Abstract
Dual cognitive and mobility impairments are associated with an increased risk of dementia. Recent studies examining temporal trajectories of mobility and cognitive function in aging found that dual decline is associated with higher dementia risk than memory decline or gait decline only. Although initial data show that individuals with dual decline or impairment have excessive cardiovascular and metabolic risk factors, the causes of dual decline or what underlies dual decline with a high risk of dementia remain largely unknown. In December 2021, the National Institute on Aging Intramural and Extramural Programs jointly organized a workshop on Biology Underlying Moving and Thinking to explore the hypothesis that older persons with dual decline may develop dementia through a specific pathophysiological pathway. The working group discussed assessment methods for dual decline and possible mechanisms connecting dual decline with dementia risk and pinpointed the most critical questions to be addressed from a translational perspective.
The core questions
Dementia is generally considered a disease of the brain that causes impairment in cognitive domains. Recent studies have found that mobility decline or impairment may reveal brain abnormalities early in the aging process and may predict future cognitive impairment and dementia. More recent studies of older populations performed across different countries have consistently demonstrated that older persons with dual decline in memory and mobility simultaneously, in particular gait speed, have a very high risk of developing dementia1–3. However, little is known about the pathophysiological changes that link the age-accelerated dual decline in memory and gait speed (from now defined as “dual decline”) with future dementia risk. For example, why do some older persons show accelerated dual decline in memory and gait compared to the average population? And, perhaps even more important, why do these individuals have a high risk of developing dementia? An emerging hypothesis in the literature proposes that (1) older persons who experience dual decline have specific risk factors, and (2) dual decline is the clinical manifestation of the pathology connected with those risk factors, and this specific pathology eventually causes dementia. Despite the fact that robust literature demonstrates that mobility impairment is an early sign of brain changes on the pathway to dementia, the assessment of mobility impairment is often omitted from the clinical evaluation of older patients4. On December 7th, 2021, the NIA Intramural and Extramural Programs jointly organized a workshop on Biology Underlying Moving and Thinking to extend the discussion of the possible mechanisms that connect age-related dual cognitive and mobility decline with future dementia risk and to identify the most critical questions to be addressed that may stimulate the application of this new knowledge to the diagnosis and care of older patients.
Why is it important to study dual decline?
In aging, mobility and cognitive function are correlated, and, in most instances, gait speed declines faster than cognitive function, especially memory5, 6. Over the past two decades, neuroimaging and fluid biomarker research has led to a sequential model of the events that are biologically detected on the pathways to Alzheimer’s disease and dementia7. Overall, β-amyloid deposition in the brain is the initial pathological change, followed by tau deposition, and subsequently neuronal injury and dysfunction and brain atrophy, which lead to changes in memory and cognitive-related competence and behavior (Figure 1). Few studies have examined their joint clinical evolution or simultaneous change. It is noteworthy that in this widely acknowledged model, the occurrence of mobility impairment was not considered. Therefore, at what stage mobility impairment occurs is not fully clarified. In this sequence of the pathway to dementia, it remains unclear whether mobility impairment occurs prior to or after cognitive impairment. It is also unknown whether the occurrence of mobility impairment early in the pathway before clear signs of β-amyloid deposition or late in the pathway at the time of tau deposition and/or neuronal injury represents different pathophysiological routes to dementia (Figure 1).
Figure 1. Hypothesized conceptual framework of mobility in the clinical disease stage of dementia, modified from Jack et al. 20107.
