947 |
| 0–90 days | 274 (11.6) | 208 (10.9) | 66 (14.4) | |
| 91–360 days | 339 (14.3) | 272 (14.3) | 67 (14.6) | |
| No prior positive urine culture | 1753 (74.1) | 1428 (74.8) | 325 (71.0) | |
| Prior Negative Urine Culture | … | … | … | .0410 |
| 0–90 days | 108 (4.6) | 79 (4.1) | 29 (6.3) | |
| 91–360 days | 133 (5.6) | 101 (5.3) | 32 (7.0) | |
| No prior negative urine culture | 2125 (89.8) | 1728 (90.6) | 397 (86.7) | |
| Prior Oral Abx (Any) | … | … | … | <.0001 |
| 0–90 days | 708 (29.9) | 526 (27.6) | 182 (39.7) | |
| 91–360 days | 572 (24.2) | 454 (23.8) | 118 (25.8) | |
| No prior PO Abx dispensed | 1086 (45.9) | 928 (48.6) | 158 (34.5) | |
| Prior Hospitalization | … | … | … | .0563 |
| 0–90 days | 89 (3.8) | 67 (3.5) | 22 (4.8) | |
| 91–360 days | 313 (13.2) | 240 (12.6) | 73 (15.9) | |
| No prior hospitalization | 1964 (83.0) | 1601 (83.9) | 363 (79.3) | |
Abbreviations: Abx, antibiotic; AMR, antimicrobial resistance; ESBL+, extended-spectrum beta-lactamase producing; FQ, fluoroquinolone; NFT, nitrofurantoin; NS, not susceptible; PO, oral; SXT, trimethoprim-sulfamethoxazole.
Health Outcomes
After univariate analysis, only the variable of prior hospitalization with an inpatient antibiotic prescription was excluded from the multivariable model, because it did not reach the statistical threshold for inclusion (Supplementary Tables 1 and 2).
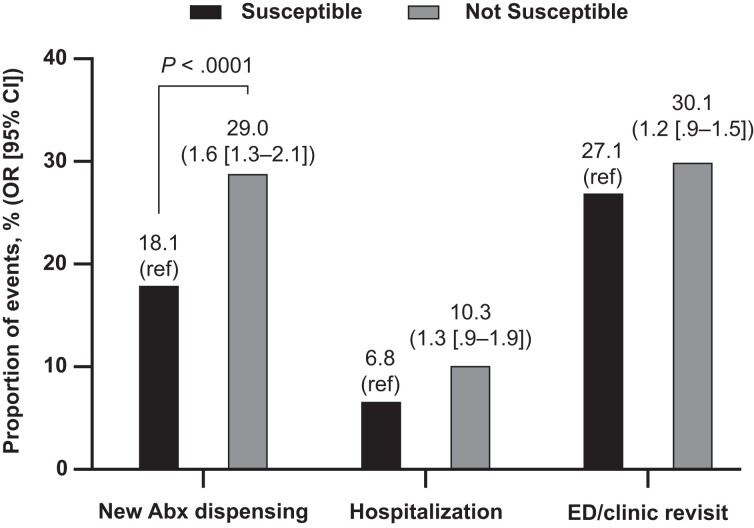
After multivariable adjustment, uUTI episodes with isolates that were not susceptible to the antimicrobial initially dispensed were 60% more likely to be followed by a new antimicrobial within 28 days versus episodes with susceptible isolates (29.0% vs 18.1%; OR, 1.6; 95% CI, 1.3–2.1; P < .0001) (Figure 1).
Figure 1.
Association of 28-day health outcomes by isolate susceptibility status to initial antimicrobial therapy. Note: Susceptible or not susceptible to antimicrobial dispensed at index. Abx, antibiotic; CI, confidence interval; ED, emergency department; OR, odds ratio; ref, reference group.
New antimicrobial dispensing within 28 days of index culture was more likely for uUTI episodes in patients aged >50 years, compared with the youngest patient group of 12–17 years (OR, 1.7; 95% CI, 1.0–3.1; P < .0001) (Table 4). New antimicrobial dispensing within 28 days was also associated with the following: prior NFT-not-susceptible urinary isolates found in the 0 (index date)–90 days before index culture (OR, 2.2; 95% CI, 1.3–4.0; P = .0195); oral non-FQ antimicrobial dispensing in the 0–90 days before index culture (OR, 2.4; 95% CI, 1.2–4.5; P = .0037); or dispensing of any oral antimicrobial in the 91–360 days before index culture (OR, 1.8; 95% CI, 1.0–3.0; P = .0371) (Table 4).
