Abstract
Purpose:
The increasing availability of clinical imaging tests (especially CT and MRI) that directly quantify adipose tissue has led to a rapid increase in studies examining the relationship of visceral, subcutaneous, and overall adiposity to cancer survival. To summarize this emerging body of literature, we conducted a systematic review and meta-analysis of imaging-measured as well as anthropometric proxies for adipose tissue distribution and cancer survival across a wide range of cancer types.
Methods:
Using keywords related to adiposity, cancer and survival, we conducted a systematic search of the literature in PubMed and MEDLINE, Embase, and Web of Science Core Collection databases from database inception to June 30, 2021. We used a random-effect method to calculate pooled hazard ratios (HR) and corresponding 95% confidence intervals (CI) within each cancer type, and tested for heterogeneity using Cochran’s Q test and the I2 test.
Results:
We included 203 records for this review, of which 128 records were utilized for quantitative analysis among 10 cancer types: breast, colorectal, gastroesophageal, head and neck, hepatocellular carcinoma, lung, ovarian, pancreatic, prostate, and renal cancer. We found that imaging-measured visceral, subcutaneous, and total adiposity were not significantly associated with increased risk of overall mortality, death from primary cancer, or cancer progression among patients diagnosed with these 10 cancer types; however, we found significant or high heterogeneity for many cancer types. For example, heterogeneity was similarly high when the pooled HRs (95% CI) for overall mortality associated with visceral adiposity were essentially null as in 1.03 (0.55, 1.92; I2 = 58%) for breast, 0.99 (0.81, 1.21; I2 = 71%) for colorectal, versus when they demonstrated a potential increased risk 1.77 (0.85, 1.60; I2 = 78%) for hepatocellular carcinoma and 1.62 (0.90, 2.95; I2 = 84%) for renal cancer.
Conclusion:
Greater adiposity at diagnosis (directly measured by imaging) is not associated with worse survival among cancer survivors. However, heterogeneity and other potential limitations were noted across studies, suggesting differences in study design and adiposity measurement approaches, making interpretation of meta-analyses challenging. Future work to standardize imaging measurements and data analyses will strengthen research on the role of adiposity in cancer survival.
Keywords: Cancer, Survival, Adiposity, Computed Tomography, Body Composition, Waist-Hip Ratio
BACKGROUND
Excessive body weight (overweight or obesity) has been associated with increased incidence of 13 types of cancers [1], which are estimated to account for over 40% of all cancers diagnosed in the United States in 2022 [2]. Excessive body weight is associated with a number of systemic and local changes that are hypothesized to promote cancer initiation and progression, for example, increased circulating levels of insulin and glucose as well as adipose tissue-derived hormones and inflammatory mediators [3–6]. Yet, paradoxically, often overweight (body mass index [BMI]: 25–29.9 kg/m2) and sometimes obesity (BMI ≥ 30 kg/m2) are reported to be associated with more favorable outcomes among many of these 13 cancers as well as other cancer types [7, 8]. A potential reason for this is that although BMI is correlated with overall body fatness [9], it does not distinguish muscle from adipose tissue nor quantify specific adipose tissue depots. While, on average, all of these tissues increase in quantity with increasing body size, the relationships of muscle and visceral and subcutaneous adipose tissue to cancer survival often differ [10–12]; and there is substantial variation in body composition between individuals with identical body size, particularly among older patients with chronic conditions such as cancer [10, 11, 13]. Therefore, studies using more precise measures of overall body fatness that can also distinguish specific adipose tissue depots are needed to understand the relationship of adiposity to cancer survival.
The increasing availability of clinical imaging has led to a rapid increase in research using more precise measures of body fatness [14]. Imaging tests such as computed tomography (CT) are frequently performed among cancer patients for diagnostic purposes. Reference methods to directly quantify both total adipose tissue and specific adipose tissue depots from partial fields of view have been developed, and measurements correlate well with whole body volumes on magnetic resonance imaging (MRI) [15]. To summarize this emerging literature on adiposity and cancer survival, we conducted a meta-analysis of imaging-measured adiposity and survival in multiple cancer types. Given that waist-based anthropometric measures are commonly used as surrogates for visceral adiposity and triceps skinfolds for subcutaneous adiposity, we also included these anthropometric measures in our review and meta-analysis.
METHODS
We registered this review (No. CRD42021262968) a priori at PROSPERO, an international database of prospectively registered systematic reviews with health-related outcomes [16]. This review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [17]. Ethical approval was not applicable due to not involving human participants.
Data Sources and Searches
A systematic search of the literature was conducted in PubMed and MEDLINE, Embase, and Web of Science Core Collection databases from database inception to June 30, 2021. Key Medical Subject Headings (MeSH) terms were predefined for adiposity and cancer survival, and details of the full searching strategy are listed in Supplementary Table 1.
Inclusion Criteria
We included prospective studies that estimated associations of adiposity (measured by imaging and anthropometry) and survival after cancer diagnosis among adults (≥18 years). Only studies published in English were included. Case reports, case-control studies, cross-sectional studies, ecologic studies, conference abstracts, reviews, guidelines, perspectives, editorials, letters, and non-human research were excluded. Given that BMI has been the subject of several prior systematic reviews [18–40], and our focus is specifically adiposity and adipose tissue distribution rather than body size, we excluded studies that solely focused on BMI. We also excluded studies that used bioelectrical impedance (BIA) since it is not an imaging technique for estimating total body fat and is based on the assumption of constant hydration status that cancer patients may not meet, which may produce biased estimates of total body fat [41, 42]. Also, studies combining multiple different cancers in analysis were excluded.
Data Extraction and Quality Assessment
Titles and abstracts were screened independently by two reviewers (E. Cheng and J. Kirley) to identify articles related to adiposity and cancer survival. Afterwards, the full texts of those considered eligible were also independently reviewed by the same two authors. Any discrepancies were evaluated by another author (E. M. Cespedes Feliciano) and discussed among these three authors. We extracted the following information from each study: descriptive characteristics of the study population (sample size, country, age, sex, stage, and race and ethnicity); follow-up; adiposity assessment method (for imaging studies) or assessment time (for anthropometry studies); adiposity classification (how adiposity was measured and compared); outcome results; and covariates if multivariable models were applied. For publications using duplicate or overlapping cohorts, we selected the publications with the largest sample size. We also contacted study authors when we needed clarification and additional information not available in the online publications and supplementary materials.
Two reviewers (E. Cheng and J. Kirley) independently assessed the quality of the studies using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies developed by the National Heart, Lung, and Blood Institute (NHLBI) [43]. Any discrepancies were settled after further discussion among these two authors. This tool includes 14 questions focusing on key concepts for evaluating internal validity including considerations regarding the study population, sample size, exposure and outcome assessment, timeframe, loss to follow-up and control for confounding. Rather than creating a list that can simply add up to judge quality, NHLBI encourages investigators to examine the study comprehensively with this tool and then give overall quality rating (good, fair, or poor). If the articles were finally evaluated as poor, we excluded them from systematic review and meta-analysis.
Primary Exposure
In addition to measuring total adipose tissue (TAT), imaging via CT, MRI or Dual X-Ray absorptiometry (DXA) can further separately measure or estimate visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).
Visceral adiposity was typically measured via 1) VAT area [cm2] on a single-slice abdominal scan, 2) VAT area scaled by height squared [cm2/m2], 3) VAT-related ratios such as VAT/SAT and VAT/TAT, 4) VAT volume [cm3] across multiple slices or estimated from three-dimensional imaging models, 5) VAT mass (kg) calculated from estimation formulas [44], and 6) VAT thickness [mm] defined as the imaging distance between abdominal wall and an abdominal organ of interest [45, 46].
Subcutaneous adiposity was typically measured via 1) SAT area [cm2] at a single-slice abdominal scan, 2) SAT area scaled by height squared [cm2/m2], 3) SAT-related ratios such as SAT/VAT and SAT/TAT, 3) subcutaneous volume [cm3] across multiple slices or estimated from three-dimensional imaging models, and 4) subcutaneous mass (kg) calculated from estimation formulas [44].
Total adiposity was generally considered as a sum of SAT and VAT, and was typically measured via 1) TAT area [cm2] at a single-slice abdominal scan, 2) TAT area scaled by height squared [cm2/m2], 3) TAT volume [cm3] across multiple slices or three-dimensional imaging models, and 4) TAT mass (kg) calculated from estimation formulas [15, 47–49].
As for anthropometric measures, waist-hip ratio (WHR), waist circumference (WC), and waist-height ratio (WHtR) were usually measured as surrogates for visceral adiposity [50, 51], and triceps skinfold thickness has been widely used as an measurement of subcutaneous fat [52, 53]. For meta-analysis in anthropometric measures, when a study investigated multiple measures of visceral adiposity, we would prioritize the findings of WHR over WC and WHtR.
The primary exposure of interest is adiposity, defined as greater quantity of adipose tissue in two main depots (visceral and/or subcutaneous) as well as total body fat (sum of visceral, subcutaneous, and other adipose tissue depots, if measured). For studies categorizing adiposity measures, we used the risk estimate that compared the highest and lowest quantiles, representing patients with most excessive adipose tissue (highest quantile) and least adipose tissue (lowest quantile). For studies (N = 32) analyzing adiposity measures as continuous variables, their findings were reported in different ways (such as one unit increase and one standard deviation increase) and included in a sensitivity analysis for meta-analysis.
Primary Outcomes
For this analysis, three primary outcomes of interest were defined as follows. Overall survival (OS) was defined as the time from cancer diagnosis until death from any cause. Cancer-specific survival (CSS) was defined as the time from cancer diagnosis until death from the primary cancer. Progression-free survival (PFS) was defined as the time from cancer diagnosis until cancer recurrence, metastasis, or other events (such as new lesions and second primary tumors) suggesting cancer growth.
Statistical Analysis
For each cancer, at least two studies were needed for a meta-analysis [54, 55], and we calculated a pooled hazard ratio (HR) with 95% confidence interval (CI) using the DerSimonian-Laird method for random-effects meta-analysis [56]. Heterogeneity between the studies was calculated both using Cochran’s Q test and the I2 test [57]. A p value <0.05 or I2 value >75% suggested significant evidence of heterogeneity, whereas for I2 values, ≤25%, 26–50%, and 51–75% suggested low, moderate, and high heterogeneity [57, 58]. For each cancer, we assessed publication bias with funnel plots if there were ≥10 studies [59, 60], and further examined it with Begg and Mazumdar rank correlation test [61]. All statistical analyses were conducted using R statistical software version 4.1.2 (R Project for Statistical Computing) from November 19, 2021 to December 16, 2021. All p values were 2-sided, and the significance level was set at p = 0.05.
RESULTS
The searching and screening process is shown in Figure 1: we included 202 records for this review, of which 128 records were utilized for quantitative analysis. For imaging, there were 10 cancer types with ≥2 eligible studies for at least one outcome (OS, CSS, or PFS) to conduct a meta-analysis, including breast, colorectal, gastroesophageal, head and neck, hepatocellular carcinoma, lung, ovarian, pancreatic, prostate, and renal cancer. In contrast, for anthropometry, there were three cancer types eligible for meta-analysis, including breast, colorectal, and prostate cancer.
Figure 1.
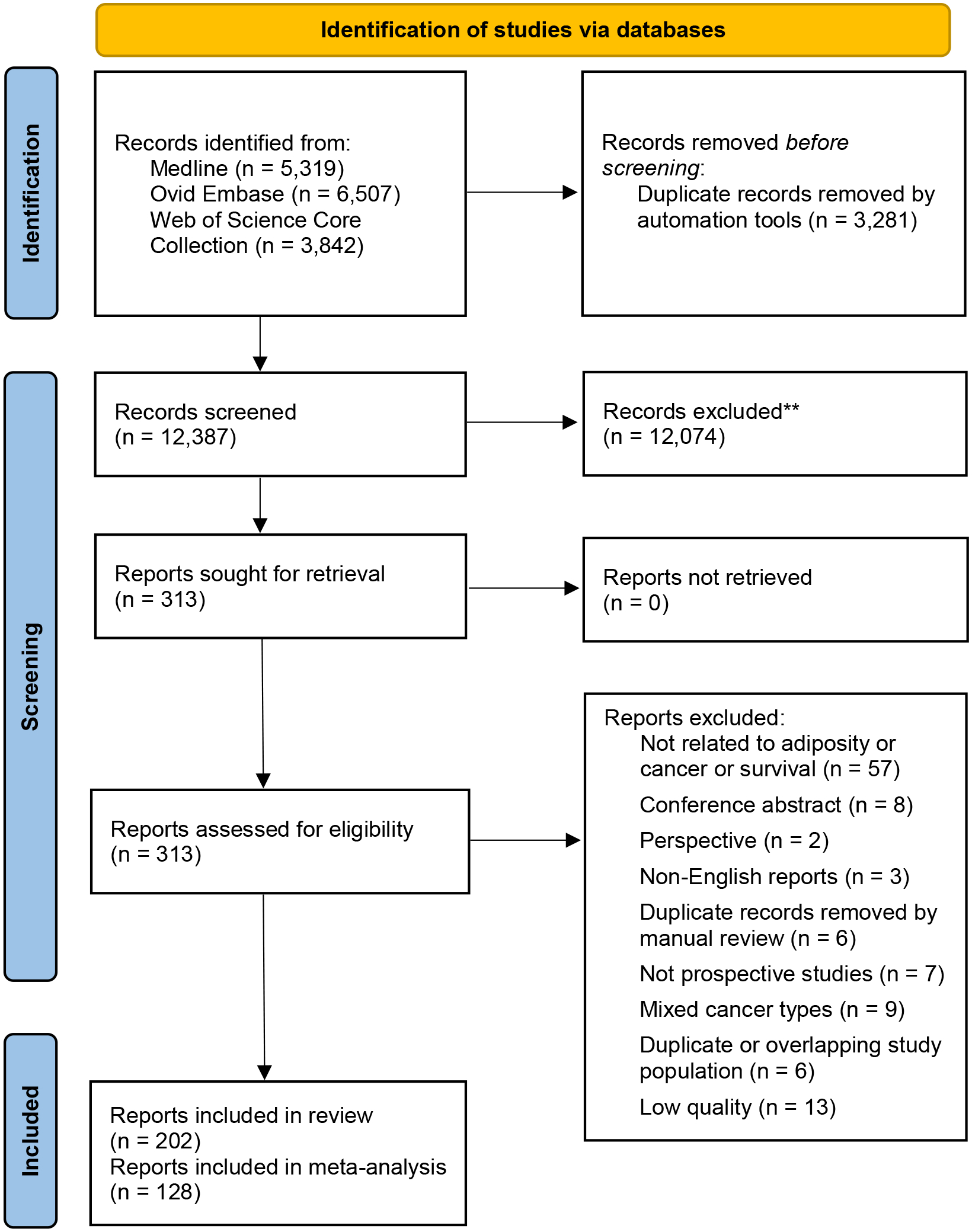
PRISMA 2020 Flow Diagram for the Systematic Review Which Included Searches of Databases Only
IMAGING
Breast
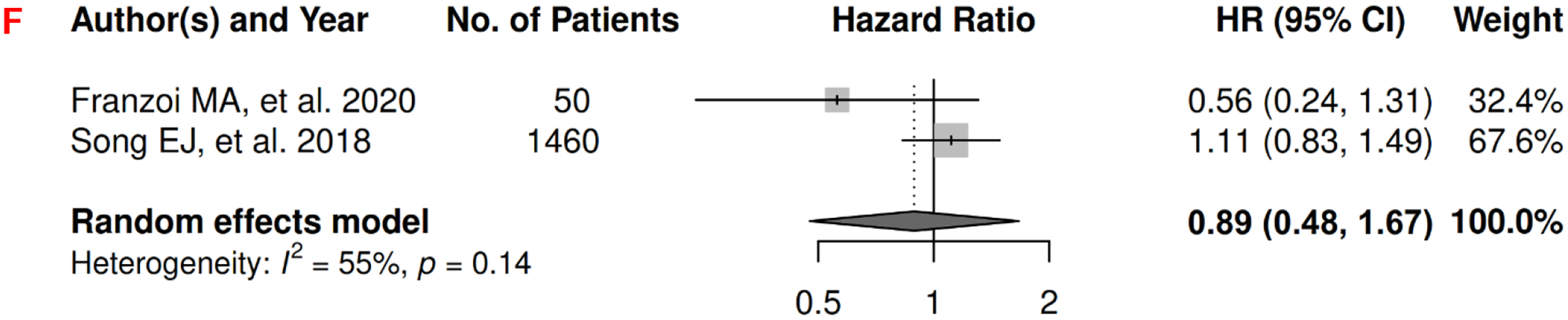
In breast cancer, there were 7 records included for meta-analysis and summary estimates are presented in Figure 2. More details of published records included into the meta-analysis are in Supplementary Table 2 [62–68]. Visceral, subcutaneous, and total adiposity were not significantly associated with OS among breast cancer patients (HRs for visceral: 1.03 [0.55, 1.92]; subcutaneous: 1.36 [0.90, 2.05]; total: 1.14 [0.77, 1.69]), and corresponding heterogeneity was high for visceral and total adiposity, but low for subcutaneous adiposity. No meta-analysis could be performed for CSS, but one record suggested that visceral adiposity was associated with increased risk of death from breast cancer (HR: 1.18 [1.02, 1.37]) and subcutaneous was not (HR: 0.92 [0.78, 1.08]) [69]. No records in total adiposity and CSS have been published. Visceral, subcutaneous, and total adiposity were not significantly associated with risk of breast cancer progression (HRs for visceral: 1.20 [0.40, 3.57]; subcutaneous: 1.01 [0.53, 1.93]; total: 0.89 [0.48, 1.67]), and corresponding heterogeneity was significant for visceral adiposity, low for subcutaneous, and high for total adiposity.
Figure 2.
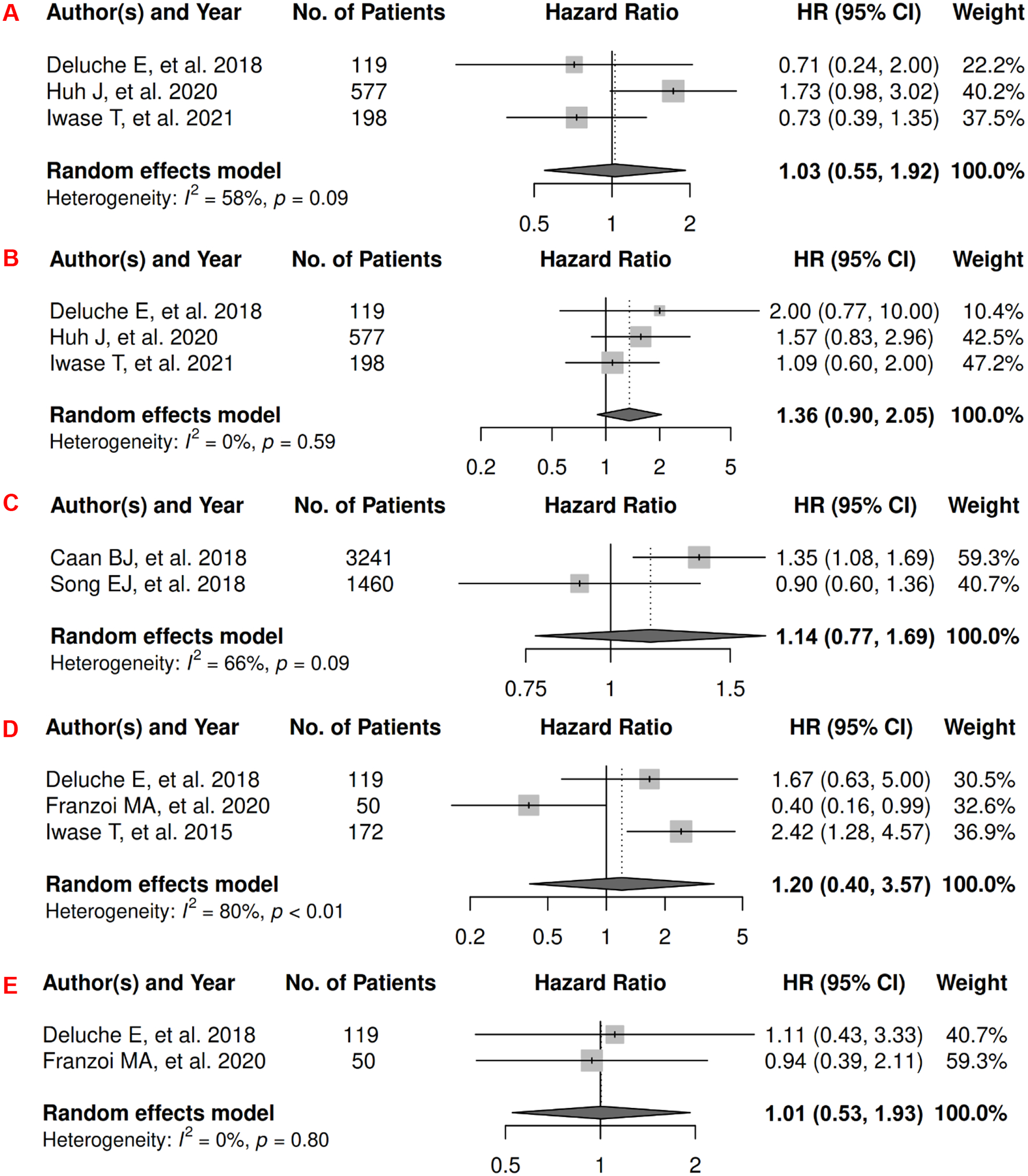
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Breast Cancer
2A: visceral adiposity and overall survival.
