Abstract
Background:
The aim of this meta-analysis and systematic review was to evaluate the association between intraoperative bile cultures (IOBC) and postoperative complications of patients undergoing pancreaticoduodenectomy.
Methods:
A detailed literature search was performed from 1/2015–7/2022 in PubMed, Web of Science, Google Scholar, and EMBASE for related research publications. The data were extracted, screened, and graded independently. Analysis of pooled data was performed, and a Risk Ratio (RR) with corresponding confidence intervals (CIs) was calculated and summarized.
Results:
A total of 8 articles were included with 1,778 pancreaticoduodenectomy patients who had an IOBC performed. A systematic review demonstrated that some of the most common organisms isolated in a positive IOBC were Enterococcus species, Klebsiella species, and E. Coli. Four studies also showed that specific micro-organisms were associated with specific postoperative complications (SSI and IAA). The postoperative complications that were evaluated for an association with a positive IOBC were surgical site infections (SSI) (RR=2.33 CI 95% [1.47–3.69], p<0.01), delayed gastric emptying (DGE) (RR=1.23 CI 95% [0.63–2.38], p=n.s.), 90-day mortality (RR=0.68, CI 95% [0.01–52.76], p=n.s.), postoperative pancreatic hemorrhage (POPH) (RR=1.70, CI 95% [0.33–8.74], p=n.s.), intra-abdominal abscess (IAA) (RR=1.70, CI 95% [0.38–7.56], p=n.s.), and postoperative pancreatic fistula (POPF) (RR=0.97, CI 95% [0.72–1.32], p=n.s.).
Conclusions:
The cumulative data suggest that a positive IOBC has no association with predicting the postoperative complications of DGE, 90-day mortality, POPH, IAA, or POPF. However, the data also suggest that a positive IOBC was associated with a patient developing a SSI.
Keywords: Pancreas, Intraoperative Bile Cultures, Oncology, Postoperative Pancreatic Fistula, Delayed Gastric Emptying, Surgical Site Infections, Intra-abdominal abscess, 30-day Mortality
Introduction:
Pancreaticoduodenectomy (PD) is a complicated procedure that is associated with a high postoperative morbidity rate (30–60%) (1). Some of the postoperative complications include superficial surgical site infections (SSI), delayed gastric emptying (DGE), mortality, postoperative pancreatic hemorrhage (POPH), organ space intra-abdominal abscess, and postoperative pancreatic fistula (POPF). Postoperative complications are associated with a prolonged hospital stay, higher treatment costs, increased readmission, reoperations, and delay in further cancer treatment (2), (3). In the past, Significant bacterial concentrations within bile have been associated with an increased risk of post-operative complications (4). Thus, intraoperative bile cultures (IOBC) for stented patients have commonly been performed to help guide postoperative antibiotic management, especially in the treatment of SSIs (5). The intraoperative bile culture is performed due to the proximity of bile to the site of the PD and is used to detect bacterial colonization, which allows for an early targeted systemic antibiotic treatment in the event of infectious complications (5). The association between bacterobilia (positive IOBC) and the post-operative complications described above has never been well-discussed, and whether the presence of bacterobilia increases the risk or severity of the SSI, DGE, 90-day mortality, POPH, IAA, and POPF remains unclear (3), (5), (6), (7). The aim of this meta-analysis and systematic review is to clarify the impact of bacterobilia on the development of these postoperative complications as well as to characterize the infectious pathogens between bile and specific postoperative complication.
Methods:
Literature Search
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using PubMed, Web of Science, Google Scholar, and EMBASE from the dates of 1/2015–7/2022. The initial search was conducted using the terms “pancreatic resection”, “intraoperative bile cultures”, “postoperative pancreatic fistula”, “delayed gastric emptying”, “surgical site infections”, and “Intra-abdominal abscess”, “90-day Mortality”, and “oncology” in all fields. The initial search yielded 103 results (Figure 1). An additional 8 articles were hand-selected and added to the search. From the combined 111 articles, 11 articles were removed before the screening. Of the remaining 100 articles, 94 were excluded after being screened for the following inclusion criteria: English only, human subjects only, Intraoperative bile cultures reported, and intraoperative bile cultures evaluating postoperative complications and 2015–2022. Duplicates, meta-analyses, and systematic reviews were also removed, yielding 8 total articles. The remaining 8 articles were examined in their entirety and searched for quality reporting data related to the key inclusion criteria, which are outlined in the section below. After this review, the 8 articles remained, and the predictive nature that intraoperative bile culture has on specific postoperative complications (POPF, DGE, IAA, POPF, Mortality, and SSI) was extracted (Figure 1).
Figure 1.
