Abstract
The poor generalizability of clinical research data due to the enrollment of highly educated, non-Latinx White participants hampers development of therapies for Alzheimer’s disease (AD). Black and Latinx older adults have a greater risk for dementia, yet it is unclear how health care disparities and sociocultural factors influence potential AD therapies and prognosis. Low enrollment of under-represented populations may be attributable to several reasons including greater exclusion due to higher rates of comorbidities, lower access to AD clinics, and the legacy of unethical treatment in medical research. This perspective outlines solutions tested in the Brain Health Registry and the Alzheimer’s Disease Neuroimaging Initiative, including culturally-informed digital research methods, community-engaged research strategies, leadership from under-represented communities, and the reduction of exclusion criteria based on comorbidities. Our successes demonstrate that it is possible to increase inclusion and engagement of under-represented populations into U.S-based clinical studies thereby increasing the generalizability of their results.
Introduction
Alzheimer’s disease (AD) is a heterogeneous neurodegenerative condition, underlain by complex neurobiological mechanisms. There is substantial variability in clinical manifestations, age of onset, levels of biomarkers, neuropathological features, and genetic risk factors, all of which may influence response to treatment [1].From a biopsychosociocultural perspective [2] multiple factors influence an individual’s risk, presentation, and course of neurologic conditions such as AD. These include biological and psychological risk factors and comorbidities, as well as sociocultural factors (e.g., ethnocultural status, quality of education, socioeconomic status, acculturation) and cognitive reserve/resilience. A greater understanding of how these factors interact to predict disease in individuals is required to implement precision medicine approaches.
A decade ago, following the passing of the United States’ National Alzheimer’s Project Act, the need for a large, population-based, longitudinal cohort to study healthy aging and preclinical dementia was identified [3]. Such a cohort would provide a setting in which biological markers for the early detection of neurodegeneration and underlying pathologies could be validated, allowing comparison of results across different studies. It would provide a diverse population for genomic studies to identify the genetic architecture of AD, enable a better understanding of underlying pathogenic mechanisms, and allow the development of disease models and accurate risk prediction. A similar Framingham like international study was proposed by an OECD working group [1]. At the core of these proposals was that genetic and cultural diversity of cohorts were essential to investigate how the interaction of genetic, lifestyle, and environmental factors affect the risk and clinical course of AD and dementia.. However, as yet, our understanding of the contribution of these factors has emerged, with a few notable exceptions, from observational studies and randomized clinical trials of predominantly highly educated, non-Latinx White samples [4, 5] that are not representative of the wider population [6]. Results obtained from current clinical AD research cohorts may lack external and internal validity due to limited generalizability to the wider population [7]. They likely provide an incomplete picture of how sociocultural factors influence AD diagnosis and the safety and efficacy of potential AD treatments. Therefore, it is critical to expand research participation and provide comprehensive characterization of populations who experience disproportionate disease impact but who have been historically under-included in AD research [8].
People of minoritized ethnocultural backgrounds and those from low resource settings, who are under-served and under-included in clinical research, differ in dementia risk, prevalence, and incidence [9]. In the United States, Black and Latinx populations have a greater prevalence and incidence of dementia clinically diagnosed as AD [10], while low socioeconomic status is associated with worse cognitive outcomes [11] including a greater risk of dementia [12]. Biologically, studies of CSF and blood biomarkers of AD in different ethnocultural populations have reported variable results [6, 9], but consistently find that biomarker cut points established in predominantly non-Latinx White populations are not always generalizable to other ethnocultural populations l[13]. Black Americans had lower levels of the AD pathological biomarkers, CSF phosphorylated and total tau compared to non-Latinx White Americans, despite similar cognition [14]. Intriguingly, these lower levels of AD biomarkers were only observed in carriers of the APOE ε4 AD risk allele. While the frequency of the APOE ε4 risk allele was approximately 50% higher in Black Americans and Native Hawaiians compared to other ethnocultural groups in the Multiethnic Cohort Study [15], it did not fully account for the racial/ethnic disparities in AD incidence However, among APOE ε4 non-carriers, Black American and Latinx populations had a significantly higher incidence of dementia. These studies suggest that the APOE ε4 allele and traditional AD pathological biomarkers do not fully account for the observed differences in prevalence, incidence, and risk of AD in minoritized ethnocultural populations in the United States, and that the APOE ε4 allele may have differential effects within these populations. Sociocultural and environmental factors associated with racial and structural inequities that confer increased AD vulnerability may instead account for this elevated risk. It is also possible that differences between ethnocultural groups in as yet unknown genetic risk factors beyond the APOE ε4 allele contribute to the observed differences in prevalence, incidence, and risk of dementia.
The increased risk of dementia in these under-included populations [6, 9, 16] may be partially attributable to unequal exposures to a variety of environmental, sociocultural, and behavioral factors that exert their effect “upstream” of biological factors [6, 9, 16], as suggested by the application of the National Institute on Aging Health Disparities Research Framework [6, 17] to AD. Exposure to pollution, smoking, and chronic stress result in health inequities via probable epigenetic mechanisms [18], which may act in concert with factors such as inequitable access to a healthy diet, healthcare, and education [6]. People from minoritized and marginalized communities differ in their rates of comorbidities, such as cerebrovascular disease and psychiatric disorders, that are commonly associated with AD pathology [19, 20] and that affect AD disease susceptibility and progression. The synergistic interaction of these factors may influence the traditional AD pathological cascade, or affect other dementia risk or protective factors such as comorbidities, immune function, and resilience [6, 9].
Given the need for more diverse cohorts and the failure of many clinical studies to engage and retain these populations,, it is critical to understand historical and contemporary factors contributing to the inadequate representation of these populations, and to develop strategies to engage them. There are several reasons for the under-inclusion of various groups in AD clinical research in the United States [21].