2B: subcutaneous adiposity and overall survival.
2C: total adiposity and overall survival.
2D: visceral adiposity and progression-free survival.
2E: subcutaneous adiposity and progression-free survival.
2F: total adiposity and progression-free survival.
Colorectal Cancer (CRC)
In CRC, there were 27 records included for meta-analysis and summary estimates are presented in Figure 3. More details of published records included into the meta-analysis are in Supplementary Table 3 [70–96]. Visceral, subcutaneous, and total adiposity were not significantly associated with OS among CRC patients (HRs for visceral: 0.99 [0.81, 1.21]; subcutaneous: 1.01 [0.77, 1.32]; total: 0.89 [0.58, 1.37]), and corresponding heterogeneity were significant for all three adiposity measures. Similarly, visceral, subcutaneous, and total adiposity were not significantly associated with risk of death from CRC (HRs for visceral: 0.92 [0.77, 1.08]; subcutaneous: 0.78 [0.57, 1.07]; total: 1.11 [0.83, 1.49]), and corresponding heterogeneity was low for visceral and subcutaneous adiposity, but high for total adiposity. Visceral, subcutaneous, and total adiposity were not significantly associated with risk of CRC progression (HRs for visceral: 1.07 [0.75, 1.54]; subcutaneous: 0.78 [0.48, 1.25]; total: 0.69 [0.21, 2.30]), and corresponding heterogeneity was significant for visceral adiposity, and high for subcutaneous and total adiposity.
Figure 3.
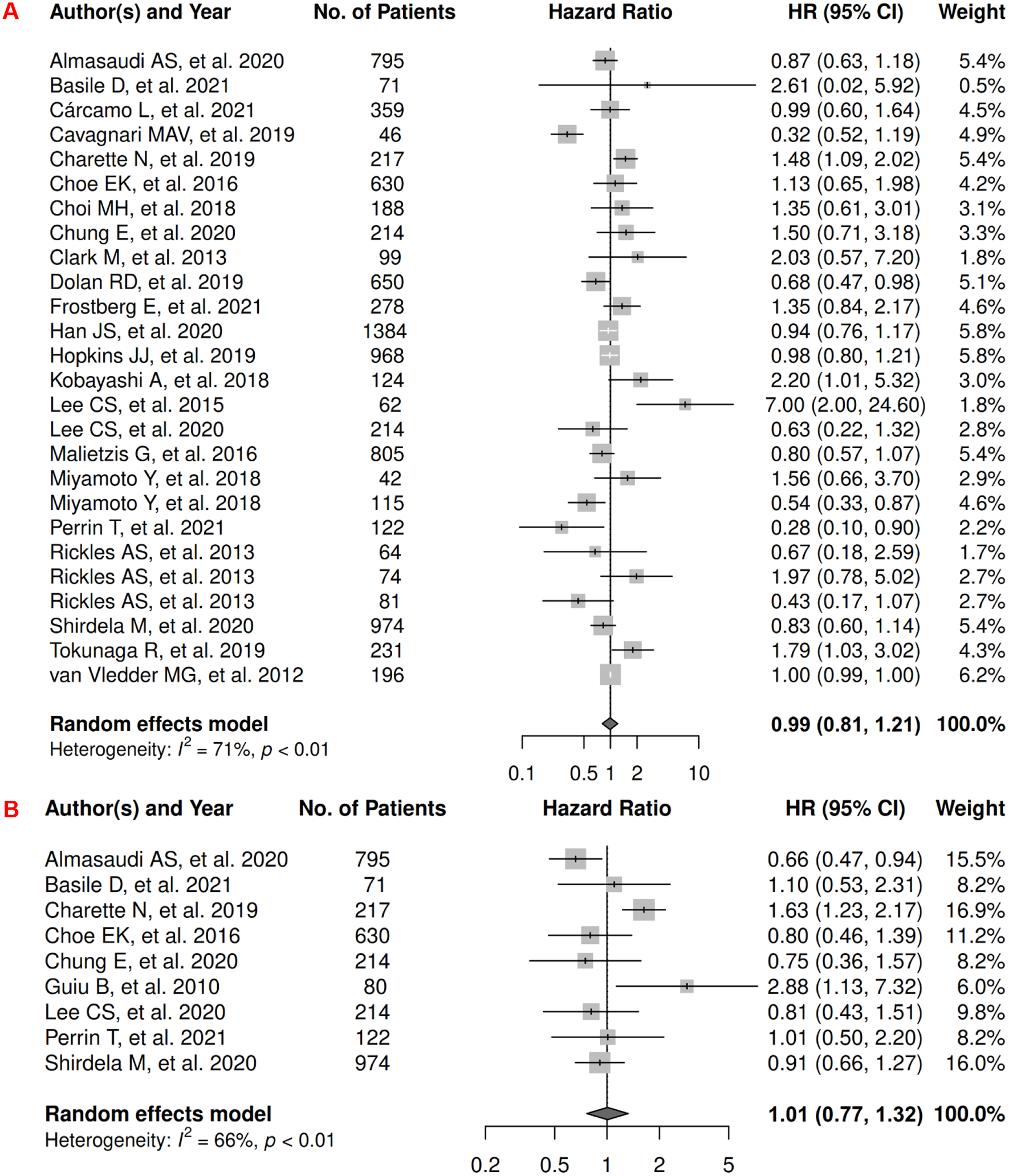
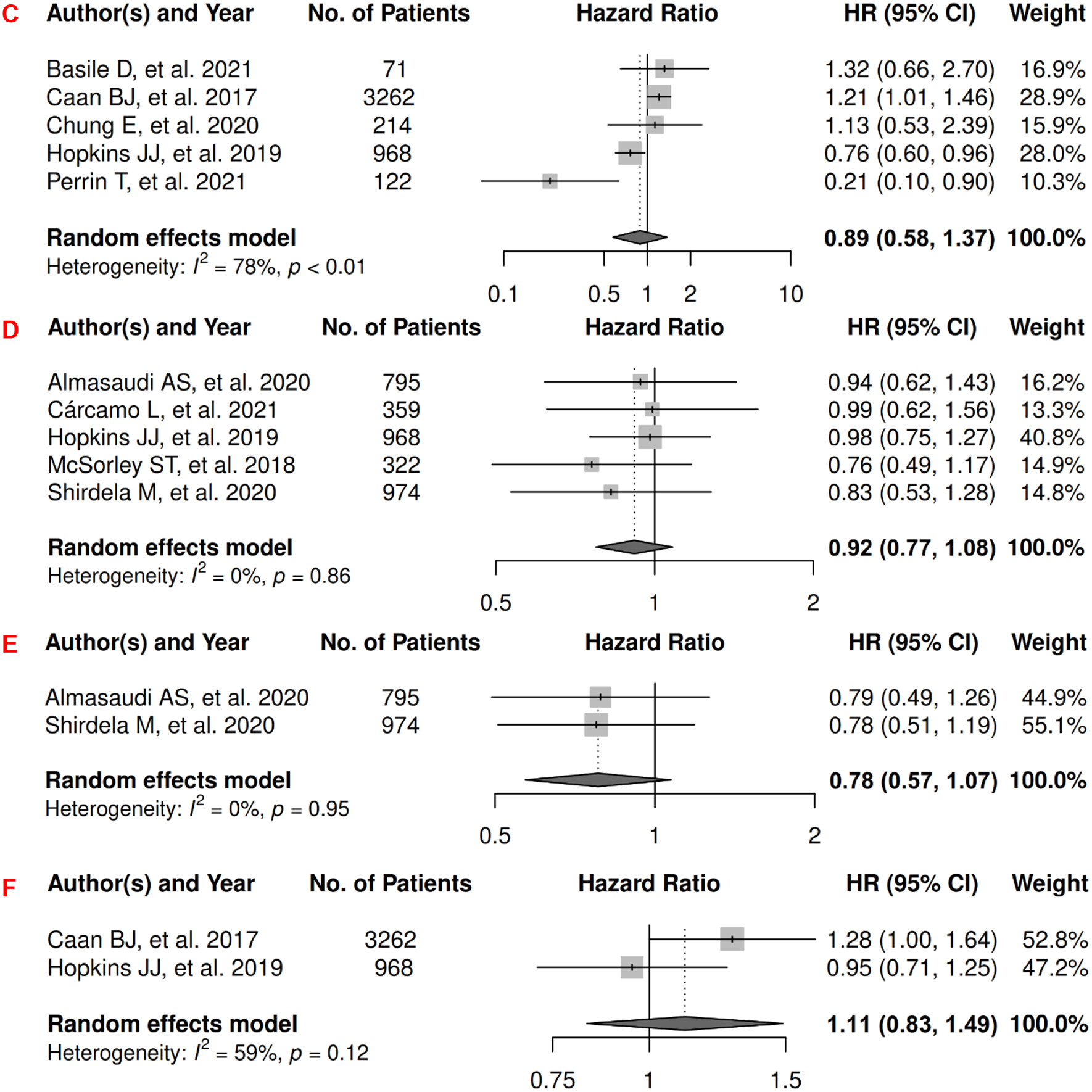
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Colorectal Cancer
3A: visceral adiposity and overall survival.
3B: subcutaneous adiposity and overall survival.
3C: total adiposity and overall survival.
3D: visceral adiposity and cancer-specific survival.
3E: subcutaneous adiposity and cancer-specific survival.
3F: total adiposity and cancer-specific survival.
3G: visceral adiposity and progression-free survival.
3H: subcutaneous adiposity and progression-free survival.
3I: total adiposity and progression-free survival.
Gastroesophageal
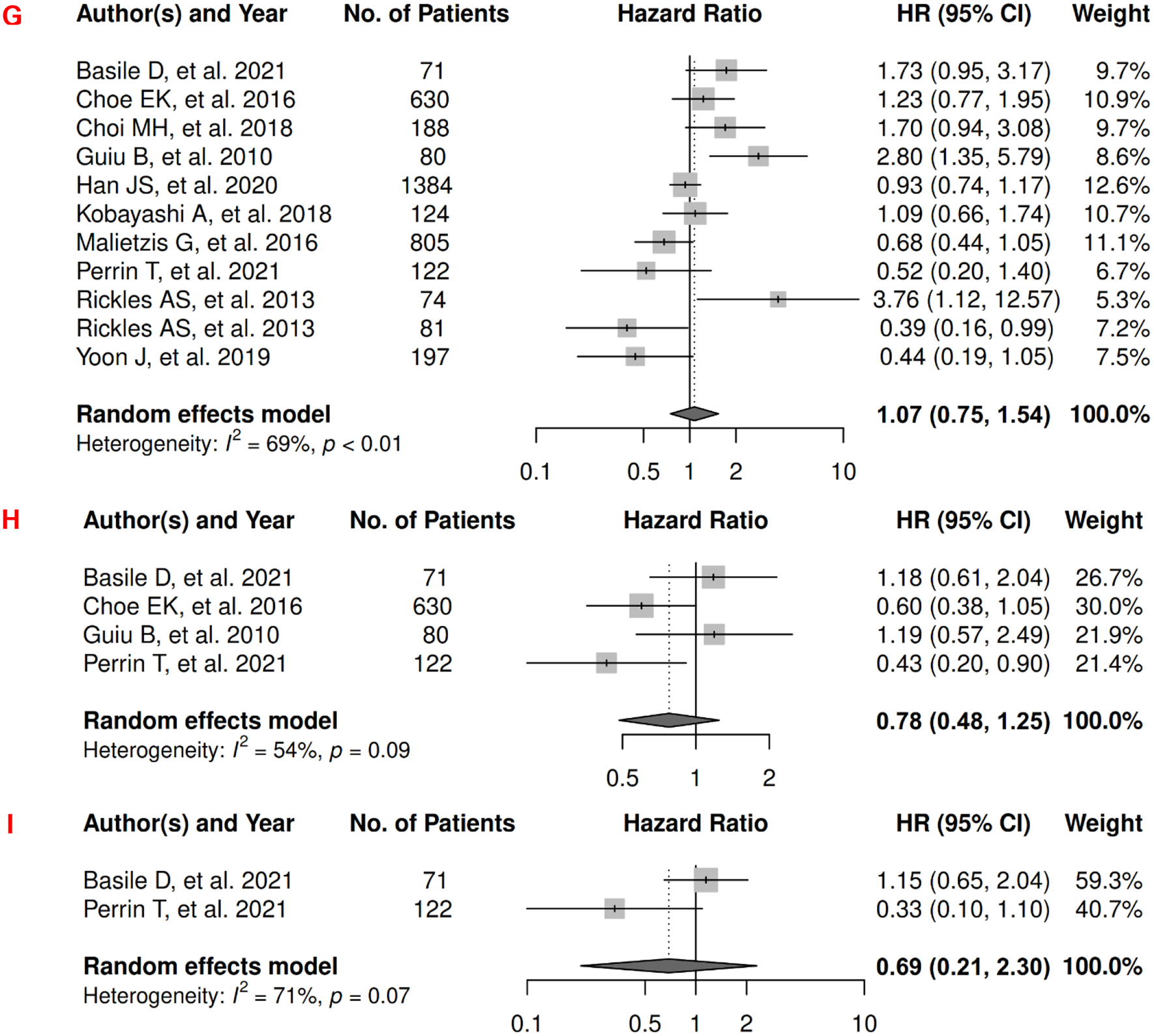
In gastroesophageal cancer, there were 15 records included for meta-analysis and summary estimates are presented in Figure 4. More details of published records included into the meta-analysis are in Supplementary Table 4 [97–111]. Visceral adiposity was not significantly associated with OS among gastroesophageal cancer patients (HR: 0.87 [0.67, 1.14]), whereas subcutaneous adiposity was associated with a decreased risk of mortality (HR: 0.64 [0.46, 0.90]) and no study was done for total adiposity and OS. Corresponding heterogeneity statistics were significant for both visceral and subcutaneous adiposity measures. No records have been published for CSS. Visceral adiposity was not significantly associated with risk of gastroesophageal cancer progression (HR: 0.89 [0.33, 2.42]), and corresponding heterogeneity was significant. No PFS meta-analysis could be performed for subcutaneous and total adiposity, but two studies suggested non-significant associations [112, 113]: HR (1.001 [0.998, 1.004]) for subcutaneous adiposity (measured as continuous SAT index); p = 0.47 for total adiposity analyzed using the Kaplan-Meier estimator.
Figure 4.
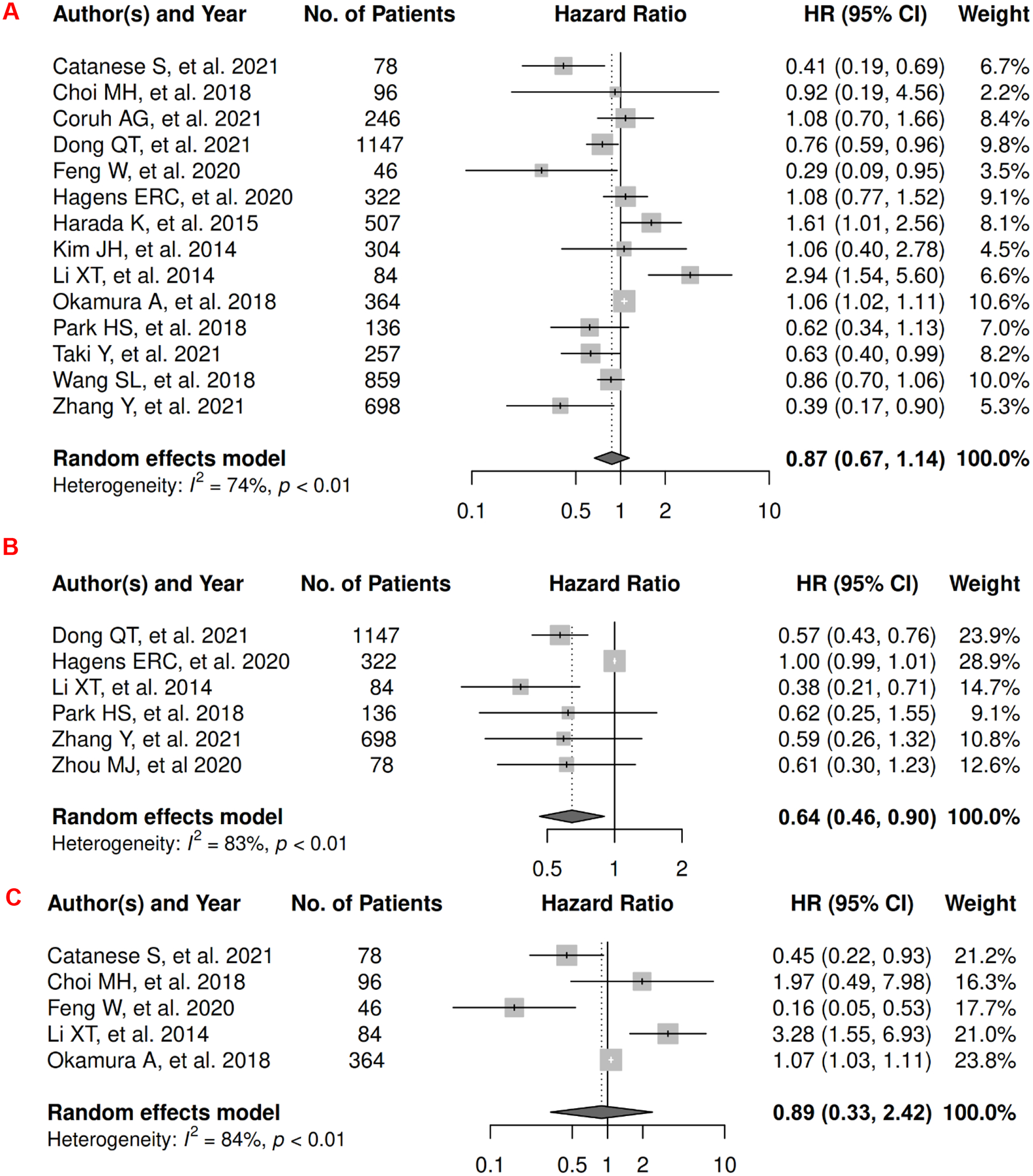
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Gastroesophageal Cancer
4A: visceral adiposity and overall survival.
4B: subcutaneous adiposity and overall survival.
4C: visceral adiposity and progression-free survival.
Head and Neck
In head and neck cancer, there were 3 records included for meta-analysis and summary estimates are presented in Figure 5. More details of published records included into the meta-analysis are in Supplementary Table 5 [114–116]. No meta-analysis could be performed for OS, but three studies investigated the associations of visceral, subcutaneous, and total adiposity, respectively. Visceral adiposity was not significantly associated with risk of mortality (HR: 0.35 [0.09, 1.43]) [114], whereas subcutaneous and total adiposity were associated with a decreased risk of mortality (HRs for subcutaneous: 0.60 [0.48, 0.76]); total: 0.29 [0.10, 0.83]) [116, 117]. No records have been published for CSS. Visceral and subcutaneous adiposity were not significantly associated with risk of head and neck cancer progression (HRs for visceral: 1.36 [0.28, 6.58]; subcutaneous: 1.00 [0.49, 2.03]), and heterogeneity was significant for visceral adiposity and high for subcutaneous adiposity. No PFS meta-analysis could be performed for total adiposity, but one suggested a significant association with lower risk of progression (HR: 0.27 [0.10, 0.71]) [117].
Figure 5.