Table 4.
Independent Covariates of 28-Day Health Outcomes (Modeled) After Adjustment for Relevant Covariatesa
| New Antibiotic Dispenses | Hospitalization | ED/Clinic Revisit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Yes (n, [%]) | OR (95% CI) | P Value | Yes (n, [%]) | OR (95% CI) | P Value | Yes (n, [%]) | OR (95% CI) | P Value |
| Age Group, Years | |||||||||
| 12–17 | 15 (15.0) | ref | < .0001 | 1 (1.0) | ref | .0004 | 17 (17.0) | ref | .4389 |
| 18–24 | 61 (16.1) | 1.1 (0.6–2.0) | 17 (4.5) | 4.7 (0.6–36.1) | 95 (25.1) | 1.5 (0.8–2.7) | |||
| 25–50 | 131 (15.4) | 1.0 (0.5–1.8) | 47 (5.5) | 5.4 (0.7–40.0) | 222 (26.1) | 1.5 (.8–2.6) | |||
| >50 | 272 (26.3) | 1.7 (1.0–3.1) | 112 (10.8) | 10.0 (1.4–73.8) | 321 (31.0) | 1.6 (.9–2.8) | |||
| Prior ESBL+ Isolatesb | |||||||||
| 0–90 days | 8 (33.3) | .9 (.3–2.3) | .0513 | 7 (29.2) | 2.9 (1.0–8.3) | .0114 | 8 (33.3) | .3 (.1–.8) | .0180 |
| 91–360 days | 4 (11.8) | .3 (.1–.8) | 7 (20.6) | 3.4 (1.2–9.2) | 7 (20.6) | .4 (.2–1.1) | |||
| No prior AMR | 467 (20.2) | ref | 163 (7.1) | ref | 640 (27.7) | ref | |||
| Prior FQ-NS Isolatesb | |||||||||
| 0–90 days | 19 (29.2) | Not included in final model | 11 (16.9) | Not included in final model | 34 (52.3) | 2.3 (1.2–4.5) | .0406 | ||
| 91–360 days | 24 (26.4) | 11 (12.1) | 31 (34.1) | 1.3 (.7–2.3) | |||||
| No prior AMR | 436 (19.7) | 155 (7.0) | 590 (26.7) | ref | |||||
| Prior NFT-NS Isolatesb | |||||||||
| 0–90 days | 30 (46.2) | 2.2 (1.3–4.0) | .0195 | 11 (16.9) | Not included in final model | 23 (35.4) | .9 (.5–1.6) | .3749 | |
| 91–360 days | 23 (24.2) | .9 (.5–1.5) | 9 (9.5) | 36 (37.9) | 1.4 (.9–2.3) | ||||
| No prior AMR | 426 (19.3) | ref | 157 (7.1) | 596 (27.0) | ref | ||||
| Prior Oral FQ (Any) | |||||||||
| 0–90 days | 43 (23.6) | Not included in final model | 24 (13.2) | Not included in final model | 53 (29.1) | .7 (.5–1.0) | .0293 | ||
| 91–360 days | 87 (29.5) | 25 (8.5) | 104 (35.3) | 1.3 (.9–1.8) | |||||
| No prior PO FQ dispensed | 349 (18.5) | 128 (6.8) | 498 (26.4) | ref | |||||
| Prior Oral Non-FQ (Any) | |||||||||
| 0–90 days | 188 (30.7) | 2.4 (1.2–4.5) | .0037 | 60 (9.8) | Not included in final model | 203 (33.1) | Not included in final model | ||
| 91–360 days | 112 (20.3) | .8 (.5–1.4) | 36 (6.5) | 161 (29.1) | |||||
| No prior PO non-FQ dispensed | 179 (14.9) | ref | 81 (6.8) | 291 (24.3) | |||||
| Prior Oral Abx (Any) | |||||||||
| 0–90 days | 204 (28.8) | .9 (.4–1.6) | .0371 | 71 (10.0) | Not included in final model | 228 (32.2) | 1.5 (1.1–1.9) | .0130 | |
| 91–360 days | 121 (21.2) | 1.8 (1.0–3.0) | 34 (5.9) | 164 (28.7) | 1.3 (1.0–1.6) | ||||
| No prior PO Abx dispensed | 154 (14.2) | ref | 72 (6.6) | 263 (24.2) | ref | ||||
| Prior Hospitalizationc | |||||||||
| 0–90 days | 21 (23.6) | .9 (.5–1.6) | .2192 | 15 (16.9) | 2.5 (1.3–4.7) | .0005 | 25 (28.1) | .8 (.5–1.4) | .3167 |
| 91–360 days | 91 (29.1) | 1.3 (1.0–1.7) | 44 (14.1) | 2.0 (1.3–2.9) | 110 (35.1) | 1.2 (.9–1.6) | |||
| No prior hospitalization | 367 (18.7) | ref | 118 (6.0) | ref | 520 (26.5) | ref | |||
Abbreviations: Abx, antibiotic; CI, confidence interval; ED, emergency department; ESBL+, extended-spectrum beta-lactamase producing; FQ, fluoroquinolone; NFT, nitrofurantoin; NS, not susceptible; OR, odds ratio; PO, oral; ref, reference.