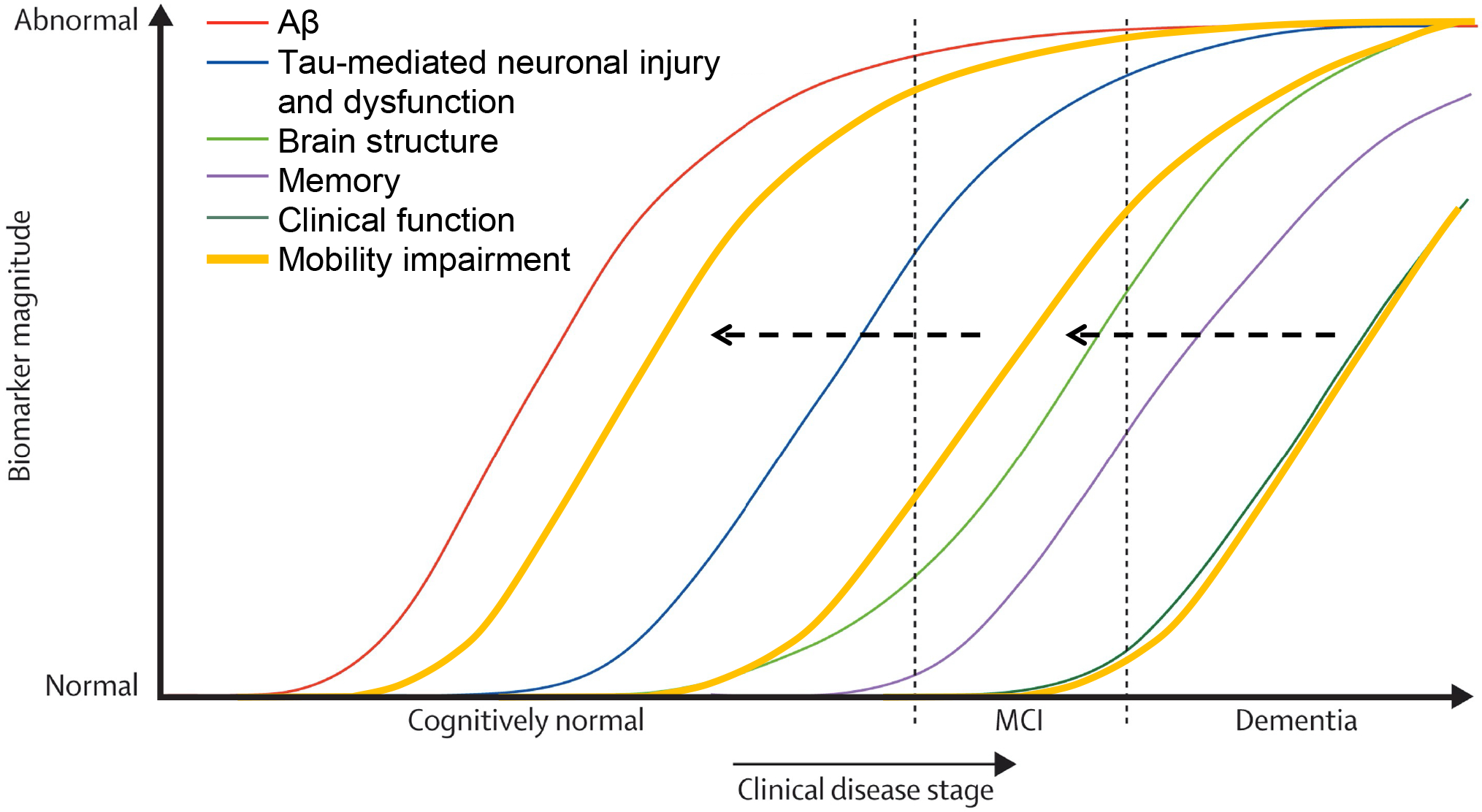
The added curves of mobility impairment are hypothesized to occur either before or after brain structural changes. The occurrence of mobility impairment at different stages may indicate different types of pathologies. (Obtaining permission from the publisher Elsev
While studies have described the appearance of mobility impairment across the clinical disease stages, from early normal aging to symptomatic dementia, initial research in this field focused almost exclusively on early mobility changes in normal aging without overt neurological symptoms. For example, mobility impairment, such as slow gait or mild parkinsonian signs, is associated with faster cognitive decline and a higher risk of incident mild cognitive impairment or dementia. However, the strength of the reported associations between mobility impairment and future dementia risk varies across studies8–10. Recent studies have considered dual cognitive and mobility impairments, a condition where both mobility and cognition, especially memory, decline or impair together. For instance, motoric cognitive risk (MCR) syndrome, first proposed in 2013, considers the co-occurrence of cognitive complaints and slow gait and has been assessed in different population studies. MCR is more strongly associated with dementia risk compared to its individual criterion of subjective cognitive complaints or slow gait only11, 12. Older adults with gait abnormalities are twice as likely to develop dementia than those without gait abnormalities, whereas older adults with MCR are more than three times more likely to develop dementia than those without MCR. A recent concept of physio-cognitive decline syndrome (PCDS) includes slow gait and/or muscle weakness for mobility impairment13. Of note, both MCR and PCSD have been defined based on cross-sectional data. More recently, researchers operationalized “dual decline” using longitudinal trajectories of mobility and cognition over time before dementia3, 14 (Table 1). In line with previous findings on MCR and dementia risk, those with dual decline in gait speed and cognitive function, especially memory, had a higher dementia risk than those with gait or memory decline only1, 3, 14. These studies also suggested that tracking trajectories may provide an additional predictive value for future dementia risk than assessment at one point in time and is less subject to selection and attrition biases. Examining trajectories of dual decline over time and tracking pathophysiological and biomarker changes that occur simultaneously with dual decline may allow more robust inferences about the pathophysiology implicated, both in terms of the study of risk factors and outcomes.
