Abstract
3D-printed microfluidic chips have been attracting a lot of attention as a way to realize rapid fabrication from digital 3D designs and to achieve ready-to-use products with simple procedures that minimize human intervention. The ability to print high-resolution chips that are at the same time transparent, biocompatible, and contain regions of dissimilar materials has been an ongoing challenge. 3D-printing transparent chips allow for the optical inspection of specimens containing cells and labelled biomolecules inside the chip. Multimaterial printing enables the choice of materials in every layer and can be applied in the fields of basic biology, biomedicine, and pharmacology. In this “print-pause-print” (PPP) protocol, we describe detailed strategies for fabricating transparent bio-microfluidic devices and multimaterial chips using stereolithographic 3D-printing. To print transparent bio-microfluidic chips, we developed a transparent resin based on poly(ethylene glycol) diacrylate (Mw: 258, PEG-DA-258) and a smooth chip surface technique; cells are successfully cultured on PEG-DA-258 prints and inside PEG-DA-258 microchannels. To demonstrate the multimaterial potential of the technique, we designed a molecular diffusion device that comprises parts made of two different materials: the channel walls, which are water-impermeable, and a porous barrier structure, which is permeable to small molecules that diffuse through it. The two materials were prepared from two different molecular-weight PEG-DA-based printing resins. Alignment of the two dissimilar-material structures is performed automatically by the printer during the printing process, which only requires a simple pause step to exchange the resins. The convenience and simplicity of microfabrication, amounting to a protocol that lasts less than 1 hr, could facilitate a variety of chip-based applications such as biomolecule analysis, cell biology, organ-on-a-chip, and tissue engineering.
INTRODUCTION
In the last three decades, microfluidics has been utilized in various areas including biomolecule analysis, cell biology, tissue engineering, chemical synthesis, and diagnostics making use of advantages such as small reagent consumption, short diffusion paths leading to rapid reaction times, integration capability with upstream and downstream steps, and portability.1–4 Microfluidic chips are generally fabricated with replica-molding and bonding methods based on soft lithography using poly(dimethoylsiloxane) (PDMS).5,6 Although PDMS-based molding techniques are able to provide micron-level features, complex fabrication steps result in time-consuming and labor-intensive procedures, especially when multilayer alignment is involved.7,8 In addition, microfabrication equipment (e.g. in a cleanroom facility) is often needed to produce the PDMS mold, which adds to the cost and complexity. In order for molded devices to be cost-effective, they also need to be mass-produced, which is a challenge for the customization of products in a short time. We and others have proposed that the adaptation of additive manufacturing technology to the production a microfluidic device will allow for the automated, assembly-free, customized production of complex 3D microdevices.9–12 In this protocol, we introduce the fabrication processes of two types of 3D-printed microfluidic devices (a transparent bio-microfluidic chip and a multimaterial molecule diffusion microchip) developed in our lab.13,14
Transparent 3D-printed bio-microfluidic chip
In bio-microfluidics, transparency is important for the detection of cellular processes (e.g. fluorescence reporters, immunohistochemistry, phase microscopy, etc.), measuring the progress and outcome of biochemical reactions (e.g. colorimetry), and real-time tracking of entities present in the channels (e.g. cells, droplets, organisms, etc.). A common problem of acrylate resins for stereolithography has been the addition of absorbers or photosensitizers that also confer an orange or yellow tint to the prints,15–17 although more advanced formulations with improved absorbers have addressed this problem recently.18,19 In addition, even when the resin is transparent, the roughness of the vat surface and that of the build plate becomes imprinted on every layer of the chip, causing light scattering and resulting in a translucent chip appearance. In our laboratory, we have developed a method for the stereolithographic 3D-printing of transparent microfluidic devices based on printing between two smooth surfaces. The two smooth surfaces could be either two glass plates13 or one glass plate and a Teflon film.18
Multimaterial 3D-printing for microfluidics
Microfluidic devices have been used for the precise manipulation of cell behavior with signaling molecules in various fields such as tissue engineering, cell biology, and drug delivery.20–22 Often these molecules are delivered through a porous barrier to control the rate of delivery; by fabricating a porous barrier in between impermeable channels in a microfluidic chip, precise transfer of the target molecule can be carried out through the porous barrier from a drug delivery channel into a tissue area where the cell behavior can be monitored.23–26 However, the fabrication process of these multimaterial microfluidic device includes a complicated, time-consuming, and expensive process of assembly and bonding of microchannels with the porous microstructure. Incorporation of various materials into/with microfluidics using extrusion-based printing has been demonstrated but lacks high resolution.27–35 Stereolithographic multimaterial 3D-printing provides an alternative high-resolution fabrication route. Advances in multimaterial stereolithography have been reported using multiple resins,36 elastomers,37 ceramics,38 and different cell-encapsulating resins39 or porous scaffolds,40 but with no specific application to microfluidics.
In this protocol, we demonstrate a fabrication method for stereolithographically printed multimaterial microfluidic devices. As a proof-of-concept application, we demonstrate a device featuring a porous barrier between two water-impermeable microfluidic channels for the selective diffusion of small molecules such as hydrogen ions or fluorescein molecules through the porous barrier.14 This multimaterial printing process shortens the manufacturing time of the porous barrier chip, eliminates bonding failures, and reduces overall chip fabrication costs. We expect multimaterial stereolithography to be of wide applicability in areas such as biochemical separations, drug delivery, diagnostics, and regenerative and personalized medicine.
Experimental design for 3D-printing transparent bio-microfluidic chip and multimaterial microfluidic chip
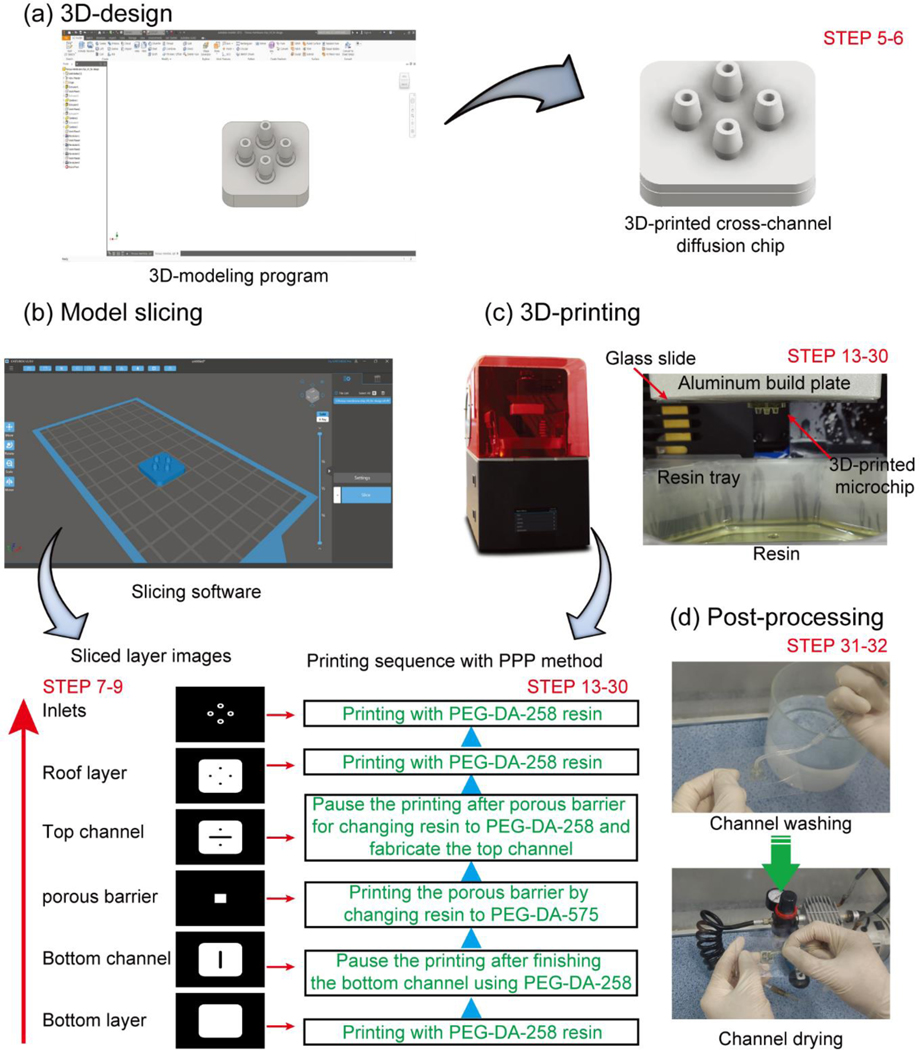
Fig. 1 shows the stereolithographic 3D-printing procedure to fabricate a transparent bio-microfluidic chip and a multimaterial molecule diffusion microchip. Prior to printing, a 3D-design of the microchip is drawn using a 3D-modeling program (Fig. 1a, Steps 5–6). The 3D-design is then digitally sliced into layers of a designated thickness using a slicing program. The sliced 2D-image files and printing conditions such as UV exposure time and light power intensity are sent to the 3D-printer to fabricate the microchip (Fig. 1b, Steps 7–9).