1. Exclusion of people with comorbidities
Many studies of AD and other dementias, and almost all clinical trials, explicitly exclude people with comorbidities or on medications known to affect cognition. These include substance abuse, cerebrovascular disease including stroke, psychiatric conditions such as severe depression, and medical comorbidities, and together, they act to exclude the majority of older adults [22–24]. Higher rates of comorbidities in Black Americans and adults with low education [20] result in even greater exclusion from clinical studies. In a recent preliminary analysis [25], we examined exclusion rates of adults from under-represented communities based on the operationalized medical condition exclusion criteria of the A4 study using data from the Health and Retirement Study [26], a representative population-based sample in the US, with linked Medicare administrative data. The rates of exclusion of Black and Latinx adults were significantly higher than that of non-Latinx White adults. Likewise, those with fewer years of education and lower incomes also had higher rates of exclusion.
2. Lower access to tertiary clinics at academic medical centers
The historic legacy of structural racism, through policies such as redlining, disinvestment for minority communities, and resource zoning, continues to systematically contribute to AD health inequities by creating a compounding context of inaccessibility over time. Specifically, a key barrier to AD research participation for adults belonging to marginalized and underserved groups is lower access to AD clinics in or near their communities[27]. The need for accessible clinics to build ties with these communities was identified by participants of the “Diagnosis and Assessment of Alzheimer’s Disease in Diverse Populations” workshop in Chicago, IL [28]. Furthermore, evidence from a meta-analysis of ethnocultural differences in dementia care [29] suggests that diagnostic services are accessed later in the disease process by Black and Latinx older adults with dementia compared to non-Latinx White older adults with dementia.
3. A legacy of unethical treatment and cultural bias in medical research
The legacy of unethical and abusive treatment of people from under-served and marginalized communities in medical research is profound, pervasive, and well documented [30]. Historic and contemporary exemplars of such mistreatment, in concert with the lack of evidence-based, culturally-responsive approaches to medical research with these groups, help to contextualize the hurdles that researchers must overcome to become trustworthy [31–33]. Given this legacy, mistrust of medical research is understandable and borne out in multiple studies. The 2020 survey of the Alzheimer’s Association [27] reported that the most prevalent reason for the failure to engage people from under-served populations in clinical trials was the belief that the medical research is biased against people of color, held by the majority of Black Americans, and higher proportions of Latinx, Asian and Native Americans compared to non-Latinx White Americans. Black Americans also had the greatest mistrust of medical research, fearing unfair treatment, that the treatment might cause sickness or that a cure would not be shared equally. Thus, it is incumbent on researchers to acknowledge and understand this legacy in order to earn the trust of these under-served populations and safely facilitate their participation in clinical research and trials. As such, the National Institute on Aging released a strategy focusing on practical approaches to improving engagement of these groups [34].
Some groups have successfully enrolled adults from under-represented and under-served populations in their studies of aging, demonstrating the ability to make their studies generalizable. These include the Health and Retirement Study [35], Multiethnic Study of Atherosclerosis [36], the Rush Memory and Aging Project [37], the Baltimore Longitudinal Study of Aging [38], the Hispanic Community Health Study/Study of Latinos [39], the Washington Heights – Hamilton Heights – Inwood Columbia Aging Project [40], the Chicago Health and Aging Project [41], the Collaborative Approach for Asian Americans and Pacific Islanders Research and Education study [42], the Knight Alzheimer’s Disease Research Center study [43], the Minority Aging Research Study [44], the Sacramento Area Latino Study on Aging [45], the Health & Aging Brain among Latino Elders (HABLE) study [46], and the Boston Puerto Rican Health Study [47] among others [48]. Despite these notable successes, it has been difficult for multisite clinical studies, especially randomized controlled trials of AD treatments to enroll a generalizable population.
Our recent work in both the Brain Health Registry (BHR) [49] and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [50] highlights a variety of innovative approaches aimed at increasing the inclusion and engagement of people from under-served, under-represented, and other marginalized communities into online registries and multi-center clinical research for AD.
The Brain Health Registry
The BHR is an online website and registry established in 2014 aimed at the recruitment, assessment, and longitudinal monitoring of participants for neuroscience research [49]. There are currently over 100,000 registrants who have provided self-report information on health and cognitive complaints, and who take ongoing online cognitive tests and health assessments. Registrants may be referred to various clinical studies across the U.S. and the cohort has been used for the development and validation of online assessments. However, non-Latinx White BHR registrants are over-represented, and Black and Latinx registrants under-represented compared to the general U.S. population (Figure 1). For registrants aged 60+ years compared to the same age group from U.S. Census data, only 3% versus 9% self-identify as Black and 7% versus 9% self-identify as Latinx, whereas 78% versus 75% self-identify as non-Latinx White, and 12% versus 7% self-report other races/ethnicities (Asian, Native American, Pacific Islander, Other, and Multiple). The disparities are even more apparent when considering all BHR registrants versus the U.S. adult population (18+ years). Overall, since inception, BHR has enrolled 7730 (9.6 %) fewer Black older adults, 7167 (8.9%) fewer Latinx older adults, 2 657 (3.3 %) fewer non-Latinx Asian older adults, and 23,110 (28.7%) fewer older adults with lower education (<12 years) than would be representative of this population. The latter discrepancy is consistent with the finding that social determinants of health are important determinants of the risk for AD and related dementias [51]. The BHR has employed the following novel approaches to address these issues:
Figure 1.