Table 1.
Operational definitions of dual cognitive and mobility decline or impairment
| Syndrome | Motoric cognitive risk syndrome (Verghese et al. 2013) | Motor and cognitive trajectories before dementia (Montero-Odasso et al. 2018) | Physio-cognitive decline syndrome (Chen et al. 2019) | Dual decline in memory and gait (Tian et al. 2020) |
|---|---|---|---|---|
| Cognitive function | Subjective cognitive complaint | Faster cognitive decline (MoCA) | Poor cognitive function (any domain) | Faster memory decline (CVLT) |
| Mobility | Slow gait speed | Faster gait decline | Slow gait speed or weakness | Faster gait decline |
| Number of assessments overtime required | 1 | 2+ | 1 | 2+ |
Emerging hypotheses
As the current evidence shows that dual decline is strongly related to aging and future dementia, factors contributing to the aging process and deficits in the central nervous system (CNS) may underlie the relationship of dual decline with high dementia risk. This is consistent with the geroscience hypothesis that the biological changes of aging are at the root of age-associated chronic diseases as well as physical and cognitive impairments. With this hypothesis in mind, we first discussed how pathophysiological changes in the CNS can cause dual decline, and then discussed causes that were not primarily neurological but may still lead to the same outcome.
Putative mechanisms linking dual decline and dementia
Pathophysiological changes that connect dual decline and dementia may include vascular burden, inflammation15, cellular senescence in the brain – autophagy affecting both cognition and mobility, mitochondrial dysfunction, and neuropathology such as glial dysfunction, and amyloid and tau deposition (Figure 2). Initial cognitive and neuroimaging studies have revealed specific brain characteristics associated with dual impairment, although definitions of dual impairment are somewhat heterogeneous across studies. Those with dual impairment, such as MCR and dual decline, showed structural brain abnormalities related to locomotion, sensorimotor integration, and cognition, including specific regions in the frontal, parietal, and temporal areas as well as cerebellum16, 17. Those with dual decline also showed cognitive declines in multiple domains compared to others who had memory or gait decline only and no decline, such as verbal fluency, attention, and sensorimotor function17. Studies searching for other characteristics associated with dual impairment have reported associations with demographic factors, cardiometabolic comorbidities, health-related biomarkers, and genetics. Those who experienced dual decline, compared to those who did not, were more frequently men and Apolipoprotein E ε4 carriers and those with diagnoses of hypertension or dyslipidemia2, 17. Those with dual decline also showed the most extensive alterations in metabolomic profiling of lipids and lipid metabolism, especially lysophosphatidylcholines (lysoPCs)18. A recent systematic review showed metabolites from sphingolipids and the sphingolipid metabolism pathway are important for both memory and gait impairments19. In agreement with the metabolic origin of dual decline, MCR is found to be associated with obesity-related polygenetic risk scores for body mass index and waist circumference20. It is worth noting that the lysoPCs are the main building blocks for cardiolipin, an essential lipid for the regulation and assemblage of electron transport complexes and mitochondrial morpho-functional architecture. Mitochondrial dysfunction can also lead to inflammatory pathways, and inflammation has been associated with impairment of cognition and mobility15. Thus, this observation led to the hypothesis that dual decline may be a clinical manifestation of accelerated mitochondrial dysfunction due to excessive ROS-induced cardiolipin oxidation and impaired resynthesis21. Two primary drivers of cognition and mobility are the CNS and the skeletomuscular system. Both systems are among the most energy-demanding tissues and are susceptible to a discrepancy between energy demand and availability. Age-related mitochondrial stress, production of reactive oxidant species (ROS), excessive ROS-induced cardiolipin oxidation, and activation of multiple inflammatory pathways can lead to impaired energy metabolism in the CNS and skeletomuscular system, ultimately leading to dual decline.
Figure 2. Emerging hypotheses of mechanisms connecting dual decline and impairment to future risk.