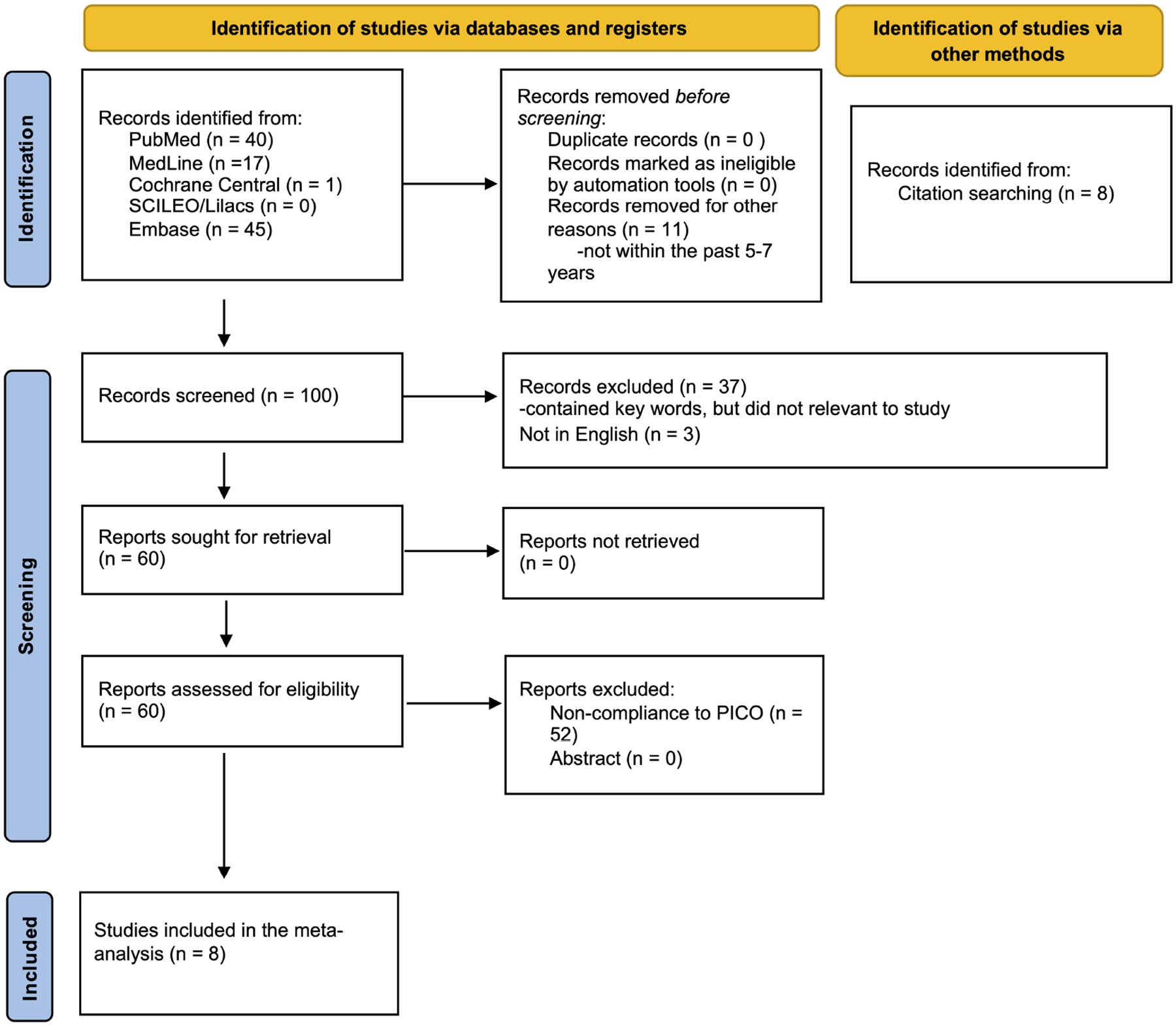
PRISMA diagram of literature search.
Inclusion and Exclusion Criteria
Inclusion was limited to English articles and included observational and comparative cohort studies. Original articles that focused on intraoperative bile cultures identifying certain postoperative complications (SSI, IAA, POPF, POPH, DGE, 90-day mortality) were identified and included. Their reference lists were further examined to identify additional studies not captured by the primary literature search. Papers discussing the specific micro-organism identified in the intraoperative bile culture as well as the micro-organism associated with a specific postoperative complication were included. Exclusion criteria included non-English studies, reviews, letters, abstracts, studies in animals, and laboratory studies. Additionally, any articles that did not examined preoperative bile cultures, postoperative bile cultures, and biliary drainage were excluded (Figure 1).
Data Extraction and Quality Assessment
One author independently screened the titles and abstracts of all articles identified in the primary search strategy. Based on the inclusion and exclusion criteria, the author assessed the full text of 8 articles, and then subsequently performed the data extraction (AF). Enduring conflicts and questions were resolved following the senior author (RM) review. Extracted perioperative and operative variables included the incidence of IAA, Incidence of POPH, the incidence of POPF, the incidence of DGE, the incidence of SSI, and postoperative mortality. The primary endpoint was if a positive intraoperative bile culture was associated with either POPF, DGE, IAA, POPH, Mortality, or SSI.
Study Scoring
A scoring system adapted from the Practicing Chiropractors’ Committee on Radiology Protocols was developed, and a “Strength Score” based on experimental design and number of participants was assigned to each study (8). Randomized controlled trials were assigned a value of +4.0 towards their Strength Score total. Non-randomized controlled trials received a value of +3.0 points.
Observational studies with controls were given a value of +2.0 points, and observational studies without controls were scored with +1.0 points. Studies with more than 100 participants were given (+0.3) points, between 50 and 100 participants were given (+0.2) points, and less than 50 participants received (+0.1) points. As an example of the scoring system, a randomized controlled trial with 125 participants would receive a total Strength Score of (+4.3) (Table 1).
Table I.
Outcomes reported in all included studies
| Author | Publication year | Type of study | Strength score | Total no. of patients | Positive Intraoperative bile culture n (%) | Surgical site infections n (%) | P value | Delayed gastric emptying n (%) | P value | 90-day mortality N (%) | P value | Postoperative pancreatic hemorrhage n (%) | P value | Intra-abdominal abscess N (%) | P value | Postoperative pancreatic fistula N (%) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parapini et al | 2022 | Retrospective | 1.3 | 162 | 136 (84%) | 77 (56.6%) | .010 | 38 (27.9% | .274 | - | - | - | - | 31 (22.8%) | .137 | ||
| Mohan et al | 2022 | Prospective | 1.1 | 50 | 23 (46%) | 14 (60.8%) | .002 | 17 (73.9%) | .309 | - | - | 3 (13.0%) | 1.06 | - | 9 (31.9%) | .522 | |
| Pretzsch et al | 2021 | Retrospective | 1.3 | 182 | 110 (60%) | 31 (28%) | .001 | 10 (10%) | n.s. | 8 (7%) | n.s. | 9 (9%) | n.s. | 20 (18%) | n.s. | 27 (24%) | .033 |
| Lin et al | 2021 | Prospective | 1.3 | 539 | 433 (80%) | 14 (3.2%) | .780 | - | - | - | - | 74 (17.1%) | < .001 | 110 (25.4%) | .610 | ||
| Bortolotti et al | 2021 | Retrospective | 1.2 | 129 | 69 (53%) | 10 (14.5%) | .850 | - | - | - | - | 0 (0%) | n.s. | 32 (46.4%) | .329 | ||
| Maatman et al | 2020 | Prospective | 1.3 | 162 | 89 (55%) | 11 (13%) | .400 | - | 1 (1%) | .06 | - | - | 32 (36%) | .300 | |||
| Scheufele et al | 2017 | Retrospective | 1.3 | 290 | 189 (65%) | 39 (21%) | .002 | - | 3 (1.6%) | .242 | 31 (16.4%) | 1.00 | 22 (11.6%) | .699 | 20 (10.6%) | 1.000 | |
| Ohgi et al | 2016 | Retrospective | 1.3 | 264 | 151 (57%) | 43 (28%) | < .001 | 27 (18%) | .222 | - | - | 10 (7%) | .026 | 34 (23%) | .804 | 73 (48%) | .534 |
Definitions
This systematic review follows the definitions of POPF and DGE as defined by the International Study Group of Pancreatic Fistula (ISGPF) (Bassi) (9). The ISGPF definitions were used as the updated definitions were published in 2017 (Bassi) (10). Surgical site infections (SSI) were defined according to the standardized American College of Surgeons National Surgical Quality Improvement Program (NSQIP) criteria (11). Hemorrhages and intra-abdominal abscesses were systematically identified. Postoperative mortality was defined as death occurring within 90 days of the procedure.