Bold denotes significant values (P < .05).
Organism isolated from urine.
Prior hospitalization was for all-cause hospital admissions, and not specifically admission for a urinary tract infection.
All-cause hospitalization within 28 days of index culture was more likely in patients aged >50 years compared with the youngest patient group (10.8% vs 1.0%; OR, 10.0; 95% CI, 1.4–73.8; P = .0004) (Table 4). All-cause hospitalization was also associated with a history of infection with ESBL+ isolates (P = .0114), both when recorded 91–360 days (20.6% vs 7.1% for no prior AMR; OR, 3.4; 95% CI, 1.2–9.2) and 0–90 days before index culture (29.2% vs 7.1%; OR, 2.9; 95% CI, 1.0–8.3). Hospitalization was more likely (P = .0005) if prior hospitalization had occurred 91–360 days (14.1% vs 6.0% for no prior hospitalization; OR, 2.0; 95% CI, 1.3–2.9), or 0–90 days (16.9% vs 6.0%; OR, 2.5; 95% CI, 1.3–4.7) before index uUTI (Table 4).
All-cause outpatient visits to ED or clinic within 28 days of index culture were less likely in patients with prior ESBL+ isolates (OR, 0.3; 95% CI, 0.1–0.8; P = .0180), but these patients were more likely to be hospitalized (OR, 2.9; 95% CI, 1.0–8.3; P = .0114) when isolates were found within 0–90 days before index culture, compared with patients without prior ESBL+ isolates (Table 4). All-cause outpatient visits to ED or clinic were associated with the presence of FQ-not-susceptible isolates (0–90 days before index culture; OR, 2.3; 95% CI, 1.2–4.5; P = .0406), and prior oral antibiotic dispensing within 0–90 days (OR, 1.5; 95% CI, 1.1–1.9; P = .0130), or 91–360 days before index (OR, 1.3; 95% CI, 1.0–1.6; P = .0130).
DISCUSSION
This study evaluated the associations between inadequate antibiotic coverage of the organism causing uUTI (where the organism was not susceptible to initial treatment) in female patients and adverse short-term health outcomes. New antimicrobial dispensing within the 28-day follow-up period was significantly more likely in patients who were initially treated with empiric therapy that did not cover the causative pathogen compared with those where initial therapy was adequate for the uropathogen. Overall, 69% of patients with isolates not susceptible to the antimicrobial dispensed at index did not require a new antimicrobial. This suggests that either many uUTIs are self-limiting or that the high concentration of antibiotics in the urine was sufficient to overcome bacterial resistance in some cases. Of the cases analyzed in this study, 19.4% of the causative uropathogens were not susceptible to the initial antimicrobial dispensed. Previous work, focusing on E coli specifically rather than a range of isolates, found a similar rate of isolates that were not susceptible to SXT and FQ in the United States [8]. Other studies have also found adverse outcomes for patients with uUTI where index episode isolates were not susceptible to the initial antimicrobial dispensed. For example, a study examining SXT treatment for uUTI among young women (aged 16–49 years) found a significantly lower rate of bacteriologic cure in uUTIs caused by SXT-resistant isolates at 5–9 and 28–42 days after treatment cessation, compared with those caused by susceptible isolates (42% vs 86%, respectively) [9]. Another study examining the use of trimethoprim to treat women with uUTI found that women with trimethoprim-resistant isolates were more likely to have significant bacteriuria 1 month after receiving trimethoprim than those with susceptible isolates (42% vs 20%, respectively) [10]. More recently, a Singapore-based study found patients with UTIs caused by isolates not susceptible to the antibiotic received were significantly less likely to have their symptoms resolved within 3 days of receipt, compared with patients with susceptible isolates (45% not susceptible vs 67% susceptible) [11].