Fig. 1.
Stereolithographic 3D-printing procedure for fabricating a microfluidic chip. (a) 3D-design of a microfluidic chip using a 3D-modelling program from step 5 to 6. (b) Layer slicing of the 3D-designed object with a slicing program and selective sliced layer images from step 7 to 9. (c) Stereolithographic printing of the microfluidic chip with a photocurable resin on the surface-treated glass slide and printing sequence with PPP method from step 13 to 30. (d) Post-processing of the 3D-printed microfluidic chip using D.I. water and pressurized air from step 31 to 32.
The resin formulation and the roughness of the two surfaces that intervene in the fabrication of every layer (the vat surface and the surface of the build plate) both contribute to the transparency of the final print. The main component of the three 3D-printing resins used in this protocol is the PEG-DA monomer with a molecular weight of 258, 575, or 700, respectively. These monomers are fully transparent regardless of their molecular weight between 200 nm and 700 nm wavelength, but both the photoinitiator and the absorber/photosensitizer can have an absorption spectrum that affects the final color of the printed chip.41 The concentration of photoinitiator and absorber/photosensitizer can also affect the biocompatibility of the chip due to chemical leaching, so great care must be taken to thoroughly rinse the chips after printing (see section on post printing procedure from step 31 to 34).13,14,18
To create smooth printing surfaces, we adhere a surface-treated glass slide to the build plate of the 3D-printer and we retrofit our vat surface (originally PDMS-coated) with a glass surface. The build plate glass is treated with 3-(trimethoxysilyl)propyl methacrylate (TMSPMA) to ensure adhesion of the prints to the glass slide. In order to ensure that prints attach only to the glass build plate, surface of the resin vat needs to be hydrophobic.13 In our previous report, the vat glass surface is treated with SIGMACOTE® (Sigma-Aldrich), a hydrophobic silane. The other way to ensure the attachment of the printing object only to the build plate is to utilize a resin vat surface that consists of a Teflon film.18,42 After pouring the PEG-DA-258 resin into the resin vat, a transparent bio-microfluidic chip is fabricated by sending the sequence of sliced 2D-images to the stereolithographic 3D-printer.
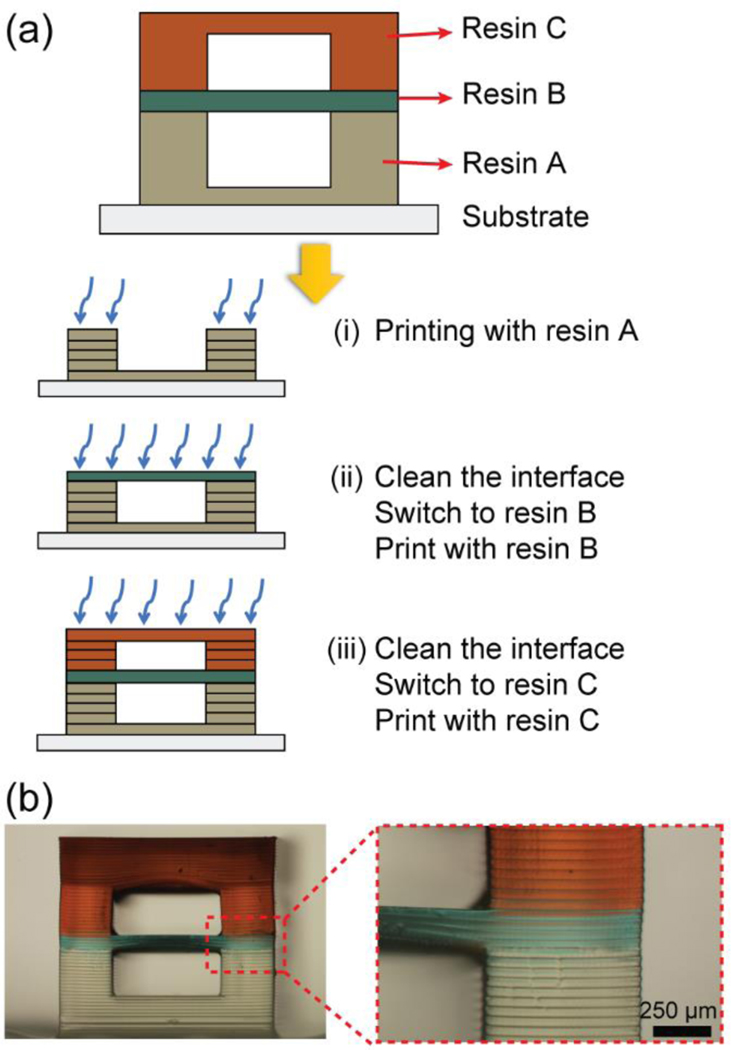
To fabricate the 3D-printed multimaterial chip, two different kinds of photocurable resin must be prepared, as the two resins will form materials with different properties – in this case, different porosity. The microfluidic channel in which fluid flows must be water-impermeable, whereas the porous barrier must be a porous material with a pore size that matches the molecular species to be filtered (Steps 3–4). Resin switching is carried out with a simple “print-pause-print” (PPP) process (Fig. 2), which entails the change of resin from one vat to another container at the appropriate time after pausing the print (when the build plate is separated from the vat). The PPP process can be carried out independently of the type of printer used.
Fig. 2.
Print-Pause-Print (PPP) protocol for 3D-printing microfluidic devices by stereolithography. (a) Schematic images of the multimaterial 3D-printing procedure with three different resins by using the PPP protocol. (b) Photograph of a 3D-printed stacked channel with PPP protocol.
The mechanical strength of the chip can be compromised if any given layer of the chip is entirely fabricated in the porous material. Therefore, we photocure the porous material only in the areas where it is needed as a barrier for molecular transport. Necessarily, this strategy leaves a gap in the subsequent layer of non-porous material, which requires a long UV exposure in order to complete the photocuring and fill the gap. To complete the microchip fabrication, uncured resin inside of the microchannel is washed away with D.I. water and pressurized air.