Statistical Methodology and Risk of Bias Assessment
Meta-analysis was performed to obtain combined estimates across manuscripts for the following six outcomes: postoperative pancreatic fistula, surgical site infections, delayed gastric emptying, postoperative pancreatic hemorrhage, intra-abdominal abscess, and 90-day mortality. The six outcomes are binary, and percentages were extracted from the manuscripts. Sensitivity analyses were performed by excluding potential non-representative manuscripts and investigating any differences in the results. To account for known differences in the design and data collection across studies, a random-effects model is used throughout to account for this known source of study heterogeneity; analysis using the alternative fixed effect model (results not shown) did not lead to any substantive differences in results. Statistical analysis was performed using R statistical software, version 4.1.2.
Results:
Eight articles met the inclusion criteria and were included in the meta-analysis (Figure 1). These studies were largely observational and comparative cohort studies (Table 1). A total of 1,778 patients with an intraoperative bile culture performed were subjects for analysis in these studies. Patients included in this meta-analysis were undergoing pancreaticoduodenectomy procedures. The decision to obtain an intraoperative bile culture was at the physician’s discretion. An IOBC was obtained based on various preoperative and intraoperative events such as antibiotic prophylaxis, placement of a pre-operative bile stent, and transection of the CBD (12), (6), (13), (7), (3), (14), (5), (15).
Microbiological analysis of bile cultures
In a systematic review of the microbiological analysis of bile cultures from the eight studies, Bortolotti et al. found that out of the patients with a positive IOBC (n=69), the most common organism isolated was gram-negative bacilli (57%) and The next most common were Gram-positive cocci (GPC) (43%) (Table 2) (12). Lin et al. not only found that the most common micro-organism, but further concluded that the Candida species, Enterococcus species, Citrobacter species, Streptococcus species, Enterobacter species, and Escherichia coli are significantly associated with IAA (Table 2) (6). Maatman et al. results were similar and found that Enterococcus was associated with an increased incidence of incisional SSI (p=0.01) (Table 2) (13). Mohan et al. findings followed the trend of the studies mentioned above (Table 2) (7). Ohgi et al., Parapini et al. and Pretzsch et al. results differed in that most of their IOBCs were polymicrobial (Table 2), (3), (14), (5). Parapini et al. found that patients with Enterococcus (p=0.005) and Streptococcus (p=0.002) in bile cultures had increased rates of SSI (Table 2) (14). Finally, Scheufele et al. found that the presence of E. faecium and Citrobacter species in the bile was associated with an increased risk of wound infection (Table 2) (15).
Table II.
Top micro-organisms isolated in a positive intraoperative bile culture and postoperative complications associated with specific micro-organisms
| Author | Publication year | Total no. of patients | Positive Intraoperative bile culture n (%) | Polymicrobial growth n (%) | Monomicrobial growth n (%) | Predominant Gram-positive bacteria isolated in IOBC n (%) | Predominant Gram-negative bacteria isolated in IOBC n (%) | Postoperative complication | Micro-organism associated with postoperative complication | Treatment algorithm | Antibiotic Resistance n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parapini et al | 2022 | 162 | 136 (84%) | 100 (74%) | 36 (27%) | Enterococcus 38 (28%), Streptococcus 21 (15%), Staphylococcus 8 (6%), Streptococcus viridans 4 (3%), other gram-positives 4 (3%) | Klebsiella 19 (14%), Enterobacter 16 (12%), E. coli 13 (10%), VRE8 (5%), ESBL 2 (2%), other Gram-negatives 25 (18%) | SSI (SSI and OSI) | Enterococcus species (P = .005), Streptococcus species (P = .002) | Perioperative antibiotic prophylaxis (cefazolin) | 112 (82%) |
| Mohan et al | 2022 | 50 | 23 (46%) | 3 (13%) | 20 (87%) | Enterococcus spp. 7 (30.4%) | E. coli 15 (65.2%), Klebsiella pneumoniae 3 (13%), Citrobacter 1 (4.3%) | - | - | Perioperative antibiotic prophylaxis (piperacillin-tazobactum) and Postoperative antibiotic therapy × 3 postoperative days | - |
| Pretzsch et al | 2021 | 182 | 110 (60%) | - | - | E. faecalis 47 (43%), e. Faecium 35 (32%), Streptococcus spp. 18 (16%), Staphylococcus spp. 12 (11%), e. Casseliflavus 6 (5%), Clostridium spp. 5 (5%), other 18 (16%) | E. coli 21 (19%), Klebsiella spp. 20 (18%), E. cloacae 14 (13%), Hafnia 5 (5%), Pseudomonas aeruginosa 3 (3%) | - | - | Perioperative antibiotic prophylaxis (cefuroxime) | 82 (75%) |
| Lin et al | 2021 | 539 | 433 (80%) | - | - | Enterococcus species 163 (37.6%) | Enterobacter 107 (24.7%), E. coli 102 (23.6%), Klebsiella 83 (19.2%) | IAA (no subclassification) | Candida species (OR = 4.994; 95% CI: [1.837−13.572]; P = .002), Enterococcus species (OR = 4.156; 95% CI: [2.661–6.491]; P < .001), Citrobacter species (OR = 3.376; 95% CI: [1.686–6.762]; P= .001), Streptococcus species (OR = 2.796; 95% CI: [1.502–5.206]; P = .001), Enterbacter species (OR = 2.515; 95% CI: [1.546–4.093]; P < .001), Escherichia coli (OR = 2.333; 95% CI: [1.837–13.572]; P = .002) | Perioperative antibiotic prophylaxis (cefmetazole) and Postoperative antibiotic therapy × 5 postoperative days | - |
| Bortolotti et al | 2021 | 129 | 69 (53%) | 57 (83%) | 12 (17%) | Enterococcus 48 (29%), staphylococcus 6 (4%), Streptococcus 17 (10%) | E. coli 32 (19%), Klebsiella 24 (14%), proteus 4 (2%), Enterobacter 11 (7%), | - | - | Perioperative antibiotic prophylaxis (cefazolin) and postoperative antibiotic therapy (broad-spectrum) | - |
| Maatman et al | 2020 | 162 | 89 (55%) | 57 (64%) | 32 (36%) | Enterococcus 48 (54%), Streptococcus 31 (27%), Lactobacillus 13 (15) | Klebsiella 24 (35%), Enterbacter 17 (19%), E. coli 16 (18%) | SSI (incisional) | Enterococcus species (P = .01) | Perioperative antibiotic prophylaxis (ceftriaxone or metronidazole) | 14 (16%) |
| Scheufele et al | 2017 | 290 | 189 (65%) | - | - | Enterococcus species 100 (52%), Streptococcus species 21 (11%) | E. coli 42 (22%), Enterobacter cloacae 34 (18%), Citrobacter 18 (9.5%) | SSI (superficial and deep infections) | Enterococcus faecium (OR = 2.83; 95% Cl: [1.17–6.84], Citrobacter species (OR = 5.09; 95% [1.65–15.71] | Perioperative antibiotic prophylaxis (ampicillin-sulbactam) and postoperative antibiotic therapy (piperacillin-tazobactam) × 7 days | 107 (57%) |
| Ohgi et al | 2016 | 264 | 151 (57%) | 101 (67%) | 50 (33%) | Enterococcus species 69 (46%), Streptococcus 54 (36%), Staphylococcus 20 (13%), MRSA 3 (2%) | Klebsiella species 59 (39%), Enterobacter species 31 (21%), Escherichia 24 (16%), Citrobacter 22 (15%), Pseudomonas species 6 (4%), Bacteroides species 6 (4%), Serratia species 4 (3%), Candida species 13 (9%) | - | - | Perioperative antibiotic prophylaxis (cephalosporin) | - |
ESBL, xxx: IAA, intra-abdominal abscesses; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio: OSI, operative site infection; SSI, surgical site infection.
