Abstract
Objectives—
Patellar tendon injuries occur via various mechanisms such as overuse, or due to surgical graft harvest for anterior cruciate ligament reconstruction (ACLR). Quantified patellar tendon stiffness after injury may help guide clinical care. Continuous shear wave elastography (cSWE) allows for the assessment of viscosity and shear modulus in tendons. The reliability of the measure, however, has not been established in the patellar tendon. The purpose of this study was to investigate the interrater reliability, intrarater reliability, and between-day stability of cSWE in both healthy and pathological patellar tendons.
Methods—
Participants with patellar tendinopathy (n = 13), history of ACLR using bone-patellar tendon-bone autograft (n = 9), and with no history of patellar tendon injury (n = 13) were recruited. cSWE was performed 4 times by multiple raters over 2 days. Intraclass correlations (ICC) and minimum detectable change (MDC95%) were calculated.
Results—
Good to excellent between-day stability were found for viscosity (ICC = 0.905, MDC95% = 8.3 Pa seconds) and shear modulus (ICC = 0.805, MDC95% = 27.4 kPa). The interrater reliability measures, however, were not as reliable (ICC = 0.591 and 0.532).
Conclusions—
cSWE is a reliable assessment tool for quantifying patellar tendon viscoelastic properties over time. It is recommended, however, that a single rater performs the measure as the interrater reliability was less than ideal.
Keywords: anterior cruciate ligament, bone-patellar tendon-bone graft, continuous shear wave elastography, patellar tendon, tendinopathy, viscoelastic properties
Patellar tendon injuries are common amongst athletes of all levels (recreational to professional) and age groups, and may become a significant burden for those who suffer such injuries.1,2 One of the themes observed across pathological tendons, regardless of the source of injury, are morphological alterations (eg, tendon thickening, larger cross-sectional areas, or hypoechoic regions identified on sonographic evaluations) to the tendon.3 Tendon structure is associated with function and outcomes after injury, and may be readily assessed in a clinical setting.4–8 Evaluating the structural changes in the injured patellar tendon may assist in identifying the underlying pathophysiology as well as guide clinical care after injury.
Patellar tendinopathy (PT) is an overuse injury of the patellar tendon typically associated with activities involving plyometrics.9,10 Prevalence of PT has been reported as high as 45% in higher level jumping athletes.2 Tendon injury may also be of iatrogenic nature after procedures such as graft harvest of the bone-patellar tendon-bone (BPTB) autograft during anterior cruciate ligament reconstruction (ACLR).11 The BPTB autograft, harvested from the central third of the patellar tendon, is frequently used due to the low re-rupture rates reported in active young adults compared to alternative graft types.12,13 The use of a BPTB autograft, however, comes with secondary impairments specific to the graft harvest site. Prolonged quadriceps weakness,14,15 a metric associated with successful outcomes after anterior cruciate ligament surgery has been identified,16 likely because of the additional trauma to the extensor mechanism. Anterior knee pain also persists after using BPTB autografts17,18 and rates of post-traumatic knee osteoarthritis may also be elevated.19,20 In both populations described above, understanding the injured tendon’s structure may assist in improving our knowledge on the underlying pathophysiology and assist in guiding postinjury or postoperative care to optimize outcomes.
Morphological (eg, thickness and cross-sectional area) changes to injured tendons assessed using B-mode ultrasound and magnetic resonance imaging have been observed with time after surgery and rehabilitation in patients after tendon pathology.5,21–24 It is also known, however, that improvements in symptoms and function can happen without morphological changes.25 The variability and mismatch in morphological and symptom presentation along the course of rehabilitation, may in part be due to changes in the underlying viscoelastic properties (ie, stiffness) of the tendon which may not be captured with traditional imaging modalities.7,26–29 Assessing viscoelastic properties of the patellar tendon may provide additional knowledge beyond measuring morphology alone when identifying pathological tendons and tracking changes over time.
Methods using shear wave elastography to quantify stiffness in the musculoskeletal system have become increasingly popular in recent years due to the low risk and noninvasive approach to quantify and track tissue mechanical properties.29–32 Continuous shear wave elastography (cSWE) is an ultrasound-based method to evaluate tendon viscoelastic properties as biomarkers for injury and recovery.33 cSWE involves measuring wave speeds of shear waves transduced through a tendon using an external actuator34–36 to calculate two coefficients of interest in viscoelastic materials, shear modulus (kPa) and viscosity (Pa seconds). The method has been validated in the Achilles tendon and demonstrated fair to excellent intrarater reliability.37 The reliability of cSWE in the patellar tendon, tendons with pathology (tendinopathy or graft harvest), or the interrater reliability of cSWE, however, has not been established. The purpose of this study was to investigate the interrater reliability, intrarater reliability, and between-day stability of cSWE in both healthy and pathological patellar tendons.