In our study, both new antimicrobial dispensing and all-cause hospitalizations within the 28-day follow-up period were more likely to occur in patients >50 years old. Earlier studies have similarly found older age to be a risk factor for UTIs; this could be due to cumulative antibiotic exposure, which increases with age as UTIs become more prevalent, and could contribute to the accumulation of AMR [2, 12]. Over time, this accumulation may be worsened by recurrent UTI requiring antimicrobial treatment, which also shares an association with older age [13, 14]. Furthermore, the reduction in estrogen in postmenopausal women and age-related depletion of hydrogen peroxide-producing lactobacilli in the vaginal flora, which reduces natural protection against UTIs, are associated with an increased tendency towards infection [14]. As such, the increased likelihood of all-cause hospitalization with older age is to be expected as a typical consequence of aging.
All-cause hospitalization was more likely in patients with prior ESBL+ isolates within the 0–90 or 91–360 days before index culture. Other studies have highlighted that ESBL+ isolates are particularly challenging in terms of AMR, with multiple antibiotic classes failing to treat UTIs caused by ESBL+ isolates, thus a worse health outcome for patients with ESBL+ isolates was unsurprising [15, 16]. As a result of the AMR associated with ESBL+ isolates, uUTIs caused by these organisms are often treated using IV antibiotics such as carbapenems, requiring inpatient stays [17]. Furthermore, ESBL+ isolate carriage has been associated with comorbidities that may have increased the likelihood of hospitalization, such as pulmonary disease, cerebrovascular disease, and liver impairment [18, 19]. All-cause hospitalizations were also more likely if patients had been admitted to hospital in the prior 0–90 or 91–360 days. Ho et al [11] found a similar association and suggested that it could have been related to a substantially higher AMR rate in hospital settings, although it may be due to other factors leading to poorer health as our data include hospitalizations for reasons other than UTI.
All-cause outpatient visits to ED or clinic within the follow-up period were associated with prior FQ-not-susceptible isolates. Patients with ESBL+ isolates at index were more likely to be hospitalized compared with patients with non-ESBL+ isolates, which may have impacted ED and clinic visit results; It is interesting to note that outpatient visits were approximately 70% less likely to occur for patients with prior ESBL+ isolates. The increased likelihood of hospitalization is a reasonable explanation for the reduction in outpatient visits. However, this could have been influenced by other factors; prior ESBL+ isolates in patient histories may have prompted physicians to prescribe therapies with greater efficacy against ESBL+ isolates at index. Another possible factor is simply if the ESBL+ urine isolates were susceptible to NFT, which is one of the first-line recommended agents active against uUTI [20]. Although NFT is not necessarily a first-line therapy for uUTI caused by ESBL+ uropathogens, its use as an empiric therapy may have positively affected the short-term outcomes if the subsequent uUTI was also caused by an ESBL+ isolate. Finally, this result could also have been impacted by the small sample size; only 24 patients had prior ESBL+ isolates.
A key strength of this study was the focus on specific outcomes, rather than specific treatments for uUTI. This allowed for the results to be better generalized across susceptibility to initial treatment, regardless of the treatment selected. The use of microbiology data to confirm prior nonsusceptible uropathogens, pharmacy prescription history to confirm prior treatments, and records of previous healthcare exposure(s) improved the validity of the data used. The use of patient histories up to 360 days before index culture demonstrated covariates associated with adverse health outcomes, regardless of antimicrobials prescribed or isolate susceptibility at index. It is notable that prior resistance phenotypes (except for SXT-not susceptible) and prior dispensing of an oral antibiotic were covariates of multiple adverse outcomes, in some cases even when occurring up to 12 months before index uUTI. This emphasizes the potential efficiency of our approach. With outpatient antimicrobial stewardship programs gaining support [21], the covariate-based risk stratification insights presented in this study may be an important contribution to helping reduce antimicrobial misuse and overuse. The high proportion of isolates not susceptible to the initial antimicrobial may lead to an increase in the total course of antimicrobial drugs as well as additional exposure to multiple classes of agents, both of which have been linked to increasing resistance [22] and increased risk for Clostridioides difficile [23]. As electronic medical records become more ubiquitous in healthcare, the use of these to aid outpatient stewardship efforts might become more feasible. Collated data containing the clinical covariates presented here may help identify at-risk uUTI populations during the initial clinic visit. Furthermore, the consolidation of patient characteristic data to monitor infections on a community-level can inform clinical management of community-acquired uUTIs. This, along with an individualized risk-stratification approach, may be more conducive to effective treatment of uUTIs compared with those which do not consider patient characteristics or clinical history [7].