Limitations of our protocol
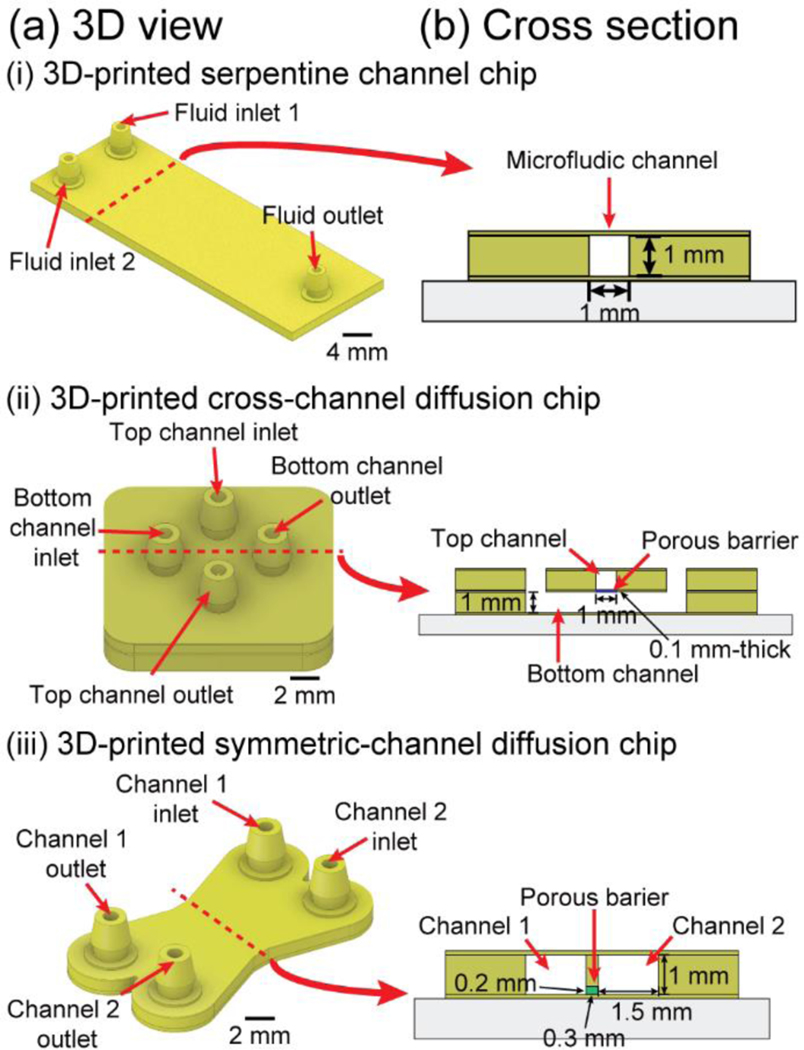
The 3D-printed microfluidic chip fabrication method is simple, rapid, inexpensive, and robust. However, as with any process, some limitations exist. The limitations are (i) printing resolution, (ii) limited resin selection, and (iii) the necessity of human intervention for multimaterial printing. Photolithographic and molding processes still achieve higher resolutions, as SLA-DLP 3D printing resolution is fundamentally limited by the printer’s pixel size, and resin formulations.15,18 In our lab, using a commercial printer with 27 μm projected pixel size and high-absorption transparent resins, we have achieved single-pixel-wide microchannels.18,42 For all practical purposes, since in stereolithography the resolution that can be achieved by any given resin is a function of the speed of the reaction (slower reactions result in more diffusive spread of the reactants), resin selection for high-resolution microfluidics is limited to acrylate resins because acrylates react very fast. In our previous research, we were able to print single-pixel-wide capillary channels (27 μm) with a 37 : 1 aspect ratio, not involving manual intervention.42 However, the “manual intervention” involved in multimaterial printing does not exert forces on the printed parts and only involves the liquids in the resin vat (not the part), while the part goes back to the original location using the same motors that are used in normal printing mode. A potential source of resolution loss could come from the time delays during the print-pause-print (PPP) process, since resolution in stereolithography depends on the (time-dependent) diffusion of reactants. If no action is taken to remove excess reactants from the part (e.g. washing, wiping, wicking), the features could broaden during the pause. Ongoing development in stereolithography resins is expected to yield more biocompatible formulations.18,19 Although, in the present form of this protocol, human intervention is required to print a multimaterial device, it is a simple procedure that could in principle be automated and already replaces a whole set of alignment and bonding processes.
MATERIALS
REAGENTS
Poly(ethylene glycol) diacrylate (MW=258) (PEGDA-258; Sigma Aldrich) ! CAUTION May cause skin irritants. Wear protective gloves and a lab coat while handling.
Poly(ethylene glycol) diacrylate (MW=575) (PEGDA-575; Sigma Aldrich) ! CAUTION May cause skin irritants. Wear protective gloves and a lab coat while handling.
Poly(ethylene glycol) diacrylate (MW=700) (PEGDA-700; Sigma Aldrich) ! CAUTION May cause skin irritants. Wear protective gloves and a lab coat while handling.
Irgacure 819 (IRG, BASF Corporation) ! CAUTION May cause an allergic skin reaction. Avoid skin contact to wear gloves.
2-isopropyl thioxanthone (ITX; Tokyo Chemical Industry) ! CAUTION ITX may be harmful if swallowed. Wear protective gloves and wash hands thoroughly after handling them.
Acetone (Sigma Aldrich) ! CAUTION Toxic, and unstable explosive. Handle it in the fume hood by wearing protective gloves and avoid flames.
Isopropyl alcohol (Sigma Aldrich) May cause eye irritation. Wear protective gloves and glasses while handling.
Deionized water (D.I. water)
3-(trimethoxysilyl)propyl methacrylate (TMSPMA; Sigma Aldrich)
SIGMACOTE® (Sigma Aldrich) ! CAUTION SIGMACOTE® is a flammable liquid that may cause skin corrosion/irritation, and serious eye damage/irritation. Handle it in the fume hood by wearing protective gloves and glasses and keep away from flames, and hot surfaces.
Hydrochloric acid (HCl; Sigma Aldrich) ! CAUTION Toxic and fume production reagent. HCl is harmful if exposed to the skin or inhaled. Use only in the fume hood and wear eye protection, lab coat, and gloves to avoid exposure.
Phenol red (Sigma Aldrich)
Fluorescein (Sigma Aldrich)
Phosphate buffered saline (PBS)
EQUIPMENT
Stereolithography 3D-printer (e.g., Ember DLP 3D-printer (Autodesk), Pico2-HD (Asiga), and ILIOS HD SLA printer kit)
3D modeling software (e.g., Inventor® (Autodesk), Solidworks (Dassault Systèmes Solidworks Corp.), and Rhinoceros (Robert McNeel & Associates))
Model slicing software (e.g., Print Studio (Autodesk), Chitubox (Chitubox), and Asiga Composer (Asiga))
UV lamp (B100 A, UVP)
Convection oven
50 mL Conical tube (SPL)
Aluminum foil
1 mm-thick glass slide (Fisher Science)
Hot plate
Oxygen plasma processing reactor (e.g., CUTE plasma system (FemtoScience Inc.))
Air compressor
Water bath (25 °C, still)
1 mL syringe
Silicon tube (I.D. 1.5 mm, O.D. 3.0 mm)
PROCEDURE
Resin preparation for transparent bio-microfluidic device ● TIMING 1 h
▲ CRITICAL STEP Perform STEP 1 through STEP 2 in the darkroom.
-
1
To make a UV-curable photoresin for stereolithographic 3D-printer, pour 40 g PEG-DA-258 monomer into a 50 mL conical tube wrapped with aluminum foil.
-
2
In our experiment for printing the bio-microfluidic device, 0.4% (w/w) IRG is chosen in terms of balancing resolution and absence of color. Vigorously stir the photoresin using a mini-vortexer to dissolve the initiator powder in the PEG-DA-258 monomer and then heat the mixture in a 70 °C oven for 30 min to completely dissolve IRG. ■ PAUSE POINT Addition of higher concentration of photoinitiator leads to the enhancement of radical reaction during 3D-printing, but it causes yellowing of the device and toxic chemical leaching after printing which affects cell viability on the 3D-printed device. Photoinitiator concentrations in excess of 1% can cause the reaction to be so exothermic as to cause fumes and “burn” the resin. On the other hand, insufficient photoinitiator causes the print to fail. ■ PAUSE POINT There are dozens of resin formulations that can be used in SLA/DLP 3D printers, but most of them are proprietary. The key parameter that allows for the generalization of a method is being open-source. One of the few resins that is open-source is PEG-DA, and as such it allows for dissemination of designs reproducibly across labs. If such resin formulations are disclosed, it can be actively used for various studies based on 3D printers, and it will be possible to accelerate the development of related industries.
Resin preparation for multimaterials 3D-printing ● TIMING 1 h
▲ CRITICAL STEP Perform STEP 3 through STEP 4 in the darkroom.