Materials and Methods
Participants
An a priori power analysis was completed using methods based on a lower acceptable limit.38 Fourteen participants were required with two raters, a desired reliability of ICC = 0.9, lower acceptable limit of ICC = 0.6, β = 0.8, and α = 0.05. Thirteen participants with PT, 9 participants with a history of BPTB graft harvest for ACLR, and 13 participants with no current or history of patellar tendon injury (Healthy) were recruited for this study (Table 1). Participants in the tendinopathy group all had symptomatic PT confirmed by a licensed physical therapist (A.S.L.) at the time of data collection, and the participants in the BPTB group were included in the study regardless of patellar tendon symptoms at the time of testing. This study was approved by the institutional review board at the University of Delaware and participants provided written informed consent.
Table 1.
Participant Demographics by Group
| Group | Sex (Women:Men) | Age (Years) | Height (m) | Weight (kg) | Symptom Duration, Months (Range) | Time From Surgery, Months (Range) |
|---|---|---|---|---|---|---|
|
| ||||||
| PT | 4:9 | 31.2 ± 9.4 | 1.79 ± 0.10 | 80.8 ± 17.0 | 33.8 (1.0–93.1) | – |
| BPTB | 5:4 | 22.9 ± 5.6 | 1.73 ± 0.13 | 72.7 ± 19.9 | – | 28.2 (1.6–91.7) |
| Healthy | 6:7 | 26.2 ± 2.4 | 1.76 ± 0.09 | 76.0 ± 8.4 | – | – |
| All | 15:20 | 27.2 ± 7.2 | 1.76 ± 0.11 | 76.9 ± 15.2 | – | – |
PT, patellar tendinopathy; BPTB, bone-patellar tendon-bone graft; Healthy, healthy control; All, all participants combined.
Study Design
cSWE was performed by 4 different raters (Table 2) of varying levels of experience performing cSWE. Rater A, the most experienced rater, and rater B, the rater with the least amount of experience who received 2 hours of training for familiarization of the methods performed all data collections for the PT group. Rater C and D performed all data collections for the healthy and BPTB group. Rater A and C were assigned as the primary rater to compare for intrarater reliability and between-day stability, while measurements from rater B and D were assigned as secondary raters used to calculate interrater reliability.
Table 2.
Clinical and Ultrasound Experience of Each Rater
| Raters | Clinical Credentials | Years Since Licensure | Experience With Ultrasound Imaging | Experience With cSWE |
|---|---|---|---|---|
|
| ||||
| A | Physical therapist | 4 years | 4 years | 4 years |
| B | None | – | No experience | 2 hours |
| C | Physical therapist | 1 year | 2 years | 1 year |
| D | Physical therapist | 12 years | 2 years | 1 year |
cSWE was performed in two sessions approximately 24 hours apart (Figure 1). Participants were encouraged to avoid any strenuous exercise 24 hours prior to each test session. On day 1, the primary raters (A and C) performed 1 round of cSWE. On day 2, the primary raters performed 2 rounds of cSWE followed by the secondary raters (B and D) performing 1 round of cSWE. Between trials on day 2, participants were asked to stand up and walk a single loop around the room to reset positioning for the test, and all markings indicated on the participants’ knees were removed using alcohol wipes (Figure 2). The patellar tendon of interest (right versus left) was determined using the surgical knee for the BPTB group, the symptomatic side (or the most symptomatic side if the participant experienced bilateral symptoms) for the tendinopathy group, and a random number generator was used to choose the side of interest in the healthy control group.
Figure 1.
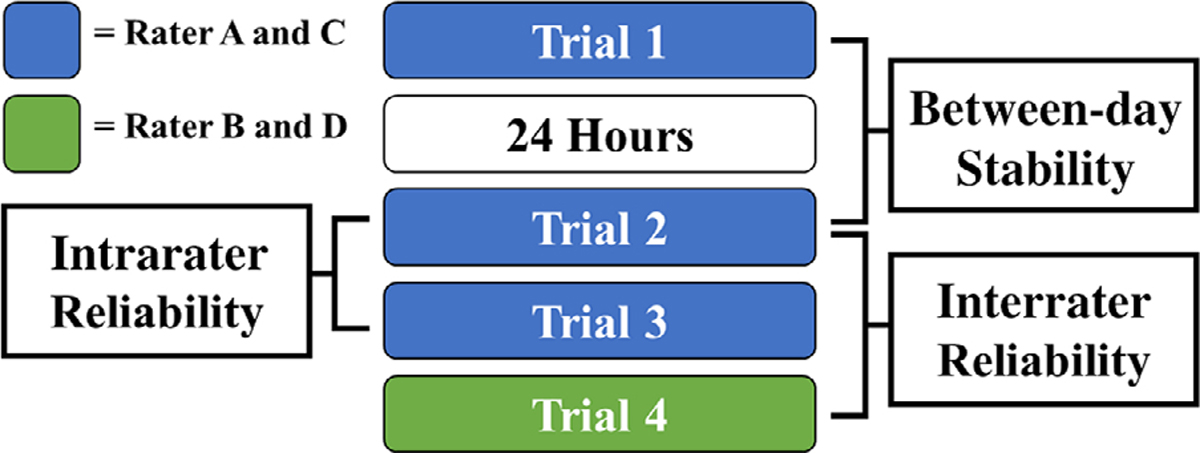
Reliability measures of interest. Inter- and intrarater reliability was assessed within the same day and between-day intrarater stability was assessed 24 hours apart.
