Abstract
During lytic replication, herpesviruses express their genes in a temporal cascade culminating in expression of “late” genes. Two subfamilies of herpesviruses, the beta- and gammaherpesviruses (including human herpesviruses cytomegalovirus, Epstein Barr virus, and Kaposi’s sarcoma-associated herpesvirus), use a unique strategy to facilitate transcription of late genes. They encode six essential viral transcriptional activators (vTAs) that form a complex at a subset of late gene promoters. One of these vTAs is a viral mimic of host TATA-binding protein (vTBP) that recognizes a strikingly minimal cis-acting element consisting of a modified TATA box with a TATTWAA consensus sequence. vTBP is also responsible for recruitment of cellular RNA polymerase II (Pol II). Despite extensive work in the beta/gammaherpesviruses, the function of the other five vTAs remains largely unknown. The vTA complex and Pol II assemble on the promoter into a viral preinitiation complex (vPIC) to facilitate late gene transcription. Here, we review the properties of the vTAs and the promoters on which they act.
Keywords: late gene transcription, betaherpesviruses, gammaherpesviruses, vTA, vTBP
1. Introduction
1.1. Double-stranded DNA viruses use diverse strategies for temporal control of gene expression
Double-stranded DNA viruses (dsDNA) undergo a temporal cascade of viral gene expression to ensure that genes required for the final stages of the life cycle are not expressed before they are needed. Adenoviruses, bacteriophages, baculoviruses, herpesviruses, papillomaviruses, polyomaviruses, and poxviruses control viral gene expression at the level of transcription. Expression of the last class of viral genes – called the “late” genes – occurs after the viral genome has been replicated. The mechanisms by which late genes are expressed, and the mechanisms of dependence on viral genome replication, vary widely.
Viral genome replication can mediate late gene transcription through titration of a repressive factor, titration of a repressive modification, or alteration of the viral genome state. Additionally, viruses may encode transcription factors that bind late gene promoter elements to drive transcription. Polyomavirus Simian virus 40 (SV40) is an example of a dsDNA virus in which replication-dependent late gene transcription is a consequence of titration of a repressive factor. The SV40 late gene promoter is bound by host proteins in the steroid/thyroid hormone receptor family; multiple rounds of viral genome replication results in lower occupancy of the repressive factor and subsequent de-repression of late gene transcription [1, 2]. In the alphaherpesvirus Herpes Simplex Virus-1 (HSV-1), a single round of genome replication is accompanied by a change in the state of the genome such that transcription initiation complexes can form on previously silenced late gene promoters [3].
Baculoviruses, T4 bacteriophages, poxviruses, and the beta/gammaherpesviruses all encode factors that promote transcription of viral genes. These factors can be directly responsible for transcription (as in the case of DNA-dependent RNA polymerases) or can modulate the host machinery to enable efficient late transcript synthesis [4-11]. The diverse array of transcriptional strategies is likely heavily influenced by the cell type (senescent vs. dividing) and cellular compartment where viral replication occurs (nucleus vs. cytoplasm). In this review we will focus on late gene transcriptional regulation for the beta/gammaherpesviruses, which encode six viral factors that hijack the host RNA polymerase to facilitate late gene transcription.
1.2. Herpesviruses are important human pathogens with a biphasic replication cycle
Nine herpesviruses from three subfamilies – the alpha, beta, and gammaherpesviruses – are known to infect humans. All herpesviruses have large (125-240 kilobase pairs) dsDNA genomes and replicate entirely within the host nucleus. They employ a biphasic life cycle, which includes a lytic (replicative) phase and a latent (quiescent, immune evasive) phase. This allows the virus to infect a host, persist for their lifetime, and reactivate periodically to assorted stimuli. Due to their ability to establish latency, herpesviruses are highly prevalent, with most individuals testing seropositive for at least one of the nine species by adulthood [12-15].
Chronic herpesvirus infections are usually asymptomatic; however, in immunocompromised patients – such as transplant recipients, neonates, and those with untreated HIV-1 infection – these viruses can have devastating effects. The betaherpesvirus subfamily includes human cytomegalovirus (HCMV), which can cause congenital birth defects [16] and human herpesviruses HHV-6A, HHV-6B, and HHV-7 [17]. The human gammaherpesviruses Epstein Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) can cause cancers [18]. Additionally, EBV has recently been associated with multiple sclerosis [19]. Herpesviruses remain a major public health concern, as we lack therapeutic agents capable of targeting the latent viral reservoir and vaccines for any of the beta/gammaherpesviruses.
1.3. The beta/gammaherpesviruses transcribe late genes in a manner distinct from alphaherpesviruses
Herpesviruses do not encode their own RNA polymerase and thus depend on host RNA Polymerase II (Pol II) to produce the >100 viral transcripts expressed during lytic infection. Expression of viral mRNA follows a temporally coordinated sequence, such that their gene products are expressed at the appropriate time in the life cycle of the virus (Figure 1). Immediate early (IE) gene products include immunomodulatory proteins and transcription factors that enable the efficient expression of early (E) genes. Early genes facilitate viral genome replication and formation of membraneless viral replication compartments within the nucleus where replication and late gene transcription occur [20-24]. Viral genome replication triggers expression of the late (L) genes, which encode proteins required for virion assembly and release such as capsid and tegument proteins. This temporal coordination ensures that viral antigens that may be detected by the host immune system are minimized during early stages of infection. Once the virus and host cell are primed, genome replication ensues and dozens of previously silent genes are transcribed.
Figure 1. Herpesviruses undergo a temporal cascade of lytic gene expression.