-
3
For multimaterial 3D-printing (for a cross-channel diffusion chip and a symmetric-channel diffusion chip), three different resins are prepared using PEG-DA-258 and PEG-DA-575, and PEG-DA-700. Use PEG-DA-258 for fabricating a microfluidic channel layer and apply both PEG-DA-575 and PEG-DA-700 to print a porous barrier. With PEG-DA-258 or PEG-DA-575 monomer, pour 40 g of each monomer into 50 mL conical tube which is covered with aluminum foil. Add 0.6% (w/w) IRG as a photoinitiator and 0.6% (w/w) ITX as a photosensitizer into each conical tube and stir the mixtures using a mini-vortexer. Dissolve the mixtures completely in the 70 °C oven in 30 min. ▲ CRITICAL STEP Insertion of photosensitizer leads to enhance the UV light absorbance. This phenomenon interrupts light penetration through the printing resin that improves the Z-axis resolution during the 3D-printing.
-
4
Prepare 40% (w/w in water) PEG-DA-700 resin by adding 40 g of PEG-DA-700 monomer into an aluminum foil-covered conical tube to protect UV light. Insert 0.6% (w/w) IRG into PEG-DA-700 resin and dissolve with a mini-vortex. Store the mixture in the 70 °C oven for 30 min. Blend 20 g of the PEG-DA-700 resin mixture with 30 g of distilled water to make 40% (w/w in water) PEG-DA-700 resin.
■ PAUSE POINT PEG-DA-258 has a single PEG repeating unit and has the smallest molecular weight among PEG-DAs, so the pores in the 3D-printed object are very small, and the water molecules cannot diffuse into the object, making it suitable for printing the impermeable walls of a microfluidic chip. On the other hand, molecules (including water) can diffuse through the pores of PEG-DA with MW > 300 (i.e. PEG-DA-575 and higher, which form hydrogels). The limitations of using PEG-DA with high MW are mechanical: hydrogels are brittle and/or soft, and only hold low-pressure fluid flow.
Design a microfluidic device ● TIMING 1–3 h
-
5
Use any commercial 3D-modeling program (e.g. Inventor, Solidworks, or Rhino) to draw a 3D chip layout (Fig. 1a). Beginners may need to familiarize themselves with 3D-modeling software by practicing several days to complete the tutorials. ▲ CRITICAL STEP In the case of multimaterial printing, the “print-pause-print” (PPP) method is applied to fabricate the porous barrier with another material. Therefore, the user is responsible for knowing when to stop the print by watching the layer number appear at the LCD panel on the 3D-printer. When the printer has stopped, the user changes the resin vat in order to fabricate a porous barrier (Fig. 1b).
-
6
After drawing the 3D object, the design is saved in the original format of the 3D-modeling program (e.g. .ipt for Autodesk Inventor) for the possibility of future revisions and exports in the appropriate format (.stl) for model slicing before 3D-printing.
Slice the designed object ● TIMING 10 min
-
7
Load the 3D object (in .stl format) on the slicing software (e.g. Creation Workshop®, Print Studio, or Chitubox) as shown in Fig. 1b. Some 3D-printer companies provide slicing software that is configured for their resin and printer. To print multiple numbers of the same model on a single print session, duplicate the model and paste on the XYZ space of the software. Avoid any overlap between the models.
-
8
After slicing the object with 25 μm-thick layers, the sliced image files obtained as a digital photomask and the layersetting.csv file for the setting of the UV exposure time are compressed together into a zipped folder. Printing resolution is determined by the reactivity of the resin but is also affected by the thickness of the sliced layer, UV exposure time, and the power of the UV lamp. In general, a thinner thickness layer needs a short UV exposure time and results in higher-resolution prints. ? TROUBLESHOOTING The details of troubleshooting is stated at Table 1. In our case, to print a transparent object using the PEG-DA-258 resin, UV exposure time sets to 0.3 s with 25 μm-thick layers at 32.21 mW/cm2. Some commercial printers provide customized software that can control slicing layer thickness, layer exposure time, and UV lamp power. The customized software also has a function to send slice images and printing conditions to the printer, so you can print more conveniently.
Table 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| Step 8 | The printed object features low resolution. | 1. Excessive UV exposure time while fabricating channel layers. 2. Thick layer slicing thickness for the channel layers. 3. Excessive UV lamp power of the printer. |
1. Reduce the UV exposure time. Excessive UV exposure time causes the resin to overcure, making the printed object thicker than designed. 2. In a thick layer, more light diffraction occurs compared with thin sliced printing layer. Therefore, the printing resolution could be poor. If the slicing thickness is decreased, the printing resolution could be increased. 3. Excessive UV lamp power leads to the overcuring of resin and more light diffraction. Reduce the lamp energy density to avoid low resolution prints. |
In some slicing software such as Composer (Asiga 3D-printers), we can set the location (layer height, Z) at which the printer must pause for switching the resin vat during multimaterial printing. This allows automated pausing by the printer, reduces user intervention, and simplifies the PPP process. To achieve this, follow these steps:
-
8.1.
Slice the 3D object with the customized software up until the desired layer height Z1 at which the printer must pause. In doing so, set the print range from Z=0 to Z1 in the software, and submit the sliced images to the 3D-printer.
-
8.2.
Re-load the 3D object (in .stl format) into the customized slicing software, and re-slice it from Z=Z1 to the next Z location where you want the printer to pause for switching the resin vat. Once done, submit the sliced images to the 3D-printer.
-
8.3.In case several resin vats are to be used for building a multimaterial 3D object, re-do STEP 8.2 until the entire object is sliced into a set of complete digital photomasks.
-
9Send the zipped folder file to the stereographic 3D-printer to fabricate the object.
-
9
Silanized the surface of the glass slide ● TIMING 10 h
-
10
Wash a glass slide thoroughly from top to down and front to back with acetone, IPA, and D.I. water. Using a plastic washing bottle, spray the washing solutions to completely clean the impurities adhering to the glass surface. Dry the glass slide with pressurized air and store it in the 70 °C oven overnight.
-
11
An oxygen plasma system is used for activating the glass surface. Pump the air out from the plasma chamber until the pressure reaches 5 × 10−2 Torr. Then introduce oxygen gas into the plasma chamber. When the chamber pressure is stabilized at 5 × 10−1 Torr, activate the oxygen plasma for 210 s. The required duration of the plasma depends on the actual plasma power and chamber configuration, which are model-dependent.
-
12
To silanize the glass surface, spray 0.8 mL TMSPMA onto a piece of paper. Place the TMSPMA soaked paper on the 85 °C hot plate in the vacuum chamber. Keep activated glass slides upright in a bottomless rack and lay the rack on top of the TMSPMA-soaked paper overnight. The TMSPMA vapors functionalize both sides of the glass surface with methacrylated silane. ▲ CRITICAL STEP Keep the silanized glass slide in the desiccator until use. As the surface of the glass slide becomes inactivate over time, it is recommended to use the surface-treated glass within one week.
Printing the transparent microfluidic chip ● TIMING 1–2 h
-
13
In our previous report, we improved the smoothness of printed layers using a glass surface for the vat to obtain high transparency prints.13 If a glass vat is used, treat the vat glass surface with SIGMACOTE® (Fig. S1) to prevent the attachment of the printed object to the glass surface of the vat. Prior to coating the glass surface with SIGMACOTE®, clean the glass and blow-dry it in the hood. Recover excess of SIGMACOTE® and store it at 4 °C for reuse. Instead of a glass vat and this hydrophobic coating step, a commercially-available, reusable Teflon vat or PDMS film vat can also be used.18,42 All three types of 3D-printing resin vat are displayed in Fig. S2. These three materials appear to have in common a high hydrophobicity and sufficiently low surface energy, which promotes the release of the printed chip from the vat surface and, consequently, its adhesion to the printing head. The PDMS film vat needs to be replaced when its transparency is reduced due to the resin permeation in between PDMS pores. Resin permeation causes a reduction in printing resolution, and even printing failure. In the case of the Teflon film vat, it should be replaced when the film surface becomes wrinkled due to repeated printing, otherwise the wrinkles can cause inaccurate printing.