Figure 2.
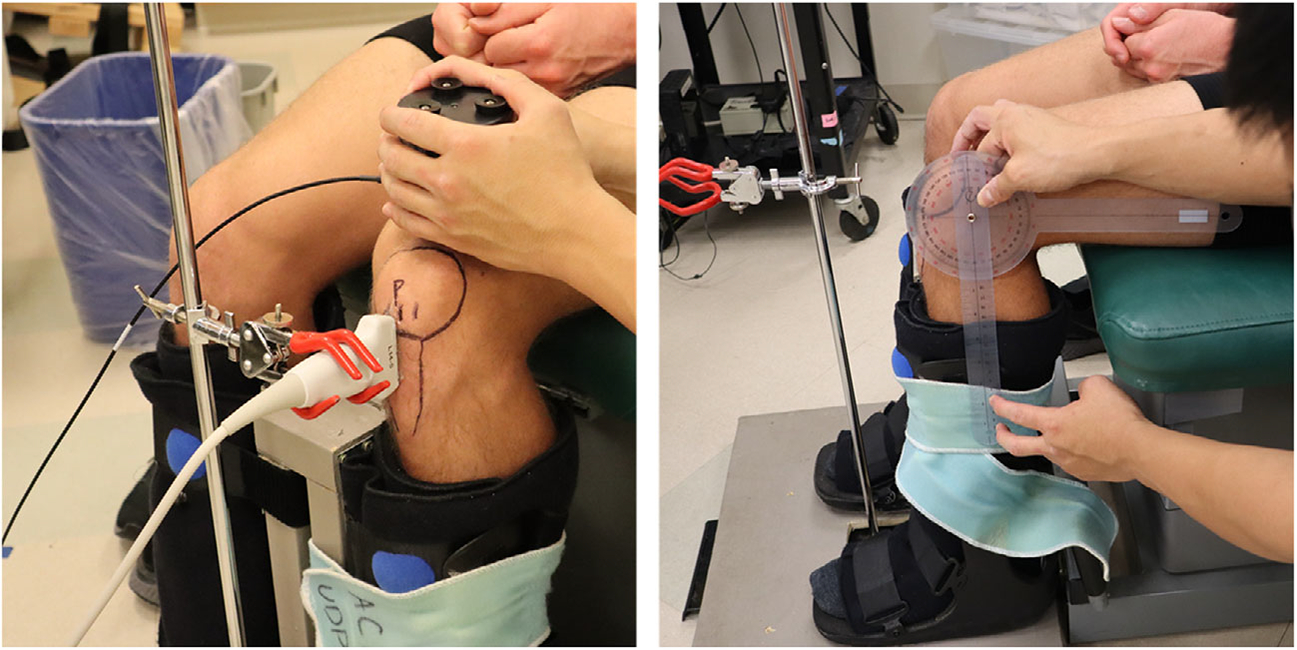
Continuous shear wave elastography (cSWE) setup. Participants were seated with their hips and knees at 90° of flexion with their lower leg stabilized in a custom boot (right). The ultrasound probe was placed on a custom clamp after the region of interest was determined, and the external actuator was placed on the quadriceps tendon above the knee held by the rater (left).
Continuous Shear Wave Elastography
Participants were positioned in an upright chair with both hips and knees position at 90° of flexion. Both feet were strapped in a custom setup made of controlled ankle motion boots for stabilization and to limit muscle contractions and movement artifact (Figure 2). The region of interest over the patellar tendon was marked using a marker after inspecting the patellar tendon using ultrasound imaging (Figure 2). For the BPTB group, the region of interest was over the central third where the graft was harvested. For the tendinopathy group, the measurement was taken 1 cm distal to the inferior pole of the patella. For the healthy control group, the patellar tendon was trisected vertically, and the central third of the patellar tendon was measured. An ultrasound scanner (Ultrasonix, Vancouver, BC, Canada) with a L14-5/38 probe was used to capture raw radiofrequency data over the region of interest (6438 frames per second), while an external actuator (Minshaker Type 4810, Bruel and Kjaer, Norcross, GA, USA) was placed over the quadriceps tendon just superior to the patella to produce shear waves. The ultrasound probe was placed parallel with the longitudinal axis of the region of interest and stabilized using a 3-prong clamp (Figure 2).
Shear waves at 11 fixed frequencies (322, 339, 358, 379, 402, 429, 460, 495, 536, 585, and 643 Hz) were produced at the quadriceps tendon while the raw radiofrequency data were collected simultaneously (Figure 3). All 11 frequencies were produced and captured independently while the knee and ultrasound probe were secured in the same position. Ultrasound data were collected for 10 msec for each of the frequencies. Three successful trials were collected during each test while the ultrasound probe was removed and repositioned between each trial to confirm proper contact and placement over the region of interest. A custom MATLAB script was used to calculate static shear modulus and viscosity using data from all 11 frequencies using the Voigt model for viscoelasticity which has been detailed in previous work.33 The average shear modulus and viscosity of the three trials collected were calculated and reported.