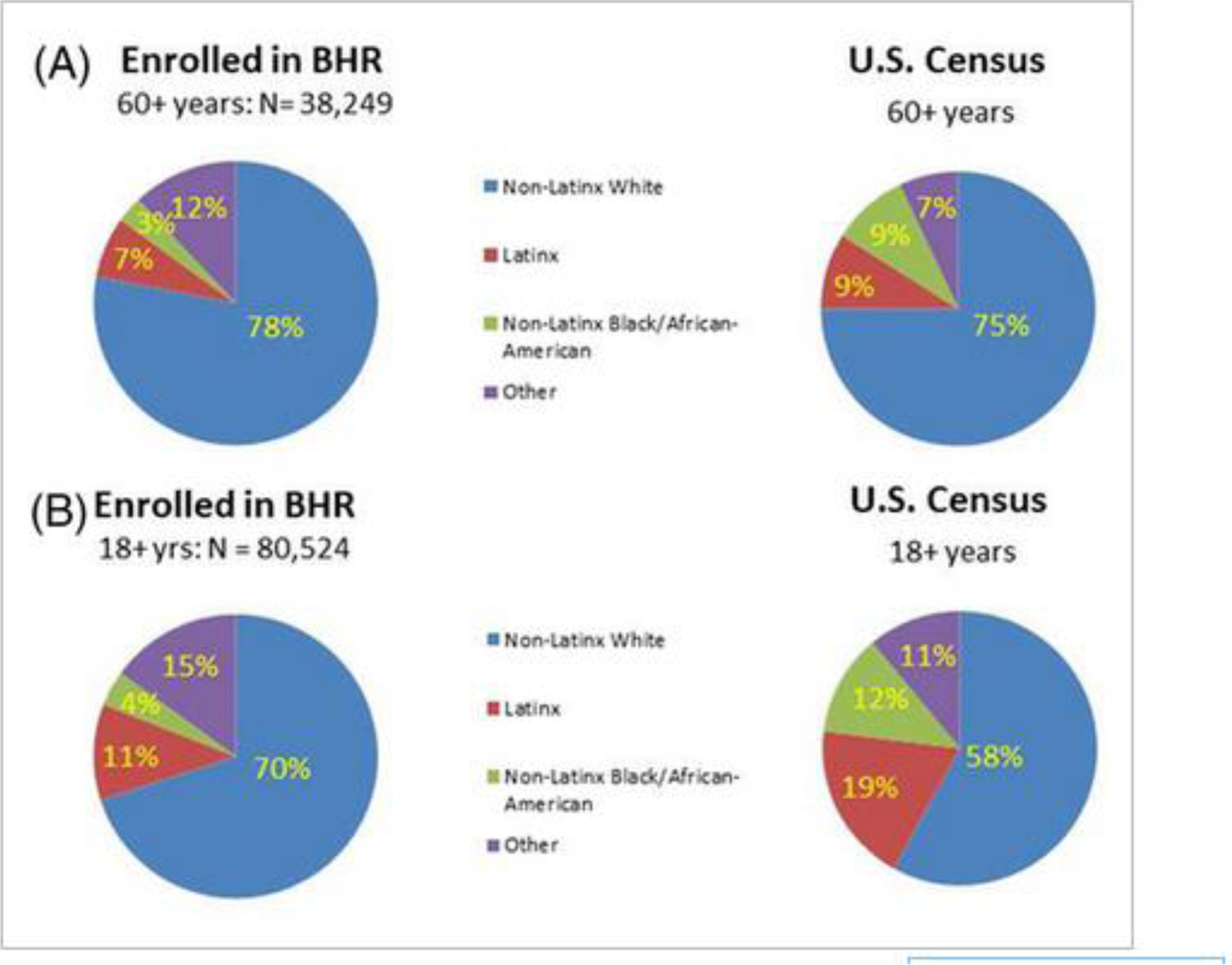
Enrollment of under-represented populations into the Brain Health Registry compared to community proportions. (A) Proportions of BHR participants aged 60+ by race/ethnicity compared to the general population aged 60+ from the U.S. Census. (B) Proportions of all BHR participants by race/ethnicity compared to the adult general population from the U.S. Census.
1. B. Smith campaign tailored for Black older adults
In 2015, B. Smith, a Black former model, restauranteur, and TV host promoted the BHR on the Today Show in a campaign aimed at increasing enrollment of Black participants. As a result, almost 6000 new BHR participants were recruited, including 872 Black older adults. This increased the percentage of Black participants in the BHR cohort from 1.5% to 4.9% and demonstrated the effectiveness of a focused, high visibility appeal to a specific under-represented group. We were very fortunate to have a nationally recognized spokesperson to facilitate our recruitment efforts. Since our experience with B. Smith, who was engaged via a personal connection, we have made several efforts to engage celebrities or nationally recognized figures, with little success to date. This underscores the difficulty of this approach.
2. The California Latino Brain Health Registry
A second novel approach used by the BHR was to launch the California Latino Brain Health Registry study (CAL-BHR) [4, 52] in 2020 which featured recruitment and engagement content culturally tailored to the Latinx population. To achieve its goal of increasing Latinx recruitment and enrollment, CAL-BHR established a Community Advisory Board, in addition to the Scientific Advisory Board, to solicit input on digital advertising themes and messaging, digital engagement and participant communication strategies, and to provide Spanish translation. CAL-BHR also contracted with a Latina-owned marketing firm with expertise in this area, Alaniz Marketing, to create culturally-tailored English and Spanish landing pages and community-informed digital outreach and engagement campaigns primarily on Facebook but also on other platforms (YouTube, Google, and Bing). Some examples of CAL-BHR advertising in both languages are shown in Figure 2A. For an investment of around $320,000, over 14 million people were exposed to the advertising material, over 170,000 clicked on the links, and ultimately 8687 people enrolled, including 6691 Latinx Americans, at a cost of $40 per participant (Figure 2B). However, the cost of enrolling older Latinx registrants was roughly double, and registrants were well educated and predominantly (77%) female. To complement these efforts, the BHR established a Spanish language BHR in which the entire experience (including questionnaires and cognitive tests) is in Spanish to facilitate engagement by Spanish speaking registrants. This succeeded in enrolling 919 Latinx participants of whom 689 were aged 55+ and 60% were female. However, our efforts were less effective in enrolling both English- and Spanish-speaking male Latinx participants.
Figure 2.