-
14
Attach the silanized glass slide from STEP 12 to the aluminum build plate by covering one side of it with PEG-DA-258 resin with 0.4 % (w/w) IRG. Expose it to UV light for 5 s to photocure the resin. This step effectively “glues” the slide to the build plate with resin.
-
15
To calibrate the 3D-printer, place the resin tray on the printer and attach the glass slide to the aluminum build plate. Lower the build plate down until the glass slide reaches the resin tray. ▲ CRITICAL STEP After the calibration step, the glass slide should be changed to a new, clean slide glass to avoid the possibility of surface contamination on the glass slide during the calibration step when it touches the vat surface.
-
16
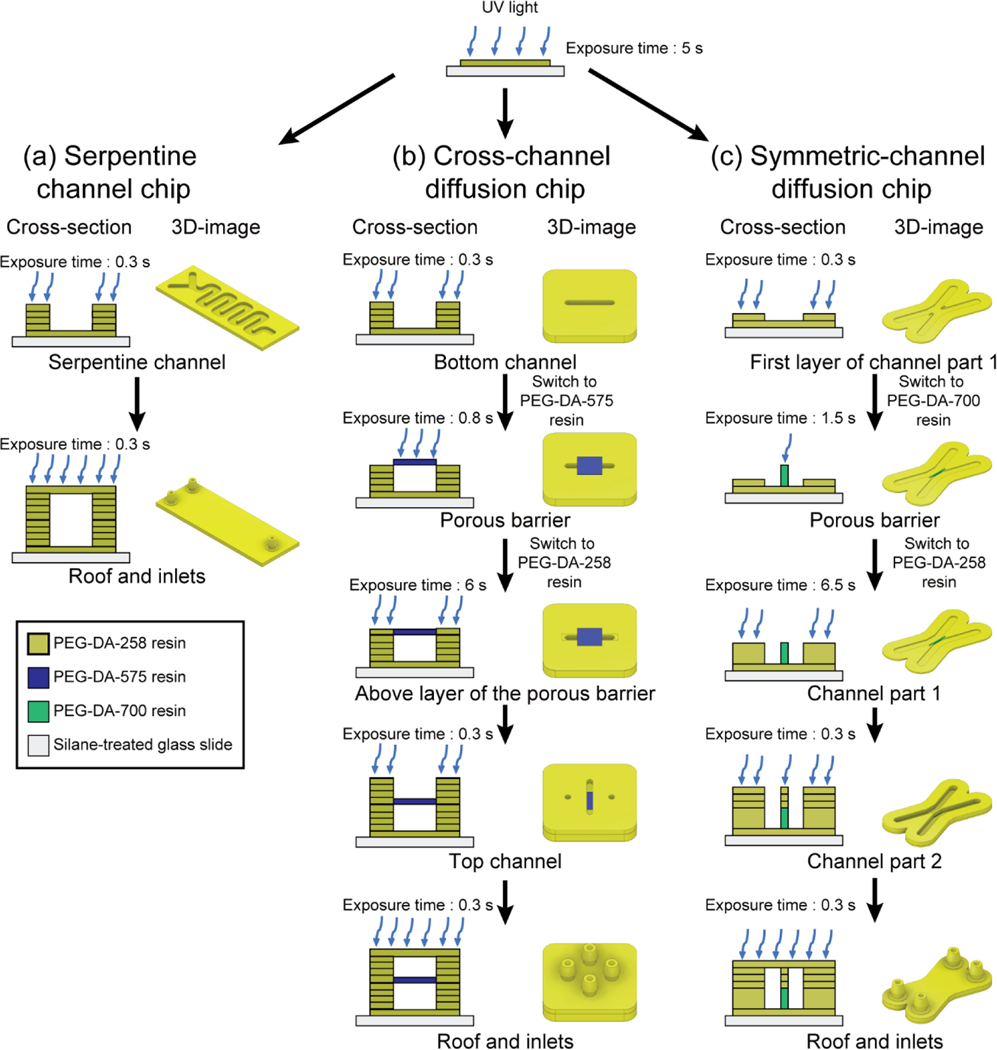
Pour the PEG-DA-258 resin into the vat and press the start button to print a transparent serpentine channel chip (Fig. 3a(i)). To induce the complete binding of the printing object to the silanized glass slide, increase the UV exposure time for the first layer of the serpentine channel microchip to 5 s; expose the remaining layers for 0.3 s (Fig. 4a). Detailed printing conditions for each layer of the transparent serpentine channel chip are shown in Fig. S3a. To avoid microchannel blockage during the first layer fabrication, we start the devices with a 100 μm-thick adhesion layer at the bottom of the channel. With this step, channel clogging does not occur when printing the channel layers.
-
17
Fabricate the object by repeating the following steps of approaching the aluminum build plate to the resin tray, curing the resin by UV light, separating the build plate from the current printing position, overlifting the Z-axis, and approaching the new printing position (Fig. 1c). The repeating steps are proceeded until finalizing the whole object printing.
-
18
After finishing the 3D-printing process, cover the build plate with aluminum foil to prevent the printed channel from photocuring by ambient light, as this would cause clogging of the channel. Then remove the object from the aluminum build plate by cutting the backside of the glass slide using a razor.
Fig. 3.
Schematic drawing of the 3D view and cross section of the 3D-printed microfluidic chips. (a) CAD design of 3D-printed microfluidic chips and (b) its cross section view. (i) 3D-printed serpentine channel chip, (ii) 3D-printed cross-channel diffusion chip, and (iii) 3D-printed symmetric-channel diffusion chip.
Fig. 4.
Schematic images of the fabrication process of 3D-printing multi-material microfluidic chips. (a) Cross-sectional view (left) and 3D view (right) of a 3D-printing process for the 3D-printed serpentine channel chip. (b) Cross-sectional view (left) and 3D view (right) of a 3D-printing process for the 3D-printed cross-channel diffusion chip with a PEG-DA-575 porous barrier. (c) Cross-sectional view (left) and 3D view (right) of a 3D-printing process for the 3D-printed symmetric-channel diffusion chip containing a 40% (w/w) PEG-DA-700 porous barrier.
Printing the cross-channel diffusion chip ● TIMING 1–2 h
-
19
To print a multimaterial microfluidic chip on the glass slide, attach the silanized glass slide to the aluminum build plate following STEP 14; continue with calibration STEP 15.
-
20
Schematic image (Fig. 3a(ii)) and the cross-sectional view (Fig. 3b(ii)) of a 3D-printed cross-channel diffusion chip are shown. To fabricate the cross-channel diffusion chip (Fig. 4b), prepare two PDMS-coated resin vats: one resin to build the walls of the microfluidic channels (PEG-DA-258 resin) and another resin to build the porous barrier (PEG-DA-575 resin). Pour each resin into their corresponding vat and completely cover the resin vats with aluminum foil to avoid light.
-
21
Details of the printing sequence for the 3D-printed cross-channel diffusion chip are shown in Fig. 4b. First, insert the PEG-DA-258 resin vat in the 3D-printer to print a bottom channel. Set the UV exposure time for the first layer as 5 s to induce the stable attachment of the printed layer to the silanized glass slide on the build plate; the rest of the layers prior to the porous barrier layer are exposed for 0.3 s using 25 μm-thick layers. Detail printing conditions for each layer of the cross-channel diffusion chip are presented in Fig. S3b. The sliced layer image is displayed in Fig. 5a. The printing sequence is the same as explained in STEP 17.
