Tweet:
AI analysis of HCM ECGs correlates with longitudinal hemodynamic, cardiac structural and laboratory markers in obstructive HCM patients.
Keywords: hypertrophic cardiomyopathy, electrocardiogram, artificial intelligence, machine learning, mavacamten
Though hypertrophic cardiomyopathy (HCM) causes significant morbidity and is a leading cause of sudden death in adolescents, initial detection remains difficult. While echocardiography is an important diagnostic modality for HCM, the electrocardiogram (ECG) is more widely accessible. Artificial intelligence (AI)-based analysis of standard 12-lead ECGs (AI-ECG) has achieved accurate fully-automated HCM diagnosis (1,2). However, it is unknown whether AI-ECG can track disease status, including cardiac structural and hemodynamic changes over time, to inform disease-specific clinical decision making.
We applied two AI-ECG algorithms to pre-treatment and on-treatment ECGs from the phase 2 PIONEER-OLE trial(3) of the cardiac myosin inhibitor mavacamten in patients with obstructive HCM. The two AI-ECG algorithms were independently developed and trained at the University of California San Francisco (UCSF; San Francisco, CA, US)(1) and Mayo Clinic (Rochester, MN, US)(2) as previously described; institutional review board approval was obtained from both institutions. PIONER-OLE patients had 12-lead ECGs, echocardiography, and drug plasma and N-terminal pro B-type natriuretic peptide (NT-proBNP) measurements at day 0 (pre-treatment), weeks 4, 8, and 12, and every 12 weeks thereafter. Both algorithms were first validated in cohorts constructed by combining pre-treatment ECGs from PIONEER-OLE patients (n=13) and age- and sex-matched control ECGs from subjects without HCM from Mayo Clinic and UCSF (n=2,600 each) in a 1:200 ratio to approximate HCM prevalence. Both algorithms were then applied to all ECGs (n=216) from each PIONEER-OLE patient acquired pre-treatment and on-treatment through January 29, 2020. We examined longitudinal associations of AI-ECG-predicted HCM scores with echocardiographic and laboratory metrics important to HCM clinical decision making.
Mean age of the PIONEER-OLE cohort at baseline was 57.8 years; 69.2% of patients were male and 92.3% had New York Heart Association class II symptoms. Median follow-up was 79 weeks (range 25.0–90.1). To discriminate PIONEER-OLE pre-treatment HCM ECGs from ECGs of age/sex-matched non-HCM controls, area under the receiver operating characteristic curve (AUC) was 0.938 (95% CI, 0.924–0.950) for the UCSF algorithm and 0.979 (95% CI, 0.942–1.000) for the Mayo algorithm. Sensitivity and specificity were 84.6% and 96.3% for the UCSF algorithm, and 92.3% and 94.1% for the Mayo algorithm, respectively. When applied to all PIONEER-OLE ECGs (n=216), both algorithms demonstrated decreases in mean HCM scores during mavacamten treatment averaged across each time point, with a mean score reduction of 43% (0.67 pre-treatment to 0.38 at 72 weeks) for UCSF and a reduction of 56% (0.85 pre-treatment to 0.37 at 72 weeks) for the Mayo algorithm. The longitudinal AI-ECG HCM score trends generally mirrored the decreasing trends over time in LVOT gradient with Valsalva and NT-proBNP (Figure 1). Mavacamten pharmacokinetic levels increased on initiation then remained stably elevated over time in most patients.
Figure 1: Longitudinal changes in averaged AI-ECG scores and cardiac metrics.