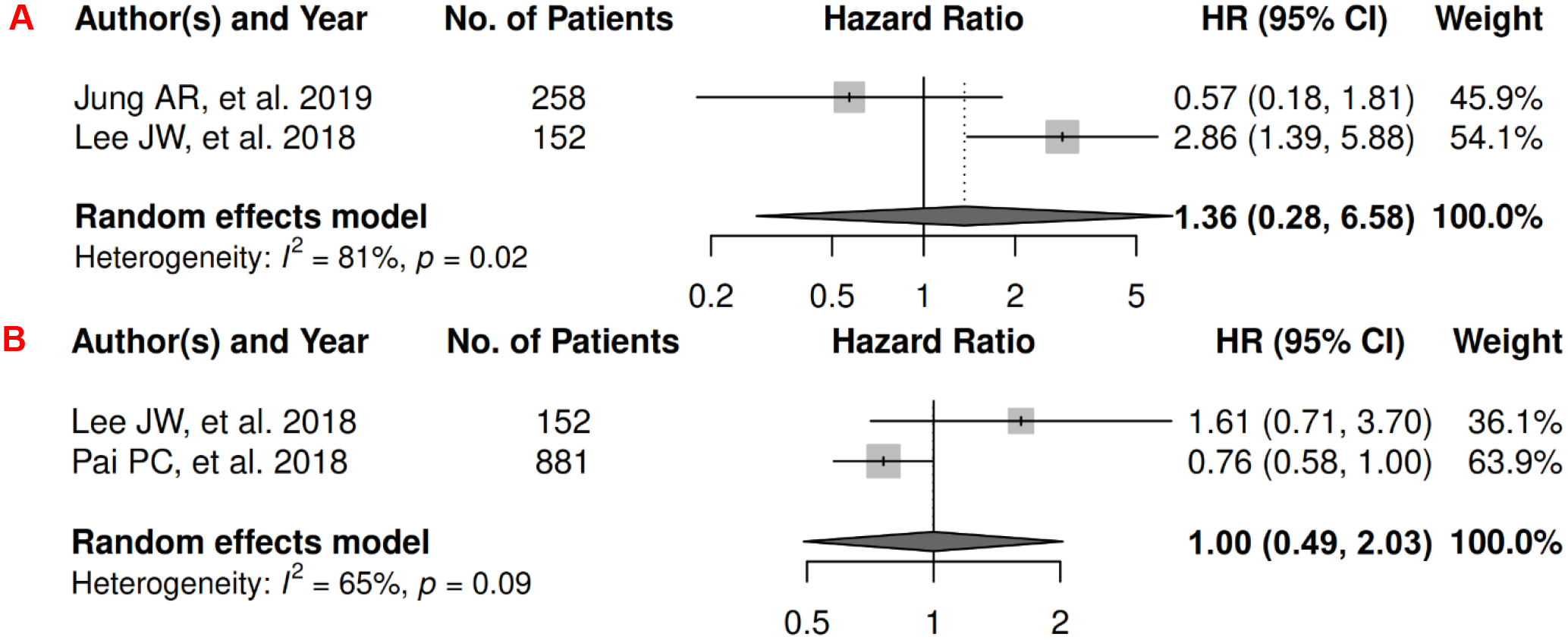
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Head and Neck Cancer
5A: visceral adiposity and progression-free survival.
5B: subcutaneous adiposity and progression-free survival.
Hepatocellular Carcinoma (HCC)
In HCC, there were 11 records included for meta-analysis and summary estimates are presented in Figure 6. More details of published records included into the meta-analysis are in Supplementary Table 6 [118–128]. Visceral, subcutaneous, and total adiposity were not significantly associated with risk of mortality among HCC patients (HRs for visceral: 1.17 [0.85, 1.60]; subcutaneous: 1.10 [0.55, 2.21]; total: 1.05 [0.44, 2.48]), and corresponding heterogeneity were significant for all three adiposity measures. No records have been published for CSS. Visceral adiposity was not significantly associated with risk of HCC progression (HR: 0.96 [0.73, 1.28]), and heterogeneity was significant for visceral adiposity. No PFS meta-analysis could be performed for subcutaneous and total adiposity. Two studies reported subcutaneous adiposity (measured as continuous SAT index) was inconsistently associated with PFS: one was significant (HR: 1.03 [1.01. 1.05]) whereas the other was not (HR: 1.00 [0.99, 1.01]) [129, 130], and one study reported total adiposity (measured as continuous TAT index) was significantly associated with increased risk of progression (HR: 1.03 [1.01. 1.05]) [129].
Figure 6.
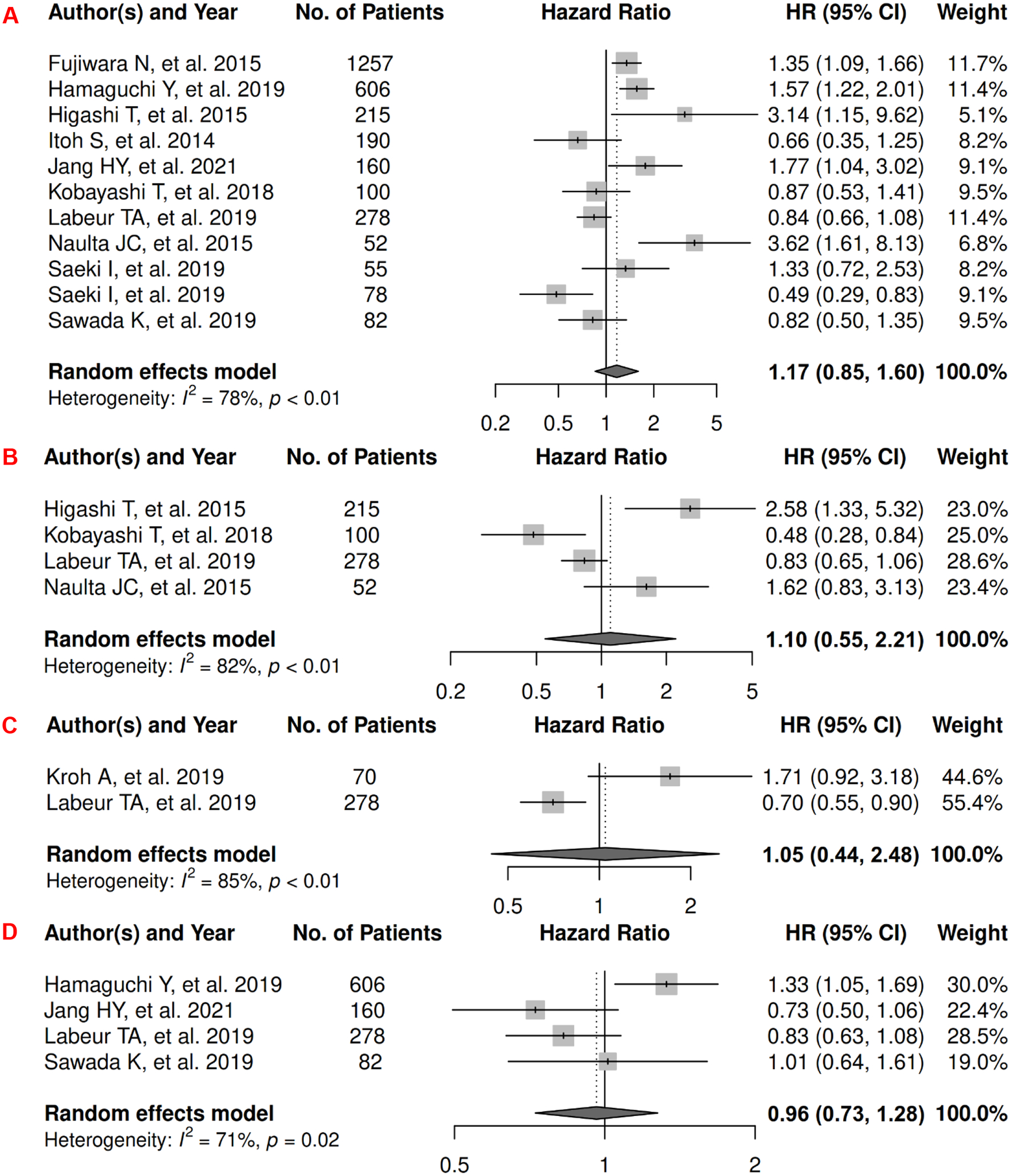
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Hepatocellular Carcinoma
6A: visceral adiposity and overall survival.
6B: subcutaneous adiposity and overall survival.
6C: total adiposity and overall survival.
6D: visceral adiposity and progression-free survival.
Lung
In lung cancer, there were 7 records included for meta-analysis and summary estimates are presented in Figure 7. More details of published records included into the meta-analysis are in Supplementary Table 7 [44, 131–136]. Visceral and subcutaneous adiposity were not significantly associated with OS among lung cancer patients (HRs for visceral: 1.05 [0.90, 1.22]; subcutaneous: 0.74 [0.45, 1.20]), and corresponding heterogeneity was low for visceral and high for subcutaneous adiposity. Only one study investigated total adiposity and OS and reported no association (HR: 0.99 [0.68, 1.46]) [137]. No records have been published for CSS. Visceral adiposity was not significantly associated with risk of lung cancer progression (HR: 1.06 [0.85, 1.33]), and heterogeneity for studies was low. No records have been published for subcutaneous and total adiposity and PFS.
Figure 7.
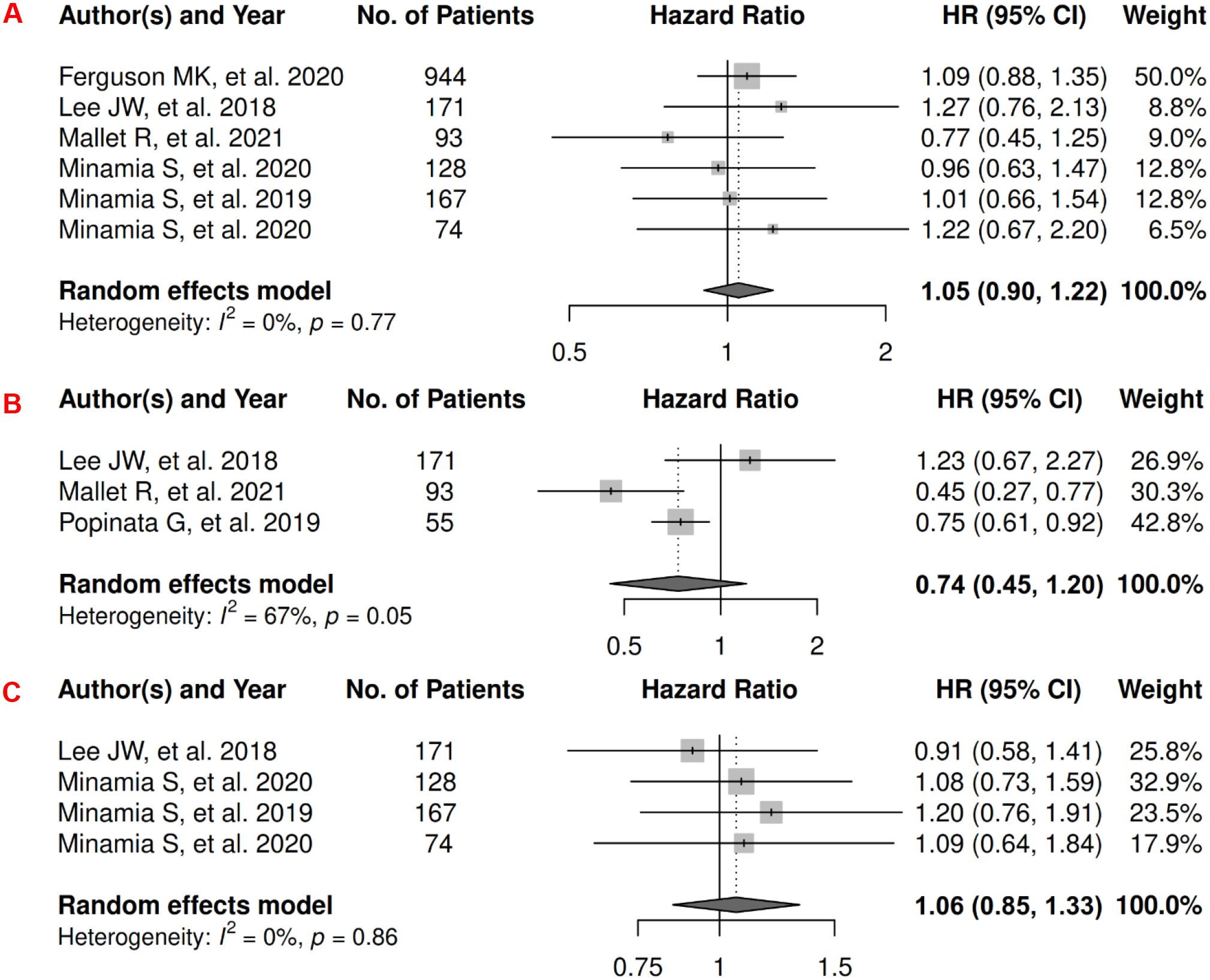
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Lung Cancer
7A: visceral adiposity and overall survival.
7B: subcutaneous adiposity and overall survival.
7C: visceral adiposity and progression-free survival.
Ovarian
In ovarian cancer, there were 4 records included for meta-analysis and summary estimates are presented in Figure 8. More details of published records included into the meta-analysis are in Supplementary Table 8 [138–141]. Visceral and subcutaneous adiposity were not significantly associated with OS among ovarian patients (HRs for visceral: 0.53 [0.11, 2.66]; subcutaneous: 0.65 [0.19, 2.19]), and corresponding heterogeneity was significant for both visceral and subcutaneous adiposity. Only one study investigated total adiposity (tertiles) and OS with only an insignificant p value (p = 0.33) reported for the Kaplan-Meier estimator [142]. No records have been published for CSS. Visceral adiposity was not significantly associated with risk of ovarian cancer progression (HR: 0.76 [0.44, 1.30]) but subcutaneous adiposity was associated with a borderline decreased risk (HR: 0.76 [0.58, 1.00]), and corresponding heterogeneity was high for visceral adiposity and low for subcutaneous adiposity. No records have been published for total adiposity and PFS.
Figure 8.
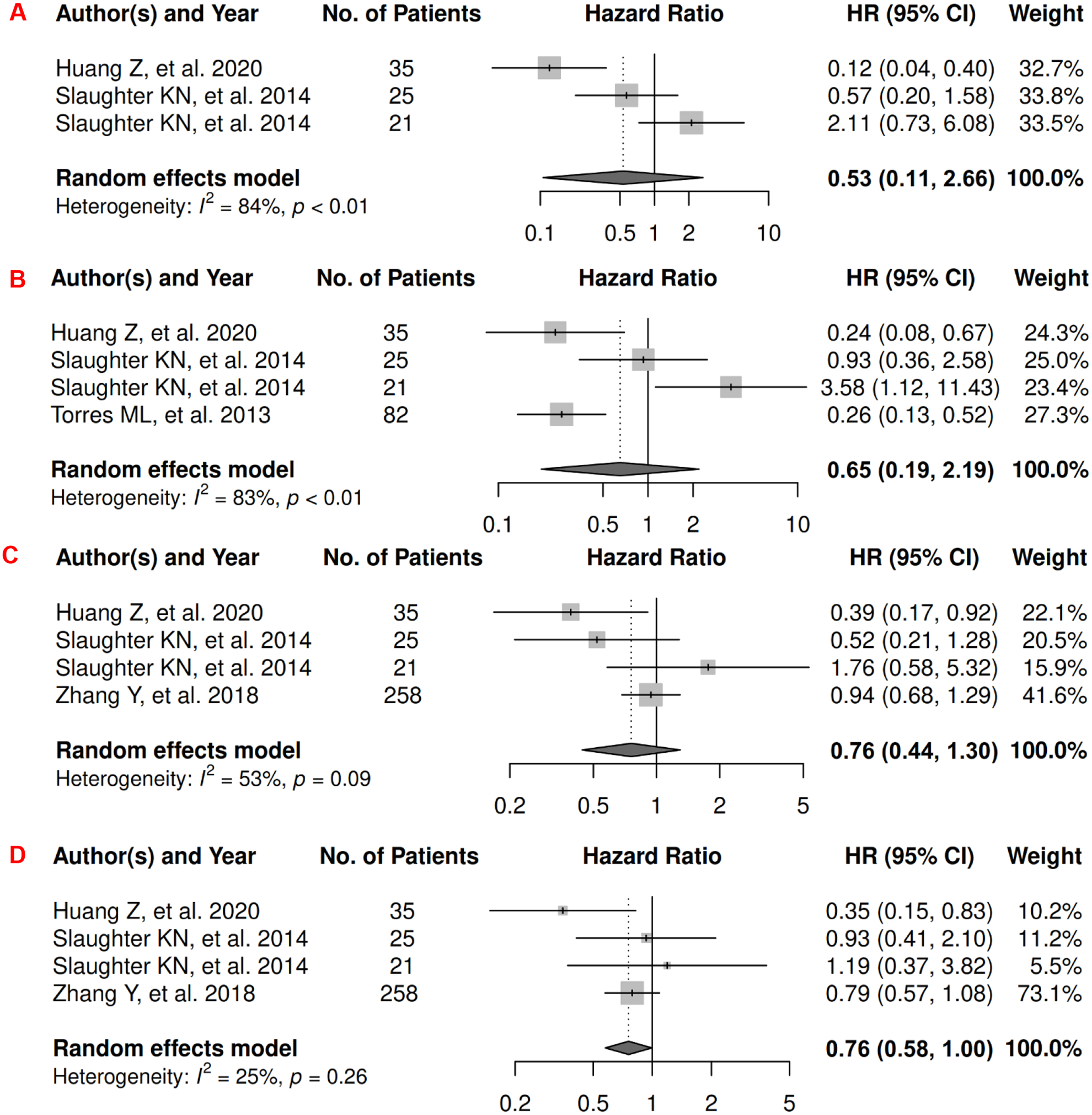
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Ovarian Cancer
8A: visceral adiposity and overall survival.
8B: subcutaneous adiposity and overall survival.
8C: visceral adiposity and progression-free survival.
8D: subcutaneous adiposity and progression-free survival.
Pancreatic
In pancreatic cancer, there were 7 records included for meta-analysis and summary estimates are presented in Figure 9. More details of published records included into the meta-analysis are in Supplementary Table 9 [45, 143–148]. Visceral and subcutaneous adiposity were not significantly associated with OS among pancreatic cancer patients (HRs for visceral: 1.05 [0.88, 1.26]; subcutaneous: 0.81 [0.44, 1.49]), and corresponding heterogeneity was low for visceral adiposity and moderate for subcutaneous adiposity. Only one study investigated total adiposity and OS, and reported an insignificant association [149]. No records have been published for CSS. No meta-analysis could be performed for visceral and subcutaneous adiposity and PFS, and no records have been published for total adiposity and PFS. However, four studies investigated visceral adiposity and PFS, and two reported significant, adverse associations (HR: 1.01 [1.00, 1.02] for continuous VAT measures; and p = 0.04 for the Kaplan-Meier estimator) whereas the others did not (HR: 1.00 [0.99, 1.01] for continuous VAT measures; and p >0.05 for the Kaplan-Meier estimator) [150–153]. Two studies investigated subcutaneous adiposity and PFS, and both reported no association (HR: 0.98 [0.83, 1.15] for continuous SAT measures; and p >0.05 for the Kaplan-Meier estimator) [150, 153].
Figure 9.
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Pancreatic Cancer
9A: visceral adiposity and overall survival.
9B: subcutaneous adiposity and overall survival.
Prostate
In prostate cancer, there were 12 records included for meta-analysis and summary estimates are presented in Figure 10. More details of published records included into the meta-analysis are in Supplementary Table 10 [154–165]. Visceral and total adiposity were not significantly associated with OS among prostate cancer patients (HRs for visceral: 1.07 [0.84, 1.35]; total: 0.88 [0.65, 1.20]), but subcutaneous adiposity was associated with a decreased risk: 0.69 [0.57, 0.84]; corresponding heterogeneity was high for visceral adiposity, and low for subcutaneous and total adiposity. Visceral adiposity was not significantly associated with risk of death from prostate cancer (HR: 1.02 [0.76, 1.35]), whereas subcutaneous adiposity was associated with a decreased risk (HR: 0.73 [0.55, 0.98]). Heterogeneity was low for both visceral and subcutaneous adiposity. No records have been published for total adiposity and CSS. Visceral and total adiposity were not significantly associated with prostate cancer progression (HRs for visceral: 1.04 [0.87, 1.24]; total: 0.95 [0.73, 1.24]), and subcutaneous adiposity was significantly associated with decreased prostate cancer progression (HR: 0.81 [0.68, 0.97]). Heterogeneity was moderate for visceral adiposity, and low for subcutaneous and total adiposity.