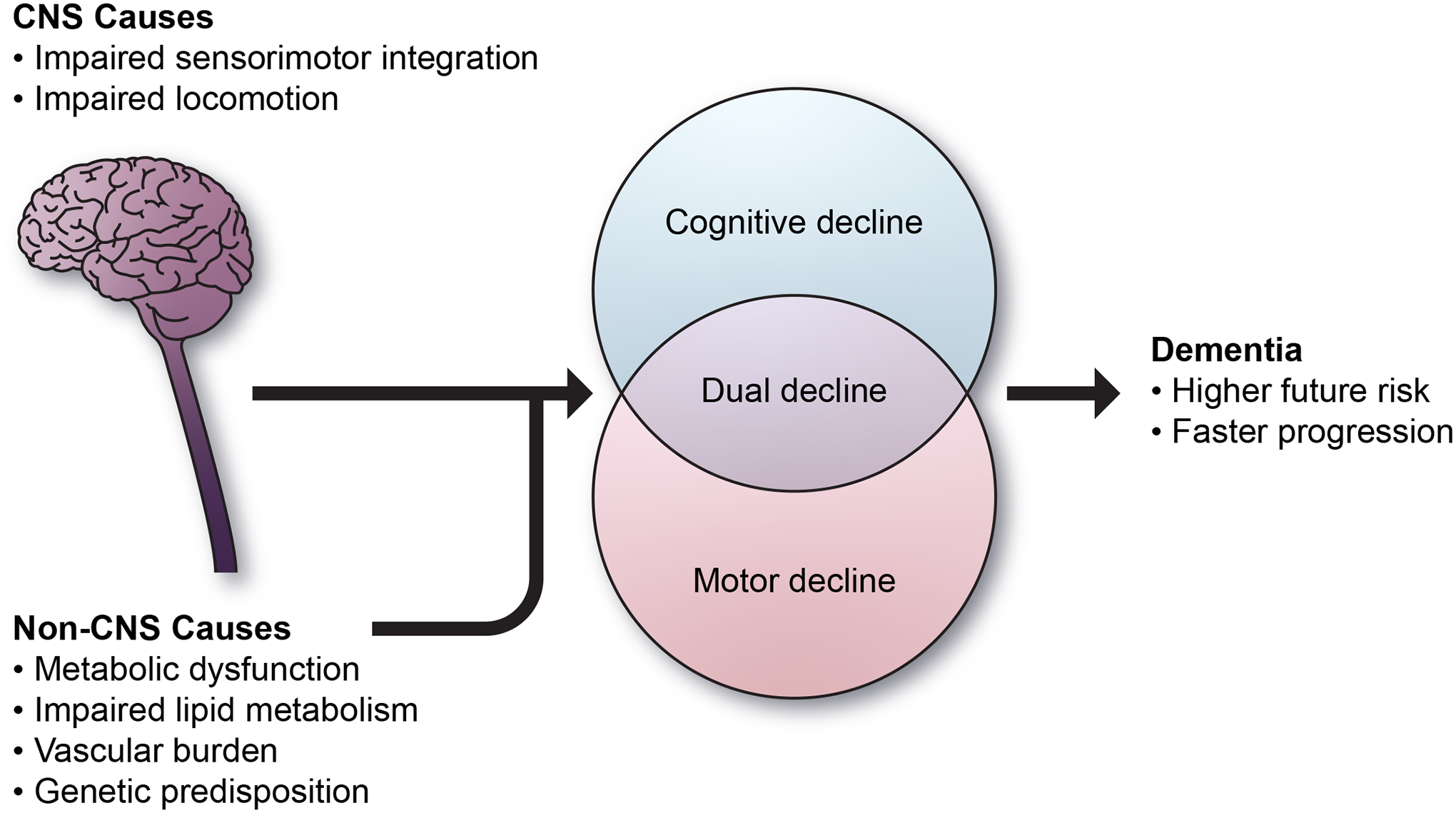
The accumulating burden of ADRD pathologies outside the cerebrum may be sufficient to account for motor impairment and serve as a proxy for a higher burden of total CNS pathologies.
What are the questions to be addressed and what does the field need?
Emerging research is aimed at understanding mechanisms underlying dual impairment in the attempt to further elucidate pathophysiological pathways to better inform early detection, prevention, and effective intervention for dual decline and impairment. An overarching question is whether the identification of dual decline is just a screening target or may also provide a subclassification of dementia that may hypothetically respond to different interventions. Gait speed and verbal memory can be assessed clinically on a routine basis and identification of dual decline may be seen as early signs of an underlying dementia process. Older persons with dual decline may need additional medical attention. Data from the literature suggest that dual decline is associated with excessive cardiovascular and metabolic risk factors and diseases. Thus, it is possible that the identification and treatment of cardiovascular and metabolic risk factors in this population reduce the risk of dementia, and this hypothesis should be addressed in future studies. This and other critical questions were discussed at the workshop from three perspectives: 1) Develop a standard operational definition; 2) describe the biological and physiological systems underlying dual decline; 3) start developing strategies for dementia prevention in those with dual decline and impairment.