Treatment algorithm
Out of the eight studies evaluated, four treated patients with perioperative antibiotic prophylaxis (14), (5, 13), (3). The other four studies used postoperative antibiotic therapy in addition to perioperative antibiotic prophylaxis (12), (6), (7), (15). The postoperative antibiotic selection was guided by the results of the IOBC. The selection of antibiotic and duration of postoperative treatment was institution-specific (Table 2).
Surgical Site infections
Surgical site infections were defined in all eight studies; however, these definitions varied. Three studies reported surgical site infections as “wound infections” (3), (5), (15). Four studies reported surgical site infections as SSIs (6), (13), (7), (14). Maatman et al. further subclassified SSIs into incisional SSIs and organ-space SSIs. The two sub-categories were combined Field (12) for the statistical analysis. And one study referred to SSI as an “intra-abdominal infection” (Bortolotti) (12). All 1,778 patients from all eight studies were screened for developing an SSI with an IOBC. 1200 out of 1778 patients had a positive IOBC. Of those 1200 patients, 239 patients went on to develop an SSI post-operation. 578 out of 1,778 patients had a normal IOBC. 49 of those 578 patients developed an SSI post-operation (Table 1). On meta-analysis, a patient with a positive IOBC was 2.33 more times likely to develop an SSI than a patient with a negative IOBC (RR=2.33, 95% CI 1.47–3.69, p < 0.01) (Figure 2). The heterogeneity of the study was I2=37% and the trend of the data showed that a patient with a positive IOBC was more likely to develop an SSI (Figure 2). Overall, three out of the eight studies evaluated showed that there is no association between a positive IOBC and a patient developing a SSI (Table 1) (12), (6), (13). However, the remaining five studies (7), (3), (14), (5), (15), and this meta-analysis showed that there is a significant association (Table 1).
Figure 2.
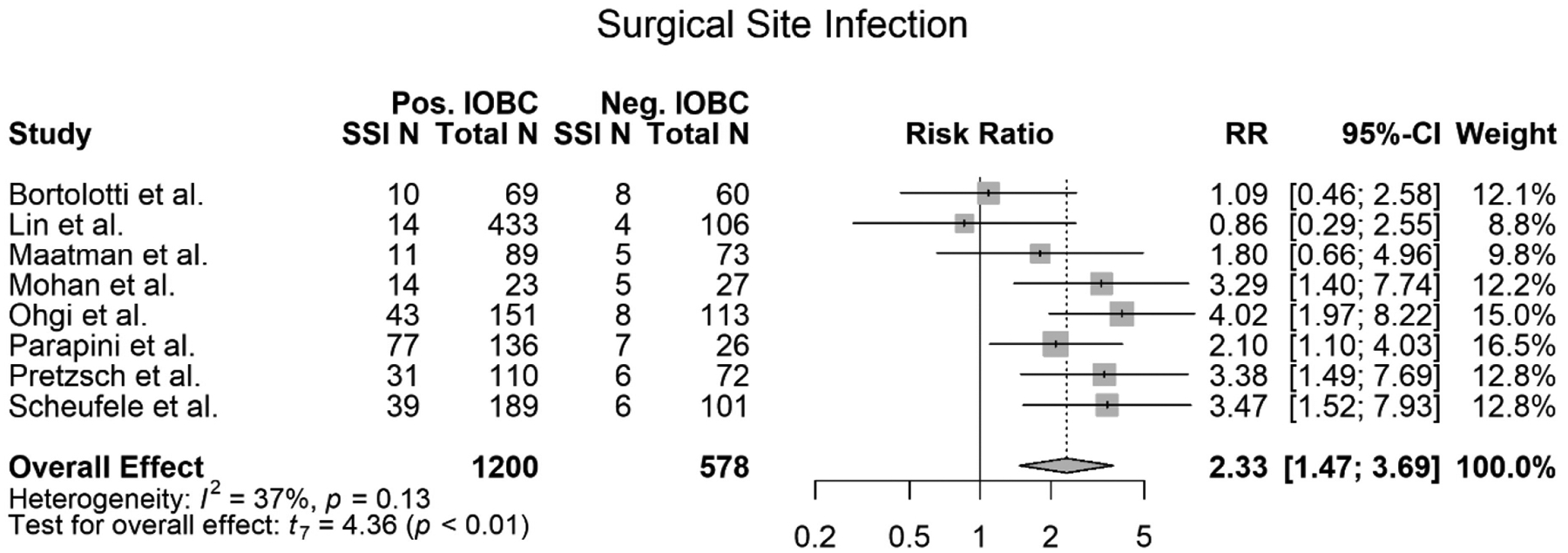
Forest plot of meta-analysis of surgical site infections (SSI) association with a positive IOBC.