Figure 3.
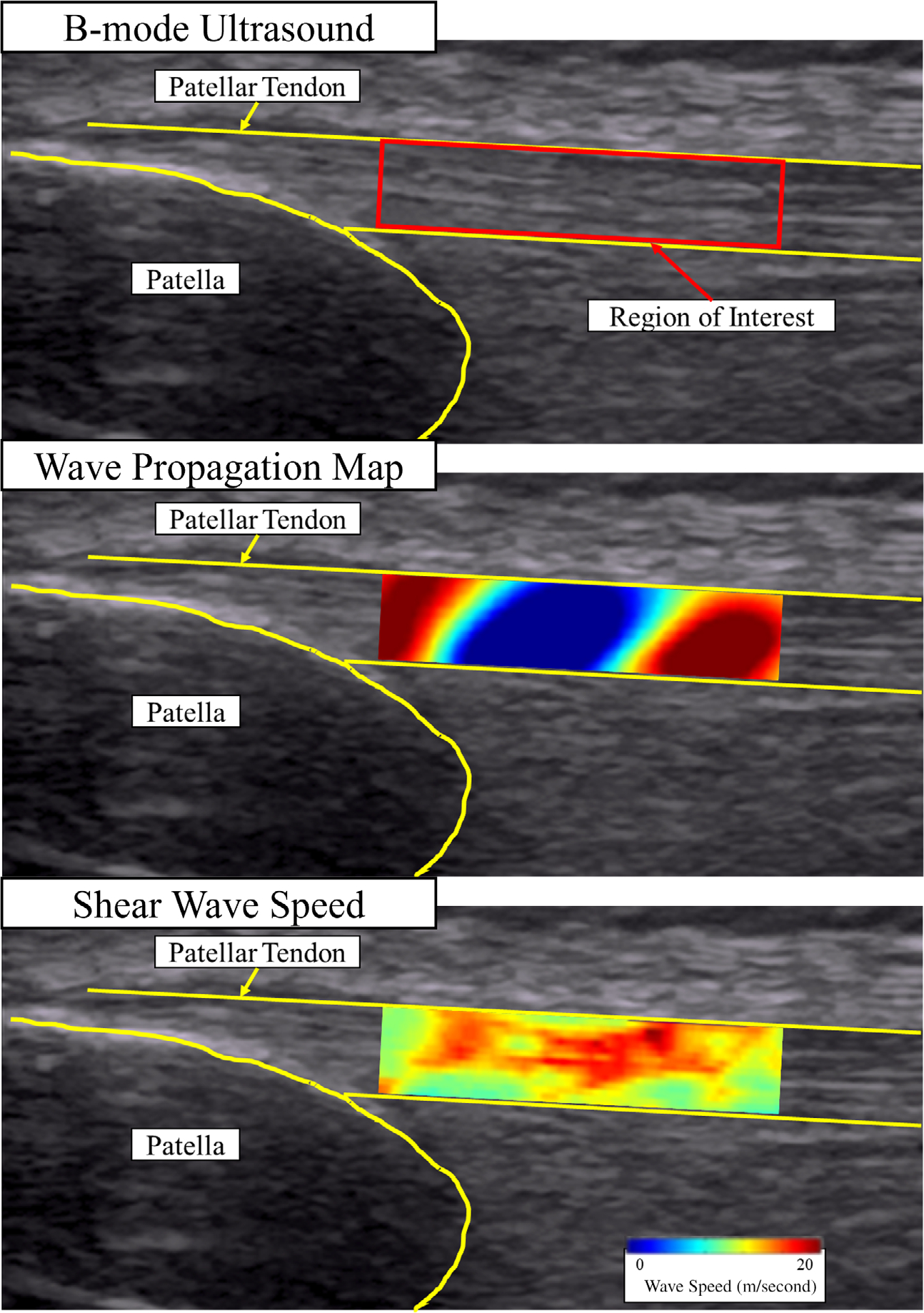
Shear wave propagation through the patellar tendon. Shear wave speeds measured within the region of interest for the 11 frequencies were then used to calculate viscosity and shear modulus using the Voigt model.33 The images reflect an example trial from one of the healthy patellar tendons at the 322 Hz frequency.
Statistics
Three separate reliability measures for both shear modulus and viscosity were calculated using intraclass correlations (ICC). Between-day intrarater stability (Trial 1 versus Trial 2) and within-day intrarater reliability (Trial 2 versus Trial 3) were calculated using a mean-rating, absolute agreement, two-way mixed effects model. Within-day interrater reliability (Trial 2 versus Trial 4) was calculated using a mean-rating, absolute agreement, one-way random effects model.39 ICCs were calculated for the complete sample combining data from both healthy and pathological patellar tendons (n = 35), and separately using the data from only the pathological patellar tendons (n = 22) for comparison. The standard error of the measurement and individual minimum detectable change (MDC95%) at the 95% confidence interval were calculated using ICC values obtained from the two cohorts.