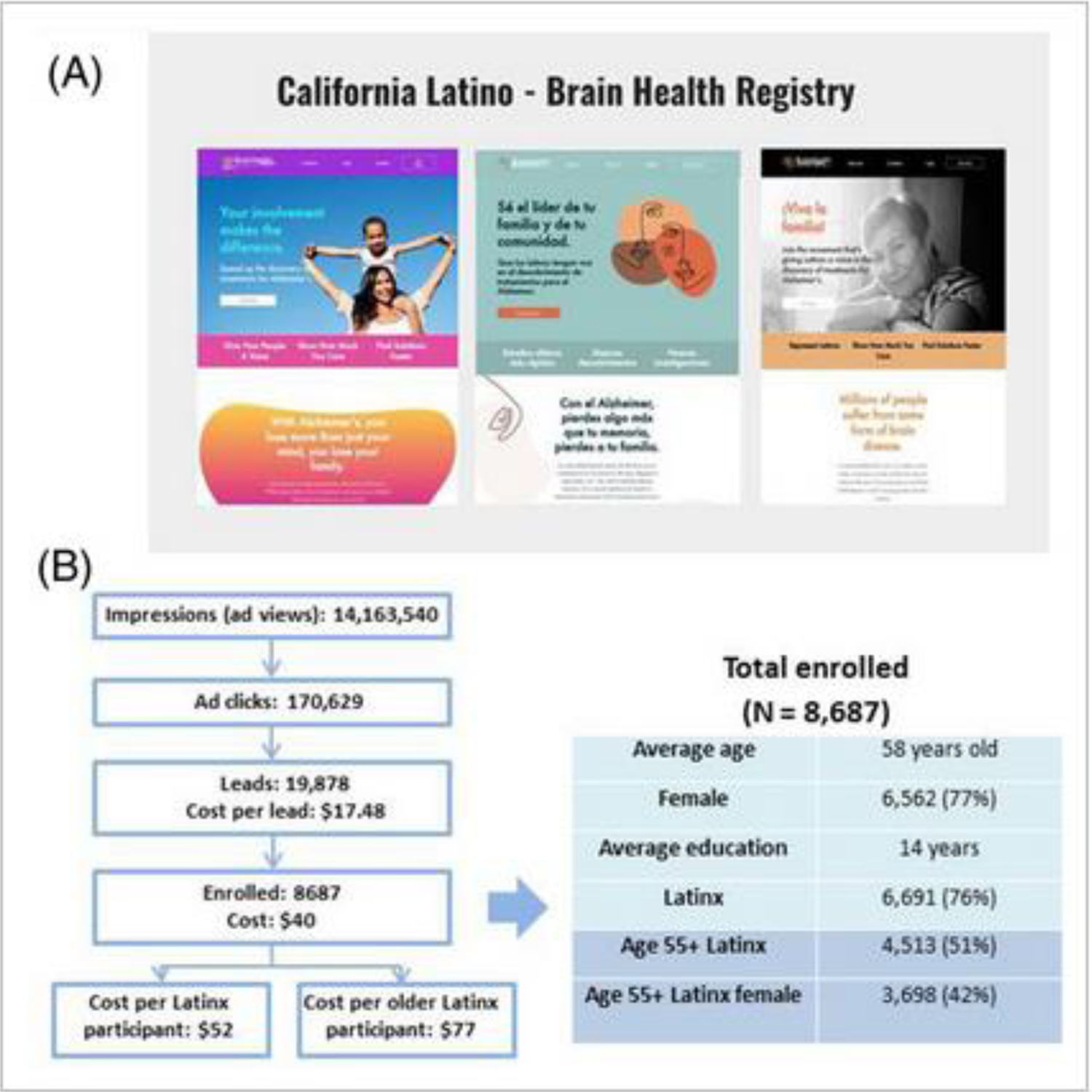
Latinx focused recruitment strategies used by the California Brain Health Registry. (A) Latinx focused advertising in English and Spanish. (B) recruitment of Latinx participants into CAL-BHR.
It should be noted that all recruitment activities require funding resources. The Latino Brain Health Registry and associated marketing efforts were funded by the California Department of Public Health and this grant has been renewed. However, many research grants emphasize funding of data collection and analysis, and often recruitment efforts are underfunded, presenting a barrier to the use of this approach by researchers limited by funding availability..
3. Community-Engaged Digital Alzheimer’s Research (CEDAR) project
A similar approach has been taken to increase engagement (e.g., retention, task completion) of Black BHR participants in the BHR CEDAR project. Using digital community-engaged research (CER) methods, CEDAR has invited over 3,000 Black BHR participants to join since August 2021. Of these, as of April 2022, 280 (7%) have enrolled, and 198 (71% of those enrolled) have completed a cross-sectional mixed methods online survey aimed at increasing our understanding of motivators and barriers to BHR participation and preferences for communication channels and engagement strategies. In September 2021, a CEDAR Community-Science Partnership Board (CSPB), comprising 20 Black BHR participants and Project Investigators, was established. CSPB meetings to date have introduced community members to CER methods, the study team, and the project goals. They have also included multiple community listening sessions and offered CSPB members the opportunity to provide study guidance and feedback on proposed digital outreach strategies and materials (e.g., website, social media strategy, images). Of note, all community members are compensated for their time for all CSPB meetings and related work.
Alzheimer’s Disease Neuroimaging Initiative (ADNI)
ADNI, a longitudinal naturalistic study of AD progression established in 2004 [53], has had difficulty in enrolling people with low education and from ethnoculturally under-represented populations [21]. Of all ADNI participants in 2019 compared to the same age group (60+years) from 2019 U.S. Census data, 88% versus 76% self-identified as non-Latinx White, 5% versus 10% self-identified as Black, 4% versus 9% self-identified as Latinx, and 2% versus 5% self- identified as Asian [54]. Moreover, of ADNI3 participants, only 15% had fewer than or equal to 12 years of education compared to 44% of those aged 60+ according to the 2019 U.S. Census. In an attempt to rectify this imbalance, two years into the enrollment for the ADNI3 cohort in 2018, we closed enrollment of cognitively unimpaired non-Latinx White older adults but continued with the enrollment of all other ethnocultural groups (we were also trying to recruit more individuals with MCI and AD across all ethnocultural groups). However, rates of enrollment of Black and Latinx participants were not increased by this restriction, although their percentages increased due to the enrollment of fewer non-Latinx White older adults.