Limitations of this study included the data having been gathered from only 9 outpatient facilities from hospital networks in the United States, although these were distributed across all major regions of the United States, which improved the generalizability of the results. Given that uUTIs are typically treated empirically, without the use of urine culture to inform choice of therapy [5, 6], the inclusion only of patients with a positive urine culture may have biased the study population towards inclusion of patients with recurrent uUTI and potentially a higher percentage of not susceptible isolates, reducing the generalizability of the study results. Furthermore, although the outcomes chosen as the focus of the study represent common markers of treatment failure, other clinically relevant outcomes, such as time to symptom resolution, were not available. Although the observed associations between adverse short-term health outcomes, age, and prior all-cause hospitalization were expected, these could have been confounded by unmeasured variables. In bivariate analysis, we found a significant difference in organism distribution between susceptible and not susceptible groups; this was adjusted for in the multivariable model but does raise the possibility that observed differences may have been influenced by the presence of E coli versus non-E coli isolates. Given that this was a database study, we do not have data on why new antibiotics were given (whether a not-susceptible isolate was found, or whether patient symptoms indicated a different drug would be more suitable than the initial antimicrobial prescribed), nor do we know the reasons for all-cause hospitalizations and ED/clinic visits, because these included causes other than uUTI. Patients were excluded if they received IV therapy at baseline for their index uUTI; however, IV therapy was evaluated in the 28-day follow-up period if a patient was hospitalized and then received IV antibiotics. A limitation of the study is that if IV therapy was received in the follow-up period but without hospital admission (in an ED visit, for example) this would not be captured, unlike any oral antibiotic received that would have been included in the data. The study did not utilize International Classification of Disease (ICD) diagnostic codes for uUTI diagnosis, which may have led to inclusion of patients with complicated UTI in the study cohort despite the exclusion criteria applied, potentially biasing results towards higher rates of resistance. Likewise, without ICD diagnostic codes, it was not possible to identify patients with immunosuppressed conditions beyond evaluating use of an immunosuppressive medication, which could also result in bias. Finally, data on confirmed antibiotic dispensing did not provide information about whether the treatments were used as prescribed.
CONCLUSIONS
This study found that approximately 1 in 5 uUTI episodes were caused by isolates that were not susceptible to the initial antimicrobial prescribed. In 29.0% of these cases, a new antimicrobial was dispensed within the 28-day follow-up period. Such cases demonstrated where treatments may fail despite guidelines being generally followed, suggesting adjustment of some guidelines to accommodate the growing issue of AMR might be required. The use of detailed, patient-specific data repositories was valuable in capturing the associations between adverse short-term health outcomes and patient-level factors such as older age, prior not-susceptible uropathogens, prior antimicrobial treatment, and prior hospitalization. These patient-level factors were significantly associated with adverse outcomes, demonstrating the need for careful consideration of individual patient histories in the treatment of uUTI. Furthermore, these data encourage physicians to consider patient-specific risk factors during initial clinical assessment, because this could help improve empiric treatment choices for uUTI, as well as reduce antimicrobial overuse and potentially AMR over time.
Supplementary Material
Acknowledgments
We thank Latha Vankeepuram (Program Manager, Data Science and Analytics at Becton, Dickinson and Company) for her dedicated contribution to database management for this study. Medical writing support, under the guidance of the authors, was provided by Dr. Fraser Shearer and Leen Al-Mohammad of Ashfield MedComms, an Inizio company, and was funded by GSK. Trademarks are owned by or licensed to their respective owners (the GSK group of companies or Becton, Dickinson and Company).
Author contributions. All authors had access to the study data, take responsibility for the accuracy of the analysis, contributed to data interpretation, reviewed and contributed to the content of the manuscript, and had authority in the decision to submit the manuscript.
Financial support. This study, including study design, data collection, analysis, and interpretation, and medical writing and submission support for the manuscript, was funded by GSK (Study 212502).
Contributor Information
Barbara W Trautner, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, Texas, USA.
Keith S Kaye, Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA.
Vikas Gupta, Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA.
Aruni Mulgirigama, GSK, Brentford, London, United Kingdom.
Fanny S Mitrani-Gold, GSK, Collegeville, Pennsylvania, USA.
Nicole E Scangarella-Oman, GSK, Collegeville, Pennsylvania, USA.