As a secondary purpose, one-way analysis of variance (α = 0.05) was used to compare shear modulus and viscosity among the three groups (PT, BPTB, and Healthy). The assumptions for normality and homogeneity were met. Pair-wise comparisons were performed for significant main effects of group. All statistics were performed using R.40
Results
For the complete sample, excellent between-day stability was observed for viscosity (ICC = 0.905), and good between-day stability was observed for shear modulus (ICC = 0.805). Good within-day intrarater reliability was observed for both viscosity and shear modulus (ICC = 0.839 and 0.751). Moderate interrater reliability was observed for both viscosity and shear modulus (ICC = 0.591 and 0.532). The individual MDC95% of the measures were identified to be 8.3 Pa seconds for viscosity and 27.4 kPa for shear modulus (Table 3). ICC outcomes using only the pathological patellar tendons (Table 4) identified similar reliability measures compared to the complete sample including healthy patellar tendons.
Table 3.
Reliability and minimum detectable change of the complete sample (n = 35)
| Shear Modulus (kPa) | ICC |
95% CI |
Pooled Mean | Pooled SD | SEM | MDC95% Individual | |
|---|---|---|---|---|---|---|---|
| Average Measures | Lower | Upper | |||||
|
| |||||||
| Stability | 0.805 | 0.657 | 0.890 | 68.2 | 22.4 | 9.9 | 27.4 |
| Intrarater | 0.751 | 0.562 | 0.859 | 65.8 | 22.8 | 11.4 | 31.6 |
| Interrater | 0.532 | 0.176 | 0.735 | 67.0 | 21.1 | 14.4 | 40.0 |
| Viscosity (Pa × s) | |||||||
| Stability | 0.905 | 0.833 | 0.946 | 25.3 | 9.7 | 3.0 | 8.3 |
| Intrarater | 0.839 | 0.705 | 0.910 | 24.1 | 9.7 | 3.9 | 10.8 |
| Interrater | 0.591 | 0.279 | 0.769 | 25.5 | 9.1 | 5.8 | 16.1 |
ICC, intraclass correlation; 95% CI, 95% confidence interval; SD, standard deviation; SEM, standard error of measure; MDC95%, minimum detectable change; Bold values indicate ICC and MDC relevant for assessing tendon changes over time. Excellent reliability: ICC > 0.9; good reliability: 0.75 < ICC <0.9; moderate reliability: 0.5 < ICC <0.75; poor reliability: ICC < 0.5.39
Table 4.
Reliability and Minimum Detectable Change of the Pathological Tendons Only (ie, PT and BPTB Groups) (n = 22)
| Shear Modulus (kPa) | ICC |
95% CI |
Pooled Mean | Pooled SD | SEM | MDC95% Individual | |
|---|---|---|---|---|---|---|---|
| Average Measures | Lower | Upper | |||||
|
| |||||||
| Stability | 0.817 | 0.624 | 0.912 | 68.6 | 22.8 | 9.8 | 27.1 |
| Intrarater | 0.813 | 0.616 | 0.909 | 68.4 | 22.9 | 9.9 | 27.4 |
| Interrater | 0.424 | −0.185 | 0.722 | 65.8 | 20.0 | 15.2 | 42.0 |
| Viscosity (Pa × s) | |||||||
| Stability | 0.933 | 0.863 | 0.968 | 27.3 | 10.4 | 2.7 | 7.4 |
| Intrarater | 0.880 | 0.753 | 0.942 | 26.0 | 10.4 | 3.6 | 10.0 |
| Interrater | 0.644 | 0.266 | 0.828 | 26.3 | 9.8 | 5.9 | 16.3 |
ICC, intraclass correlation; 95% CI, 95% confidence interval; SD, standard deviation; SEM, standard error of measure; MDC95%, minimum detectable change. Bold values indicate ICC and MDC relevant for assessing tendon changes over time. Excellent reliability: ICC > 0.9; good reliability: 0.75 < ICC <0.9; moderate reliability: 0.5 < ICC <0.75; poor reliability: ICC <0.5.39
Main effects of group were observed for both shear modulus (F(2, 32) = 4.43, P = .020, ) and viscosity (F(2, 32) = 4.33, P = .022, ) (Table 5). Pairwise comparisons identified for viscosity, that the PT group presented with higher viscosity compared to both the BPTB (P = .013) and Healthy (P = .025) groups. No differences were found between the BPTB and Healthy groups (P = .612). For shear modulus, the PT group presented with higher shear modulus compared to the BPTB group (P = .007) and the difference between the Healthy group trended toward significance (P = .052). No differences were found between the BPTB and Healthy groups (P = .308).
Table 5.
Shear Modulus and Viscosity by Group
| Group | Shear Modulus (kPa) | Viscosity (Pa seconds) |
|---|---|---|
|
| ||
| PT | 80.3 ± 16.4 | 31.3 ± 10.5 |
| BPTB | 57.1 ± 16.3 | 20.1 ± 7.9 |
| Healthy | 65.5 ± 22.1 | 22.9 ± 8.6 |
PT, patellar tendinopathy; BPTB, bone-patellar tendon-bone graft; Healthy, healthy control.