In working to understand the reason behind low enrollment of people from under-represented communities, we hypothesized that they would have higher screen fail rates due to their probable higher rate of comorbidities which would lead to greater exclusion under ADNI3 inclusion/exclusion criteria. However, ADNI3 screen fail rates were in fact higher in the non-Latinx White group (33%) than in the Latinx (23%) or Black (18%) groups [21]. The low enrollment of these communities might alternatively be attributed to current recruitment strategies that do not include them in the initial approach or to the barriers to their participation that lie in the prescreening process. To improve these recruitment strategies, we assembled teams of diversity and inclusion experts:
1. ADNI3 Diversity Task Force
In a concerted effort to increase enrollment of adults from under-represented and under-served communities, we formed an ADNI3 Diversity Task Force (DVTF) in July 2020, co-led by Drs. Ozioma Okonkwo and Monica Rivera Mindt. They utilized an evidence-based, CER approach with an emphasis on culturally-informed methods [8]. The DVTF assembled an External Advisory Board comprised of leading scientists in brain health and AD inequities research and provided supplemental funding to 13 ADNI sites to accelerate enrollment of Black and Latinx participants. The ADNI protocol was modified to allow for: a) return of amyloid PET results to participants; b) greater flexibility by making lumbar puncture optional; and c) remote visits to allay pandemic-related concerns regarding in-person assessments. They also provided extensive training and support to the 13 ADNI DVTF sites and staff to implement their approach. Moreover, the DVTF engaged Alaniz Marketing to develop customized inclusion and engagement approaches and materials for these participants, paralleling the approach of CAL-BHR. Together, they set up locally branded, culturally-informed social media posts and landing pages centered around each of these 13 clinical centers that connected with specific groups. An example tailored to Black or Latinx older adults in the vicinity the ADNI3 clinic at Duke University in Durham, North Carolina is shown in Figure 3. A key aspect of this approach was focusing on areas in close proximity to clinical sites to overcome the issue of enrolling participants who then face difficulties in traveling for assessment. Last but not least, an ADNI CSPB has been launched, similar in function to the CEDAR CSPB detailed above.
Figure 3.
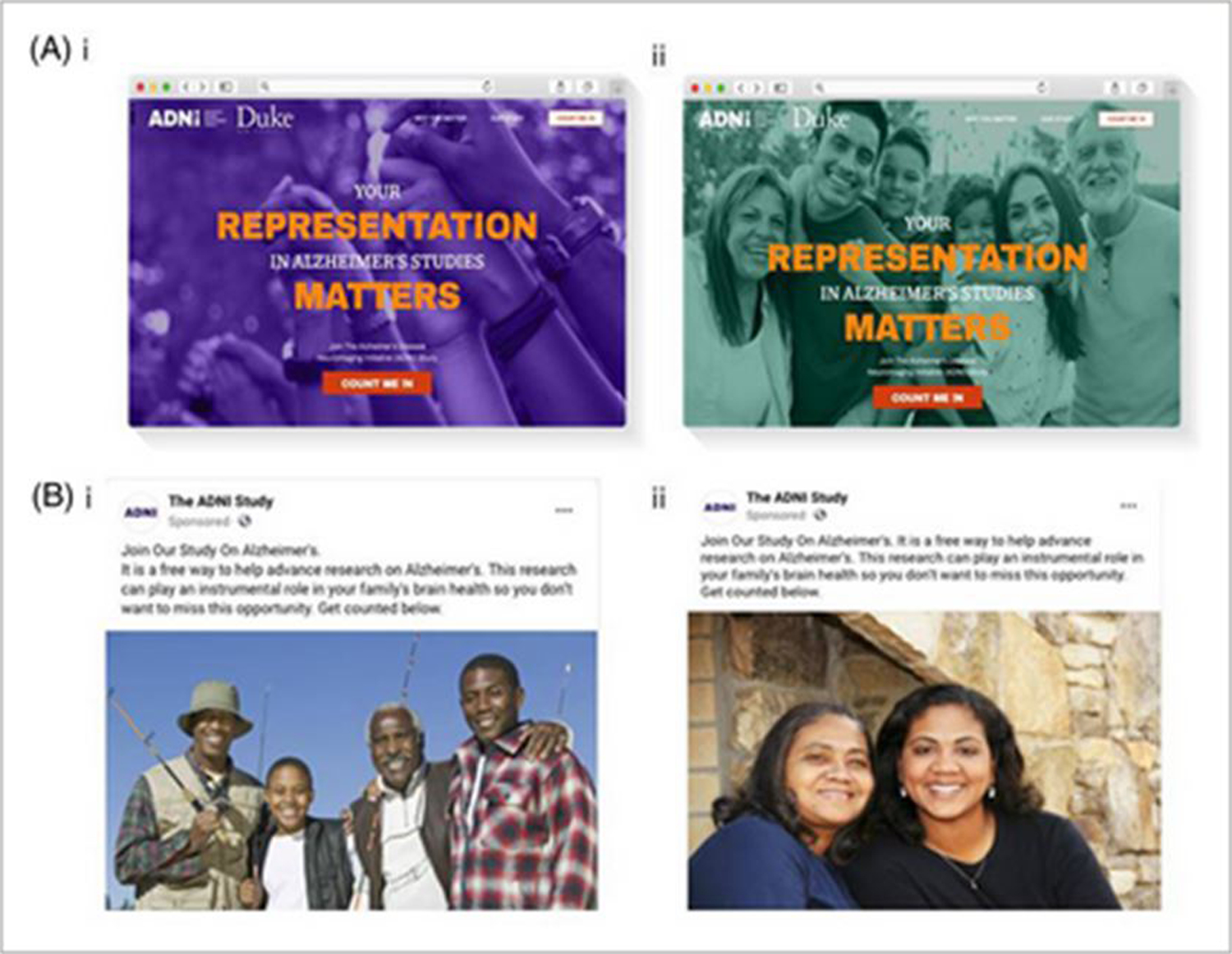
Culturally branded and locally tailored social media advertisements and landing pages developed by the ADNI 3 diversity task force. (A) ADNI landing pages tailored for Black (i) and Latinx older adults in the vicinity of the ADNI clinic at Duke University, Durham, North Carolina (ii). (B) Social media advertisements tailored for Black (i) and Latinx (ii) older adults
Since the start of the culturally-informed social media engagement campaign in May 2021, more than 1600 Black and 1500 Latinx older adults completed the ADNI pre-screener which included contact forms customized to the clinical DVTF sites, boosting new enrollments of Black and Latinx participants from 14.4% to 22%, and of participants from all under-represented groups to 26.7% of the ADNI3 cohort. The number of Black and Latinx participants increased from 93 to 177 in the year after the implementation of DVTF strategies, equating to an increase in the rate of enrollment of adults from these populations across all ADNI sites from 2.34 per month to 5.49 per month. Thus, this community-engaged and culturally-informed engagement approach in the pre-screening process succeeded in increasing enrollment of elders from under-represented communities where previous strategies failed, consistent with our findings in CAL-BHR.