Define the concept:
Previous studies have used various methods to operationally define and detect dual decline, but none of these methods is free of problems and questions remain (Table 1). What mobility measure should we focus on? Gait speed is one commonly used mobility measure due to its easy access to clinics, cost-effectiveness, and robustness, but because slow gait may be caused by a wide variety of impairments in different physiological systems, one key question is how to redefine slow gait as a more direct indicator of the CNS problems than impairments in other systems. As an example, we recently found that a combination of slow gait and low activity fragmentation is more strongly associated with dementia risk than slow gait alone22. Low activity fragmentation, derived from the accelerometry in a free-living environment, may indicate a lack of the use of compensation strategies and thus identify those with compromised brain health. In addition, dual-task gait, walking while performing a cognitively demanding task such as talking, is a marker of the motor-cognitive interface because it isolates the cognitive control of gait. Low performance in dual-task gait has been associated with future dementia23. Other measures, such as step-to-step gait parameters, or behavioral compensation for slow gait, are worth investigating and may be used in conjunction with slow gait speed to identify subgroups whose slow gait is due to CNS problems. What cognitive measure or domain should we focus on? One recent study examined the relationships between dual decline in gait speed and multiple cognitive domains and found that dual decline in gait and memory was associated with the highest dementia risk than dual decline in other cognitive domains1. Among those who show early signs of dual decline, would specific blood biomarkers help identify those at high risk of developing dementia? Initial genetic-based and omics-based data have shown plasma metabolomic profiles of dual decline, the polygenic inheritance of MCR, and shared cortical proteins to both motor and cognitive resilience18, 20, 24. Specifically, certain metabolites of lysoPCs important for cardiolipin, multifunctional proteins, and obesity-related genetic traits may be early biomarkers of dual decline.
Understand mechanisms:
Establishing a unifying definition for dual impairment that can be extensively used in clinical and epidemiological studies is a research priority, and understanding its relationship with dementia and the mediating effect of biomarkers may provide insights into potential mechanisms. What are the pathophysiological profiles of dual impairment? Brain damage that may cause dual cognitive and mobility impairments include neuroinflammatory processes, microstructural integrity, cerebral microbleeds, cerebral amyloid angiopathy, microinfarcts, and braking in specific brain functional networks25, 26. Other contributing factors, such as vascular burden, inflammation, and mitochondrial dysfunction, need further support from empirical data, especially longitudinal data to track pathophysiological changes over time. What are the personal characteristics of dual impairment? Given known sex differences in mobility disability, brain aging, and dementia risk, it will be important to determine whether risk factors, biological changes, and outcomes associated with dual decline are different in men and women. Other stratification characteristics that will be important to study include race, personality traits, and physical activity levels. Knowledge of these characteristics may both improve the predictive value of a dual decline diagnosis and provide clues about possible underlying pathophysiology. How can the system biology approach further contribute to our understanding of underlying mechanisms? Multi-omics data as well as their interaction and integration may advance our understanding of complex biology and pathways using artificial intelligence or machine learning. In this context, blood biomarkers related to amyloid and tau deposition and macro- and microvascular systems, such as trafficking metabolites and microbiome, are possible targets of future studies.