Discussion
The purpose of this study was to investigate the interrater reliability, intrarater reliability, and between-day stability of cSWE in both healthy and pathological patellar tendons. Our study findings indicate good to excellent reliability for both viscosity and shear modulus measures obtained via cSWE when repeat measures were collected by a single rater. The interrater reliability of the measure, however, only demonstrated moderate reliability, emphasizing the importance of a single rater performing cSWE when used in any study design.
The good to excellent reliability found in this study is comparable to what has been reported in the Achilles tendon.37 Additionally, this study supports the reliability of using cSWE not only in healthy tendons but also in pathological tendons as comparable ICCs were observed (Tables 3 and 4). The comparison between groups identified that the PT group may present with higher shear modulus and viscosity compared to the 2 other groups in this study, possibly indicating the underlying source of pathology and symptoms (Table 5). No differences were found between the BPTB and Healthy groups, and this finding is likely due to the sampling strategy for the BPTB group which was not optimized for detecting group differences (Table 1). Symptoms were not a part of inclusion criteria for the BPTB group as the primary objective of the study was to establish reliability, and participants from a large range of time from surgery were included to strengthen external validity for the reliability measure in this population. A more homogeneous sample in the BPTB group such as those with symptomatic patellar tendons or those acutely after ACLR is necessary to establish clinical meaning of the mechanical properties measured using cSWE. While a larger sample size is needed to further establish group differences in tendon viscoelastic properties, our data from a limited sample provides promising insight for future use of cSWE. cSWE can be used as a reliable tool in future studies investigating long term changes in the patellar tendon over the course of treatment for PT or recovery after BPTB autograft harvest for ACLR.
The relatively low interrater reliability compared to intrarater reliability was an unexpected finding. While no definitive conclusions can be drawn from our data, we have identified potential sources of error that may explain this finding. The initial scanning of the patellar tendon to identify region of interest is the first likely reason. The ultrasound probe is only able to image a 1 mm width region (credit card thickness), and it is likely the region selected varied between raters. The variability in alignment of the probe with the tendon fibers may also affect values recorded for shear wave speed due to tendon anisotropy. We do, however, report the average of 3 repositioned trials to limit this error, and the slight variation within the region captured is intended to capture a more representative image of each region. To improve interrater reliability of cSWE, an average of more than 3 trials may be necessary. Other sources of error may be from the amount of pressure applied at the ultrasound probe on the patellar tendon or the external actuator at the quadriceps tendon. Especially at the quadriceps tendon, since the rater manually held the actuator, there may have been variability in the pressure or steadiness of the hand holding the actuator resulting in slightly different shear waves transferred through the tendon. Future studies may investigate the influence of taking the average of more trials or controlling for ultrasound probe and actuator pressure and steadiness, to see if interrater reliability can be improved. Lastly, exercise between the 2 days of study visits were not tightly controlled due to ethical reasons, since participation in rehabilitation was a priority for the participants who were currently undergoing treatment. The participants’ activity level between study days may have influenced reliability in tendon mechanical properties.
cSWE is a reliable assessment tool of the viscoelastic properties in the patellar tendon with pathology. It is recommended, however, that a single rater performs all tests, as the interrater reliability of the measure was found to be less reliable in this study.
Acknowledgments
Funding was provided by the National Institutes of Health (NIH) including the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and Eunice Kennedy Shriver National Institute of Child Health and Human Development: R37-HD037985, R01-AR072034, T32-HD007490. ALS’s work was supported in part by Florence P. Kendall and Promotion of Doctoral Studies I scholarships from the Foundation for Physical Therapy Research.
Abbreviations
- ACLR
anterior cruciate ligament reconstruction
- BPTB
bone-patellar tendon-bone
- cSWE
continuous shear wave elastography
- ICC
intraclass correlations
- MDC
minimum detectable change
- PT
patellar tendinopathy
Footnotes
The authors of this manuscript have no conflicts of interest to disclose.
Contributor Information
Naoaki Ito, Biomechanics and Movement Science Program, University of Delaware, Newark, Delaware, USA; Department of Physical Therapy, University of Delaware, Newark, Delaware, USA.
Haraldur B. Sigurðsson, Biomechanics and Movement Science Program, University of Delaware, Newark, Delaware, USA; School of Health Sciences, University of Iceland, Reykjavik, Iceland.
Ryan T. Pohlig, Biostatistic Core Facility, College of Health Sciences, University of Delaware, Newark, Delaware, USA.
Daniel H. Cortes, Department of Mechanical and Nuclear Engineering, Penn State University, State College, Pennsylvania, USA.
Karin Grävare Silbernagel, Biomechanics and Movement Science Program, University of Delaware, Newark, Delaware, USA; Department of Physical Therapy, University of Delaware, Newark, Delaware, USA.