2. ADNI4 Engagement Core
The success of ADNI3’s DVTF resulted in the creation of a new ADNI Engagement Core under the same leadership. The Engagement Core is at the center of our renewed grant cycle, ADNI4 (expected to be funded by the NIA beginning in September 2022) which aims to enlist around 20,000 older adults to complete an online, digital screener, initiating engagement and assessment with minimal barriers. Critically, the goal is that 50 to 60% of participants will come from multiple under-served ethnocultural groups e.g., Black, Latinx, American Indian and Alaska Native, Asian, Native Hawaiian and Other Pacific Islander, and from communities and persons from low education backgrounds. They will be engaged with central and local CER and culturally-informed approaches guided by ADNI4’s Engagement Core and CSPB. These include a culturally-informed digital engagement and social media campaign, and the use of Community Research Liaisons supporting enrollment in under-represented communities. Of these 20,000 people, we aim to collect plasma from 4000, either by directing them to get their blood drawn at a local phlebotomy center or by sending a phlebotomist to their home. From these 4000, participants from under-represented communities will be guided through the ADNI process to in clinic enrollment by Community Research Navigators. From these 4000 and based on the clinical characteristics determined by the online screener together with the risk of amyloid elevation in brain (as determined by plasma biomarkers), we aim to enroll 500 new participants into ADNI clinical sites, 250–300 of whom will be from under-represented populations.
Current conclusions
On our journey to improve enrollment and engagement of people from under-represented, under-served, and other marginalized communities, we have learned some important lessons that may be applicable to the strategic goal of establishing longitudinal, population-based cohorts:
1. Leadership
It is critical to have and to adequately support dedicated leadership, investigators, and staff from under-represented communities who also have the requisite expertise and knowledge to lead and conduct evidence-based, community-engaged, and culturally-informed research in an ethical manner that includes and empowers these communities.
2. Community-engaged science
It is equally critical to recognize input from these communities and to have that voiced in a Community-Science Partnership Board (CSPB), which engages both community members and scientists in an equitable process of collaboration and co-learning to provide guidance and carefully review all aspects of the study (e.g., recruitment and retention approaches, study materials, graphics and language, dissemination).
3. Culturally-informed study designs and implementation
Study design changes, for example, reduction of exclusion criteria based on comorbidities (particularly cerebrovascular disease), implementation of remote engagement and assessment procedures, and increased flexibility in required study procedures (e.g., lumbar punctures) and study implementation (e.g., assessment hours, support for travel) may help lower barriers to participation for people from minoritized, under-represented populations. Equally important, more inclusive studies will need to ensure culturally- and linguistically competent staff and utilize evidence-based, culturally valid assessments to comprehensively evaluate and characterize the diverse populations we seek to study, understand, and serve.
4. Culturally-informed digital engagement
While in-person inclusion and engagement (e.g., retention, task completion) efforts certainly remain important, community-engaged digital engagement and communications, including social media and paid advertisements, serve as an influential adjunct to scale up large cohort studies. Working with a marketing firm with community ownership and experience in reaching people from under-represented communities for clinical studies using digital social media greatly enhances their engagement. Culturally-informed, locally-branded websites that outreach to communities surrounding local clinical sites may bring more study candidates from under-represented populations for enrollment. While data suggest that digital outreach and engagement is more cost-efficient than in-person recruitment, it is unclear whether this approach would be similarly effective for retention and task engagement of participants. Thus, further research is needed to better understand the individual and potentially synergistic impact of traditional, in-person (“boots on the ground”) and digital engagement efforts.
We hope that our recent experiences in the BHR and ADNI demonstrates that, using culturally informed, CER efforts, it is possible to overcome the hurdle of engaging with and including a diverse range of older adults from communities that have been historically under-served, under-represented, and marginalized in medical research into online and clinical studies. These early successes demonstrates that strategic goals identified in response to NAPA within the US, are is possible with evidence-based approaches, expertise, leadership, and dedication, and our experiences may represent a stepping stone en route to the establishment of even larger, longitudinal, diverse cohorts better powered to investigate the underpinnings and implications heterogeneity in AD and dementia.
It must be noted that our experiences in improving engagement with underserved populations in the BHR and ADNI are recent - within the last two years - and therefore the effectiveness of our strategies to retain participants from diverse backgrounds over the timeframe of 5 to 10 years required to track asymptomatic in preclinical participants is unknown. Possibly, additional approaches will be required to encourage participants to remain within the study in the long-term. We anticipate that these will be developed in conjunction with community advisors [this is entirely guesswork Mike!] As this Perspective is intended to share our experiences to date, is expected that will report more fully on this issue at a future time.