Kalvin Yu, Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA.
Gang Ye, Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA.
Ashish V Joshi, GSK, Collegeville, Pennsylvania, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13:269–84. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol 2019; 11. doi: 10.1177/1756287219832172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. Am Fam Physician 2005; 72:451–6. [PubMed] [Google Scholar]
- 4. Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol 2010; 7:653–60. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 5. Colgan R, Williams M. Diagnosis and treatment of acute uncomplicated cystitis. Am Fam Physician 2011; 84:771–6. [PubMed] [Google Scholar]
- 6. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 7. King LM, Hersh AL, Hicks LA, Fleming-Dutra KE. Duration of outpatient antibiotic therapy for common outpatient infections, 2017. Clin Infect Dis 2021; 72:e663–6. doi: 10.1093/cid/ciaa1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaye KS, Gupta V, Mulgirigama A, et al. Antimicrobial resistance trends in urine Escherichia coli isolates from adult and adolescent females in the United States from 2011–2019: rising ESBL strains and impact on patient management. Clin Infect Dis 2021; 73:1992–9. doi: 10.1093/cid/ciab560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raz R, Chazan B, Kennes Y, et al. Empiric use of trimethoprim-sulfamethoxazole (TMP-SMX) in the treatment of women with uncomplicated urinary tract infections, in a geographical area with a high prevalence of TMP-SMX-resistant uropathogens. Clin Infect Dis 2002; 34:1165–9. doi: 10.1086/339812. [DOI] [PubMed] [Google Scholar]
- 10. McNulty CA, Richards J, Livermore DM, et al. Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J Antimicrob Chemother 2006; 58:1000–8. doi: 10.1093/jac/dkl368. [DOI] [PubMed] [Google Scholar]
- 11. Ho HJ, Tan MX, Chen MI, et al. Interaction between antibiotic resistance, resistance genes, and treatment response for urinary tract infections in primary care. J Clin Microbiol 2019; 57:e00143–19. doi: 10.1128/JCM.00143-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65:1–12. doi: 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]
- 13. McMurdo MET, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother 2008; 63:389–95. doi: 10.1093/jac/dkn489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aydin A, Ahmed K, Zaman I, Khan MS, Dasgupta P. Recurrent urinary tract infections in women. Int Urogynecol J 2015; 26:795–804. doi: 10.1007/s00192-014-2569-5. [DOI] [PubMed] [Google Scholar]
- 15. Osthoff M, McGuinness SL, Wagen AZ, Eisen DP. Urinary tract infections due to extended-spectrum beta-lactamase-producing gram-negative bacteria: identification of risk factors and outcome predictors in an Australian tertiary referral hospital. Int J Infect Dis 2015; 34:79–83. doi: 10.1016/j.ijid.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 16. Hayakawa K, Gattu S, Marchaim D, et al. Epidemiology and risk factors for isolation of Escherichia coli producing CTX-M-type extended-spectrum beta-lactamase in a large U.S. medical center. Antimicrob Agents Chemother 2013; 57:4010–8. doi: 10.1128/AAC.02516-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pana Z, Zaoutis T. Treatment of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) infections: what have we learned until now? [version 1; peer review: 2 approved]. F1000Res 2018; 7:F1000 Faculty Rev-1347. doi: 10.12688/f1000research.14822.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ben-Ami R, Rodríguez-Baño J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 2009; 49:682–90. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- 19. Briongos-Figuero LS, Gómez-Traveso T, Bachiller-Luque P, et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int J Clin Pract 2012; 66:891–6. doi: 10.1111/j.1742-1241.2012.02991.x. [DOI] [PubMed] [Google Scholar]
- 20. Tulara NK. Nitrofurantoin and fosfomycin for extended spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniae. J Glob Infect Dis 2018; 10:19–21. doi: 10.4103/jgid.jgid_72_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The Joint Commission . R3 Report Issue 23: Antimicrobial stewardship in ambulatory health care. Available at: https://www.jointcommission.org/standards/r3-report/r3-report-issue-23-antimicrobial-stewardship-in-ambulatory-health-care. Accessed 16 December 2021.
- 22. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 23. Tartof SY, Rieg GK, Wei R, Tseng HF, Jacobsen SJ, Yu KC. A comprehensive assessment across the healthcare continuum: risk of hospital-associated Clostridium difficile infection due to outpatient and inpatient antibiotic exposure. Infect Control Hosp Epidemiol 2015; 36:1409–16. doi: 10.1017/ice.2015.220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.