Figure 10.
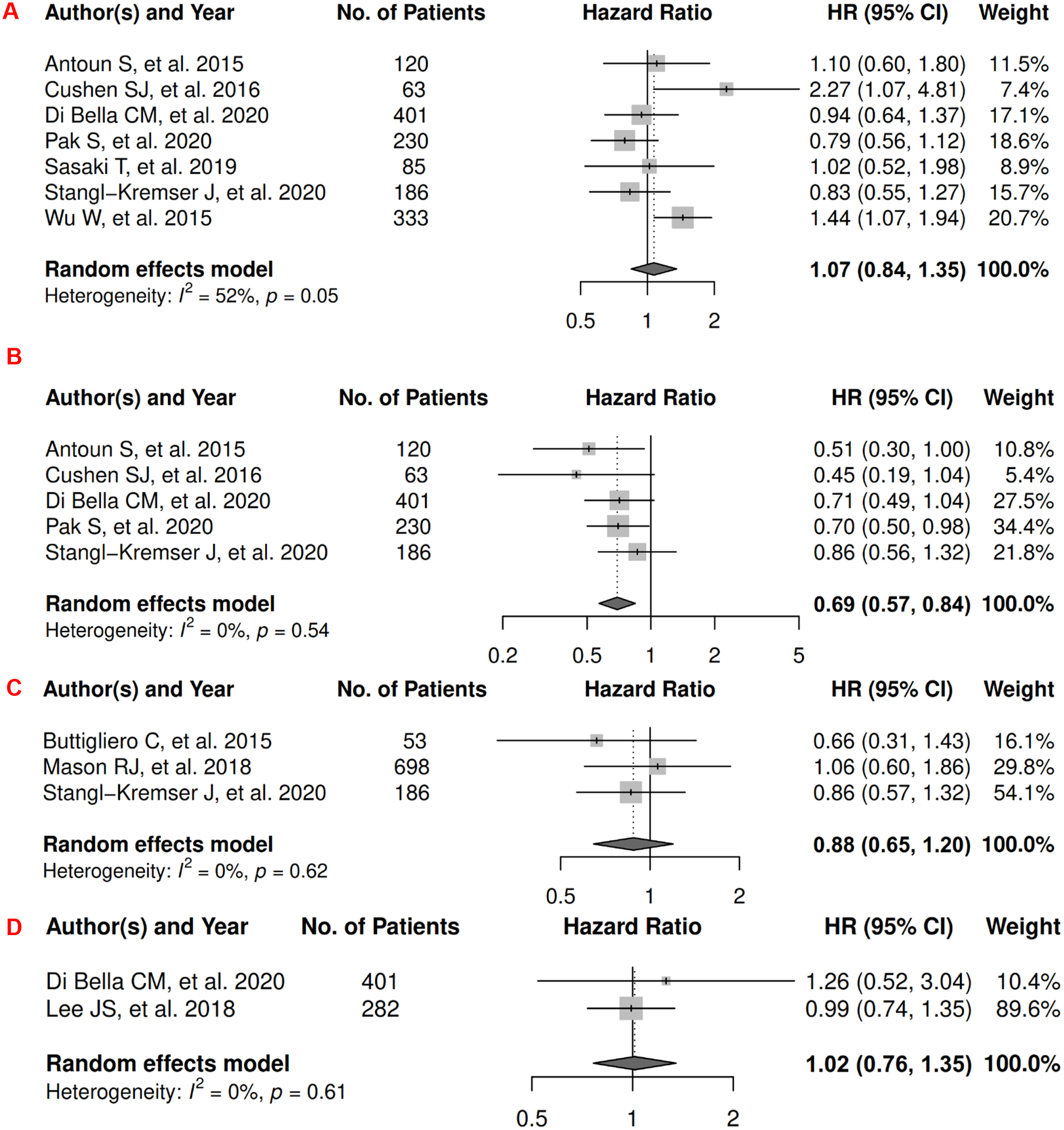
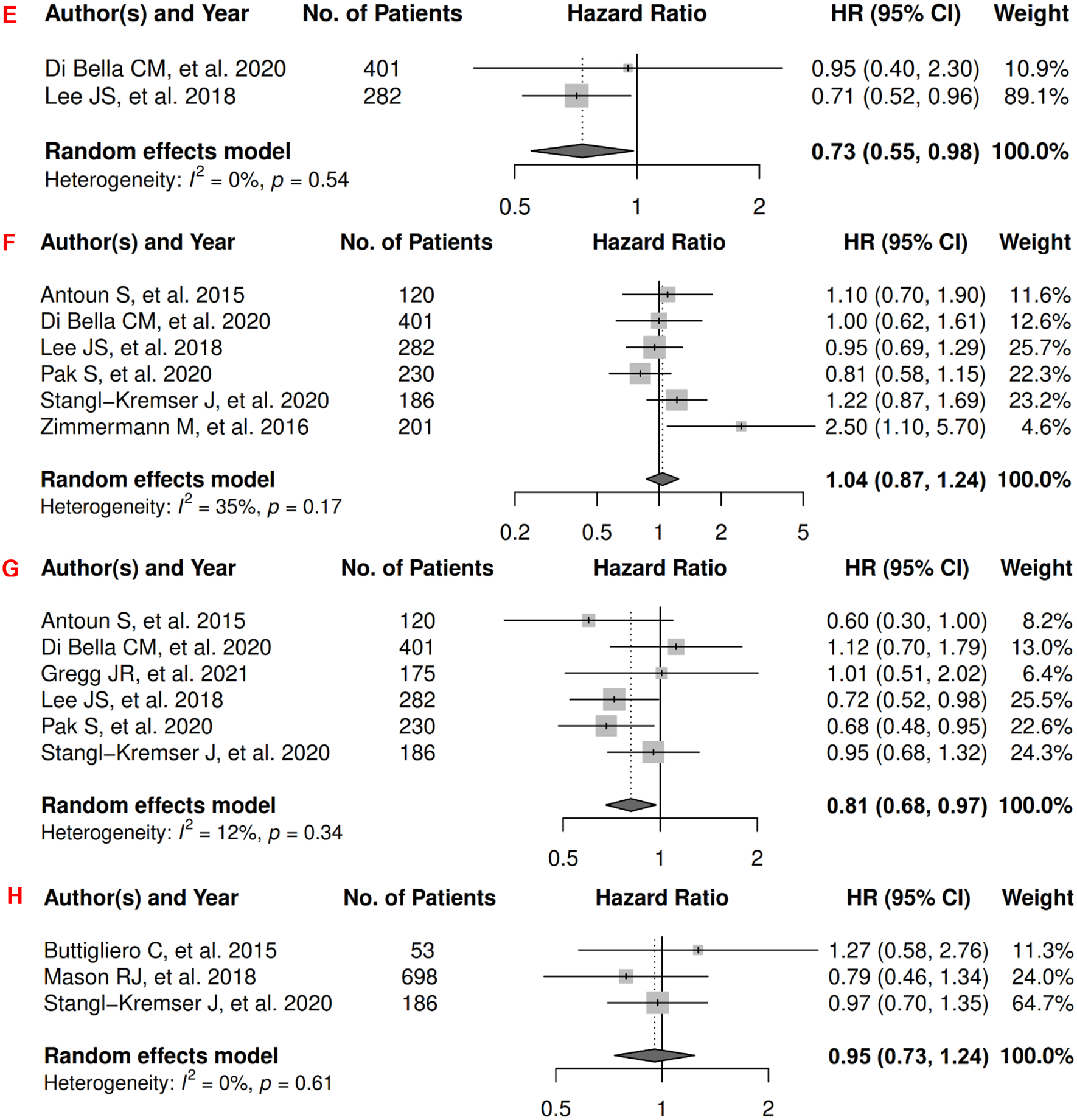
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Prostate Cancer
10A: visceral adiposity and overall survival.
10B: subcutaneous adiposity and overall survival.
10C: total adiposity and overall survival.
10D: visceral adiposity and cancer-specific survival.
10E: subcutaneous adiposity and cancer-specific survival.
10F: visceral adiposity and progression-free survival.
10G: subcutaneous adiposity and progression-free survival.
10H: total adiposity and progression-free survival.
Renal
In renal cancer, there were 13 records included for meta-analysis and summary estimates are presented in Figure 11. More details of published records included into the meta-analysis are in Supplementary Table 11 [46, 166–177]. Visceral, subcutaneous, and total adiposity were not significantly associated with OS among renal cancer patients (HRs for visceral: 1.62 [0.90, 2.95]; subcutaneous: 0.90 [0.25, 3.27]; total: 0.87 [0.65, 1.18]), and corresponding heterogeneity was significant for visceral and subcutaneous adiposity, and low for total adiposity. Visceral adiposity was not significantly associated with increased risk of death from renal cancer (HR: 2.47 [0.09, 67.43]) in the two studies examining this association, and heterogeneity was significant. No records have been published for subcutaneous and total adiposity and CSS. Visceral and subcutaneous adiposity were not significantly associated with renal caner progression (HRs for visceral: 0.85 [0.32, 2.27]; subcutaneous: 1.29 [0.31, 5.37]), and heterogeneity was significant for both. No records have been published for total adiposity and PFS.
Figure 11.
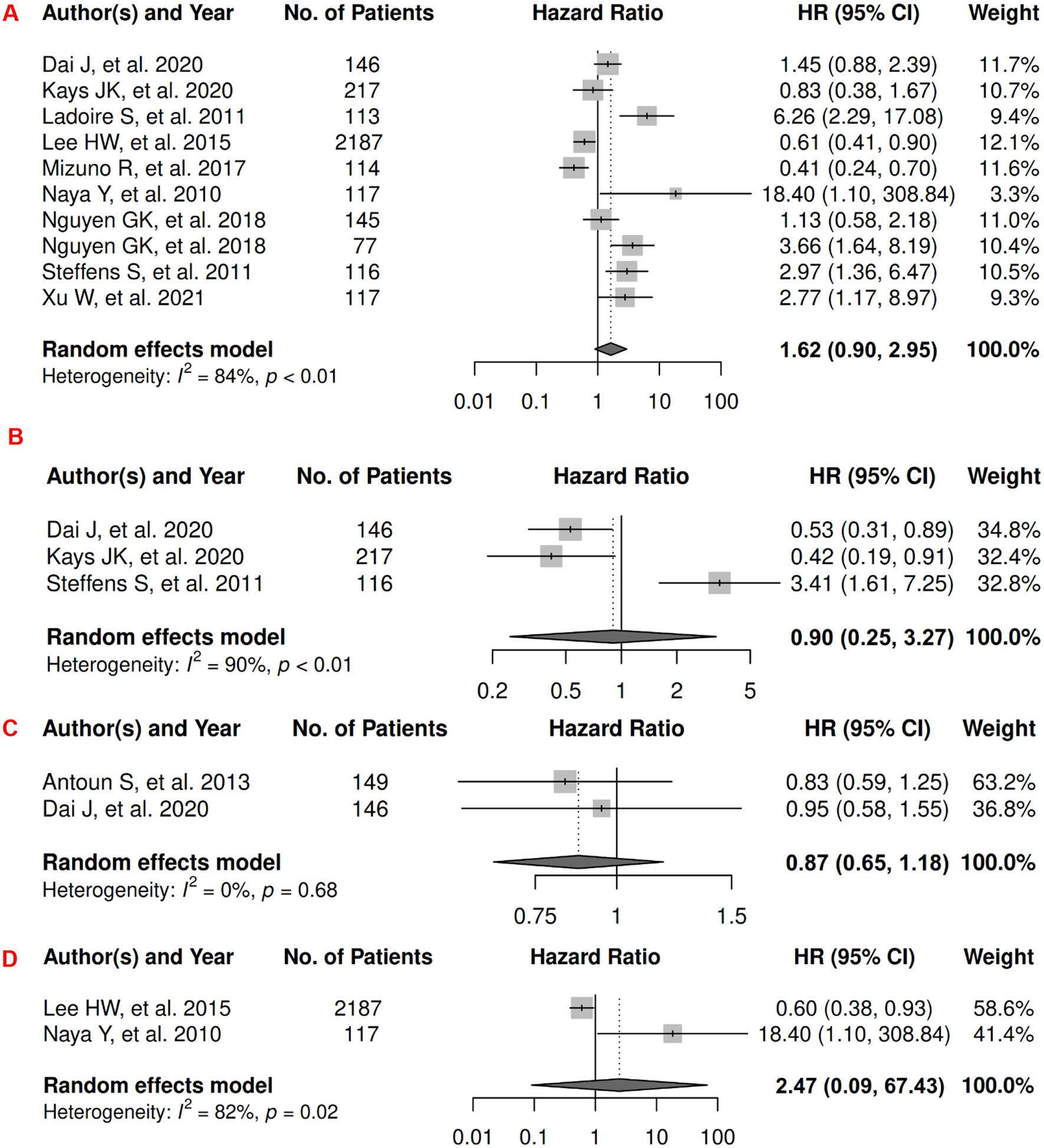
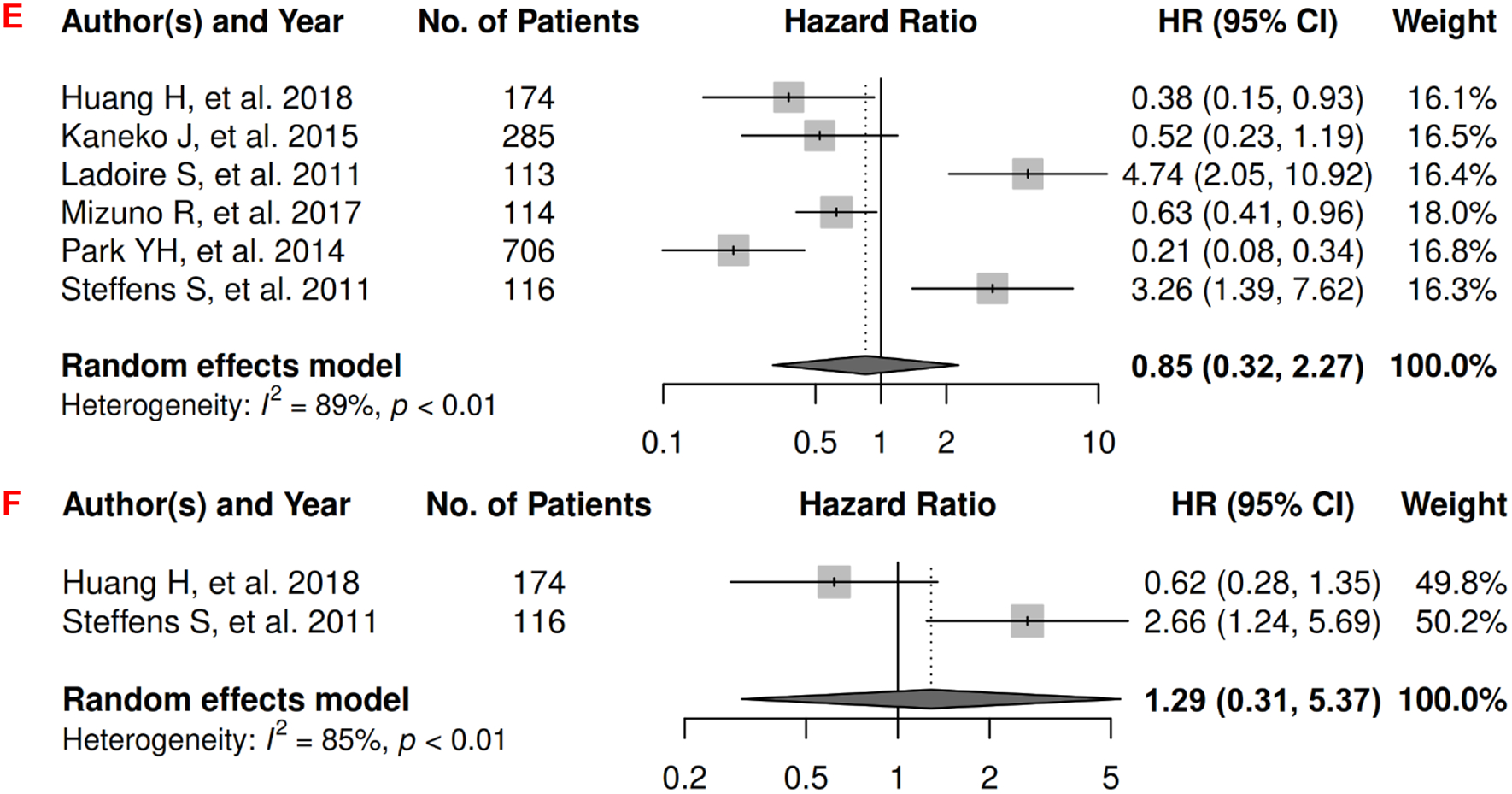
Forest Plots of Assessing the Associations between Imaging-Measured Adiposity and Survival among Renal Cancer
11A: visceral adiposity and overall survival.
11B: subcutaneous adiposity and overall survival.
11C: total adiposity and overall survival.
11D: visceral adiposity and cancer-specific survival.
11E: visceral adiposity and progression-free survival.
11F: subcutaneous adiposity and progression-free survival.
Other Cancers
There were 18 records in adiposity and survival in 12 cancer types for which no meta-analysis could be conducted. These cancer types were adrenocortical, acute myeloid leukemia (AML), biliary, bladder, cholangiocarcinoma, endometrial, lymphoma, melanoma, multiple myeloma, nasopharyngeal, sarcoma, and urinary tract. More details of these published records are in Supplementary Table 12 [178–195]. Most studies reported insignificant associations or p values suggesting that greater adiposity may not be associated with worse survival among most of these cancer types.
Sensitivity Analysis
There were 52 records not included into the above meta-analyses due to 1) analyzing adiposity measure as continuous variables, or 2) only reporting p values (most were >0.05). More details are presented in Supplementary Tables 13–22 [69, 112, 113, 117, 129, 130, 137, 142, 149–153, 196–234]. After including studies in meta-analysis that analyzed adiposity measure as continuous variables, the results of the overall pattern remained almost same: imaging-measured adiposity was not significantly associated with risk of mortality or progression (Supplementary Table 23). However, three associations became significant: higher subcutaneous adiposity was significantly associated with worse OS (HR: 1.14 [1.03, 1.27]) and worse PFS (HR: 1.02 [1.01–1.03]) in breast cancer; and higher visceral adiposity was significantly associated with worse OS (HR: 1.14 [1.01–1.29]) in lung cancer. The inverse association of higher subcutaneous adiposity and OS remained significant in prostate cancer, but the magnitude of HR was slightly attenuated from 0.73 (0.55, 0.98) to 0.77 (0.61, 0.96).
Publication Bias
Funnel plots and Begg and Mazumdar rank correlation tests suggested no publication bias, except for visceral adiposity and overall survival in renal cancer (p = 0.04). More details are in Supplementary Figure 1 and Supplementary Table 24.
ANTHROPOMETRY
Breast
In breast cancer, there were 14 records for meta-analysis and summary estimates are presented in Figure 12. More details of published records included into the meta-analysis are in Supplementary Table 25 [235–248]. Visceral adiposity assessed by the proxy measure of waist-related anthropometric measures was significantly associated with increased risk of mortality (HR: 1.30 [1.15, 1.46]) and death from breast cancer (HR: 1.26 [1.03, 1.55]), but not with breast cancer progression (HR: 1.17 [0.88, 1.55]). Heterogeneity was moderate for all three estimates. No records have been published on subcutaneous adiposity and breast cancer survival assessed by anthropometric proxies.
Figure 12.