Delayed Gastric Emptying
Four out of the eight studies included in this meta-analysis met the criteria to be evaluated for DGE (7), (3), (14), (5). All four of these studies relied upon the same definition for DGE. 658 total patients were screened for the developing DGE. Of those 658 patients, 420 patients had a positive IOBC. Of those 420 patients with positive IOBCs, 92 developed DGE. Of those 658 screened patients, 238 had a normal IOBC. Of those 238 patients, 44 patients developed DGE. The meta-analysis showed that there was no difference between a positive and normal IOBC being able to predict a DGE (RR=1.23, 95% CI 0.63–2.38, p=0.40) (Figure 3). The meta-analysis also showed a mildly high heterogeneity (I2=28). The trend of the data did show that a patient with a positive IOBC was more likely to develop DGE however, the data was not significant (Figure 3). All four studies evaluated for DGE agreed with the findings of this meta-analysis that there was no association between a positive IOBC and the patient developing DGE (Table 1) (7), (3), (14), (5).
Figure 3.
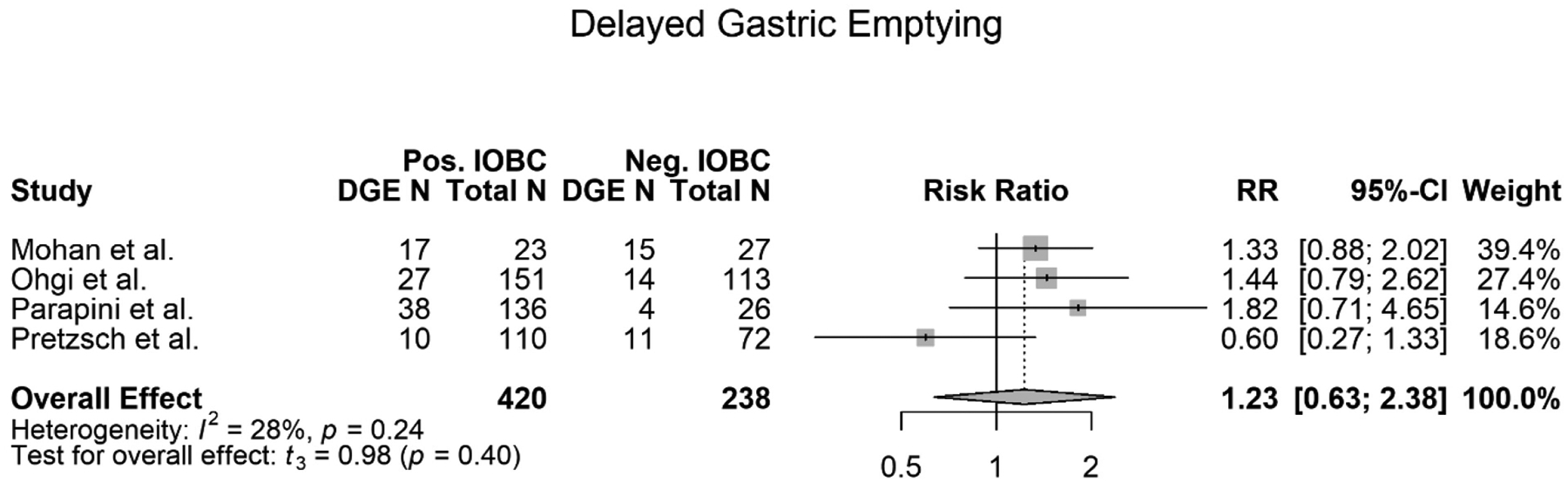
Forest plot of meta-analysis of delayed gastric emptying (DGE) association with a positive IOBC.
90-day Mortality
Three of the eight studies included in this meta-analysis met the criteria to be evaluated for an association between a positive IOBC and an increase in 90-day mortality (13), (5), (15). Due to the high heterogeneity of this analysis (I2=66%), lack of power, as well as the insignificance of this data, the figure will not be included with the other figures and instead will be a supplemental figure (Supplemental Figure 1). All three of the studies relied on the same definition of 90-day mortality. 634 total patients were evaluated across these three studies. Of the 634 total patients, 388 patients had a positive IOBC; of those 388 patients, 12 died within 90 days. Of the 634 total patients, 246 patients had a normal IOBC and of those 246 patients, 10 died within 90 days. The meta-analysis showed that there was no association between a positive IOBC and 90-day mortality (RR=0.66, 95% CI 0.01–52.76, p=0.74) (Supplemental Figure 1). Our meta-analysis and the three studies all concluded that there was no association between a positive IOBC and an increase in 90-day mortality (Table 1) (13), (5), (15).
Postoperative Pancreatic Hemorrhage
Four of the eight studies included in this meta-analysis met the criteria to be evaluated for positive IOBCs association with a POPH (3), (5), (15), (7). 786 total patients across these four studies were evaluated for an association between a positive IOBC and POPH. Of the 786 total patients, 473 patients had a positive IOBC. Of those 473 patients, 53 patients developed POPH. Of the 786 total patients, 313 patients had a normal IOBC. Of those 313 patients, 23 patients developed a POPH. The meta-analysis showed no association between a positive IOBC and developing a POPH (RR=1.70, 95% CI 0.33–8.74, p=0.38) (Figure 4). Three of the four studies evaluated agreed with the findings of this meta-analysis (Table 1) (7), (5), (15). However, Ohgi et al. disagreed with these findings and stated that there is an association between a positive IOBC and a patient developing a POPH (Table 1) (3).
Figure 4.