-
22
To print the porous barrier in the cross-channel diffusion chip, physically press the pause button on the 3D-printer and switch the PEG-DA-258 resin vat to PEG-DA-575 resin vat. The water-soluble PEG-DA-258 resin residue on the build plate was washed with D.I. water. Due to the smaller reactivity of the PEG-DA-575 resin compared to that of PEG-DA-258 resin, the UV exposure time for PEG-DA-575 resin must be increased to 0.8 s. Press the resume button on the 3D-printer and print the four layers of porous barrier layers. The image of the sliced porous barrier layer is shown in Fig. 5b. PEG-DA-258 and PEG-DA-575 resins are hydrophilic and have low viscosity, which makes them highly chemically compatible. Due to these characteristics, there is no bubble trapping during the resin tray change. The printing sequence is the same as explained in STEP 17. ■ PAUSE POINT To enhance the physical integrity of the barrier, the porous barrier should have sufficient thickness in Z. In our case, we decided that the total thickness of the porous layer be 100 μm to prevent the breakage of the barrier during the diffusion test. While printing the porous barrier, uncured resin remains inside the bottom channel. Irradiating UV light on the remaining resin (part of which can seep into the porous barrier) can cause the resin to block the bottom channel. We solved this problem by adding a photosensitizer (0.6 % (w/w) ITX) to the PEG-DA-575 resin, which increases the absorbance of light by the porous barrier. With the addition of ITX, the danger of clogging the bottom channel is minimized even when using long UV exposure times.
-
23
After rinsing the build plate using D.I. water to avoid cross contamination of the resin, switch the PEG-DA-575 resin vat to the PEG-DA-258 resin vat to fabricate the rest of the top channel, roof, and inlets for the cross-channel diffusion chip by repeating the printing sequence in STEP 17. The 2D layer just above the porous barrier needs to over-exposure with 6 s to fill the 100 μm-thick space around the porous barrier (The sliced image of the above layer of the porous barrier is shown in Fig. 5c) and the rest of the layers expose 0.3 s until whole printing is finished (The sliced image of the top channel is shown in Fig. 5d).
-
24
Cover the printed cross-channel diffusion chip with aluminum foil and detach it from the build plate by cutting using a razer.
Fig. 5.
Selected sliced layer images of the 3D-printed cross-channel diffusion chip. (a) Bottom channel. (b) PEG-DA-575 porous barrier. (c) Above the layer of the PEG-DA-575 porous barrier for the UV over-exposure. (d) Top channel.
Printing the symmetric-channel diffusion chip ● TIMING 1–2 h
-
25
A 3D CAD representation (Fig. 3a(iii)) and a cross-sectional view (Fig. 3b(iii)) of the 3D-printed symmetric-channel diffusion chip are displayed. Attach the silanized glass slide from STEP 14 to the aluminum build plate and perform the calibration with STEP 15 to fabricate the diffusion chip.
-
26
Use two PDMS-coated resin vats for fabricating the 3D-printed symmetric-channel diffusion chip. One resin vat is for the PEG-DA-258 resin to print a microfluidic channel, the other one is for the 40% (w/w in water) PEG-DA-700 resin to fabricate a porous barrier. Pour each resin into a vat and cover it completely to avoid the photopolymerization reaction.
-
27
The total printing process of the 3D-printed symmetric-channel diffusion chip is presented in Fig. 4c. First, load the PEG-DA-258 resin to the 3D-printer to fabricate the bottom layer (exposure time: 5 s) and print the first layer of channel part 1 with 0.3 s exposure time with a sliced image at Fig. 6a.
-
28
Pause the printing process to print the porous barrier and wash the build plate with D.I water to remove the resin residue and then equip the 40% (w/w in water) PEG-DA-700 resin vat to the 3D-printer. Resume for next layer printing with the sliced image in Fig. 6b. Fabricate 8 layers of the porous barrier by exposing UV light for 1.5 s per layer with the repeating steps explained in STEP 17.
-
29
To print the rest of the object, wash the build plate with D.I. water after pausing it and place the PEG-DA-258 resin vat on the 3D-printer. Press the resume button of the 3D-printer. The layer just above the porous barrier (Fig. 6c) needs to be over-exposed to the UV light for 6.5 s to create the microchannel walls beside the porous barrier. Fabricate the rest of the channel parts with a sliced image at Fig. 6d. and the roof and inlets with 0.3 s UV exposure time. Detail printing conditions for each layer of the symmetric-channel diffusion chip are showned in Fig. S3c. ■ PAUSE POINT Note that, in Fig. 6c, there are no microchannel features above the porous barrier layer to avoid pore blocking on the barrier due to residual light traversing the resin during exposure.
-
30
Wrap the printed object with aluminum foil to avoid ambient light exposure and remove the object with the glass slide by wedging into the backside of the glass slide with a razor.
Fig. 6.
Selected sliced layer images of the 3D-printed symmetric channel diffusion chip. (a) The first layer of the symmetric channel, (b) 40% (w/w) PEG-DA-700 porous barrier, (c) Above the layer of the 40% (w/w) PEG-DA-700 porous barrier for the UV over-exposure to create the channel part 1 of the symmetric channel, and (d) Channel part 2.
Post printing procedure ● TIMING 10 min
▲ CRITICAL STEP Perform STEP 31 through STEP 32 in the darkroom.
-
31
Unwrap aluminum foil from the printed object in the darkroom and deep the whole device into the D.I. water bath. Wash the surface of the object and glass slide and flush out the microchannels several times with D.I. water using a syringe to remove the remaining resin in the microchannels (Fig. 1d). Pay particular attention to clean the microchannel. ! CAUTION Less washing provokes a channel clogging with resin residue and excessive washing may cause the break of the porous barrier.
-
32
Clean and dry the printed object with pressurized air (Fig. 1d).
-
33
In the case of the transparent bio-microfluidic chip, perform the over-curing process to prevent leaching a cytotoxic uncured material during the cell culture. Keep the cleaned chip in water and leave it in the UV gel box overnight. The UV gel box could accelerate polymerization of uncured resin. At the same time, uncured PEG-DA-258 monomers and photoadditives could leach out while the chip stays in the water bath. ■ PAUSE POINT After the first washing of the chip, we perform an over-curing step and the removal of photoadditives (step 33), then confirm the absence of cytotoxicity by growing cells on the chip to determine the optimal post-treatment time.43,44
-
34
All the printed chips are inspected using a microscope. In the case of the porous barrier chips, keep the chip in the D.I. water to avoid the drying of the porous barrier which causes a break of the barrier until it uses. ! CAUTION PEG-DA-258 has swelling resistance in water for up to 2 weeks. Molecule diffusion test should be accomplished in 2 weeks after fabricating a porous barrier chip.
ANTICIPATED RESULTS
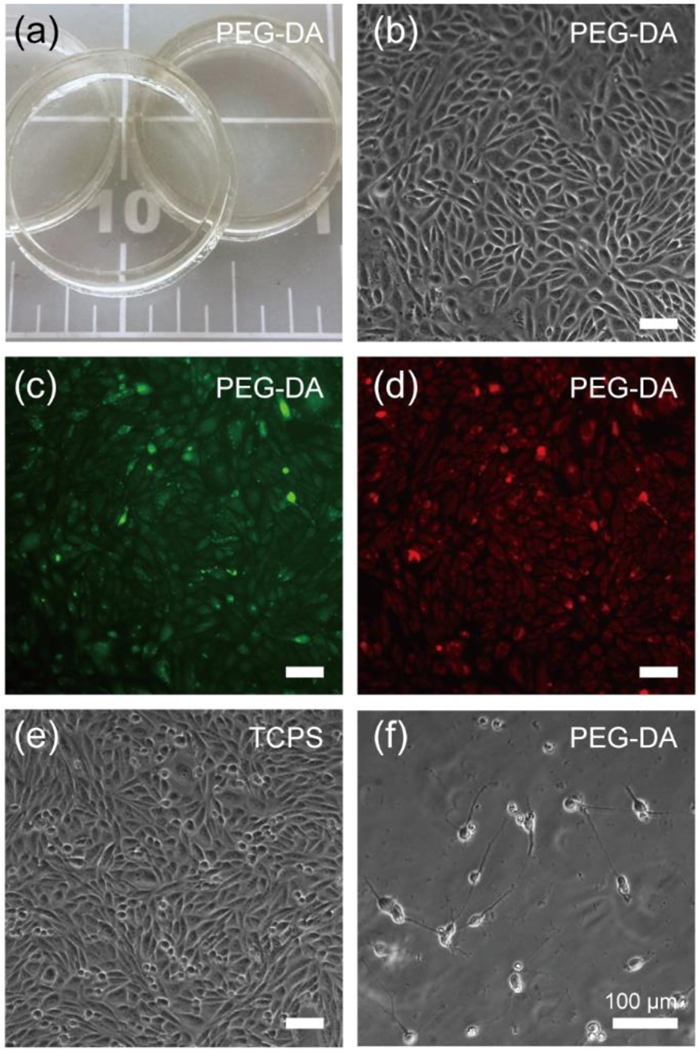
Fig. 7 illustrates the cytocompatibility and transparency to be expected from the 3D-printing process when using PEG-DA-258 resin with 0.4 % (w/w) IRG. For maximum surface smoothness we used glass surfaces for both the build plate and the resin vat, resulting in very transparent Petri dishes (Fig. 7a). This digital manufacturing technique can be applied to 3D-print transparent microfluidic chips18 for a variety of areas requiring the optical observation/analysis of a target such as a live cell, live tissue, or the concentration of biomolecules inside the chip. In Fig. 7a, stereolithographically printed PEG-DA-258 Petri dishes (25 mm-diameter, 1 mm-wide and 5 mm-height walls) show high transparency. We performed cytocompatibility tests using the printed Petri dish by seeding CHO cells (Fig. 7b–d) and embryonic primary hippocampal neurons (Fig. 7f). A 12-hr post-curing/washing of the 3D-printed Petri dishes ensured the high cell viability and proliferation rate of the cells as compared to a commercial polystyrene cell culture dish.