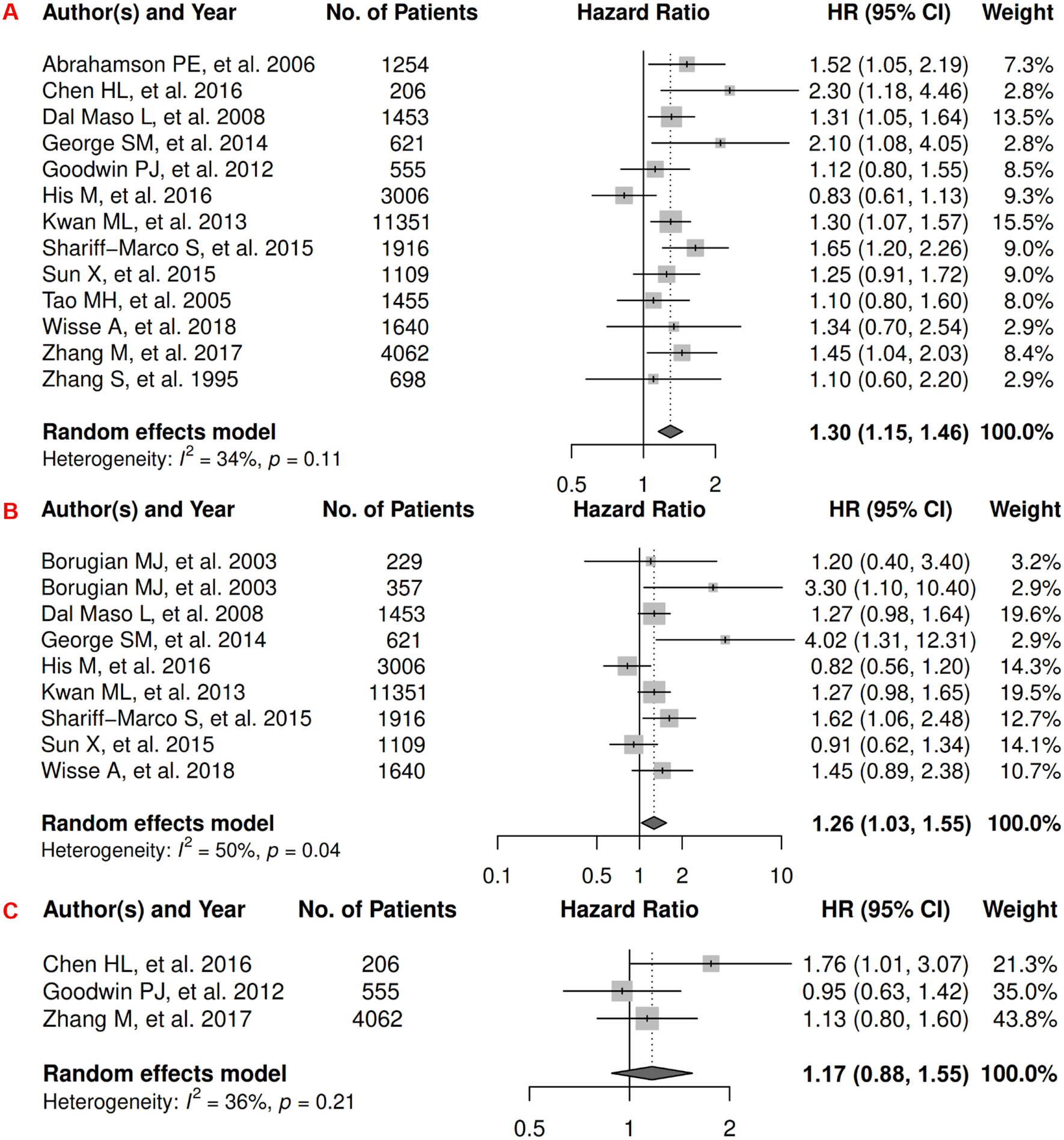
Forest Plots of Assessing the Associations between Anthropometry-Measured Visceral Adiposity and Survival among Breast Cancer
12A: visceral adiposity and overall survival.
12B: visceral adiposity and cancer-specific survival.
12C: visceral adiposity and progression-free survival.
Colorectal Cancer (CRC)
In colorectal cancer, there were 5 records for meta-analysis and summary estimates are presented in Figure 13. More details of published records included into the meta-analysis are found in Supplementary Table 26 [249–253]. Waist-related anthropometric measures were significantly associated with increased risk of mortality (HR: 1.24 [1.04, 1.47]) and death from CRC (HR: 1.27 [1.08, 1.49]), and heterogeneity was moderate and high for OS and CSS, respectively. No records have been published for 1) visceral adiposity assessed by waist-related anthropometric measure and PFS, and 2) subcutaneous adiposity assessed by anthropometric measures and CRC survival.
Figure 13.
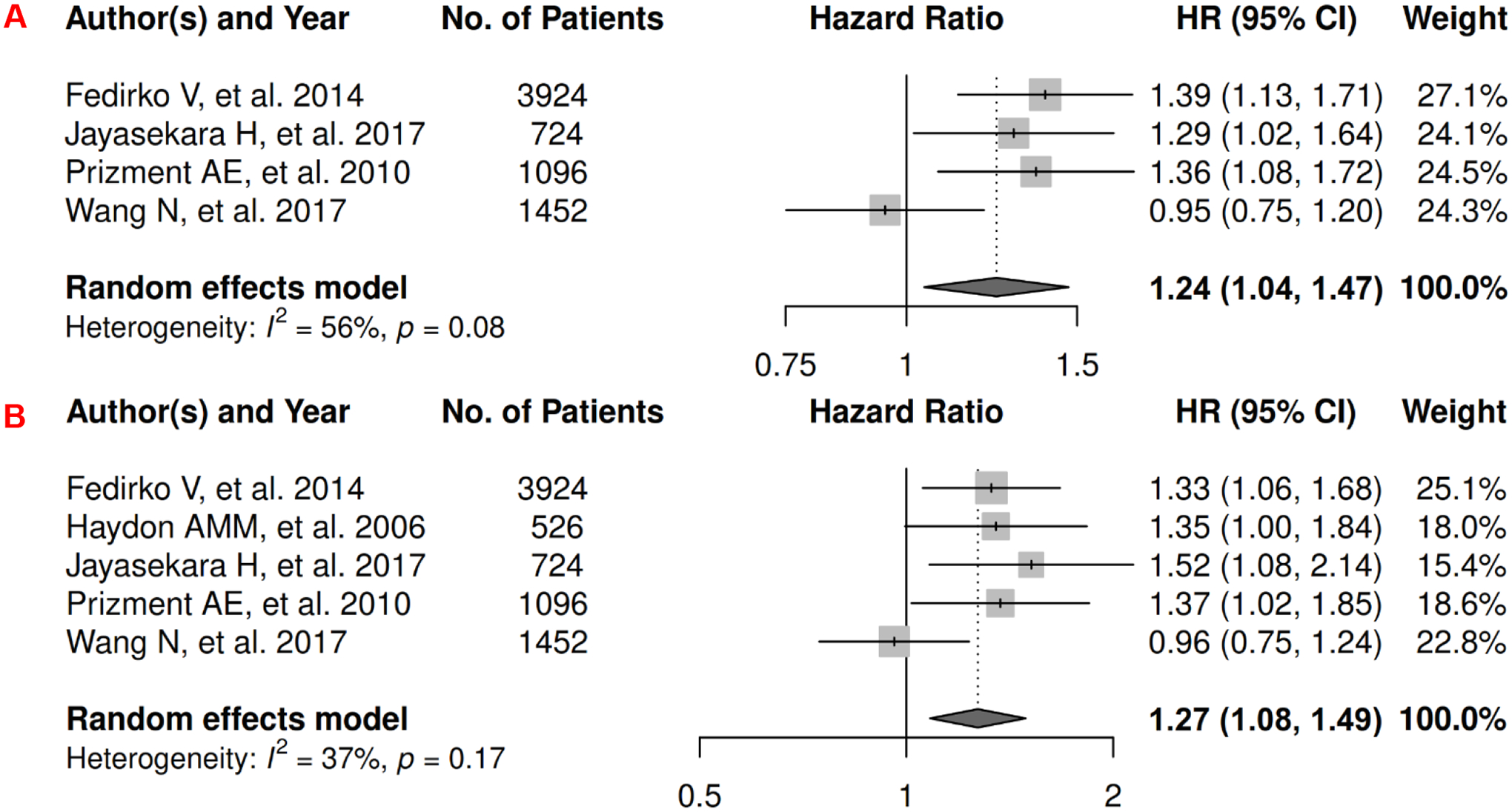
Forest Plots of Assessing the Associations between Anthropometry-Measured Visceral Adiposity and Survival among Colorectal Cancer
13A: visceral adiposity and overall survival.
13B: visceral adiposity and cancer-specific survival.
Prostate
In prostate cancer, there were 3 records for meta-analysis and summary estimates are presented in Figure 14. More details of published records included into the meta-analysis are in Supplementary Table 27 [254–256]. Waist-related anthropometric measures were not associated with increased risk of mortality (HR: 1.11 [0.90, 1.36]) or death from prostate cancer (HR: 1.02 [0.81, 1.29]), and heterogeneity was low for both OS and CSS. No records have been published for 1) visceral adiposity assessed by waist-related anthropometric measure and PFS, and 2) subcutaneous adiposity assessed by anthropometric measures and prostate cancer survival.
Figure 14.
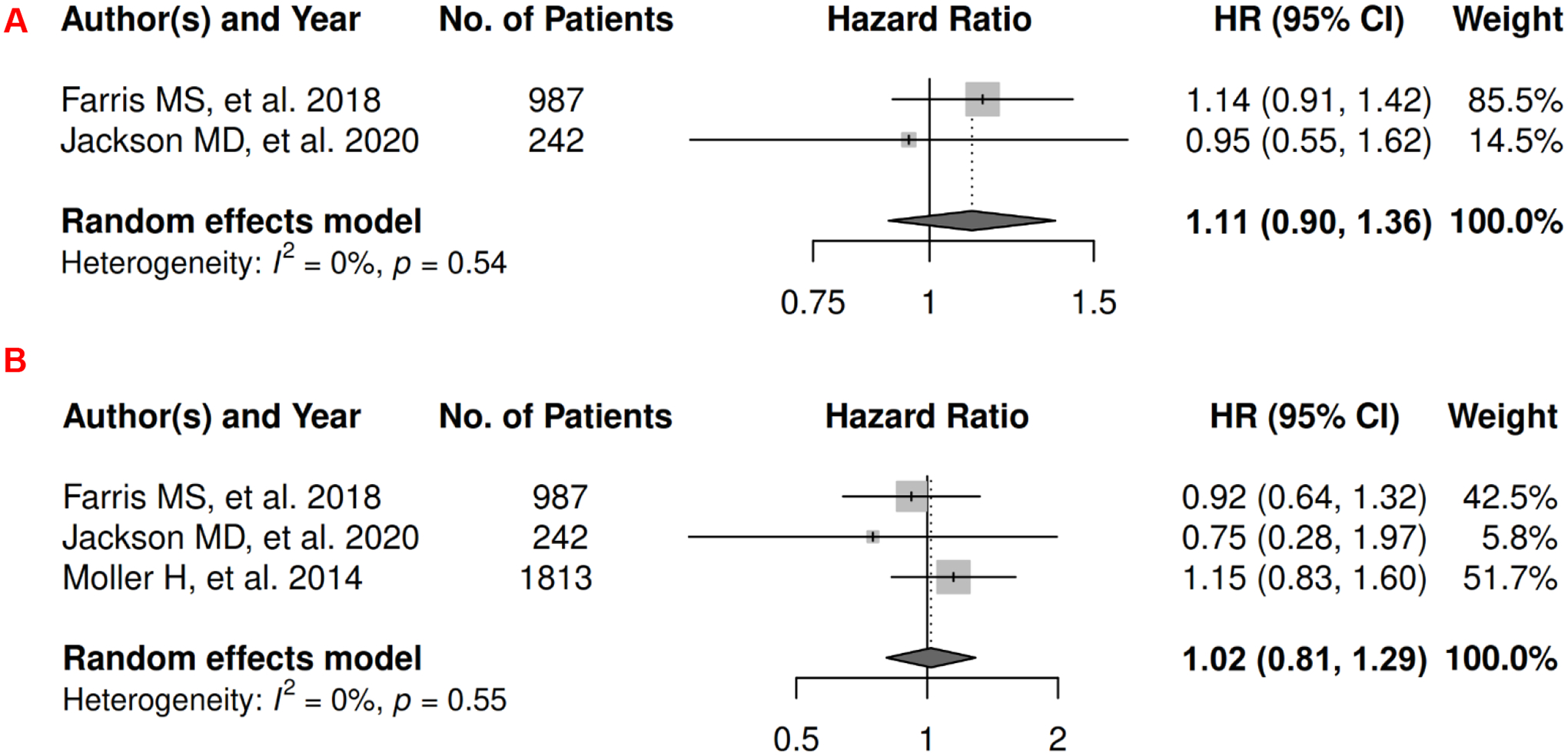
Forest Plots of Assessing the Associations between Anthropometry-Measured Visceral Adiposity and Survival among Prostate Cancer
14A: visceral adiposity and overall survival.
14B: visceral adiposity and cancer-specific survival.
Other Cancers
There were 4 records in adiposity and survival in 4 cancer types that no meta-analysis could be conducted. These cancer types were gastroesophageal, HCC, lung, and lymphoma. More details of these published records are in Supplementary Table 28 [257–260]. Most studies reported insignificant associations or p values suggesting that greater adiposity may not be associated with worse survival among these cancer types.
Publication Bias
Funnel plots and Begg and Mazumdar rank correlation tests for breast cancer suggested no publication bias (Supplementary Figure 1 and Supplementary Table 24).
Comparison of Findings in Imaging Studies vs. Anthropometry Studies
The associations of visceral adiposity with survival for meta-analysis were assessed in both imaging studies and anthropometry studies for breast, colorectal, and prostate cancer. To enable easier comparison, we summarized their findings in Supplementary Table 29.
The Impact of Stage on Findings
Since very few studies reported stage-specific estimates, the exact impact of stage on the meta-analysis of adiposity (imaging-measured or anthropometry-measured adiposity) and cancer survival could not be quantified. However, to provide some insight, we replicated meta-analyses for adiposity and overall survival by restricting to studies with only non-metastatic cancer stage (at least two studies were needed). The findings (Supplementary Table 30) were similar to those without restricting to only non-metastatic stage, except that 1) the association between imaging-measured visceral adiposity and overall survival became significant for pancreatic cancer and 2) the association between anthropometry-measured visceral adiposity and overall survival became not significant for breast cancer. Some potential explanations for such changes may be: 1) for non-metastatic pancreatic cancer patients, visceral adiposity was an indicator for increased pancreatic steatosis that was associated with increased lymphatic invasion, positive lymph nodes, and decreased survival after pancreatoduodenectomy [261]; and 2) for breast cancer, the number of anthropometric studies included for meta-analysis decreased from 13 to 3, after restricting to studies with only non-metastatic stage; however, the magnitude of the HR became larger from 1.30 (1.15, 1.46) to 1.50 (0.76, 2.98) although becoming not significant.
DISCUSSION
In the most comprehensive study to date of imaging measures of adiposity and cancer-related outcomes across 10 cancer sites (breast, colorectal, gastroesophageal, head and neck, HCC, lung, ovarian, pancreatic, prostate, and renal), we found visceral, subcutaneous, and total adiposity were not significantly associated with mortality, death from primary cancer, or cancer progression. For gastroesophageal, head and neck, ovarian and prostate cancer, subcutaneous adiposity appeared protective and was associated with significantly lower mortality risk. Conversely, anthropometric proxies for visceral adiposity were significantly associated with increased risk of overall mortality and death from primary cancer among patients with breast and colorectal cancer.
Several explanations exist for our findings. First, excess adiposity is often associated with higher levels of muscle needed to support extra weight. Patients with low muscle mass often have higher risk of recurrence, overall and cancer-specific mortality [262]. Thus, any risk due to excess adiposity, unless adiposity is extremely high, may be attenuated by the benefits of having adequate muscle which has shown to be protective [72]. In this study, out of 128 records included for meta-analysis, only 11 (8.6%) studies included muscle mass or sarcopenia as covariates for adjusted models and studies without such adjustment may underestimate the unfavorable effects of adiposity. Second, adiposity to a certain extent may provide protective nutritional reserves, especially in the form of SAT, which is considered a more inert storage depot than VAT and has not been linked as strongly to adverse metabolic sequelae [263]. A moderate amount of SAT may enable patients to survive the catabolic effects of cancer and its treatments, and the resulting weight and muscle loss that can occur [264]. Third, tumor biology may be related to excess adiposity, leading to heterogeneity in diseases previously assumed to be similar [265]. For example, in patients with clear cell renal carcinoma (ccRCC), adverse metabolic oncogene fatty acid synthase (FASN) was downregulated in obese patients with ccRCC and upregulated in those who were normal weight [265]. Furthermore, advanced ccRCC tumors of obese patients showed higher angiogenic scores than those of normal-weight patients, which may explain why obese patients survive longer when treated with antiangiogenic treatment [266]. This suggests that either ccRCC in patients with excess adiposity is more indolent, or that the adipose tissue surrounding the tumor may alter its metabolism, resulting in less aggressive disease.
Very few previous reviews or meta-analyses have examined imaging measures of adiposity and cancer survival. One systematic review included 22 prognostic studies and only examined VAT [267]. It concluded that adverse associations between VAT and survival were more frequently observed among patients with colorectal (four of six studies included in that systematic review) and pancreatic (three of five studies included in that systematic review) cancers, compared to higher VAT predicting longer survival in most studies of renal cell carcinoma patients (four of five studies included in that systematic review) [267]. While our study did not see an increased risk between VAT and survival of colorectal and pancreatic cancers, nor a decreased risk associated with renal cancer, their review included fewer studies, a number of which had a small sample size and did not distinguish different survival outcomes such as OS and PFS. As we conducted quantitative analyses by incorporating a larger number of studies, our estimates are more representative of the associations of VAT and other adiposity measures of survival among different cancer types [268].
Bias due to reverse causation or collider stratification bias could also be considered an explanation for our predominantly null findings. In the case of reverse causation, since most imaging measurements are taken during clinical cancer care for diagnostic and surveillance purposes, patients with more advanced cancers may have already lost tissue by time of diagnosis [269]; thus, lower adiposity could be caused by adipose tissue wasting due to more aggressive disease [269, 270]. Collider bias is a specific type of selection bias that occurs when analyses are restricted to a select subgroup (e.g., cancer patients) experiencing a condition that is causally influenced by two or more variables [271]: e.g., if cancer incidence is caused by excess adiposity, which also increases mortality after cancer diagnosis, but among patients with low levels of adiposity cancer incidence is due exclusively to unrelated factors that also sharply increase mortality risk (e.g., genetics predisposing to an aggressive tumor). Thus, when analyses are restricted to cancer patients, an inverse association is artificially induced between excess adiposity and cancer survival [8]. However, previous studies suggest that these biases, while plausible, are unlikely to fully explain associations [272, 273].
In our meta-analysis of studies using anthropometric measures, results align with previous meta-analyses [30, 274] and suggest that excess adiposity was associated with increased risk of mortality for both breast and colorectal cancer patients. The inconsistency of the results from these anthropometric studies that compared to those from imaging studies directly measuring VAT and SAT could be due to the use of either less precise measures, timing of assessment, or better control for confounding. As a reference standard for measuring body composition [275], CT and MRI provide more direct quantifications of adipose tissue than anthropometric measures and distinguish adipose tissue distribution [7, 8]. Second, patient populations may vary between studies using anthropometry and studies using CT. Anthropometric studies likely include a higher proportion of patients diagnosed with earlier stage disease as these measures are collected as part of research studies for which healthier individuals may volunteer, whereas imaging is most often collected as part of routine clinical care for select stages and cancer sites. For example, in breast cancer, CT scans are typically only available on stage III and IV patients and a small percentage of patients with stage II disease. In contrast, anthropometric studies likely include all stages of disease, of which stage I constitute a large proportion of the patient population. If the effect of adiposity differs by stage, which was observed in a study where BMI increased risk of mortality in CRC patients at stage I/II but not stage III/IV [276], results from anthropometric studies may demonstrate higher risk with adiposity than imaging studies. Additionally, compared to imaging studies, anthropometry studies have overall larger sample size and adjusted for potential confounders more comprehensively. For example, for breast cancer, we observed overall larger sample size and more covariates for adjustment in anthropometry studies (Supplementary Table 25) vs. imaging studies (Supplementary Table 3). In addition, when multiple waist-based measures were available, we prioritized WHR, which may reflect both abdominal adiposity (increased waist circumference) as well as a lack of gluteal muscularity (decreased hip circumference); thus, the harms of excess adiposity may be more apparent after controlling for muscularity. Third, timing of measurement could affect results in several ways. While CT and MRI, if ordered, are requested for cancer diagnostics [277, 278], many of the anthropometric measurements were done several years before cancer diagnosis. For example, in our analysis for waist-based anthropometric measures and survival in colorectal cancer, 4 out of 5 studies included into meta-analysis assessed on average 5–8 years before diagnosis. These pre-diagnosis measurements capture, in part, the increased risk of cancer incidence associated with excess adiposity, and may not accurately reflect the relationship of at-diagnosis adiposity to survival. Since clinicians are confronted with at-diagnosis adiposity when making decisions about cancer treatment and supportive lifestyle interventions, it is the relationship of at-diagnosis adiposity to cancer outcomes that is most relevant to patient care. Given that this systematic review and meta-analysis primarily focuses on single timepoint for adiposity and adiposity is dynamic and can change over time, future studies could focus on the prognostic roles of adiposity change among cancer survivors, which is also of high clinical relevance to clinicians and patients.