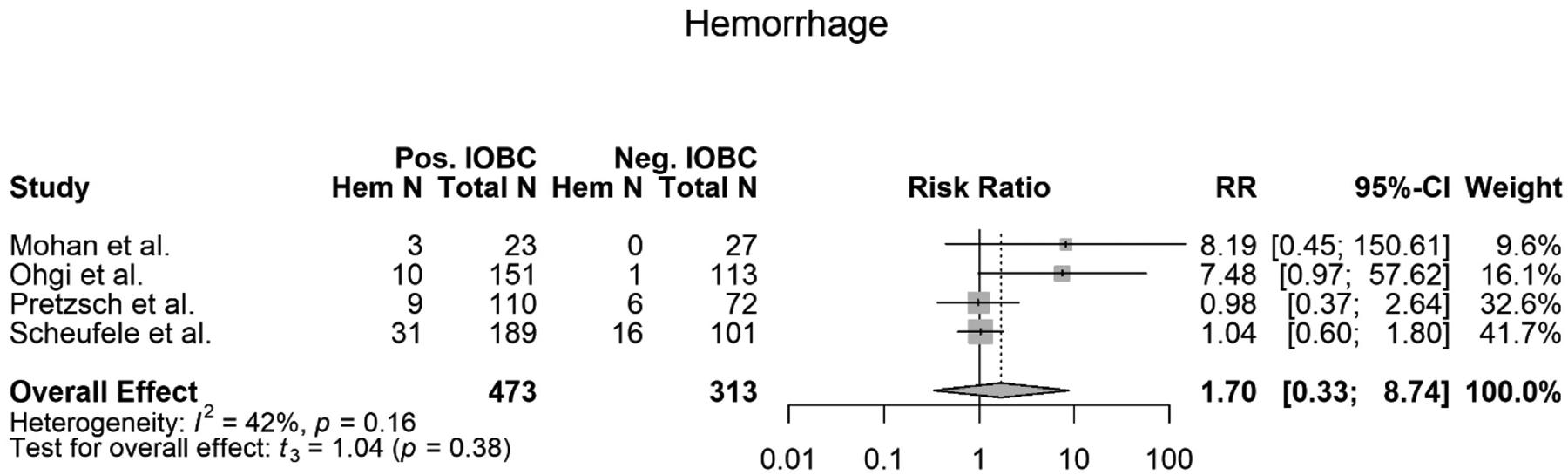
Forest plot of meta-analysis of postoperative pancreatic hemorrhage’s (POPH) association with a positive IOBC.
Intra-abdominal Abscess
Five out of the eight studies evaluated in this meta-analysis met the criteria to be evaluated for an association between a positive IOBC and developing an IAA (12), (6), (3), (5), (15). All five studies used the same definition for an IAA. From the five studies, 1404 total patients were evaluated for an association between a positive IOBC and developing IAA. Of those 1404 patients, 952 patients had a positive IOBC. Of those 952 patients, 150 patients developed an IAA. Out of the 1404 patients, 452 patients had a normal IOBC. Of those 452 patients, 43 patients developed an IAA. The meta-analysis showed that there was no association between a positive IOBC and developing an IAA (RR=1.70, 95% CI 0.38–7.56, p=0.38) (Figure 5). The meta-analysis also showed high heterogeneity (I2=57%) with a slight trend showing that a positive IOBC was associated with a patient developing an IAA. However, the data was not significant and did not support this trend (Figure 5). Overall, out of the five studies evaluated, all except one study concluded that there was no association between a positive IOBC and a patient developing an IAA (Table 1) (12), (3), (5), (15). Lin et al. disagreed with these findings and concluded that there is an association between a positive IOBC and a patient developing an IAA (Table 1) (6).
Figure 5.
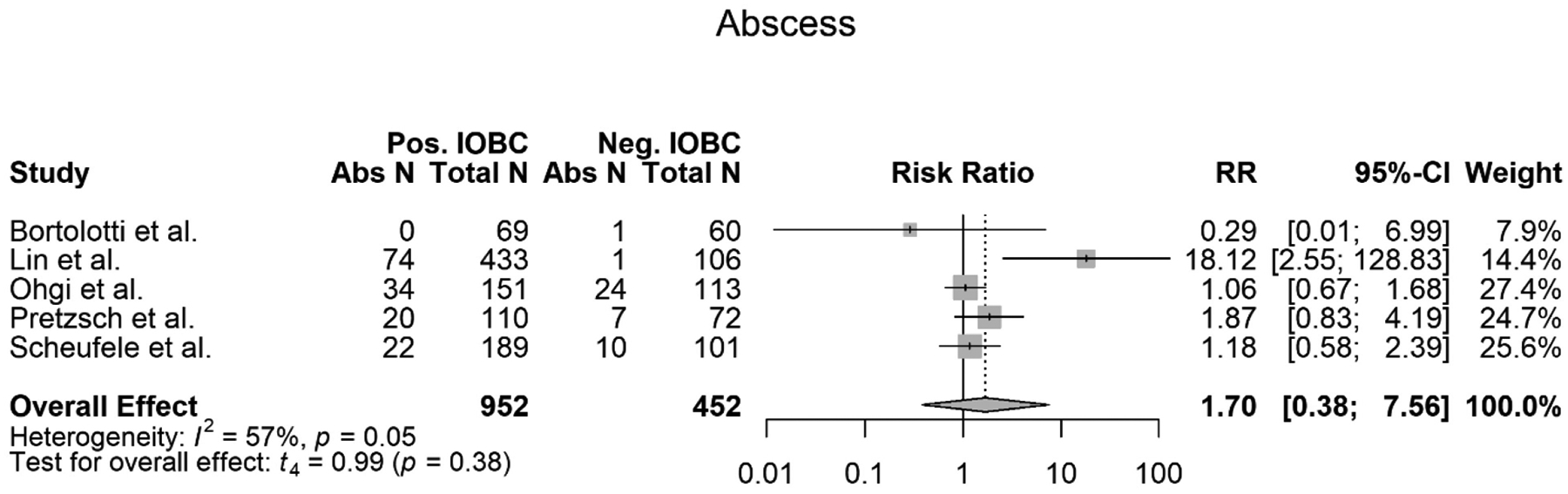
Forest plot of meta-analysis of intra-abdominal abscess’s (IAA) association with a positive IOBC.
Postoperative Pancreatic Fistula
All eight studies included in this meta-analysis met the criteria to be evaluated for an association between a positive IOBC and a patient developing a POPF (12), (6), (13), (7), (3), (14), (5), (15). All eight studies used the same definition for a POPF. However, some studies further subclassified the POPFs into “Clinically relevant POPF” (CR-POPF), POPF grade B, and POPF grade C (13), (3), (14), (5). For this meta-analysis, the POPF (all grades) data were combined for statistical analysis. From the eight studies, all 1,778 patients were evaluated. Out of the total 1,778 patients, 1200 patients had a positive IOBC. Of those 1200 patients, 334 patients went on to develop a POPF. Of the total 1,778 patients, 578 patients had a normal IOBC. Of those 578 patients, 190 patients developed a POPF. A meta-analysis of the eight studies showed that there was no association between a positive IOBC and developing a POPF (RR=0.97, 95% CI 0.72–1.32, p=0.84) (Figure 6). The meta-analysis also showed high heterogeneity (I2=54%) with a slight trend showing that a positive IOBC was not associated with a patient developing a POPF. The data supported this trend, and no association was established (Figure 6). Overall, seven of the eight studies, as well as this meta-analysis, found that there was no association between a positive IOBC and a patient developing a POPF (Table 1) (12), (6), (13), (7), (3), (14), (15). Pretzsch et al. reported that there was an association between a positive IOBC and a CR-POPF, however, the authors did note that the CR-POPF data reported was higher than the institutional average due to selection bias (Table 1) (5). It is also important to note that although Ohgi et al. did not find an association between a positive IOBC and a patient developing a POPF overall, there was an association between a positive IOBC and a patient developing a POPF (grades B/C) (p=0.025) (3). In addition a few of these papers reviewed the incidence of post-operative bilirubin and there was no correlation to positive intra-operative bile cultures and post-operative bilirubin.