Respond to the problem:
The ultimate scope of research in this field is to develop interventions for slowing down the clinical progress of dual decline and at least moderating the high risk of developing dementia. To date, there is no definitive evidence that non-pharmacological interventions may prevent cognitive decline. However, there is limited evidence that physical activity (eg. LIFE trial) and dietary intake (eg. Mediterranean diet) may slow down physical function decline with aging. We list several recent intervention studies which show beneficial effects on the CNS, skeletomuscular system or vascular system which may lead to benefits to dual decline. What non-pharmacological interventions may effectively prevent dual decline? Some non-pharmacological studies may prevent dual decline but empirical data are needed, such as transcranial direct current stimulation studies27, cognitive training, cognitive stimulation/engagement training28–34, blood pressure management35, dietary restriction, weight loss, and increased physical activity36, 37. Specific studies, such as social dance, computerized brain games, Experience Corps, and the ACTIVE (Advanced Cognitive Training for Independent and Vital Elderly) trial, were discussed at the workshop. Recent multi-modal interventions seem to link multiple systems to prevent or maintain cognitive and motor function, such as the SYNERGIC (SYNchronizing Exercises, Remedies in GaIt and Cognition) trial38, FINGER (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) intervention39, and recent World-Wide FINGERS network40. The SYNERGIC trial combines physical resistance and aerobic exercises with cognitive training and vitamin D supplementation and has shown efficacy in improving both gait speed and cognition in older adults with Mild Cognitive Impairment, a pre-dementia state. An “at home” trial, the SYNERGIC@Home trial, is being conducted and now delivering interventions remotely to accommodate COVID-19 pandemic restrictions38, 41. The FINGER intervention included multiple lifestyle components: physical exercise, cognitive training, dietary guidance, social activities, and management of metabolic and vascular risk factors. Results from this FINGER trial showed improved cognition and reduced dementia risk42, 43. What is needed for future non-pharmacological interventions? Several key elements remain unclear. For instance, the most effective features of exercise, such as type, duration, frequency, intensity, and personalization should be considered and compared. Recent data suggest that more physical activity at personalized moderate-to-high intensity levels may prevent brain microstructural decline localized to the temporal area44. Long-term maintenance effects and criteria of outcome measures to track effectiveness, such as biomarkers and brain scans, need further investigation. What drug therapies may effectively prevent dual decline? Understanding biological pathways is key to identifying therapeutic targets. Currently, microbiome-mediated therapies are being examined for aging and healthspan in marmosets45. Preliminary results from the anti-amyloid antibody Lecanemab trial show positive effects on both primary and secondary outcomes of dementia. Whether these drug therapies prevent dual decline during aging remains unknown. The novel genes and omics-based markers can be targeted in drug discovery to develop new personalized medicine therapies for dual decline and impairment46–48. Notably, while mobility is often not considered in intervention studies targeting dementia, there is enough evidence to recommend that mobility assessment could be used as a secondary endpoint to measure efficacy.
Challenges and future opportunities
An important challenge to overcome for the advancement of the field is to agree on one operational definition of dual cognitive and mobility impairment that can be applied systematically across observational cohorts and clinical studies. Understanding dual decline where declines occur simultaneously and over time will require leveraging data collected in multiple observational and intervention studies and examining similarities and differences. This work would be possible only if different studies adopt operational definitions of dual decline that are similar enough to be harmonized across studies. Validation of biomarkers and the genetic basis of dual impairment will also likely require large numbers that can only be obtained by combining data from different studies and homogeneity of the reference phenotype would greatly improve research in this field. A widely accepted definition of the syndrome will also be helpful to start designing effective and customized non-pharmacological interventions that address sensory handicaps, tech literacy, and transferring basic skills to real-world tasks49, 50.
Conclusions
Dual cognitive and mobility impairments are aging phenotypes related to a higher risk of dementia than mobility or cognitive impairment only. The root causes that are common to both are unknown. Initial evidence of the unique characteristics of dual decline and impairment, including clinical phenotype, biomarkers, and genetics, may suggest mechanisms of vascular burden, inflammation, and mitochondrial dysfunction. It may be premature to attempt interventions when knowledge gaps remain about underlying mechanisms and specificity for current outcome measures. Thus, identifying risk factors and understanding the etiology of dual decline are key routes for future prevention and intervention strategies, which warrant investigation.
Acknowledgment
We would like to acknowledge Drs. Coryse St. Hillaire-Clarke, Lyndon Joseph, and Chhanda Dutta for their participation as session chairs at the workshop. This work was supported in part by the Intramural Research Program of the National Institute on Aging, NIH, Baltimore, MD.