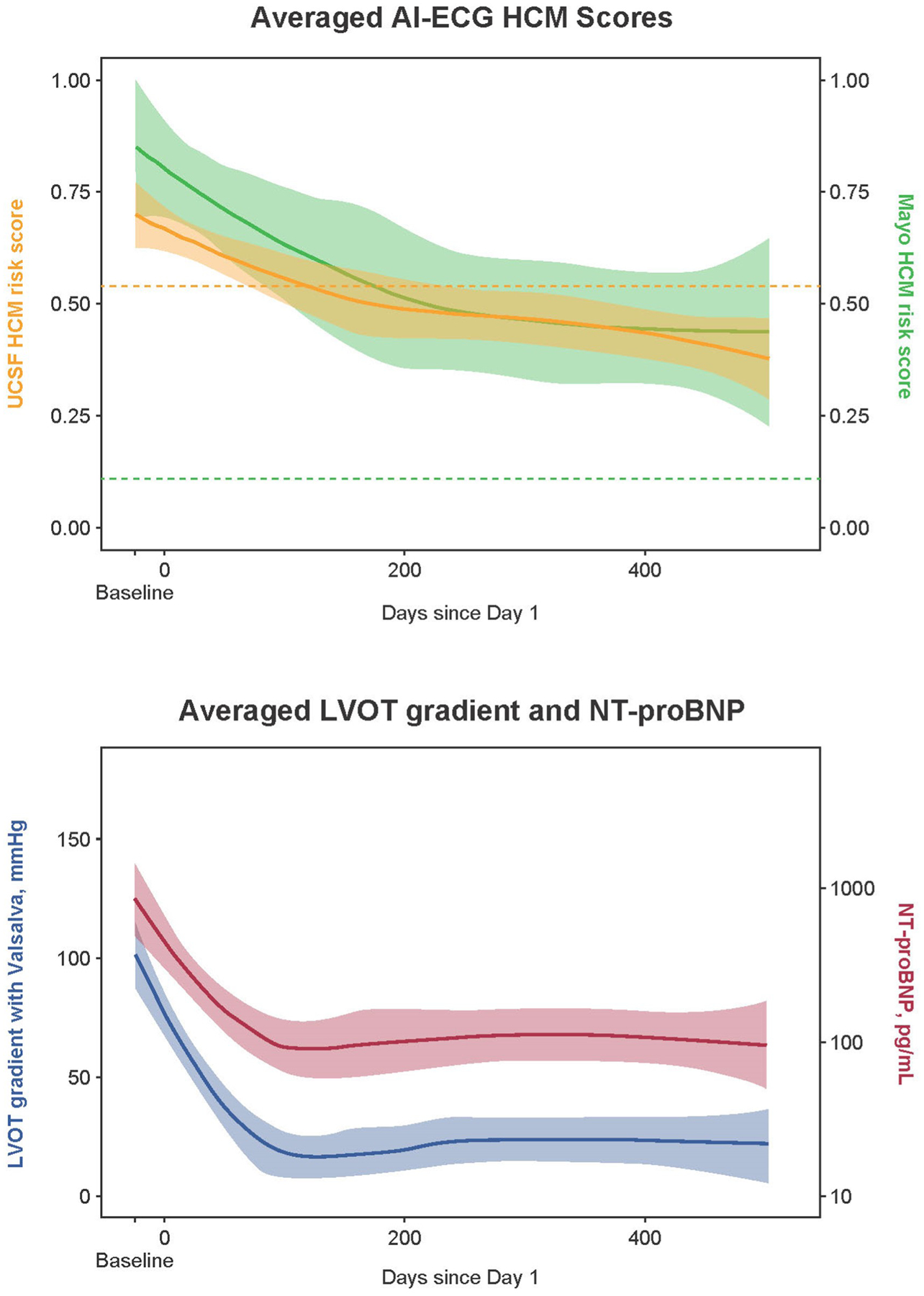
Values averaged across all patients, shown with 95% confidence interval bands. Dotted lines (upper panel) show HCM score threshold used to indicate HCM for each algorithm. AI-ECG, artificial intelligence–based electrocardiogram analysis; HCM, hypertrophic cardiomyopathy; LVOT, left ventricular outflow tract; NT-proBNP, N-terminal pro B-type natriuretic peptide.