Andrew L. Sprague, Department of Physical Therapy, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
References
- 1.Zwerver J, Bredeweg SW, Van Den Akker-Scheek I. Prevalence of jumper’s knee among nonelite athletes from different sports: a cross-sectional survey. Am J Sports Med 2011; 39:1984–1988. 10.1177/0363546511413370. [DOI] [PubMed] [Google Scholar]
- 2.Lian ØB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med 2005; 33:561–567. 10.1177/0363546504270454. [DOI] [PubMed] [Google Scholar]
- 3.Millar NL, Silbernagel KG, Thorborg K, et al. Tendinopathy. Nat Rev Dis Primers 2021; 7:1. 10.1038/s41572-020-00234-1. [DOI] [PubMed] [Google Scholar]
- 4.Sprague AL, Couppé C, Pohlig RT, Snyder-Mackler L, Silbernagel KG. Pain-guided activity modification during treatment for patellar tendinopathy: a feasibility and pilot randomized clinical trial. Pilot Feasibility Stud 2021; 7:1–17. 10.1186/s40814-021-00792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zellers JA, Cortes DH, Pohlig RT, Engineering N. Tendon morphology and mechanical properties assessed by ultrasound show change early in recovery and potential prognostic ability for 6 month outcomes. Knee Surg Sports Traumatol Arthrosc 2019; 27:2831–2939. 10.1007/s00167-018-5277-8.Tendon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrigan P, Cortes DH, Pohlig RT, Grävare SK. Tendon morphology and mechanical properties are associated with the recovery of symptoms and function in patients with Achilles tendinopathy. Orthop J Sports Med 2020; 8:1–9. 10.1177/2325967120917271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breda SJ, van der Vlist A, de Vos RJ, Krestin GP, Oei EHG. The association between patellar tendon stiffness measured with shear-wave elastography and patellar tendinopathy—a case-control study. Eur Radiol 2020; 30:5942–5951. 10.1007/s00330-020-06952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprague AL, Couppé C, Pohlig RT, Cortes DC, Silbernagel KG. Relationships between tendon structure and clinical impairments in patients with patellar tendinopathy. J Orthop Res 2022; 40:2320–2329. 10.1002/JOR.25262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott A, Squier K, Alfredson H, et al. ICON 2019: international scientific tendinopathy symposium consensus: clinical terminology. Br J Sports Med 2020; 54:260–262. 10.1136/BJSPORTS-2019-100885. [DOI] [PubMed] [Google Scholar]
- 10.Larsson MEH, Ingela K, Nilsson-helander K. Treatment of patellar tendinopathy—a systematic review of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc 2012; 20:1632–1646. 10.1007/s00167-011-1825-1. [DOI] [PubMed] [Google Scholar]
- 11.Meisterling RC, Wadsworth T, Ardill R, Griffiths H, Lane-Larsen CL. Morphologic changes in the human patellar tendon after bone-tendon-bone anterior cruciate ligament reconstruction. Clin Orthop Relat Res 1993; 289:208–212. 10.1097/00003086-199304000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Bottoni CR, Smith EL, Shaha JS, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med 2015; 43:2501–2509. 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 13.Kaeding CC, Pedroza AD, Reinke EK, et al. Change in anterior cruciate ligament graft choice and outcomes over time. Arthrosc - J Arthrosc Relat Surg 2017; 33:2007–2014. 10.1016/j.arthro.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes JD, Burnham JM, Hirsh A, et al. Comparison of short-term biodex results after anatomic anterior cruciate ligament reconstruction among 3 autografts. Orthop J Sports Med 2019; 7:1–7. 10.1177/2325967119847630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuenze C, Pietrosimone B, Lisee C, et al. Demographic and surgical factors affect quadriceps strength after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2019; 27:921–930. 10.1007/s00167-018-5215-9. [DOI] [PubMed] [Google Scholar]
- 16.Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med 2016; 50:804–808. 10.1136/bjsports-2016-096031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy A, Casabianca L, Andrieu K, Baverel L, Noailles T. Complications following harvesting of patellar tendon or hamstring tendon grafts for anterior cruciate ligament reconstruction: systematic review of literature. Orthop Traumatol Surg Res 2017; 103:S245–S248. 10.1016/j.otsr.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Poehling-Monaghan KL, Salem H, Ross KE, et al. Long-term outcomes in anterior cruciate ligament reconstruction: a systematic review of patellar tendon versus hamstring autografts. Orthop J Sports Med 2017; 5:1–9. 10.1177/2325967117709735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui C, Salmon LJ, Kok A, Maeno S, Linklater J, Pinczewski LA. Fifteen-year outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft for “isolated” anterior cruciate ligament tear. Am J Sports Med 2011; 39:89–98. 10.1177/0363546510379975. [DOI] [PubMed] [Google Scholar]