References:
- 1.Collyer TA, Murray AM, Woods RL, et al. Association of Dual Decline in Cognition and Gait Speed With Risk of Dementia in Older Adults. JAMA Netw Open. May 2 2022;5(5):e2214647. doi: 10.1001/jamanetworkopen.2022.14647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montero-Odasso M, Speechley M, Muir-Hunter SW, et al. Dual decline in gait speed and cognition is associated with future dementia: evidence for a phenotype. Age Ageing. Oct 23 2020;49(6):995–1002. doi: 10.1093/ageing/afaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian Q, Resnick SM, Mielke MM, et al. Association of Dual Decline in Memory and Gait Speed With Risk for Dementia Among Adults Older Than 60 Years: A Multicohort Individual-Level Meta-analysis. JAMA Netw Open. Feb 5 2020;3(2):e1921636. doi: 10.1001/jamanetworkopen.2019.21636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility--giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. May 2014;311(20):2061–2. doi: 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. Dec 2014;69(12):1536–44. doi: 10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayakody O, Breslin M, Ayers E, et al. Relative Trajectories of Gait and Cognitive Decline in Aging. J Gerontol A Biol Sci Med Sci. Nov 15 2021; doi: 10.1093/gerona/glab346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. Jan 2010;9(1):119–28. doi: 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauchet O, Annweiler C, Callisaya ML, et al. Poor Gait Performance and Prediction of Dementia: Results From a Meta-Analysis. J Am Med Dir Assoc. Jun 1 2016;17(6):482–90. doi: 10.1016/j.jamda.2015.12.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. Aug 2010;67(8):980–6. doi: 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Q, An Y, Resnick SM, Studenski S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: Results from the Baltimore Longitudinal Study of Aging. Age Ageing. May 1 2017;46(3):445–451. doi: 10.1093/ageing/afw185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. Nov 28 2002;347(22):1761–8. doi: 10.1056/NEJMoa020441 [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. Apr 2013;68(4):412–8. doi: 10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LK. Physio-cognitive decline syndrome: A new proposal. Aging Medicine and Healthcare; 2019:1. [Google Scholar]
- 14.Montero-Odasso M, Speechley M, Muir-Hunter SW, et al. Motor and Cognitive Trajectories Before Dementia: Results from Gait and Brain Study. J Am Geriatr Soc. Sep 2018;66(9):1676–1683. doi: 10.1111/jgs.15341 [DOI] [PubMed] [Google Scholar]
- 15.Walker KA, Basisty N, Wilson DM 3rd, Ferrucci L. Connecting aging biology and inflammation in the omics era. J Clin Invest. Jul 15 2022;132(14)doi: 10.1172/JCI158448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumen HM, Schwartz E, Allali G, et al. Cortical Thickness, Volume, and Surface Area in the Motoric Cognitive Risk Syndrome. J Alzheimers Dis. 2021;81(2):651–665. doi: 10.3233/JAD-201576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian Q, Studenski SA, Montero-Odasso M, Davatzikos C, Resnick SM, Ferrucci L. Cognitive and neuroimaging profiles of older adults with dual decline in memory and gait speed. Neurobiol Aging. Jan 2021;97:49–55. doi: 10.1016/j.neurobiolaging.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 18.Tian Q, Shardell M, Tanaka T, Kuo P, Resnick S, Ferrucci L. Metabolomic signatures of dual decline in memory and gait: An aging phenotype of high risk of dementia. 2021:e057749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian Q, Mitchell BA, Corkum AE, Moaddel R, Ferrucci L. Metabolites Associated with Memory and Gait: A Systematic Review. Metabolites. Apr 15 2022;12(4)doi: 10.3390/metabo12040356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sathyan S, Wang T, Ayers E, Verghese J. Genetic basis of motoric cognitive risk syndrome in the Health and Retirement Study. Neurology. Mar 26 2019;92(13):e1427–e1434. doi: 10.1212/WNL.0000000000007141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells. Jul 16 2019;8(7)doi: 10.3390/cells8070728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian Q, Studenski SA, An Y, et al. Association of Combined Slow Gait and Low Activity Fragmentation With Later Onset of Cognitive Impairment. JAMA Netw Open. Nov 1 2021;4(11):e2135168. doi: 10.1001/jamanetworkopen.2021.35168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. Association of Dual-Task Gait With Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA Neurol. Jul 1 2017;74(7):857–865. doi: 10.1001/jamaneurol.2017.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zammit AR, Yu L, Petyuk V, et al. Cortical Proteins and Individual Differences in Cognitive Resilience in Older Adults. Neurology. Mar 29 2022;98(13):e1304–e1314. doi: 10.1212/WNL.0000000000200017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchman AS, Yu L, Wilson RS, et al. Progressive parkinsonism in older adults is related to the burden of mixed brain pathologies. Neurology. Apr 16 2019;92(16):e1821–e1830. doi: 10.1212/WNL.0000000000007315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugenschmidt CE, Burdette JH, Morgan AR, Williamson JD, Kritchevsky SB, Laurienti PJ. Graph theory analysis of functional brain networks and mobility disability in older adults. J Gerontol A Biol Sci Med Sci. Nov 2014;69(11):1399–406. doi: 10.1093/gerona/glu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manor