Fig. 7.
Cytocompatibility study using surfaces that were 3D-printed using the PEG-DA-258 resin with 0.4% (w/w) photoinitiator (IRG). Prior to seeding, we exposed the surfaces to a UV bath in water for 12 h so as to leach out possible un-reacted PEG-DA monomers and/or photoinitiator. We then treated the surfaces with oxygen plasma right before coating proteins or seeding cells. (a) Transparent PEG-DA-258 Petri dishes (25 mm-diam., 5 mm-tall and 1 mm-thick walls). (b) Phase-contrast image of Chinese hamster ovary cells (CHO-K1) seeded in a 3D-printed Petri dish. CHO-K1 cells were cultured in DMEM media (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone) and grown in a 5% CO2 atmosphere at 37 °C. This image demonstrates that our 3D-printing technique is adequate for phase-contrast microscopy, a mode of imaging that is very sensitive to surface irregularities. (c, d) Fluorescence images of the cells cultured in the 3D-printed PEG-DA-258 Petri dish after labelling with live fluorescent tracer Cell Tracker Green (c) and Orange (d), showing that our resin yields a plastic that is appropriate for fluorescence-based observations. (e) Phase-contrast image of CHO cells seeded in a tissue culture polystyrene (TCPS) Petri dish as a control, showing no appreciable morphological differences compared to (b). (f) Phase-contrast image of primary mouse hippocampal neurons (embryonic day 818) seeded in a 3D-printed Petri dish. These surfaces were coated with poly-D-lysine (100 μg/mL) (Sigma-Aldrich) overnight and Matrigel (BD Biosciences) (diluted 1:60 with DMEM) for 1 h at 37 °C. Primary neurons were harvested from the hippocampi of embryonic day 18 mice (Brainbits), and enzymatically dissociated using a papain dissociation kit (Worthington Biochemical), following well established protocols. The dissociated cells were suspended in Neurobasal media (Invitrogen) supplemented with 1× B-27 (Invitrogen), 0.5 mM GlutaMax (Invitrogen) and 100 U/mL penicillin–streptomycin (Invitrogen), and plated onto the Matrigel-coated PEG-DA-258 surfaces.
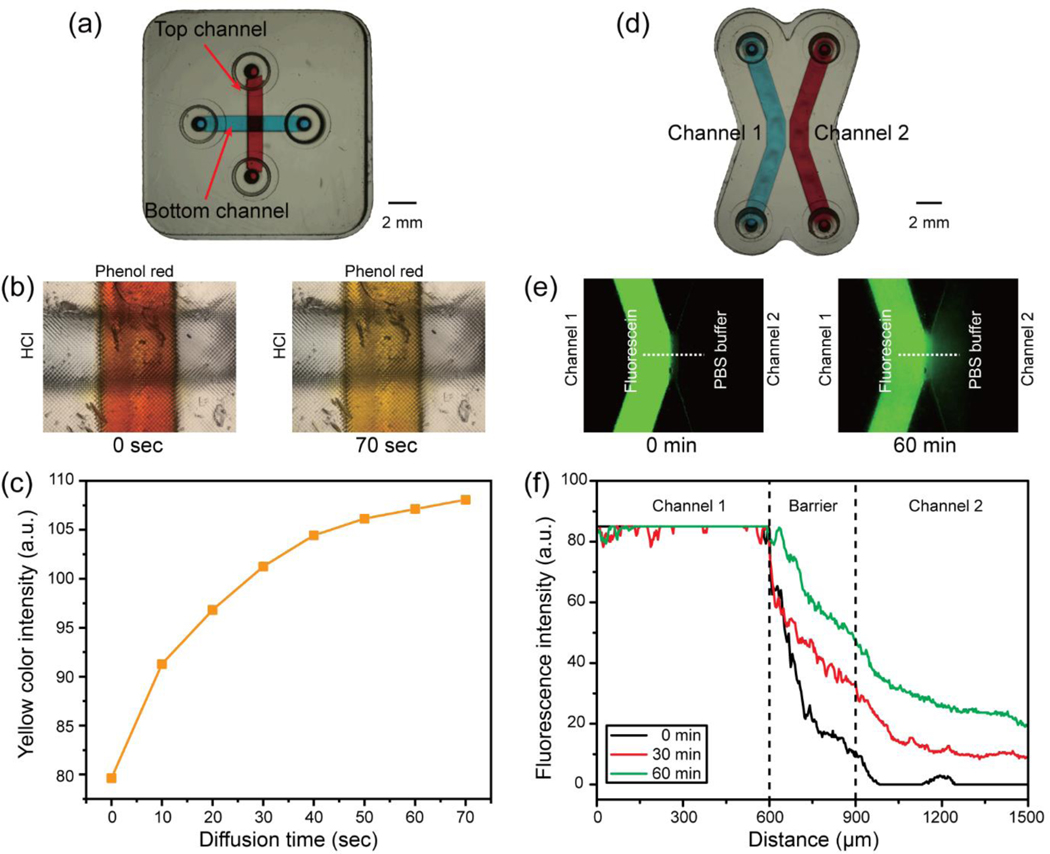
Multimaterial stereolithography was successfully demonstrated by printing a porous barrier within the transparent microchannel. The porous barriers were fabricated by using the print-pause-print (PPP) method that created the cross-channel/symmetric channel diffusion chip (Fig. 8a, d). PEG-DA-258 resin with 0.6% (w/w) IRG and 0.6% (w/w) ITX was prepared for printing a microfluidic channel and two different types of porous barrier resins were made using PEG-DA-575 with 0.6% (w/w) IRG and 0.6% (w/w) ITX to print the cross-channel diffusion chip and 40% (w/w in water) PEG-DA-700 with 0.6% (w/w) IRG to fabricate the symmetric-channel diffusion chip, respectively. To demonstrate selective diffusion of hydrogen ions through the PEG-DA-575 porous barrier, pH-sensitive phenol red dye was chosen to observe color change with pH-shift. Hydrochloric acid, as a source of hydrogen ion, was added into the bottom channel of the cross-channel diffusion chip while phenol red was loaded into the top channel. The color of the phenol red solution gradually changed from red to yellow in the top channel while the color of the bottom channel remained transparent, as shown in Fig. 8b. A graph of the evolution of the yellow color intensity profiles in the top channel is displayed in Fig. 8c, demonstrating that the yellow color intensity, and thus the hydrogen ion concentration, increases with time. Since the phenol red dyes stayed in the top channel, this measurement indicates that only hydrogen ions were able to diffuse through the barrier.