- 20.Curado J, Hulet C, Hardy P, et al. Very long-term osteoarthritis rate after anterior cruciate ligament reconstruction: 182 cases with 22-year’ follow-up. Orthop Traumatol Surg Res 2020; 106:459–463. 10.1016/j.otsr.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Bernicker JP, Haddad JL, Lintner DM, DiLiberti TC, Bocell JR. Patellar tendon defect during the first year after anterior cruciate ligament reconstruction: appearance on serial magnetic resonance imaging. Arthroscopy 1998; 14:804–809. 10.1016/S0749-8063(98)70014-3. [DOI] [PubMed] [Google Scholar]
- 22.Rispoli DM, Sanders TG, Miller MD, Morrison WB. Magnetic resonance imaging at different time periods following hamstring harvest for anterior cruciate ligament reconstruction. Arthroscopy 2001; 17:2–8. 10.1053/jars.2001.19460. [DOI] [PubMed] [Google Scholar]
- 23.Kiss ZS, Kellawayi DP, Cook JL, Khan M, Study T. Postoperative patellar tendon healing: an ultrasound study. Australas Radiol 1998; 42(February):28–32. [DOI] [PubMed] [Google Scholar]
- 24.Svensson M, Kartus J, Ejerhed L. Does the patellar tendon normalize after harvesting its central third? A prospective long-term MRI study. Am J Sports Med 2004; 32:34–38. 10.1177/0363546503258935. [DOI] [PubMed] [Google Scholar]
- 25.Drew BT, Smith TO, Littlewood C, Sturrock B. Do structural changes (eg, collagen/matrix) explain the response to therapeutic exercises in tendinopathy: a systematic review. Br J Sports Med 2014; 48:966–972. 10.1136/bjsports-2012-091285. [DOI] [PubMed] [Google Scholar]
- 26.Suydam SM, Cortes DH, Axe MJ, Snyder-Mackler L, Buchanan TS. Semitendinosus tendon for ACL reconstruction: regrowth and mechanical property recovery. Orthop J Sports Med 2017; 5. 10.1177/2325967117712944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akkaya S, Akkaya N, Agladıoglu K, Gungor HR, Ok N, Özçakar L. Real-time elastography of patellar tendon in patients with auto-graft bone–tendon–bone anterior cruciate ligament reconstruction. Arch Orthop Trauma Surg 2016; 136:837–842. 10.1007/s00402-016-2459-z. [DOI] [PubMed] [Google Scholar]
- 28.Taş S, Onur MR, Yılmaz S, Soylu AR, Korkusuz F Shear wave elastography is a reliable and repeatable method for measuring the elastic modulus of the rectus femoris muscle and patellar tendon. J Ultrasound Med 2017; 36:565–570. 10.7863/ultra.16.03032. [DOI] [PubMed] [Google Scholar]
- 29.Gulledge CM, Baumer TG, Juliano L, et al. Shear wave elastography of the healing human patellar tendon following ACL reconstruction. Knee 2019; 26:347–354. 10.1016/j.knee.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Blank J, Blomquist M, Arant L, Cone S, Roth J. Characterizing musculoskeletal tissue mechanics based on shear wave propagation: a systematic review of current methods and reported measurements. Ann Biomed Eng 2022; 50:751–768. 10.1007/s10439-022-02935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh CL, Kuo PL, Gennisson JL, Brum J, Tanter M, Li PC. Shear wave measurements for evaluation of tendon diseases. IEEE Trans Ultrason Ferroelectr Freq Control 2016; 63:1906–1921. 10.1109/TUFFC.2016.2591963. [DOI] [PubMed] [Google Scholar]
- 32.Deffieux T, Montaldo G, Tanter M, Fink M. Shear wave spectroscopy for in vivo quantification of human soft tissues visco-elasticity. IEEE Trans Med Imaging 2009; 28:313–322. 10.1109/TMI.2008.925077. [DOI] [PubMed] [Google Scholar]
- 33.Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM. Continuous shear wave elastography: a new method to measure viscoelastic properties of tendons in vivo. Ultrasound Med Biol 2015; 41:1518–1529. 10.1016/j.ultrasmedbio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto A, Yamakoshi Y, Ohsawa T, et al. Shear wave velocity measurement of upper trapezius muscle by color Doppler shear wave imaging. J Med Ultrason 2018; 45:129–136. 10.1007/s10396-017-0803-8. [DOI] [PubMed] [Google Scholar]
- 35.Chen PY, Yang TH, Kuo LC, Shih CC, Huang CC. Characterization of hand tendons through high-frequency ultrasound elastography. IEEE Trans Ultrason Ferroelectr Freq Control 2020; 67:37–48. 10.1109/TUFFC.2019.2938147. [DOI] [PubMed] [Google Scholar]
- 36.Tsuchida W, Yamakoshi Y, Matsuo S, et al. Application of the novel estimation method by shear wave elastography using vibrator to human skeletal muscle. Sci Rep 2020; 10:1–10. 10.1038/s41598-020-79215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corrigan P, Zellers JA, Balascio P, Silbernagel KG, Cortes DH. Quantification of mechanical properties in healthy Achilles tendon using continuous shear wave elastography: a reliability and validation study. Ultrasound Med Biol 2019; 45:1574–1585. 10.1016/j.ultrasmedbio.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou GY. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat Med 2012; 31:3972–3981. 10.1002/sim.5466. [DOI] [PubMed] [Google Scholar]
- 39.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15:155–163. 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Team RDC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]