Strengths and limitations
To our knowledge, our analysis provides the first and most comprehensive summary of the evidence on adiposity and cancer survival across a wide range of cancer types. In addition to meta-analysis of adiposity among 10 cancer types, we also reviewed imaging-measured and anthropometry-measured adiposity studies among varied cancers, including adrenocortical, AML, biliary, bladder, cholangiocarcinoma, endometrial, lymphoma, melanoma, multiple myeloma, nasopharyngeal, sarcoma, and urinary tract. Although these cancer types did not have sufficient studies for meta-analysis, most reported null associations of adiposity and cancer survival.
However, our study has several limitations. First, we noticed significant and high heterogeneity in meta-analyses of many cancer types. In imaging-measured studies, methods are not yet standardized, and investigators choose different anatomic landmarks to quantify body composition or apply different Hounsfield unit (HU) ranges to quantify adipose tissue. In addition, adiposity exposures were categorized in the different ways as well as being scaled as continuous variables in different forms across the studies, which may further result in heterogeneity. Second, not all studies fit multivariable models to report adjusted HRs, particularly if adiposity-related variables were not significantly associated with survival in univariate analyses. However, while unadjusted for confounders, these null HRs align with the null findings reported in our analysis. Third, due to the relatively small number of studies in some cancer types (for example, head and neck [N = 3] and ovarian [N = 4]) and potential publication bias for renal cancer, we should cautiously interpret findings in these cancers. Fourth, we did not conduct subgroup analyses, and future studies should further explore the associations between adiposity and cancer survival by demographic and clinicopathologic characteristics such as age, sex, and disease stage, which may contribute to heterogeneity. For example, stage is among key factors impacting survival, and the associations of adiposity with survival may differ by stage due to the increased prevalence of adipose tissue wasting in advanced disease. Since few studies included for this meta-analysis reported stage-specific findings, the exact impact of staging on our findings could not be calculated based on current published reports. However, to provide some insight into the impact of stage on our findings, we conducted meta-analysis for adiposity and overall survival by restricting to studies with only non-metastatic cancer stage, and the findings remained similar. Although no publication bias was observed in our analysis of waist-based measures of adiposity and decreased survival among breast cancer survivors, we note that half of studies reported insignificant associations and exposure assessments occurred pre-, peri-, and post-diagnosis, suggesting some level of heterogeneity. Fifth, only 11 (8.6%) out of 128 studies included in this review controlled for muscularity in multivariable models, but even those which did control for muscle did not find a significant increased risk of cancer-related outcomes. Sixth, there may be selection concerns for imaging-measured adiposity studies among some but not all cancer types. For example, CT scans are not routinely used for breast cancer at stage I and II, but they are commonly ordered for all stages of colorectal cancer at diagnosis and follow-up to determine cancer staging. Thus, such selection bias may affect the generalizability of our findings in some cancer types.
CONCLUSIONS
Imaging-measured visceral, subcutaneous, and total adiposity were not associated with increased risk of overall mortality, death from primary cancer, or cancer progression among patients with breast, colorectal, gastroesophageal, head and neck, HCC, lung, ovarian, pancreatic, prostate, and renal cancer. In some cancers, excess SAT adiposity was associated with better survival. However, given high heterogeneity of studies included and the rapid increase in the use of clinical imaging to examine the relationship of body composition to cancer outcomes, more scientific rigor must be employed before robust comparisons can be made, including standardized vertebral landmarks, and establishment of relevant cut points to indicate excess adiposity. The ultimate goal of standardization is to enable comparison across studies to understand the role of adiposity in cancer survival, and to provide clinicians with risk stratification tools to identify the most vulnerable patients and design appropriate interventions to enhance survivorship.
Supplementary Material
Acknowledgments
This study was supported by, in part, by grants from the National Institutes of Health: K01CA226155 (Cespedes Feliciano) and the National Cancer Institute: R01CA251589 (Cespedes Feliciano).
Funding
This study was supported by, in part, by grants from the National Institutes of Health: K01CA226155, R01CA240394, R01CA251589 and R01AG065334.
Footnotes
Competing Interests: None
Ethics approval
This study is a meta‐analysis and does not involve human subjects. IRB review is not required.
Data Availability
The datasets analyzed during the current study are publicly available via applying the searching algorithm proposed in this manuscript to PubMed, Embase and Web of Science.
REFERENCE
- 1.Lauby-Secretan B, Scoccianti C, Loomis D, et al. (2016) Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 375: 794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. (2022) Cancer statistics, 2022. CA Cancer J Clin. 72: 7–33. [DOI] [PubMed] [Google Scholar]
- 3.Brown KA. (2021) Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. 17: 350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J (2011) Multiple signal pathways in obesity-associated cancer. Obes Rev. 12: 1063–70. [DOI] [PubMed] [Google Scholar]
- 5.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. (2013) Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical cancer research. 19: 6074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan DC, Sattar N, McArdle CS. (2006) ABC of obesity. Obesity and cancer. BMJ. 333: 1109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DH, Giovannucci EL. (2019) The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr Nutr Rep. 8: 175–81. [DOI] [PubMed] [Google Scholar]
- 8.Lennon H, Sperrin M, Badrick E, Renehan AG. (2016) The Obesity Paradox in Cancer: a Review. Curr Oncol Rep. 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deurenberg P, Yap M, van Staveren WA. (1998) Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 22: 1164–71. [DOI] [PubMed] [Google Scholar]
- 10.Caan BJ, Kroenke CH. (2017) Next Steps in Understanding the Obesity Paradox in Cancer. Cancer Epidemiol Biomarkers Prev. 26: 12. [DOI] [PubMed] [Google Scholar]
- 11.Shachar SS, Williams GR. (2017) The Obesity Paradox in Cancer-Moving beyond BMI-Response. Cancer Epidemiol Biomarkers Prev. 26: 981. [DOI] [PubMed] [Google Scholar]
- 12.Caan BJ, Cespedes Feliciano EM, Kroenke CH. (2018) The Importance of Body Composition in Explaining the Overweight Paradox in Cancer-Counterpoint. Cancer Res. 78: 1906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez MC, Correia M, Heymsfield SB. (2017) A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care. 20: 314–21. [DOI] [PubMed] [Google Scholar]
- 14.Dewey M, Bosserdt M, Dodd JD, Thun S, Kressel HY. (2019) Clinical Imaging Research: Higher Evidence, Global Collaboration, Improved Reporting, and Data Sharing Are the Grand Challenges. Radiology. 291: 547–52. [DOI] [PubMed] [Google Scholar]
- 15.Shen W, Punyanitya M, Wang Z, et al. (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 97: 2333–8. [DOI] [PubMed] [Google Scholar]
- 16.University of York Centre for Reviews and Dissemination. PROSPERO. https://www.crd.york.ac.uk/prospero/. Accessed December 9, 2021. [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagheri M, Speakman JR, Shemirani F, Djafarian K. (2016) Renal cell carcinoma survival and body mass index: a dose-response meta-analysis reveals another potential paradox within a paradox. Int J Obes (Lond). 40: 1817–22. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Ma J. (2011) Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 4: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan DSM, Vieira AR, Aune D, et al. (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 25: 1901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi Y, Park B, Jeong BC, et al. (2013) Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 132: 625–34. [DOI] [PubMed] [Google Scholar]
- 22.Ejaz A, Spolverato G, Kim Y, et al. (2015) Impact of body mass index on perioperative outcomes and survival after resection for gastric cancer. J Surg Res. 195: 74–82. [DOI] [PubMed] [Google Scholar]
- 23.Fahey PP, Mallitt KA, Astell-Burt T, Stone G, Whiteman DC. (2015) Impact of pre-diagnosis behavior on risk of death from esophageal cancer: a systematic review and meta-analysis. Cancer Causes Control. 26: 1365–73. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Das A, Majumder K, et al. (2018) Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. American journal of clinical oncology. 41: 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Majumder K, Arora N, et al. (2016) Premorbid body mass index and mortality in patients with lung cancer: A systematic review and meta-analysis. Lung Cancer. 102: 49–59. [DOI] [PubMed] [Google Scholar]
- 26.Hollander D, Kampman E, van Herpen CM. (2015) Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Crit Rev Oncol Hematol. 96: 328–38. [DOI] [PubMed] [Google Scholar]
- 27.Kim LH, Doan P, He Y, Lau HM, Pleass H, Patel MI. (2021) A Systematic Review and Meta-Analysis of the Significance of Body Mass Index on Kidney Cancer Outcomes. J Urol. 205: 346–55. [DOI] [PubMed] [Google Scholar]
- 28.Majumder K, Gupta A, Arora N, Singh PP, Singh S. (2016) Premorbid Obesity and Mortality in Patients With Pancreatic Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 14: 355–68 e; quiz e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrelli F, Cortellini A, Indini A, et al. (2021) Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 4: e213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Protani M, Coory M, Martin JH. (2010) Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 123: 627–35. [DOI] [PubMed] [Google Scholar]
- 31.Protani MM, Nagle CM, Webb PM. (2012) Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila). 5: 901–10. [DOI] [PubMed] [Google Scholar]
- 32.Rong X, Wei F, Geng Q, Ruan J. (2015) The association between body mass index and the prognosis and postoperative complications of hepatocellular carcinoma: a meta-analysis. Medicine. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y-Q, Yang J, Du P, et al. (2016) Effect of body mass index on overall survival of pancreatic cancer: a meta-analysis. Medicine. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Xu H, Zhou S, et al. (2018) Body mass index and mortality in lung cancer patients: a systematic review and meta-analysis. Eur J Clin Nutr. 72: 4–17. [DOI] [PubMed] [Google Scholar]
- 35.Wu S, Liu J, Wang X, Li M, Gan Y, Tang Y. (2014) Association of obesity and overweight with overall survival in colorectal cancer patients: a meta-analysis of 29 studies. Cancer Causes & Control. 25: 1489–502. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Chen Q, Li ZM, Xu XD, Song AF, Wang LS. (2018) Association of body mass index with mortality and postoperative survival in renal cell cancer patients, a meta-analysis. Oncotarget. 9: 13959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang SS, Yang H, Luo KJ, et al. (2013) The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer. 109: 2894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao B, Zhang J, Mei D, et al. (2018) Does high body mass index negatively affect the surgical outcome and long-term survival of gastric cancer patients who underwent gastrectomy: a systematic review and meta-analysis. European Journal of Surgical Oncology. 44: 1971–81. [DOI] [PubMed] [Google Scholar]
- 39.Zhong S, Yan X, Wu Y, et al. (2016) Body mass index and mortality in prostate cancer patients: a dose-response meta-analysis. Prostate Cancer Prostatic Dis. 19: 122–31. [DOI] [PubMed] [Google Scholar]
- 40.Choi EK, Park HB, Lee KH, et al. (2018) Body mass index and 20 specific cancers: re-analyses of dose-response meta-analyses of observational studies. Ann Oncol. 29: 749–57. [DOI] [PubMed] [Google Scholar]
- 41.Deurenberg P (1996) Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am J Clin Nutr. 64: 449S–52S. [DOI] [PubMed] [Google Scholar]
- 42.Elia M (2013) Body composition by whole-body bioelectrical impedance and prediction of clinically relevant outcomes: overvalued or underused? Eur J Clin Nutr. 67 Suppl 1: S60–70. [DOI] [PubMed] [Google Scholar]
- 43.National Heart, Lung, and Blood institute. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed December 9, 2021.
- 44.Mallet R, Decazes P, Modzelewski R, et al. (2021) Prognostic value of low skeletal muscle mass in patient treated by exclusive curative radiochemotherapy for a NSCLC. Sci Rep. 11: 10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balentine CJ, Enriquez J, Fisher W, et al. (2010) Intra-abdominal Fat Predicts Survival in Pancreatic Cancer. Journal of Gastrointestinal Surgery. 14: 1832–7. [DOI] [PubMed] [Google Scholar]
- 46.Xu W, Hu X, Anwaier A, et al. (2021) Fatty Acid Synthase Correlates With Prognosis-Related Abdominal Adipose Distribution and Metabolic Disorders of Clear Cell Renal Cell Carcinoma. Frontiers in Molecular Biosciences. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prado CM, Lieffers JR, McCargar LJ, et al. (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 9: 629–35. [DOI] [PubMed] [Google Scholar]
- 48.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 33: 997–1006. [DOI] [PubMed] [Google Scholar]
- 49.Yip C, Goh V, Davies A, et al. (2014) Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 24: 998–1005. [DOI] [PubMed] [Google Scholar]
- 50.Gadekar T, Dudeja P, Basu I, Vashisht S, Mukherji S. (2020) Correlation of visceral body fat with waist-hip ratio, waist circumference and body mass index in healthy adults: A cross sectional study. Med J Armed Forces India. 76: 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.(2011) World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. [Google Scholar]
- 52.Addo OY, Himes JH. (2010) Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am J Clin Nutr. 91: 635–42. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz L, Colley JR, Hamilton PJ. (1971) Measurement of triceps skinfold thickness. An investigation of sources of variation. British journal of preventive & social medicine. 25: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochrane Consumers and Communication Review Group. Cochrane Consumers and Communication Group: meta-analysis. http://cccrg.cochrane.org. Accessed December 10, 2021.
- 55.Valentine JC, Pigott TD, Rothstein HR. (2010) How many studies do you need? A primer on statistical power for meta-analysis. Journal of Educational and Behavioral Statistics. 35: 215–47. [Google Scholar]
- 56.DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials. 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 57.Schwarzer G, Carpenter JR, Rücker G. (2015) Meta-analysis with R: Springer. [Google Scholar]
- 58.Higgins JP, Thompson SG, Deeks JJ, Altman DG. (2003) Measuring inconsistency in meta-analyses. BMJ. 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higgins JPT, Green S. Recommendations on testing for funnel plot asymmetry. https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm. Accessed December 10, 2021.
- 60.Sterne JA, Sutton AJ, Ioannidis JP, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 343: d4002. [DOI] [PubMed] [Google Scholar]
- 61.Begg CB, Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics. 50: 1088–101. [PubMed] [Google Scholar]
- 62.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. (2018) Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 4: 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N. (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer. 26: 861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franzoi MA, Vandeputte C, Eiger D, et al. (2020) Computed tomography-based analyses of baseline body composition parameters and changes in breast cancer patients under treatment with CDK 4/6 inhibitors. Breast cancer research and treatment. 181: 199–209. [DOI] [PubMed] [Google Scholar]
- 65.Huh J, Park B, Lee H, et al. (2020) Prognostic Value of Skeletal Muscle Depletion Measured on Computed Tomography for Overall Survival in Patients with Non-Metastatic Breast Cancer. J Breast Cancer. 23: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwase T, Parikh A, Dibaj SS, et al. (2021) The Prognostic Impact of Body Composition for Locally Advanced Breast Cancer Patients Who Received Neoadjuvant Chemotherapy. Cancers (Basel). 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwase T, Sangai T, Nagashima T, et al. (2016) Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med. 5: 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song EJ, Lee CW, Jung SY, et al. (2018) Prognostic impact of skeletal muscle volume derived from cross-sectional computed tomography images in breast cancer. Breast Cancer Res Treat. 172: 425–36. [DOI] [PubMed] [Google Scholar]
- 69.Bradshaw PT, Feliciano EMC, Prado CM, et al. (2019) Adipose Tissue Distribution and Survival Among Women with Nonmetastatic Breast Cancer. Obesity. 27: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. (2020) Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers (Basel). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basile D, Bartoletti M, Polano M, et al. (2021) Prognostic role of visceral fat for overall survival in metastatic colorectal cancer: A pilot study. Clinical Nutrition. 40: 286–94. [DOI] [PubMed] [Google Scholar]
- 72.Caan BJ, Meyerhardt JA, Kroenke CH, et al. (2017) Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 26: 1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carcamo L, Penailillo E, Bellolio F, et al. (2021) Computed tomography-measured body composition parameters do not influence survival in non-metastatic colorectal cancer. ANZ J Surg. 91: E298–E306. [DOI] [PubMed] [Google Scholar]
- 74.Cavagnari MAV, Silva TD, Pereira MAH, et al. (2019) Impact of genetic mutations and nutritional status on the survival of patients with colorectal cancer. BMC Cancer. 19: 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charette N, Vandeputte C, Ameye L, et al. (2019) Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: a post hoc analysis of two non-randomized phase II trials. BMC Cancer. 19: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choe EK, Park KJ, Ryoo SB, Moon SH, Oh HK, Han EC. (2016) Prognostic Impact of Changes in Adipose Tissue Areas after Colectomy in Colorectal Cancer Patients. J Korean Med Sci. 31: 1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi MH, Oh SN, Lee IK, Oh ST, Won DD. (2018) Sarcopenia is negatively associated with long‐term outcomes in locally advanced rectal cancer. Journal of cachexia, sarcopenia and muscle. 9: 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung E, Lee HS, Cho ES, et al. (2019) Changes in Body Composition During Adjuvant FOLFOX Chemotherapy and Overall Survival in Non-Metastatic Colon Cancer. Cancers (Basel). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clark W, Siegel EM, Chen YA, et al. (2013) Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 216: 1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC. (2019) The relationship between computed tomography‐derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. Journal of cachexia, sarcopenia and muscle. 10: 111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frostberg E, Pedersen MR, Manhoobi Y, Rahr HB, Rafaelsen SR. (2021) Three different computed tomography obesity indices, two standard methods, and one novel measurement, and their association with outcomes after colorectal cancer surgery. Acta Radiol. 62: 182–9. [DOI] [PubMed] [Google Scholar]
- 82.Guiu B, Petit JM, Bonnetain F, et al. (2010) Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 59: 341–7. [DOI] [PubMed] [Google Scholar]
- 83.Han JS, Ryu H, Park IJ, et al. (2020) Association of Body Composition with Long-Term Survival in Non-metastatic Rectal Cancer Patients. Cancer Res Treat. 52: 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. (2019) The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients With Stage I to III Colorectal Cancer. Dis Colon Rectum. 62: 549–60. [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi A, Kaido T, Hamaguchi Y, et al. (2018) Impact of visceral adiposity as well as sarcopenic factors on outcomes in patients undergoing liver resection for colorectal liver metastases. World journal of surgery. 42: 1180–91. [DOI] [PubMed] [Google Scholar]
- 86.Lee CS, Murphy DJ, McMahon C, et al. (2015) Visceral adiposity is a risk factor for poor prognosis in colorectal cancer patients receiving adjuvant chemotherapy. Journal of gastrointestinal cancer. 46: 243–50. [DOI] [PubMed] [Google Scholar]
- 87.Lee CS, Won DD, Oh SN, et al. (2020) Prognostic role of pre-sarcopenia and body composition with long-term outcomes in obstructive colorectal cancer: a retrospective cohort study. World J Surg Oncol. 18: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malietzis G, Currie AC, Athanasiou T, et al. (2016) Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 103: 572–80. [DOI] [PubMed] [Google Scholar]
- 89.McSorley ST, Black DH, Horgan PG, McMillan DC. (2018) The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clinical nutrition. 37: 1279–85. [DOI] [PubMed] [Google Scholar]