Acknowledgements
We would like to acknowledge and thank all of the participants, leadership, team members, and funding organizations from the following projects who made this work possible, including the: Brain Health Registry (BHR; PI: M. Weiner; NIH/NIA R33AG062867-02); California Latino Brain Health Registry study (CAL-BHR; MPIs: R. Nosheny & M. Weiner, California Department of Public Health19-10616); Community-Engaged Digital Alzheimer’s Research (CEDAR; MPIs: R. Nosheny, M. Rivera Mindt, & C. Hill Genentech Health Equity Innovations 2020 Fund #G-89294); and ADNI3 (PI: M. Weiner; NIH/NIA 5U19AG024904-14). We are especially grateful to: our CAL-BHR, CEDAR, and ADNI Community-Scientific Partnership Board (CSPB) members for their time and insightful comments to guide this work, as well as our 13 ADNI3 DVTF Study Sites for their tremendous dedication and hard work, including: Butler (PI: S. Salloway); Duke University (PI: M. Doraiswamy); Icahn School of Medicine at Mount Sinai (PI: H. Grossman); Indiana University Purdue University Indianapolis (IUPUI; PI: M. Farlow); Long Beach VA (PI: C. Reist); Lou Ruvo (PIs: M. Sabbagh, J. Shi, and D. Wint); Northwestern University (PI: E. Roglaski); Rush University (PI: R. Shah); South Carolina Institute of Brain Health (PI: J. Mintzer); University of California-Los Angeles (UCLA; MPIs: M. Beigi and K.A. Vossel); University of Southern California (USC; PI: Lon Schneider); Washington University (PI: B. Ances); and Wien (PI: R. Duara).
References
- [1].Khachaturian ZS. Emerging Trends in Biomedicine and Health Technology Innovation: Addressing the Global Challenge of Alzheimer’s: OECD Publishing Paris; 2013. [Google Scholar]
- [2].Mindt MR, Byrd D, Ryan EL, Robbins R, Monzones J, Arentoft A, et al. Characterization and sociocultural predictors of neuropsychological test performance in HIV+ Hispanic individuals. Cultural Diversity and Ethnic Minority Psychology. 2008;14:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].NAPA AsAEAWo, Khachaturian ZS. Workgroup on NAPA’s scientific agenda for a national initiative on Alzheimer’s disease. Alzheimer’s & Dementia. 2012;8:357–71. [DOI] [PubMed] [Google Scholar]
- [4].Canevelli M, Bruno G, Grande G, Quarata F, Raganato R, Remiddi F, et al. Race reporting and disparities in clinical trials on Alzheimer’s disease: A systematic review. Neuroscience and biobehavioral reviews. 2019;101:122–8. [DOI] [PubMed] [Google Scholar]
- [5].Birkenbihl C, Salimi Y, Domingo-Fernándéz D, Lovestone S, consortium A, Fröhlich H, et al. Evaluating the Alzheimer’s disease data landscape. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2020;6:e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gleason CE, Zuelsdorff M, Gooding DC, Kind AJ, Johnson AL, James TT, et al. Alzheimer’s disease biomarkers in Black and non-Hispanic White cohorts: A contextualized review of the evidence. Alzheimer’s & Dementia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gianattasio KZ, Bennett EE, Wei J, Mehrotra ML, Mosley T, Gottesman RF, et al. Generalizability of findings from a clinical sample to a community-based sample: A comparison of ADNI and ARIC. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2021;17:1265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gilmore-Bykovskyi AL, Jin Y, Gleason C, Flowers-Benton S, Block LM, Dilworth-Anderson P, et al. Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimer’s & dementia (New York, N Y). 2019;5:751–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2019;15:292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017;13:72–83. [DOI] [PubMed] [Google Scholar]
- [11].Besser LM, McDonald NC, Song Y, Kukull WA, Rodriguez DA. Neighborhood Environment and Cognition in Older Adults: A Systematic Review. American journal of preventive medicine. 2017;53:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cadar D, Lassale C, Davies H, Llewellyn DJ, Batty GD, Steptoe A. Individual and Area-Based Socioeconomic Factors Associated With Dementia Incidence in England: Evidence From a 12-Year Follow-up in the English Longitudinal Study of Ageing. JAMA psychiatry. 2018;75:723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Karikari TK. Blood Tests for Alzheimer’s Disease: Increasing Efforts to Expand and Diversify Research Participation Is Critical for Widespread Validation and Acceptance. Journal of Alzheimer’s Disease. 2022:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, et al. Assessment of Racial Disparities in Biomarkers for Alzheimer Disease. JAMA neurology. 2019;76:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lim U, Wang S, Park SY, Bogumil D, Wu AH, Cheng I, et al. Risk of Alzheimer’s disease and related dementia by sex and race/ethnicity: The Multiethnic Cohort Study. Alzheimer’s & Dementia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ (Clinical research ed). 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hill CV, Pérez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethnicity & disease. 2015;25:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vick AD, Burris HH. Epigenetics and health disparities. Current epidemiology reports. 2017;4:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Caraballo C, Herrin J, Mahajan S, Massey D, Lu Y, Ndumele CD, et al. Temporal Trends in Racial and Ethnic Disparities in Multimorbidity Prevalence in the United States, 1999–2018. The American Journal of Medicine. 2022. [DOI] [PubMed] [Google Scholar]
- [20].Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health. 2018;42:186–94. [DOI] [PubMed] [Google Scholar]