The key finding from this study is that AI-ECG HCM scores correlated with disease status as measured by decreases over time in LVOT gradients and NT-proBNP levels in patients with obstructive HCM on mavacamten treatment. Therefore, AI-ECG may hold promise as a potential tool for monitoring disease status, cardiac hemodynamics, and drug therapeutic response. These significant longitudinal associations of the AI-ECG HCM score likely reflect changes in the raw ECG waveform detectable by AI-ECG that correlate with HCM disease pathophysiology and severity. While extensive prior work has linked various characteristic ECG changes with HCM, none of these changes are unique to HCM(4), and association between ECG findings and hemodynamic or laboratory measurements, either cross-sectionally or longitudinally, has not been previously demonstrated. AI-ECG enables the capture of more information from ECGs related to obstructive HCM physiology and pathophysiology than is currently appreciated by manual interpretation. The main limitation of this study is the small sample size of PIONEER-OLE, though this was counterbalanced by the uniquely rich ECG/echocardiography phenotyping and long-term follow-up of this on-treatment HCM cohort. This work provides a novel paradigm by which the AI-ECG—which may also be implemented remotely via smartphone-enabled electrodes—may permit assessment of disease status and treatment response. Future studies can evaluate this approach as a guide for drug titration to enhance safety.
Acknowledgment
We thank the patients and their families, the investigators, and the clinical study teams for making the study possible. Editorial support was provided by Kim Fuller, PhD (Cello Health Communications/SciFluent), financially supported by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb.
Sources of Funding:
Dr. Tison received support for this work from the National Institutes of Health NHLBI K23HL135274. The Tison lab received funding from MyoKardia, Inc. a wholly owned subsidiary of Bristol Myers Squibb to support this analysis. The work to derive the Mayo Clinic AI-ECG algorithm was funded by the Louis V. Gerstner, Jr. Fund at Vanguard Charitable. The PIONEER-OLE study was funded by MyoKardia. Co-authors employed by MyoKardia were involved in study design, data analysis, data interpretation, and review of the manuscript, in collaboration with academic coauthors. All authors had full access to all the data reports from the study and had final responsibility for the decision to submit for publication.
Abbreviations and Acronyms
- AI
artificial intelligence
- AUC
area under the receiver operating characteristic curve
- ECG
electrocardiogram
- HCM
hypertrophic cardiomyopathy
- LVOT
left ventricular outflow tract
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- UCSF
University of California San Francisco
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: PAN, KCS, ZA, and PAF are coinventors of the Mayo Clinic AI-ECG algorithm utilized in this work. PAN receives research funding from National Institutes of Health (NIH, including the National Heart, Lung, and Blood Institute [NHLBI] and the National Institute on Aging [NIA]), Agency for Healthcare Research and Quality (AHRQ), Food and Drug Administration (FDA), and the American Heart Association (AHA). PAN is a study investigator in an ablation trial sponsored by Medtronic. PAN and Mayo Clinic are involved in potential equity/royalty relationship with AliveCor. GHT has previously received research grants from General Electric, Janssen Pharmaceuticals and MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb; and has received consulting fees from MyoKardia. TAD has received consulting fees from Adagio Medical and Farapulse. PA, AB, YL, AJS, and JME are employees of MyoKardia. PA, AB, AJS, and JME report stock and stock options from MyoKardia. JEO has received research funding from the NIH (U2CEB021881) and from Samsung and iBeat. All other authors have nothing to disclose.
Clinical trial: NCT03496168
References:
- 1.Tison GH, Zhang J, Delling FN, Deo RC. Automated and interpretable patient ECG profiles for disease detection, tracking, and discovery. Circ Cardiovasc Qual Outcomes 2019;12:e005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko WY, Siontis KC, Attia ZI et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol 2020;75:722–33. [DOI] [PubMed] [Google Scholar]
- 3.Heitner SB, Lester S, Wang A, et al. Precision pharmacological treatment for obstructive hypertrophic cardiomyopathy with mavacamten: one-year results from PIONEER-OLE. Circulation 2019;140:A13962. [Google Scholar]
- 4.Finocchiaro G, Sheikh N, Biagini E et al. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm 2020;17:142–51. [DOI] [PubMed] [Google Scholar]