- 90.Miyamoto Y, Oki E, Emi Y, et al. (2018) Low visceral fat content is a negative predictive marker for bevacizumab in metastatic colorectal cancer. Anticancer research. 38: 491–9. [DOI] [PubMed] [Google Scholar]
- 91.Perrin T, Lenfant M, Boisson C, Bert M, Rat P, Facy O. (2021) Effects of body composition profiles on oncological outcomes and postoperative intraabdominal infection following colorectal cancer surgery. Surg Obes Relat Dis. 17: 575–84. [DOI] [PubMed] [Google Scholar]
- 92.Rickles AS, Iannuzzi JC, Mironov O, et al. (2013) Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg. 17: 133–43; discussion p 43. [DOI] [PubMed] [Google Scholar]
- 93.Shirdel M, Andersson F, Myte R, et al. (2020) Body composition measured by computed tomography is associated with colorectal cancer survival, also in early-stage disease. Acta Oncologica. 59: 799–808. [DOI] [PubMed] [Google Scholar]
- 94.Tokunaga R, Nakagawa S, Miyamoto Y, et al. (2020) The clinical impact of preoperative body composition differs between male and female colorectal cancer patients. Colorectal Dis. 22: 62–70. [DOI] [PubMed] [Google Scholar]
- 95.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. (2012) Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 99: 550–7. [DOI] [PubMed] [Google Scholar]
- 96.Yoon J, Chung YE, Lim JS, Kim MJ. (2019) Quantitative assessment of mesorectal fat: new prognostic biomarker in patients with mid-to-lower rectal cancer. Eur Radiol. 29: 1240–7. [DOI] [PubMed] [Google Scholar]
- 97.Catanese S, Aringhieri G, Vivaldi C, et al. (2021) Role of Baseline Computed-Tomography-Evaluated Body Composition in Predicting Outcome and Toxicity from First-Line Therapy in Advanced Gastric Cancer Patients. J Clin Med. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi MH, Kim KA, Hwang SS, Byun JY. (2018) CT-quantified muscle and fat change in patients after surgery or endoscopic resection for early gastric cancer and its impact on long-term outcomes. Medicine (Baltimore). 97: e13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong QT, Cai HY, Zhang Z, et al. (2021) Influence of body composition, muscle strength, and physical performance on the postoperative complications and survival after radical gastrectomy for gastric cancer: A comprehensive analysis from a large-scale prospective study. Clin Nutr. 40: 3360–9. [DOI] [PubMed] [Google Scholar]
- 100.Feng W, Huang M, Zhao X, et al. (2020) Severe loss of visceral fat and skeletal muscle after chemotherapy predicts poor prognosis in metastatic gastric cancer patients without gastrectomy. J Cancer. 11: 3310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gursoy Coruh A, Uzun C, Akkaya Z, et al. (2021) Prognostic implications of visceral obesity on gastric adenocarcinoma: does it really matter? Clin Imaging. 76: 228–34. [DOI] [PubMed] [Google Scholar]
- 102.Hagens ERC, Feenstra ML, van Egmond MA, et al. (2020) Influence of body composition and muscle strength on outcomes after multimodal oesophageal cancer treatment. J Cachexia Sarcopenia Muscle. 11: 756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harada K, Baba Y, Ishimoto T, et al. (2015) Low visceral fat content is associated with poor prognosis in a database of 507 upper gastrointestinal cancers. Annals of surgical oncology. 22: 3946–53. [DOI] [PubMed] [Google Scholar]
- 104.Kim JH, Chin HM, Hwang SS, Jun KH. (2014) Impact of intra-abdominal fat on surgical outcome and overall survival of patients with gastric cancer. International Journal of Surgery. 12: 346–52. [DOI] [PubMed] [Google Scholar]
- 105.Li X-T, Tang L, Chen Y, Li Y-L, Zhang X-P, Sun Y-S. (2015) Visceral and subcutaneous fat as new independent predictive factors of survival in locally advanced gastric carcinoma patients treated with neo-adjuvant chemotherapy. Journal of cancer research and clinical oncology. 141: 1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okamura A, Watanabe M, Yamashita K, et al. (2018) Implication of visceral obesity in patients with esophageal squamous cell carcinoma. Langenbecks Arch Surg. 403: 245–53. [DOI] [PubMed] [Google Scholar]
- 107.Park HS, Kim HS, Beom SH, et al. (2018) Marked Loss of Muscle, Visceral Fat, or Subcutaneous Fat After Gastrectomy Predicts Poor Survival in Advanced Gastric Cancer: Single-Center Study from the CLASSIC Trial. Ann Surg Oncol. 25: 3222–30. [DOI] [PubMed] [Google Scholar]
- 108.Taki Y, Sato S, Nakatani E, et al. (2021) Preoperative skeletal muscle index and visceral-to-subcutaneous fat area ratio are associated with long-term outcomes of elderly gastric cancer patients after gastrectomy. Langenbecks Arch Surg. 406: 463–71. [DOI] [PubMed] [Google Scholar]
- 109.Wang SL, Ma LL, Chen XY, et al. (2018) Impact of visceral fat on surgical complications and long-term survival of patients with gastric cancer after radical gastrectomy. Eur J Clin Nutr. 72: 436–45. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Li Z, Jiang L, et al. (2021) Impact of body composition on clinical outcomes in people with gastric cancer undergoing radical gastrectomy after neoadjuvant treatment. Nutrition. 85: 111135. [DOI] [PubMed] [Google Scholar]
- 111.Zhou MJ, Tseng L, Guo X, et al. (2021) Low Subcutaneous Adiposity and Mortality in Esophageal Cancer. Cancer Epidemiol Biomarkers Prev. 30: 114–22. [DOI] [PubMed] [Google Scholar]
- 112.Hacker UT, Hasenclever D, Linder N, et al. (2020) Prognostic role of body composition parameters in gastric/gastroesophageal junction cancer patients from the EXPAND trial. Journal of Cachexia Sarcopenia and Muscle. 11: 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saeed N, Shridhar R, Almhanna K, Hoffe S, Chuong M, Meredith K. (2017) CT-based assessment of visceral adiposity and outcomes for esophageal adenocarcinoma. Journal of Gastrointestinal Oncology. 8: 833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jung AR, Roh JL, Kim JS, et al. (2019) Prognostic value of body composition on recurrence and survival of advanced-stage head and neck cancer. Eur J Cancer. 116: 98–106. [DOI] [PubMed] [Google Scholar]
- 115.Lee JW, Ban MJ, Park JH, Lee SM. (2019) Visceral adipose tissue volume and CT-attenuation as prognostic factors in patients with head and neck cancer. Head and Neck. 41: 1605–14. [DOI] [PubMed] [Google Scholar]
- 116.Pai PC, Chuang CC, Chuang WC, et al. (2018) Pretreatment subcutaneous adipose tissue predicts the outcomes of patients with head and neck cancer receiving definitive radiation and chemoradiation in Taiwan. Cancer Medicine. 7: 1630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi Y, Ahn KJ, Jang J, et al. (2020) Prognostic value of computed tomography-based volumetric body composition analysis in patients with head and neck cancer: Feasibility study. Head Neck. 42: 2614–25. [DOI] [PubMed] [Google Scholar]
- 118.Fujiwara N, Nakagawa H, Kudo Y, et al. (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 63: 131–40. [DOI] [PubMed] [Google Scholar]
- 119.Hamaguchi Y, Kaido T, Okumura S, et al. (2019) Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer. 8: 92–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Higashi T, Hayashi H, Kaida T, et al. (2015) Prognostic Impact of Visceral Fat Amount and Branched-Chain Amino Acids (BCAA) in Hepatocellular Carcinoma. Ann Surg Oncol. 22 Suppl 3: S1041–7. [DOI] [PubMed] [Google Scholar]
- 121.Itoh S, Shirabe K, Matsumoto Y, et al. (2014) Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann Surg Oncol. 21: 3063–8. [DOI] [PubMed] [Google Scholar]
- 122.Jang HY, Choi GH, Hwang SH, et al. (2021) Sarcopenia and visceral adiposity predict poor overall survival in hepatocellular carcinoma patients after curative hepatic resection. Translational Cancer Research. 10: 854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobayashi T, Kawai H, Nakano O, et al. (2018) Prognostic value of subcutaneous adipose tissue volume in hepatocellular carcinoma treated with transcatheter intra-arterial therapy. Cancer Manag Res. 10: 2231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kroh A, Uschner D, Lodewick T, et al. (2019) Impact of body composition on survival and morbidity after liver resection in hepatocellular carcinoma patients. Hepatobiliary Pancreat Dis Int. 18: 28–37. [DOI] [PubMed] [Google Scholar]
- 125.Labeur TA, van Vugt JL, Ten Cate DW, et al. (2019) Body composition is an independent predictor of outcome in patients with hepatocellular carcinoma treated with sorafenib. Liver cancer. 8: 255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nault JC, Pigneur F, Nelson AC, et al. (2015) Visceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitors. Dig Liver Dis. 47: 869–76. [DOI] [PubMed] [Google Scholar]
- 127.Saeki I, Yamasaki T, Maeda M, et al. (2019) Effect of body composition on survival benefit of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: A comparison with sorafenib therapy. PLoS One. 14: e0218136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sawada K, Saitho Y, Hayashi H, et al. (2019) Skeletal muscle mass is associated with toxicity, treatment tolerability, and additional or subsequent therapies in patients with hepatocellular carcinoma receiving sorafenib treatment. JGH Open. 3: 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grąt K, Pacho R, Grąt M, Krawczyk M, Zieniewicz K, Rowiński O. (2019) Impact of body composition on the risk of hepatocellular carcinoma recurrence after liver transplantation. Journal of Clinical Medicine. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Imai K, Takai K, Maeda T, et al. (2018) Increased visceral fat volume raises the risk for recurrence of hepatocellular carcinoma after curative treatment. Oncotarget. 9: 14058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferguson MK, Mitzman B, Derstine B, et al. (2020) A Morphomic Index Is an Independent Predictor of Survival After Lung Cancer Resection. The Annals of thoracic surgery. 109: 873–8. [DOI] [PubMed] [Google Scholar]
- 132.Lee JW, Lee HS, Na JO, Lee SM. (2018) Effect of adipose tissue volume on prognosis in patients with non-small cell lung cancer. Clin Imaging. 50: 308–13. [DOI] [PubMed] [Google Scholar]
- 133.Minami S, Ihara S, Komuta K. (2020) Sarcopenia and Visceral Adiposity Are Not Independent Prognostic Markers for Extensive Disease of Small-Cell Lung Cancer: A Single-Centered Retrospective Cohort Study. World Journal of Oncology. 11: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Minami S, Ihara S, Nishimatsu K, Komuta K. (2019) Low body mass index is an independent prognostic factor in patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitor. World journal of oncology. 10: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Minami S, Ihara S, Tanaka T, Komuta K. (2020) Sarcopenia and visceral adiposity did not affect efficacy of immune-checkpoint inhibitor monotherapy for pretreated patients with advanced non-small cell lung cancer. World journal of oncology. 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Popinat G, Cousse S, Goldfarb L, et al. (2019) Sub-cutaneous Fat Mass measured on multislice computed tomography of pretreatment PET/CT is a prognostic factor of stage IV non-small cell lung cancer treated by nivolumab. Oncoimmunology. 8: e1580128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Magri V, Gottfried T, Di Segni M, et al. (2019) Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Management and Research. 11: 8201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang X, Xie C, Tang J, et al. (2020) Adipose tissue area as a predictor for the efficacy of apatinib in platinum-resistant ovarian cancer: an exploratory imaging biomarker analysis of the AEROC trial. BMC medicine. 18: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Slaughter KN, Thai T, Penaroza S, et al. (2014) Measurements of adiposity as clinical biomarkers for first-line bevacizumab-based chemotherapy in epithelial ovarian cancer. Gynecologic Oncology. 133: 11–5. [DOI] [PubMed] [Google Scholar]
- 140.Torres ML, Hartmann LC, Cliby WA, et al. (2013) Nutritional status, CT body composition measures and survival in ovarian cancer. Gynecologic Oncology. 129: 548–53. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Y, Coletta AM, Allen PK, et al. (2018) Perirenal Adiposity is Associated With Lower Progression-Free Survival From Ovarian Cancer. International Journal of Gynecological Cancer. 28: 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang CY, Yang YC, Chen TC, et al. (2020) Muscle loss during primary debulking surgery and chemotherapy predicts poor survival in advanced-stage ovarian cancer. Journal of Cachexia, Sarcopenia and Muscle. 11: 534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gaujoux S, Torres J, Olson S, et al. (2012) Impact of Obesity and Body Fat Distribution on Survival After Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. Annals of Surgical Oncology. 19: 2908–16. [DOI] [PubMed] [Google Scholar]
- 144.Lee J, Lee JC, Kim HW, Kim J, Hwang JH. (2020) Postoperative muscle mass restoration as a prognostic factor in patients with resected pancreatic cancer. PLoS One. 15: e0238649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee JW, Lee SM, Chung YA. (2018) Prognostic value of CT attenuation and FDG uptake of adipose tissue in patients with pancreatic adenocarcinoma. Clinical Radiology. 73. [DOI] [PubMed] [Google Scholar]
- 146.Naumann P, Eberlein J, Farnia B, et al. (2019) Cachectic Body Composition and Inflammatory Markers Portend a Poor Prognosis in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Okumura S, Kaido T, Hamaguchi Y, et al. (2017) Visceral Adiposity and Sarcopenic Visceral Obesity are Associated with Poor Prognosis After Resection of Pancreatic Cancer. Annals of Surgical Oncology. 24: 3732–40. [DOI] [PubMed] [Google Scholar]
- 148.Ryu Y, Shin SH, Kim JH, et al. (2020) The effects of sarcopenia and sarcopenic obesity after pancreaticoduodenectomy in patients with pancreatic head cancer. HPB. 22: 1782–92. [DOI] [PubMed] [Google Scholar]
- 149.Wochner R, Clauss D, Nattenmueller J, et al. (2020) Impact of progressive resistance training on CT quantified muscle and adipose tissue compartments in pancreatic cancer patients. Plos One. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Choi MH, Yoon SB, Lee K, et al. (2018) Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. Journal of Cachexia Sarcopenia and Muscle. 9: 326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cooper AB, Slack R, Fogelman D, et al. (2015) Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann Surg Oncol. 22: 2416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim B, Chung MJ, Park SW, et al. (2016) Visceral Obesity is Associated with Poor Prognosis in Pancreatic Adenocarcinoma. Nutrition and Cancer-an International Journal. 68: 201–7. [DOI] [PubMed] [Google Scholar]
- 153.Peng YC, Wu CH, Tien YW, Lu TP, Wang YH, Chen BB. (2021) Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol. 31: 2472–81. [DOI] [PubMed] [Google Scholar]
- 154.Antoun S, Bayar A, Ileana E, et al. (2015) High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. European Journal of Cancer. 51: 2570–7. [DOI] [PubMed] [Google Scholar]
- 155.Buttigliero C, Vana F, Bertaglia V, et al. (2015) The fat body mass increase after adjuvant androgen deprivation therapy is predictive of prostate cancer outcome. Endocrine. 50: 223–30. [DOI] [PubMed] [Google Scholar]
- 156.Cushen SJ, Power DG, Murphy KP, et al. (2016) Impact of body composition parameters on clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with docetaxel. Clinical Nutrition ESPEN. 13: e39–e45. [DOI] [PubMed] [Google Scholar]
- 157.Di Bella CM, Howard LE, Oyekunle T, et al. (2020) Abdominal and pelvic adipose tissue distribution and risk of prostate cancer recurrence after radiation therapy. Prostate. 80: 1244–52. [DOI] [PubMed] [Google Scholar]
- 158.Gregg JR, Surasi DS, Childs A, et al. (2021) The Association of Periprostatic Fat and Grade Group Progression in Men with Localized Prostate Cancer on Active Surveillance. Journal of Urology. 205: 122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee JS, Lee HS, Ha JS, et al. (2018) Subcutaneous Fat Distribution is a Prognostic Biomarker for Men with Castration Resistant Prostate Cancer. Journal of Urology. 200: 114–20. [DOI] [PubMed] [Google Scholar]
- 160.Mason RJ, Boorjian SA, Bhindi B, et al. (2018) Examining the association between adiposity and biochemical recurrence after radical prostatectomy. Canadian Urological Association Journal. 12: E331–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pak S, Kim MS, Park EY, Kim SH, Lee KH, Joung JY. (2020) Association of Body Composition With Survival and Treatment Efficacy in Castration-Resistant Prostate Cancer. Frontiers in Oncology. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sasaki T, Sugino Y, Kato M, Nishikawa K, Kanda H. (2020) Pre-treatment ratio of periprostatic to subcutaneous fat thickness on MRI is an independent survival predictor in hormone-naïve men with advanced prostate cancer. International journal of clinical oncology. 25: 370–6. [DOI] [PubMed] [Google Scholar]
- 163.Stangl-Kremser J, Suarez‐Ibarrola R, Andrea DD, et al. (2020) Assessment of body composition in the advanced stage of castration-resistant prostate cancer: special focus on sarcopenia. Prostate Cancer and Prostatic Diseases. 23: 309–15. [DOI] [PubMed] [Google Scholar]
- 164.Wu W, Liu X, Chaftari P, et al. (2015) Association of body composition with outcome of docetaxel chemotherapy in metastatic prostate cancer: a retrospective review. PLoS One. 10: e0122047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zimmermann M, Delouya G, Barkati M, Campeau S, Rompotinos D, Taussky D. (2016) Impact of visceral fat volume and fat density on biochemical outcome after radical prostatectomy and postoperative radiotherapy. Hormone Molecular Biology and Clinical Investigation. 26: 173–8. [DOI] [PubMed] [Google Scholar]
- 166.Antoun S, Lanoy E, Iacovelli R, et al. (2013) Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 119: 3377–84. [DOI] [PubMed] [Google Scholar]
- 167.Dai J, Zhang X, Liu Z, et al. (2020) The prognostic value of body fat components in metastasis renal cell carcinoma patients treated with TKIs. Cancer Management and Research. 12: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang H, Chen S, Li W, Wu X, Xing J. (2018) High perirenal fat thickness predicts a poor progression-free survival in patients with localized clear cell renal cell carcinoma. Urologic Oncology-Seminars and Original Investigations. 36. [DOI] [PubMed] [Google Scholar]
- 169.Kaneko G, Miyajima A, Yuge K, et al. (2015) Visceral obesity is associated with better recurrence-free survival after curative surgery for Japanese patients with localized clear cell renal cell carcinoma. Japanese Journal of Clinical Oncology. 45: 210–6. [DOI] [PubMed] [Google Scholar]
- 170.Kays JK, Koniaris LG, Cooper CA, et al. (2020) The Combination of Low Skeletal Muscle Mass and High Tumor Interleukin-6 Associates with Decreased Survival in Clear Cell Renal Cell Carcinoma. Cancers. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ladoire S, Bonnetain F, Gauthier M, et al. (2011) Visceral Fat Area as a New Independent Predictive Factor of Survival in Patients with Metastatic Renal Cell Carcinoma Treated with Antiangiogenic Agents. Oncologist. 16: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee HW, Jeong BC, Seo SI, et al. (2015) Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. International Journal of Urology. 22: 455–61. [DOI] [PubMed] [Google Scholar]
- 173.Mizuno R, Miyajima A, Hibi T, et al. (2017) Impact of baseline visceral fat accumulation on prognosis in patients with metastatic renal cell carcinoma treated with systemic therapy. Medical Oncology. 34. [DOI] [PubMed] [Google Scholar]
- 174.Naya Y, Zenbutsu S, Araki K, et al. (2010) Influence of visceral obesity on oncologic outcome in patients with renal cell carcinoma. Urol Int. 85: 30–6. [DOI] [PubMed] [Google Scholar]
- 175.Nguyen GK, Mellnick VM, Yim AK-Y, Salter A, Ippolito JE. (2018) Synergy of Sex Differences in Visceral Fat Measured with CT and Tumor Metabolism Helps Predict Overall Survival in Patients with Renal Cell Carcinoma. Radiology. 287: 884–92. [DOI] [PubMed] [Google Scholar]
- 176.Park YH, Lee JK, Kim KM, et al. (2014) Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J Urol. 192: 1043–9. [DOI] [PubMed] [Google Scholar]
- 177.Steffens S, Gruenwald V, Ringe KI, et al. (2011) Does Obesity Influence the Prognosis of Metastatic Renal Cell Carcinoma in Patients Treated with Vascular Endothelial Growth Factor-Targeted Therapy? Oncologist. 16: 1565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Boutin RD, Katz JR, Chaudhari AJ, et al. (2020) Association of adipose tissue and skeletal muscle metrics with overall survival and postoperative complications in soft tissue sarcoma patients: An opportunistic study using computed tomography. Quantitative Imaging in Medicine and Surgery. 10: 1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Celik E, Kizildag Yirgin I, Goksever Celik H, et al. (2021) Does visceral adiposity have an effect on the survival outcomes of the patients with endometrial cancer? Journal of Obstetrics and Gynaecology Research. 47: 560–9. [DOI] [PubMed] [Google Scholar]
- 180.Chakedis J, Spolverato G, Beal EW, et al. (2018) Pre-operative Sarcopenia Identifies Patients at Risk for Poor Survival After Resection of Biliary Tract Cancers. Journal of Gastrointestinal Surgery. 22: 1697–708. [DOI] [PubMed] [Google Scholar]