B, Zhou J, Harrison R, et al. Transcranial Direct Current Stimulation May Improve Cognitive-Motor Function in Functionally Limited Older Adults. Neurorehabil Neural Repair. Sep 2018;32(9):788–798. doi: 10.1177/1545968318792616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumen HM, Ayers E, Wang C, Ambrose AF, Verghese J. A social dancing pilot intervention for older adults at high risk for Alzheimer’s disease and related dementias. Neurodegener Dis Manag. Aug 2020;10(4):183–194. doi: 10.2217/nmt-2020-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verghese J, Mahoney JR, Ayers E, Ambrose A, Wang C, Holtzer R. Computerised cognitive remediation to enhance mobility in older adults: a single-blind, single-centre, randomised trial. Lancet Healthy Longev. Sep 2021;2(9):e571–e579. doi: 10.1016/s2666-7568(21)00173-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marusic U, Verghese J, Mahoney JR. Cognitive-Based Interventions to Improve Mobility: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. Jun 2018;19(6):484–491 e3. doi: 10.1016/j.jamda.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith-Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of Cognitive Training on Balance and Gait in Older Adults. J Gerontol B Psychol Sci Soc Sci. May 2015;70(3):357–66. doi: 10.1093/geronb/gbt097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith-Ray RL, Makowski-Woidan B, Hughes SL. A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact Black older adults. Health Educ Behav. Oct 2014;41(1 Suppl):62S–9S. doi: 10.1177/1090198114537068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprague BN, Phillips CB, Ross LA. Cognitive Training Attenuates Decline in Physical Function Across 10 Years. J Gerontol B Psychol Sci Soc Sci. Jun 14 2021;76(6):1114–1124. doi: 10.1093/geronb/gbaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marusic U, Verghese J, Mahoney JR. Does Cognitive Training Improve Mobility, Enhance Cognition, and Promote Neural Activation? Front Aging Neurosci. 2022;14:845825. doi: 10.3389/fnagi.2022.845825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Downey A, Stroud C, Landis S, Leshner AI. Preventing Cognitive Decline and Dementia: A Way Forward. The National Academies Press; 2017. [PubMed] [Google Scholar]
- 36.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. Nov 2011;66(11):1226–37. doi: 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sink KM, Espeland MA, Castro CM, et al. Effect of a 24-Month Physical Activity Intervention vs Health Education on Cognitive Outcomes in Sedentary Older Adults: The LIFE Randomized Trial. JAMA. Aug 25 2015;314(8):781–90. doi: 10.1001/jama.2015.9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montero-Odasso M, Almeida QJ, Burhan AM, et al. SYNERGIC TRIAL (SYNchronizing Exercises, Remedies in Gait and Cognition) a multi-Centre randomized controlled double blind trial to improve gait and cognition in mild cognitive impairment. BMC Geriatr. Apr 16 2018;18(1):93. doi: 10.1186/s12877-018-0782-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. Nov 2013;9(6):657–65. doi: 10.1016/j.jalz.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 40.Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. Jul 2020;16(7):1078–1094. doi: 10.1002/alz.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGibbon C, Jarrett P, Handrigan G, et al. Protocol for SYNchronising Exercises, Remedies in GaIt and Cognition at Home (SYNERGIC@Home): feasibility of a home-based double-blind randomised controlled trial to improve gait and cognition in individuals at risk for dementia. BMJ Open. Mar 31 2022;12(3):e059988. doi: 10.1136/bmjopen-2021-059988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solomon A, Handels R, Wimo A, et al. Effect of a Multidomain Lifestyle Intervention on Estimated Dementia Risk. J Alzheimers Dis. 2021;82(4):1461–1466. doi: 10.3233/JAD-210331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. Jun 6 2015;385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 44.Tian Q, Schrack JA, Landman BA, Resnick SM, Ferrucci L. Longitudinal associations of absolute versus relative moderate-to-vigorous physical activity with brain microstructural decline in aging. Neurobiol Aging. Aug 2022;116:25–31. doi: 10.1016/j.neurobiolaging.2022.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross CN, Reveles KR. Feasibility of fecal microbiota transplantation via oral gavage to safely alter gut microbiome composition in marmosets. Am J Primatol. Dec 2020;82(12):e23196. doi: 10.1002/ajp.23196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu L, Petyuk VA, Gaiteri C, et al. Targeted brain proteomics uncover multiple pathways to Alzheimer’s dementia. Ann Neurol. Jul 2018;84(1):78–88. doi: 10.1002/ana.25266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mostafavi S, Gaiteri C, Sullivan SE, et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat Neurosci. Jun 2018;21(6):811–819. doi: 10.1038/s41593-018-0154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchman AS, Yu L, Oveisgharan S, et al. Cortical proteins may provide motor resilience in older adults. Sci Rep. May 28 2021;11(1):11311. doi: 10.1038/s41598-021-90859-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. Jan 2014;62(1):16–24. doi: 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. Dec 20 2006;296(23):2805–14. doi: 10.1001/jama.296.23.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]