Fig. 8.
Results of the molecule diffusion test with both cross-channel diffusion chip and symmetric channel diffusion chip. (a) Photograph of the 3D-printed cross-channel diffusion chip. (b) Hydrogen ion diffusion results through the PEG-DA-575 porous barrier. The color of phenol red in the top channel shifts from red (t = 0 s) to yellow (t = 70 s). (c) Histogram of yellow color intensity measured in the top channel which the phenol red is loaded, showing that the yellow color intensity is gradually increased in the top channel. (d) Photograph of the 3D-printed symmetric channel diffusion chip. (e) Fluorescein diffusion test using the 3D-printed symmetric channel diffusion chip. Fluorescein diffused through the 40% (w/w) PEG-DA-700 porous barrier at 0 min and 60 min. The green color intensity is increased after 60 min diffusion of fluorescein at the Channel 2. (f) Fluorescence intensity profiles graph observed across the microchannels. The fluorescent intensity is gradually increased in the Channel 2.
We also demonstrated selective diffusion of fluorescein through a PEG-DA-700 barrier. Fluorescein dye diffusion through the 40% (w/w in water) PEG-DA-700 barrier was verified by observing the increment of fluorescence intensity in channel 2 of the symmetric diffusion chip in Fig. 8e. The fluorescence intensity is also measured across the microchannels and the graph of the fluorescence intensity profiles from the symmetric diffusion chip is displayed in Fig. 8f. The results indicate that the fluorescein intensity is gradually increased in channel 2 by diffusing the fluorescein molecules through the 40% (w/w in water) PEG-DA-700 porous barrier.
Supplementary Material
ACKNOWLEDGMENTS
This work was partially supported by grants from the National Cancer Institute (5R01CA181445), the National Institute of General Medical Sciences (NIGMS R21GM137161), a Nanomedical Devices Development Project of NNFC (1711160154), Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P002007), and the GRRC program of Gyeonggi province (GRRC-KPU2020-A02).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Folch A. Introduction to BioMEMS. CRC press; (2012). [Google Scholar]
- 2.Liu P. & Mathies RA Integrated microfluidic systems for high-performance genetic analysis. Trends in Biotechnology 27, 572–581 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Duncombe TA, Tentori AM & Herr AE Microfluidics: reframing biological enquiry. Nature Reviews Molecular Cell Biology 16, 554–567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elvira KS, i Solvas XC, Wootton RCR & DeMello AJ The past, present and potential for microfluidic reactor technology in chemical synthesis. Nature Chemistry 5, 905–915 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Whitesides GM, Ostuni E, Takayama S, Jiang X. & Ingber DE Soft lithography in biology and biochemistry. Annual Reviews Biomedical Engineering 3, 335–373 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Weibel DB, Diluzio WR & Whitesides GM Microfabrication meets microbiology. Nature Reviews Microbiology 5, 209–218 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Hsu C-H & Folch A. Spatio-temporally-complex concentration profiles using a tunable chaotic micromixer. Applied Physics Letters 89, 144102 (2006). [Google Scholar]
- 8.Lam EW, Cooksey GA, Finlayson BA & Folch A. Microfluidic circuits with tunable flow resistances. Applied Physics Letters 89, 164105 (2006). [Google Scholar]
- 9.Au AK, Huynh W, Horowitz LF & Folch A. 3D-Printed microfluidics. Angewandte Chemie - International Edition 55, 3862–3881 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharjee N, Urrios A, Kang S. & Folch A. The upcoming 3D-printing revolution in microfluidics. Lab on a Chip 16, 1720–1742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen A. v, Beauchamp MJ, Nordin GP & Woolley AT 3D printed microfluidics. Annual Review of Analytical Chemistry 13, 45–65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naderi A, Bhattacharjee N. & Folch A. Digital manufacturing for microfluidics. Annual Review of Biomedical Engineering 21, 325–364 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urrios A. et al. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab on a Chip 16, 2287–2294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YT, Castro K, Bhattacharjee N. & Folch A. Digital manufacturing of selective porous barriers in microchannels using multi-material stereolithography. Micromachines 9, 125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong H, Beauchamp M, Perry S, Woolley AT & Nordin GP Optical approach to resin formulation for 3D printed microfluidics. RSC Advances 5, 106621 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong H, Woolley AT & Nordin GP High density 3D printed microfluidic valves, pumps, and multiplexers. Lab on a Chip 16, 2450–2458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MP et al. Development of a 3D printer using scanning projection stereolithography. Scientific Reports 5, 9875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo AP et al. High-precision stereolithography of biomicrofluidic devices. Advanced Materials Technologies 4, 1800395 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warr C. et al. Biocompatible PEGDA resin for 3D printing. ACS Applied Bio Materials 3, 2239–2244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi NW et al. Microfluidic scaffolds for tissue engineering. Nature Materials 6, 908–915 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Yu YJ et al. Hydrogel-incorporating unit in a well: 3D cell culture for high-throughput analysis. Lab on a Chip 18, 2604–2613 (2018). [DOI] [PubMed] [Google Scholar]
- 22.LaVan DA, McGuire T. & Langer R. Small-scale systems for in vivo drug delivery. Nature Biotechnology 21, 1184–1191 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Li L. & Luo C. Gel integration for microfluidic applications. Lab on a Chip 16, 1757–1776 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Cuchiara MP, Allen ACB, Chen TM, Miller JS & West JL Multilayer microfluidic PEGDA hydrogels. Biomaterials 31, 5491–5497 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Sip CG, Bhattacharjee N. & Albert Folch. Microfluidic transwell inserts for generation of tissue culture-friendly gradients in well plates. Lab on a Chip 14, 302–314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cate DM, Sip CG & Folch A. A microfluidic platform for generation of sharp gradients in open-access culture. Biomicrofluidics 4, 044105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colosi C. et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Advanced Materials 28, 677–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang E. et al. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nature Materials 10, 877–883 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Wehner M. et al. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 536, 451–455 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Hardin JO, Ober TJ, Valentine AD & Lewis JA Microfluidic printheads for multimaterial 3D printing of viscoelastic inks. Advanced Materials 27, 3279–3284 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Skylar-Scott MA, Mueller J, Visser CW & Lewis JA Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 575, 330–335 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Lind JU et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nature Materials 16, 303–308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson BN et al. 3D printed anatomical nerve regeneration pathways. Advanced Functional Materials 25, 6205–6217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SH et al. 3D printed polymer photodetectors. Advanced Materials 30, 1803980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W. et al. Rapid continuous multimaterial extrusion bioprinting. Advanced Materials 29, 1604630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi JW, Kim HC & Wicker R. Multi-material stereolithography. Journal of Materials Processing Technology 211, 318–328 (2011). [Google Scholar]
- 37.Zhou C, Chen Y, Yang Z. & Khoshnevis B. Digital material fabrication using mask-image-projection-based stereolithography. Rapid Prototyping Journal 19, 153–165 (2013). [Google Scholar]
- 38.Zhang X, Jiang XN & Sun C. Micro-stereolithography of polymeric and ceramic microstructures. Sensors and Actuators: Physical 77, 149–156 (1999). [Google Scholar]
- 39.Chan V, Zorlutuna P, Jeong JH, Kong H. & Bashir R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab on a Chip 10, 2062–2070 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Gauvin R. et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 33, 3824–3834 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua Gong, P. Bickham B, T. Woolley A. & P. Nordin G. Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab on a Chip 17, 2899–2909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YT, Bohjanen S, Bhattacharjee N. & Folch A. Partitioning of hydrogels in 3D-printed microchannels. Lab on a Chip 19, 3086–3093 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu F, Friedrich T, Nugegoda D, Kaslin J. & Wlodkowic D. Assessment of the biocompatibility of three-dimensional-printed polymers using multispecies toxicity tests. Biomicrofluidics 9, 061103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macdonald NP, Zhu F, Hall CJ, Reboud J, Crosier PS, Patton EE, Wlodkowic D. & Cooper JM Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab on a Chip 16, 291–297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.