- 181.De Amorim Bernstein K, Bos SA, Veld J, Lozano-Calderon SA, Torriani M, Bredella MA. (2018) Body composition predictors of therapy response in patients with primary extremity soft tissue sarcomas. Acta Radiol. 59: 478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Donkers H, Fasmer KE, McGrane J, et al. (2021) Obesity and visceral fat: Survival impact in high-grade endometrial cancer. European Journal of Obstetrics & Gynecology and Reproductive Biology. 256: 425–32. [DOI] [PubMed] [Google Scholar]
- 183.Gillen J, Mills KA, Dvorak J, et al. (2019) Imaging biomarkers of adiposity and sarcopenia as potential predictors for overall survival among patients with endometrial cancer treated with bevacizumab. Gynecologic Oncology Reports. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE. (2015) Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surgical Oncology-Oxford. 24: 353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.He WZ, Jiang C, Liu LL, et al. (2020) Association of body composition with survival and inflammatory responses in patients with non-metastatic nasopharyngeal cancer. Oral Oncol. 108: 104771. [DOI] [PubMed] [Google Scholar]
- 186.Jung J, Lee E, Shim H, Park JH, Eom HS, Lee H. (2021) Prediction of clinical outcomes through assessment of sarcopenia and adipopenia using computed tomography in adult patients with acute myeloid leukemia. Int J Hematol. 114: 44–52. [DOI] [PubMed] [Google Scholar]
- 187.Mauland KK, Eng Ø, Ytre-Hauge S, et al. (2017) High visceral fat percentage is associated with poor outcome in endometrial cancer. Oncotarget. 8: 105184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miller BS, Ignatoski KM, Daignault S, et al. (2012) Worsening central sarcopenia and increasing intra-abdominal fat correlate with decreased survival in patients with adrenocortical carcinoma. World J Surg. 36: 1509–16. [DOI] [PubMed] [Google Scholar]
- 189.Okumura S, Kaido T, Hamaguchi Y, et al. (2017) Impact of Skeletal Muscle Mass, Muscle Quality, and Visceral Adiposity on Outcomes Following Resection of Intrahepatic Cholangiocarcinoma. Annals of Surgical Oncology. 24: 1037–45. [DOI] [PubMed] [Google Scholar]
- 190.Pan Y, Chen Z, Yang L, et al. (2021) Body Composition Parameters May Be Prognostic Factors in Upper Urinary Tract Urothelial Carcinoma Treated by Radical Nephroureterectomy. Frontiers in Oncology. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Papoulas M, Weiser R, Rosen G, et al. (2015) Visceral Fat Content Correlates with Retroperitoneal Soft Tissue Sarcoma (STS) Local Recurrence and Survival. World Journal of Surgery. 39: 1895–901. [DOI] [PubMed] [Google Scholar]
- 192.Psutka SP, Boorjian SA, Moynagh MR, et al. (2015) Mortality after Radical Cystectomy: Impact of Obesity Versus Adiposity after Adjusting for Skeletal Muscle Wasting. Journal of Urology. 193: 1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shin DY, Kim A, Byun BH, et al. (2016) Visceral adipose tissue is prognostic for survival of diffuse large B cell lymphoma treated with frontline R-CHOP. Ann Hematol. 95: 409–16. [DOI] [PubMed] [Google Scholar]
- 194.Takeoka Y, Sakatoku K, Miura A, et al. (2016) Prognostic Effect of Low Subcutaneous Adipose Tissue on Survival Outcome in Patients With Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 16: 434–41. [DOI] [PubMed] [Google Scholar]
- 195.Young AC, Quach HT, Song H, et al. (2020) Impact of body composition on outcomes from anti-PD1 +/−anti-CTLA-4 treatment in melanoma. Journal for ImmunoTherapy of Cancer. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Antoun S, Bayar MA, Dyevre V, Lanoy E, Smolenschi C, Ducreux M. (2019) No evidence for changes in skeletal muscle mass or weight during first-line chemotherapy for metastatic colorectal cancer. Bmc Cancer. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bachmann R, Leonard D, Nachit M, et al. (2018) Comparison between abdominal fat measured by CT and anthropometric indices as prediction factors for mortality and morbidity after colorectal surgery. Acta Gastro-Enterologica Belgica. 81: 477–83. [PubMed] [Google Scholar]
- 198.Ballian N, Lubner MG, Munoz A, et al. (2012) Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. Journal of Surgical Oncology. 105: 365–70. [DOI] [PubMed] [Google Scholar]
- 199.Bian X, Dai H, Feng J, et al. (2018) Prognostic values of abdominal body compositions on survival in advanced pancreatic cancer. Medicine. 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Blanc-Durand P, Campedel L, Mule S, et al. (2020) Prognostic value of anthropometric measures extracted from whole-body CT using deep learning in patients with non-small-cell lung cancer. European Radiology. 30: 3528–37. [DOI] [PubMed] [Google Scholar]
- 201.Brown JC, Caan BJ, Prado CM, et al. (2020) The Association of Abdominal Adiposity With Mortality in Patients With Stage I-III Colorectal Cancer. J Natl Cancer Inst. 112: 377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Buechel ME, Enserro D, Burger RA, et al. (2021) Correlation of imaging and plasma based biomarkers to predict response to bevacizumab in epithelial ovarian cancer (EOC). Gynecologic Oncology. 161: 382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cloyd JM, Nogueras-González GM, Prakash LR, et al. (2018) Anthropometric Changes in Patients with Pancreatic Cancer Undergoing Preoperative Therapy and Pancreatoduodenectomy. J Gastrointest Surg. 22: 703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Goulart A, Malheiro N, Rios H, Sousa N, Leao P. (2019) Influence of Visceral Fat in the Outcomes of Colorectal Cancer. Digestive Surgery. 36: 33–40. [DOI] [PubMed] [Google Scholar]
- 205.Gu W, Zhu Y, Wang H, et al. (2015) Prognostic value of components of body composition in patients treated with targeted therapy for advanced renal cell carcinoma: a retrospective case series. PLoS One. 10: e0118022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ha Y, Kim D, Han S, et al. (2018) Sarcopenia Predicts Prognosis in Patients with Newly Diagnosed Hepatocellular Carcinoma, Independent of Tumor Stage and Liver Function. Cancer Research and Treatment. 50: 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Iwase T, Sangai T, Fujimoto H, et al. (2020) Quality and quantity of visceral fat tissue are associated with insulin resistance and survival outcomes after chemotherapy in patients with breast cancer. Breast Cancer Research and Treatment. 179: 435–43. [DOI] [PubMed] [Google Scholar]
- 208.Katsui K, Ogata T, Sugiyama S, et al. (2021) Sarcopenia is associated with poor prognosis after chemoradiotherapy in patients with stage III non-small-cell lung cancer: a retrospective analysis. Scientific reports. 11: 11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kuritzkes BA, Pappou EP, Kiran RP, et al. (2018) Visceral fat area, not body mass index, predicts postoperative 30-day morbidity in patients undergoing colon resection for cancer. International Journal of Colorectal Disease. 33: 1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee JW, Kim SY, Lee HJ, Han SW, Lee JE, Lee SM. (2019) Prognostic significance of abdominal-to-gluteofemoral adipose tissue distribution in patients with breast cancer. Journal of Clinical Medicine. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Looijaard S, Meskers CGM, Slee-Valentijn MS, et al. (2020) Computed Tomography-Based Body Composition Is Not Consistently Associated with Outcome in Older Patients with Colorectal Cancer. Oncologist. 25: e492–e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McDonald AM, Fiveash JB, Kirkland RS, et al. (2017) Subcutaneous adipose tissue characteristics and the risk of biochemical recurrence in men with high-risk prostate cancer. Urologic Oncology-Seminars and Original Investigations. 35. [DOI] [PubMed] [Google Scholar]
- 213.Moon HG, Ju YT, Jeong CY, et al. (2008) Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. 15: 1918–22. [DOI] [PubMed] [Google Scholar]
- 214.Nattenmueller J, Wochner R, Muley T, et al. (2017) Prognostic Impact of CT-Quantified Muscle and Fat Distribution before and after First-Line-Chemotherapy in Lung Cancer Patients. Plos One. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ninomiya G, Fujii T, Yamada S, et al. (2017) Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. International Journal of Surgery. 39: 45–51. [DOI] [PubMed] [Google Scholar]
- 216.Ohki T, Tateishi R, Shiina S, et al. (2009) Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 58: 839–44. [DOI] [PubMed] [Google Scholar]
- 217.Ohwaki K, Endo F, Hattori K. (2015) Abdominal obesity, hypertension, antihypertensive medication use and biochemical recurrence of prostate cancer after radical prostatectomy. European Journal of Cancer. 51: 604–9. [DOI] [PubMed] [Google Scholar]
- 218.Park JS, Koo KC, Chung DY, et al. (2020) Visceral Adiposity as a Significant Predictor of Sunitinib-Induced Dose-Limiting Toxicities and Survival in Patients with Metastatic Clear Cell Renal Cell Carcinoma. Cancers. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Park SW, Lee HL, Doo EY, et al. (2015) Visceral Obesity Predicts Fewer Lymph Node Metastases and Better Overall Survival in Colon Cancer. Journal of Gastrointestinal Surgery. 19: 1513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rodrigues V, Landi F, Castro S, et al. (2021) Is Sarcopenic Obesity an Indicator of Poor Prognosis in Gastric Cancer Surgery? A Cohort Study in a Western Population. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 25: 1388–403. [DOI] [PubMed] [Google Scholar]
- 221.Sabel MS, Terjimanian M, Conlon AS, et al. (2013) Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J Surg Oncol. 108: 169–75. [DOI] [PubMed] [Google Scholar]
- 222.Schaffler-Schaden D, Mittermair C, Birsak T, et al. (2020) Skeletal muscle index is an independent predictor of early recurrence in non-obese colon cancer patients. Langenbecks Archives of Surgery. 405: 469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sheikhbahaei S, Reyes DK, Rowe SP, Pienta KJ. (2021) CT-based assessment of body composition following neoadjuvant chemohormonal therapy in patients with castration-naive oligometastatic prostate cancer. Prostate. 81: 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Silva A, Gomes F, Pereira SS, Monteiro MP, Araújo A, Faria G. (2021) Visceral obesity is associated with lower stage colon tumors in males without survival advantage. Surgical Oncology. 37. [DOI] [PubMed] [Google Scholar]
- 225.Sugimoto M, Farnell MB, Nagorney DM, et al. (2018) Decreased Skeletal Muscle Volume Is a Predictive Factor for Poorer Survival in Patients Undergoing Surgical Resection for Pancreatic Ductal Adenocarcinoma. Journal of Gastrointestinal Surgery. 22: 831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba-Ssalamah A. (2016) Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. European Radiology. 26: 1359–67. [DOI] [PubMed] [Google Scholar]
- 227.van Baar H, Winkels RM, Brouwer JGM, et al. (2020) Associations of Abdominal Skeletal Muscle Mass, Fat Mass, and Mortality among Men and Women with Stage I-III Colorectal Cancer. Cancer Epidemiology Biomarkers & Prevention. 29: 956–65. [DOI] [PubMed] [Google Scholar]
- 228.van Dijk DP, Bakens MJ, Coolsen MM, et al. (2017) Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 8: 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.van Dijk DPJ, Krill M, Farshidfar F, et al. (2019) Host phenotype is associated with reduced survival independent of tumour biology in patients with colorectal liver metastases. J Cachexia Sarcopenia Muscle. 10: 123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.von Hessen L, Roumet M, Maurer MH, et al. (2021) High subcutaneous adipose tissue density correlates negatively with survival in patients with hepatocellular carcinoma. Liver Int. 41: 828–36. [DOI] [PubMed] [Google Scholar]
- 231.Xu MC, Huelster HL, Hatcher JB, et al. (2021) Obesity is Associated with Longer Survival Independent of Sarcopenia and Myosteatosis in Metastatic and/or Castrate-Resistant Prostate Cancer. Journal of Urology. 205: 800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yamamoto N, Fujii S, Sato T, et al. (2012) Impact of body mass index and visceral adiposity on outcomes in colorectal cancer. Asia-Pacific Journal of Clinical Oncology. 8: 337–45. [DOI] [PubMed] [Google Scholar]
- 233.Ying P, Jin W, Wu X, Cai W. (2021) Association between CT-Quantified Body Composition and Recurrence, Survival in Nonmetastasis Colorectal Cancer Patients Underwent Regular Chemotherapy after Surgery. Biomed Res Int. 2021: 6657566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yoo ID, Lee SM, Lee JW, Baek M-J, Ahn TS. (2018) Usefulness of metabolic activity of adipose tissue in FDG PET/CT of colorectal cancer. Abdominal Radiology. 43: 2052–9. [DOI] [PubMed] [Google Scholar]
- 235.Abrahamson PE, Gammon MD, Lund MJ, et al. (2006) General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiology Biomarkers & Prevention. 15: 1871–7. [DOI] [PubMed] [Google Scholar]
- 236.Borugian MJ, Sheps SB, Kim-Sing C, et al. (2003) Waist-to-hip ratio and breast cancer mortality. American Journal of Epidemiology. 158: 963–8. [DOI] [PubMed] [Google Scholar]
- 237.Chen H-l, Ding A, Wang M-l. (2016) Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. Springerplus. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dal Maso L, Zucchetto A, Talamini R, et al. (2008) Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 123: 2188–94. [DOI] [PubMed] [Google Scholar]
- 239.George SM, Bernstein L, Smith AW, et al. (2014) Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res Treat. 146: 647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Goodwin PJ, Ennis M, Pritchard KI, et al. (2012) Insulin- and Obesity-Related Variables in Early-Stage Breast Cancer: Correlations and Time Course of Prognostic Associations. Journal of Clinical Oncology. 30: 164–71. [DOI] [PubMed] [Google Scholar]
- 241.His M, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F, Dossus L. (2016) Prediagnostic body size and breast cancer survival in the E3N cohort study. Int J Cancer. 139: 1053–64. [DOI] [PubMed] [Google Scholar]
- 242.Kwan ML, John EM, Caan BJ, et al. (2014) Obesity and Mortality After Breast Cancer by Race/Ethnicity: The California Breast Cancer Survivorship Consortium. American Journal of Epidemiology. 179: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shariff-Marco S, Gomez SL, Sangaramoorthy M, et al. (2015) Impact of neighborhoods and body size on survival after breast cancer diagnosis. Health and Place. 36: 162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. (2015) Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes & Control. 26: 1803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tao MH, Shu XO, Ruan ZX, Gao YT, Zheng W. (2006) Association of overweight with breast cancer survival. American Journal of Epidemiology. 163: 101–7. [DOI] [PubMed] [Google Scholar]
- 246.Wisse A, Tryggvadottir H, Simonsson M, et al. (2018) Increasing preoperative body size in breast cancer patients between 2002 and 2016: implications for prognosis. Cancer Causes & Control. 29: 643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang M, Cai H, Bao P, et al. (2017) Body mass index, waist-to-hip ratio and late outcomes: a report from the Shanghai Breast Cancer Survival Study. Scientific Reports. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. (1995) Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women’s Health Study. Cancer. 76: 275–83. [DOI] [PubMed] [Google Scholar]
- 249.Fedirko V, Romieu I, Aleksandrova K, et al. (2014) Pre-diagnostic anthropometry and survival after colorectal cancer diagnosis in Western European populations. Int J Cancer. 135: 1949–60. [DOI] [PubMed] [Google Scholar]
- 250.Haydon AM, Macinnis RJ, English DR, Giles GG. (2006) Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 55: 62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jayasekara H, English DR, Haydon A, et al. (2018) Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. International Journal of Cancer. 142: 238–50. [DOI] [PubMed] [Google Scholar]
- 252.Prizment AE, Flood A, Anderson KE, Folsom AR. (2010) Survival of Women with Colon Cancer in Relation to Precancer Anthropometric Characteristics: the Iowa Women’s Health Study. Cancer Epidemiology Biomarkers & Prevention. 19: 2229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang N, Khankari NK, Cai H, et al. (2017) Prediagnosis body mass index and waist–hip circumference ratio in association with colorectal cancer survival. International Journal of Cancer. 140: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Farris MS, Courneya KS, Kopciuk KA, McGregor SE, Friedenreich CM. (2018) Anthropometric measurements and survival after a prostate cancer diagnosis. British Journal of Cancer. 118: 607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jackson MD, Tulloch-Reid MK, McCaw-Binns AM, et al. (2020) Central adiposity at diagnosis may reduce prostate cancer-specific mortality in African-Caribbean men with prostate cancer: 10-year follow-up of participants in a case–control study. Cancer Causes & Control. 31: 651–62. [DOI] [PubMed] [Google Scholar]
- 256.Moller H, Roswall N, Van Hemelrijck M, et al. (2015) Prostate cancer incidence, clinical stage and survival in relation to obesity: A prospective cohort study in Denmark. International Journal of Cancer. 136: 1940–7. [DOI] [PubMed] [Google Scholar]
- 257.Erbaycu AE, Kazanci MN, Biçmen C, et al. (2010) Initial anthropometric values and nutritional status is related to survival in advanced non-small cell lung cancer. Turkiye Klinikleri Journal of Medical Sciences. 30: 1177–84. [Google Scholar]
- 258.Ferrigno D, Buccheri G. (2001) Anthropometric measurements in non-small-cell lung cancer. Supportive Care in Cancer. 9: 522–7. [DOI] [PubMed] [Google Scholar]
- 259.Liu X, Xu J. (2015) Body Mass Index and Waistline are Predictors of Survival for Hepatocellular Carcinoma After Hepatectomy. Medical Science Monitor. 21: 2203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Park S, Han B, Cho JW, et al. (2014) Effect of Nutritional Status on Survival Outcome of Diffuse Large B-Cell Lymphoma Patients Treated with Rituximab-CHOP. Nutrition and Cancer-an International Journal. 66: 225–33. [DOI] [PubMed] [Google Scholar]
- 261.Mathur A, Hernandez J, Shaheen F, et al. (2011) Preoperative computed tomography measurements of pancreatic steatosis and visceral fat: prognostic markers for dissemination and lethality of pancreatic adenocarcinoma. HPB (Oxford). 13: 404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Anjanappa M, Corden M, Green A, et al. (2020) Sarcopenia in cancer: Risking more than muscle loss. Technical Innovations & Patient Support in Radiation Oncology. 16: 50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chait A, den Hartigh LJ. (2020) Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Booth AD, Magnuson AM, Fouts J, et al. (2018) Subcutaneous adipose tissue accumulation protects systemic glucose tolerance and muscle metabolism. Adipocyte. 7: 261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hakimi AA, Furberg H, Zabor EC, et al. (2013) An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. Journal of the National Cancer Institute. 105: 1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sanchez A, Furberg H, Kuo F, et al. (2020) Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 21: 283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xiao J, Mazurak VC, Olobatuyi TA, Caan BJ, Prado CM. (2018) Visceral adiposity and cancer survival: a review of imaging studies. European Journal of Cancer Care. 27. [DOI] [PubMed] [Google Scholar]
- 268.Akhter S, Pauyo T, Khan M. (2019) What Is the Difference Between a Systematic Review and a Meta-analysis? In: Musahl V, Karlsson J, Hirschmann MT, et al. , eds. Basic Methods Handbook for Clinical Orthopaedic Research: A Practical Guide and Case Based Research Approach. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 331–42. [Google Scholar]
- 269.Theologides A (1977) Weight loss in cancer patients. CA Cancer J Clin. 27: 205–8. [DOI] [PubMed] [Google Scholar]
- 270.Ebadi M, Mazurak VC. (2014) Evidence and mechanisms of fat depletion in cancer. Nutrients. 6: 5280–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cole SR, Platt RW, Schisterman EF, et al. (2010) Illustrating bias due to conditioning on a collider. International journal of epidemiology. 39: 417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mayeda ER, Glymour MM. (2017) The Obesity Paradox in Survival after Cancer Diagnosis: Tools for Evaluation of Potential Bias. Cancer Epidemiol Biomarkers Prev. 26: 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sperrin M, Candlish J, Badrick E, Renehan A, Buchan I. (2016) Collider bias is only a partial explanation for the obesity paradox. Epidemiology (Cambridge, Mass.). 27: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jaspan V, Lin K, Popov V. (2021) The impact of anthropometric parameters on colorectal cancer prognosis: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 159: 103232. [DOI] [PubMed] [Google Scholar]
- 275.Cornier MA, Despres JP, Davis N, et al. (2011) Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 124: 1996–2019. [DOI] [PubMed] [Google Scholar]
- 276.Kocarnik JM, Chan AT, Slattery ML, et al. (2016) Relationship of prediagnostic body mass index with survival after colorectal cancer: Stage-specific associations. Int J Cancer. 139: 1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.American Cancer Society. MRI for Cancer. https://www.cancer.org/treatment/understanding-your-diagnosis/tests/mri-for-cancer.html. Accessed December 31, 2021.
- 278.National Cancer Institute. Computed Tomography (CT) Scans and Cancer. https://www.cancer.gov/about-cancer/diagnosis-staging/ct-scans-fact-sheet. Accessed December 31, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are publicly available via applying the searching algorithm proposed in this manuscript to PubMed, Embase and Web of Science.