- [21].Ashford MT, Raman R, Miller G, Donohue MC, Okonkwo OC, Mindt MR, et al. Screening and enrollment of underrepresented ethnocultural and educational populations in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Anderson TS, Ayanian JZ, Souza J, Landon BE. Representativeness of Participants Eligible to Be Enrolled in Clinical Trials of Aducanumab for Alzheimer Disease Compared With Medicare Beneficiaries With Alzheimer Disease and Mild Cognitive Impairment. Jama. 2021;326:1627–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Franzen S, Smith JE, van den Berg E, Rivera Mindt M, van Bruchem-Visser RL, Abner EL, et al. Diversity in Alzheimer’s disease drug trials: The importance of eligibility criteria. Alzheimer’s & Dementia. 2022;18:810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Babulal GM, Franzen S, Abner EL, Smith JE, van den Berg E, Mindt MR, et al. Diversity in Alzheimer’s disease drug trials: Reflections on reporting and social construction of race. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2022;18:867–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ashford M, Kabeto M, Wixom C, Nosheny R, Weiner MW, Weir D, et al. Effects of inclusion/exclusion criteria on ethnocultural and socioeconomic composition of participants in an Alzheimer’s disease clinical trial: analysis of Health and Retirement Study (HRS) data. Clinical trials in Alzheimer’s disease 2021: The general of prevention of Alzheimer’s disease; 2021. p. S 73–S170. [Google Scholar]
- [26].Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). International journal of epidemiology. 2014;43:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Association As. 2021 Alzheimer’s disease facts and figures. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2021;17:327–406. [DOI] [PubMed] [Google Scholar]
- [28].Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S. Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2008;4:305–9. [DOI] [PubMed] [Google Scholar]
- [29].Cooper C, Tandy AR, Balamurali TB, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18:193–203. [DOI] [PubMed] [Google Scholar]
- [30].Washington HA. Medical apartheid: The dark history of medical experimentation on Black Americans from colonial times to the present: Doubleday Books; 2006. [Google Scholar]
- [31].Rivera Mindt M, Byrd D, Saez P, Manly J. Increasing culturally competent neuropsychological services for ethnic minority populations: a call to action. The Clinical neuropsychologist. 2010;24:429–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. Journal of health care for the poor and underserved. 2010;21:879–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shamoo AE. Unethical medical treatment and research in US territories. Accountability in research. 2022:1–14. [DOI] [PubMed] [Google Scholar]
- [34].NIA. National Institute on Aging: National strategy for recruitment and participation in Alzheimer’s and related dementias Clinical Research. In: https://www.nia.nih.gov/research/recruitment-strategy, editor.2018. [DOI] [PMC free article] [PubMed]
- [35].Juster FT, Suzman R. An overview of the Health and Retirement Study. Journal of Human Resources. 1995:S7–S56. [Google Scholar]
- [36].Burke G, Lima J, Wong ND, Narula J. The Multiethnic Study of Atherosclerosis. Global heart. 2016;11:267–8. [DOI] [PubMed] [Google Scholar]
- [37].Bennett, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Current Alzheimer Research. 2012;9:646–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kuo P-L, Schrack JA, Shardell MD, Levine M, Moore AZ, An Y, et al. A roadmap to build a phenotypic metric of ageing: insights from the Baltimore Longitudinal Study of Aging. Journal of Internal Medicine. 2020;287:373–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic community health study/study of Latinos (HCHS/SOL). Diabetes care. 2014;37:2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tang M-X, Cross P, Andrews H, Jacobs D, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean hispanics, and caucasians in northern Manhattan. Neurology. 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- [41].Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago health and aging project (CHAP). Journal of Alzheimer’s Disease. 2003;5:349–55. [DOI] [PubMed] [Google Scholar]
- [42].Park VT, Meyer OL, Tsoh JY, Vuong Q, Bang J, Hinton L, et al. Collaborative Approach for Asian Americans and Pacific Islanders Research and Education (CARE): A recruitment registry for Alzheimer’s disease and related dementias, aging and caregiver-related research. Alzheimer’s & Dementia. 2021;17:e053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, et al. Assessment of Racial Disparities in Biomarkers for Alzheimer Disease. JAMA neurology. 2019;76:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9:734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. Journal of the American Geriatrics Society. 2003;51:169–77. [DOI] [PubMed] [Google Scholar]
- [46].O’Bryant S, Petersen M, Hall J, Johnson L, Yaffe K, Braskie M, et al. Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: Results from the HABLE study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2022;18:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public health. 2010;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sorlie P, Wei GS. Population-Based Cohort Studies: Still Relevant? Journal of the American College of Cardiology. 2011;58:2010–3. [DOI] [PubMed] [Google Scholar]
- [49].Weiner MW, Nosheny R, Camacho M, Truran-Sacrey D, Mackin RS, Flenniken D, et al. The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2018;14:1063–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Veitch DP, Weiner MW, Aisen PS, Beckett LA, DeCarli C, Green RC, et al. Using the Alzheimer’s Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2022;18:824–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Majoka MA, Schimming C. Effect of Social Determinants of Health on Cognition and Risk of Alzheimer Disease and Related Dementias. Clinical Therapeutics. 2021;43:922–9. [DOI] [PubMed] [Google Scholar]
- [52].Camacho MR, Weiner MW, González HM, Mayeda ER, Alaniz R, Ashford MT, et al. Improved methods for recruiting Latino participants for Alzheimer’s disease research using the Brain health Registry. Alzheimer’s Association International Conference. Denver, Colorado: Alzheimer’s and Dementia; 2021. p. e056193. [Google Scholar]
- [53].Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, et al. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin N Am. 2005;15:869–77, xi–xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ashford MT, Okonkwo O, Rivera Mindt M, Raman R, Miller G, Donohue MC, et al. EFFORTS TO IMPROVE RECRUITMENT AND ENGAGEMENT OF UNDERREPRESENTED POPULATIONS IN THE ALZHEIMER’S DISEASE NEUROIMAGING INITIATIVE (ADNI) STUDY. 14th Conference Clinical Trialson Alzheimer’s Disease. Boston, MA, USA: Journal of the Prevention of Alzheimer’s Disease; 2021. p. LB05. [Google Scholar]