Figure 6.
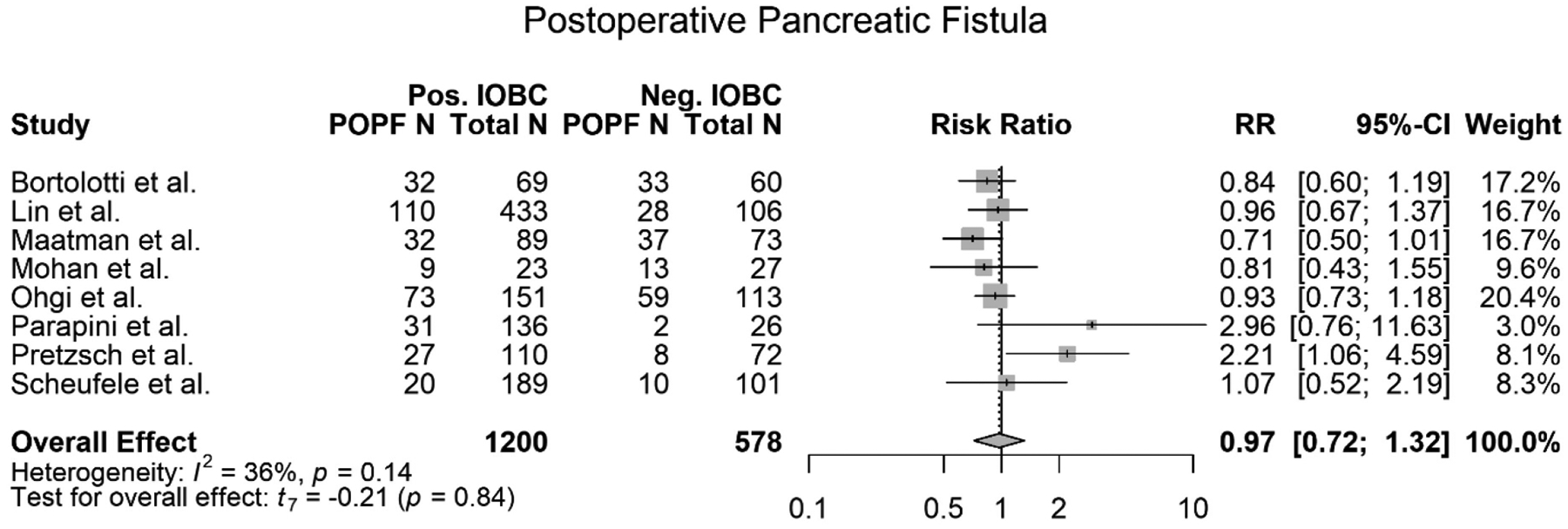
Forest plot of meta-analysis of postoperative pancreatic fistula’s (POPF) association with a positive IOBC.
Antibiotic Resistance
The role and levels of antibiotic resistance needs to also be considered as this is a key factor affecting postoperative morbidity and mortality. Four of the eight studies evaluated reported the antibiotic resistance found via the IOBCs. Parapini et al. found that 82% of the positive IOBCs (136) were resistant to the prophylactic antibiotics used (Table 2) (14). Pretzsch et al. found that 75% of the positive IOBCs (110) were resistant (Table 2) (5). Maatman et al. found a significantly less antibiotic resistance as only 16% of the 89 IOBCs were resistant (Table 2) (13) and Scheufele et al. found 57% of the positive IOBCs (189) were resistant to the first line prophylactic antibiotics.
Discussion:
Over the past decades, improvements in perioperative care have resulted in a decline in mortality rates and morbidity rates after pancreaticoduodenectomy (16–18). A decrease in mortality and morbidity rates result in improving the patients’ quality of life as well decreasing the cost of healthcare and length of hospital stay. Unfortunately, post-operative complications can arise and result in worse patient outcomes, increase cost of healthcare and hospital stay (13). Although the present study does not support a significant relationship between microbiology growth and 5 out of the 6 postoperative complications evaluated (IAA, POPH, POPF, DGE, 90-day mortality), it does show a positive relationship between relationship between microbiology growth and the patient developing a SSI post-operatively.
The IOBC’s use to guide perioperative infection management in pancreaticoduodenectomy has been documented, as the organism found in the IOBC can help guide physicians to select the appropriate antibiotics (1, 7, 19). Some studies have shown that the presence of bacteria in the biliary tract is associated with increased risk for post-operative complications, specifically intra-abdominal and wound infections following pancreaticoduodenectomy (20–22). However, there is debate about the impact of bacteria in the biliary tract on specific post-operative complications given the contradictory results of some studies. Some studies have shown that a microbiology growth from an IOBC is associated with the development of a postoperative pancreatic fistula (3). However, several other studies have shown that there is no association, including this meta-analysis (5, 13, 23). The development of a SSI is the only postoperative complication that has been repeatedly shown to have a positive association with microbiology grown via an IOBC (3, 15, 23–25). The findings of this meta-analysis agreed with these studies and add to the evidence that a positive IOBC is positively correlated with a patient developing a SSI post-operatively only.
Conclusion:
In conclusion, the cumulative data from this systematic review suggests that a positive intraoperative bile culture has no association with predicting the postoperative complications of delayed gastric emptying, 90-day mortality, postoperative pancreatic hemorrhage, intra-abdominal abscess, or postoperative pancreatic fistula. However, this meta-analysis as well as five out of the eight studies included in this meta-analysis found an association between a positive IOBC and a patient developing a SSI. Further studies are warranted to examine the findings of this study.
Supplementary Material
Figure 1. Forest plot of meta-analysis of 90-day mortality associated with a positive IOBC.
Highlights.
A patient with a positive intraoperative bile culture is 2.33 more times likely to develop a surgical site infection than a patient with a normal intraoperative bile culture.
A positive IOBC had no association with 5 out of the 6 postoperative complications evaluated (Delayed Gastric Emptying, 90-day Mortality, Postoperative Pancreatic Hemorrhage, Intra-abdominal Abscess, and Postoperative Pancreatic Fistula).
Some of the most common Microbes isolated in IOBC were Enterococcus species, E. Coli, and Klebsiella species
Funding/Financial Support:
NIH R25 CA134283-06A1
Abbreviations
- PCA
Pancreatic Cancer
- PD
pancreaticoduodenectomy
- POPF
Postoperative Pancreatic Fistula
- DGE
Delayed Gastric Emptying
- SSI
Surgical Site Infections
- IAA
Intra-abdominal abscess
- POPH
Postoperative Pancreatic Hemorrhage
- IOBC
Intraoperative Bile Cultures
- CI
confidence interval
- RR
risk ratio
- RCTs
randomized controlled trials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
This study did not require ethical approval since it was a review of published articles and did not directly involve the use of human or animal subjects.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
References
- 1.Augenstein VA, Reuter NP, Bower MR, McMasters KM, Scoggins CR, Martin RC. Bile cultures: a guide to infectious complications after pancreaticoduodenectomy. J Surg Oncol. 2010;102(5):478–81. [DOI] [PubMed] [Google Scholar]
- 2.Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260(2):372–7. [DOI] [PubMed] [Google Scholar]
- 3.Ohgi K, Sugiura T, Yamamoto Y, Okamura Y, Ito T, Uesaka K. Bacterobilia may trigger the development and severity of pancreatic fistula after pancreatoduodenectomy. Surgery. 2016;160(3):725–30. [DOI] [PubMed] [Google Scholar]
- 4.Pitt HA, Postier RG, Cameron JL. Biliary bacteria: significance and alterations after antibiotic therapy. Arch Surg. 1982;117(4):445–9. [DOI] [PubMed] [Google Scholar]
- 5.Pretzsch E, Heim A, Heiliger C, Pretzsch CM, Ilmer M, Weniger M, et al. Specific intraoperative antibiotic therapy abrogates the negative effect of biliary contamination on the Comprehensive Complication Index after pancreatic head resection. Surgery. 2021. [DOI] [PubMed] [Google Scholar]
- 6.Lin YJ, Ho TW, Wu CH, Kuo TC, Yang CY, Wu JM, et al. Specific Bile Microorganisms Caused by Intra-Abdominal Abscess on Pancreaticoduodenectomy Patients: A Retrospective Cohort Study. Curr Oncol. 2021;29(1):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohan A, Gupta R, Yadav TD, Gupta V, Sharma V, Mandavdhare H, et al. Association of Intra-Operative Bile Culture with Post-Operative Complications after Pancreaticoduodenectomy. Surg Infect (Larchmt). 2022;23(4):351–6. [DOI] [PubMed] [Google Scholar]
- 8.National Guideline C. Practicing Chiropractors’ Committee on Radiology Protocols (PCCRP) for biomechanical assessment of spinal subluxation in chiropractic clinical practice Rockville MD: Agency for Healthcare Research and Quality (AHRQ); [Available from: http://www.guideline.gov/content.aspx?id=14576. [Google Scholar]
- 9.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. [DOI] [PubMed] [Google Scholar]
- 10.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161(3):584–91. [DOI] [PubMed] [Google Scholar]
- 11.Selby LV, Sjoberg DD, Cassella D, Sovel M, Weiser MR, Sepkowitz K, et al. Comparing surgical infections in National Surgical Quality Improvement Project and an Institutional Database. J Surg Res. 2015;196(2):416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bortolotti P, Delpierre C, Le Guern R, Kipnis E, Lebuffe G, Lenne X, et al. High incidence of postoperative infections after pancreaticoduodenectomy: A need for perioperative anti-infectious strategies. Infect Dis Now. 2021;51(5):456–63. [DOI] [PubMed] [Google Scholar]
- 13.Maatman TK, Weber DJ, Qureshi B, Ceppa EP, Nakeeb A, Schmidt CM, et al. Does the Microbiology of Bactibilia Drive Postoperative Complications After Pancreatoduodenectomy? J Gastrointest Surg. 2020;24(11):2544–50. [DOI] [PubMed] [Google Scholar]
- 14.Parapini ML, Skipworth JRA, Mah A, Desai S, Chung S, Scudamore CH, et al. The association between bacterobilia and the risk of postoperative complications following pancreaticoduodenectomy. HPB (Oxford). 2022;24(2):277–85. [DOI] [PubMed] [Google Scholar]
- 15.Scheufele F, Aichinger L, Jager C, Demir IE, Schorn S, Sargut M, et al. Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br J Surg. 2017;104(2):e182–e8. [DOI] [PubMed] [Google Scholar]
- 16.Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232(6):786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236(3):355–66; discussion 66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phan GQ, Yeo CJ, Hruban RH, Littemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors: Review of 125 patients. J Gastrointest Surg. 1998;2(5):473–82. [DOI] [PubMed] [Google Scholar]
- 19.Saleh MM, Norregaard P, Jorgensen HL, Andersen PK, Matzen P. Preoperative endoscopic stent placement before pancreaticoduodenectomy: a meta-analysis of the effect on morbidity and mortality. Gastrointest Endosc. 2002;56(4):529–34. [DOI] [PubMed] [Google Scholar]
- 20.Heslin MJ, Brooks AD, Hochwald SN, Harrison LE, Blumgart LH, Brennan MF. A preoperative biliary stent is associated with increased complications after pancreatoduodenectomy. Arch Surg. 1998;133(2):149–54. [DOI] [PubMed] [Google Scholar]
- 21.van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362(2):129–37. [DOI] [PubMed] [Google Scholar]
- 22.Povoski SP, Karpeh MS Jr., Conlon KC, Blumgart LH, Brennan MF. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999;230(2):131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mussle B, Hempel S, Kahlert C, Distler M, Weitz J, Welsch T. Prognostic Impact of Bacterobilia on Morbidity and Postoperative Management After Pancreatoduodenectomy: A Systematic Review and Meta-analysis. World J Surg. 2018;42(9):2951–62. [DOI] [PubMed] [Google Scholar]
- 24.Limongelli P, Pai M, Bansi D, Thiallinagram A, Tait P, Jackson J, et al. Correlation between preoperative biliary drainage, bile duct contamination, and postoperative outcomes for pancreatic surgery. Surgery. 2007;142(3):313–8. [DOI] [PubMed] [Google Scholar]
- 25.Cortes A, Sauvanet A, Bert F, Janny S, Sockeel P, Kianmanesh R, et al. Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J Am Coll Surg. 2006;202(1):93–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Forest plot of meta-analysis of 90-day mortality associated with a positive IOBC.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