Pre-replication: In the absence of de novo viral protein synthesis, immediate early (IE) genes are transcribed. Immediate early proteins include transcriptional activators that function to promote early gene transcription. Early genes include the viral DNA replication machinery, the vTAs, and other factors that manipulate the host cell.
Post-replication: After the onset of viral genome replication and the formation of replication compartments (lighter background), dozens of previously inactive genes are transcribed. These late (L) genes include structural components of the virus and factors required for assembly of infectious virions. After sufficient viral genomes and late gene products have accumulated, capsid assembly and egress begins.
While all herpesviruses employ a late wave of transcription that is dependent on viral genome replication, the mechanism itself varies. Beta- and gammaherpesviruses encode a set of six proteins – viral transcriptional activators, or vTAs - that form a complex required for late gene expression [25]. Alphaherpesviruses do not encode such a complex, although a parallel could be drawn to the major viral transactivator (HSV-1 ICP4) which is required for both E and L gene transcription [26-31]. Another notable difference is the mechanism behind the dependence of late gene transcription on viral genome replication. Recent studies have found that in HSV-1, the act of genome replication facilitates a permanent change in transcriptomic state. Once a single round of genome replication has occurred, a preinitiation complex (PIC) containing ICP4 forms on late gene promoters. After the initial licensing event, ongoing genome replication is not required for late gene transcription [3, 26, 32, 33]. In contrast, in the gammaherpesviruses KSHV and EBV, both a lytic origin (oriLyt) in cis and continuous viral DNA replication are required for late gene transcription [22, 24, 34, 35]. These differences emphasize the complexity underlying late gene transcription regulation across the herpesviruses. Here, we focus on highlighting recent work that has enhanced our of understanding late gene transcription in the beta/gammaherpesviruses. We focus on the promoter elements and viral factors required for late gene transcription, but note that there are many open questions about the connections between late gene transcription, viral genome replication, and nuclear organization. For a review on the link between late gene transcription and viral genome replication in EBV, see ref [36], and for a review on viral replication compartments, see ref [21].
2. What are late genes in the beta/gammaherpesviruses?
2.1. Historical definition of late genes
The herpesvirus lytic transcriptional cascade was discovered almost fifty years ago. Original experiments delineated four classes of HSV-1 transcripts by adding cycloheximide [37], canavanine [38], and phosphonoacetic acid [39] to infected cells and assessing peptide and, later, RNA accumulation. This work defined the following temporal class definitions:
Immediate early (IE or α) - expressed in the absence of de novo viral protein synthesis
Early (E or β) - dependent on IE gene products and accumulate to similar or greater levels in the absence of viral genome replication
Leaky Late (L1 or γ1) - dependent on viral genome replication for increased synthesis
True Late (L2 or γ2) - dependent on viral genome replication to initiate synthesis
While the names of these gene classes suggest a relative time of expression, it needs to be emphasized that temporal kinetics is not strictly what defines each class. The classes are defined by their relationships to viral transcription factors and genome replication.
2.2. An updated definition of late genes
Since these original experiments, technology has evolved and re-characterization of herpesvirus transcript identity, kinetics, and phenotypes has been performed [40-43]. The wealth of new data these provide calls for a reevaluation of gene classification. Additionally, the original definitions of IE, E, and L do not account for transcription factors specific for late genes. This is because late gene licensing is fundamentally different for alphaherpesviruses, where the classes were originally defined. To add to the confusion, beta- and gammaherpesvirus researchers use different language to refer to the set of six proteins which facilitate late gene transcription. This includes the CMV late transcription factors (LTFs), EBV viral preinitiation complex (vPIC), and KSHV viral transcriptional activators (vTAs). As described in subsequent sections of this review, these protein complexes are homologous, and universal language can be used to describe them across viruses. Furthermore, the terms “late gene” and “vTA-dependency” are not interchangeable, as not all genes transcribed at late time points are vTA-targets [44, 45]. Thus, we propose that in addition to the canonical IE, E, L1, and L2 terminology, the subset of late genes that are dependent on the vTAs are called “vTArgets”.
Viral genes that are dependent on the vTAs can be identified through the study of vTA deletion viruses. However, in KSHV, vTA deletion results in lower expression for almost all viral genes and may also result in defects in viral genome replication [34, 46-48]. This could be a result of indirect dependence on the vTAs – i.e., a vTA-dependent gene affects stability or expression of other transcripts or processes. Thus, pairing transcriptomic analysis from vTA deletion viruses with vTA promoter binding data is essential to separate the contribution of genuine versus off-target replication effects. One caveat to this approach is that the vTAs are relatively low abundance proteins [49, 50], and that approaches like chromatin immunoprecipitation (ChIP) may not be sufficiently sensitive to identify all of the vTA-bound genomic regions.
We summarize vTArgets identified for CMV, EBV, and KSHV in Table 1. For CMV and KSHV, transcriptomic analysis of vTA-dependency was integrated with evidence of vTA promoter binding [34, 51]. We predict that the list of EBV genes will be reduced if information about vTA promoter occupancy is incorporated. Using these criteria, 42 CMV genes, 21 EBV genes, and 13 KSHV genes are vTArgets. In terms of vTA-dependence and vTBP binding, CMV UL18 (MHC homolog) and UL98 (alkaline nuclease) also fit the criteria to be called vTArgets. On these genes, both host and viral TBP bound the promoter, with host TBP bound more prominently (host/viral TBP ratio of 1.2 and 2.9) [51]. For this reason, UL18 and UL98 were excluded from the table. Five of the 21 EBV vTArgets listed (BXLF2, BPLF1, BOLF1, BFRF0.5, and BFLF1) were not annotated as vTA-dependent from an independent study where vTA knockdown was performed [45]. As Djavadian et al. 2018 used a more sensitive technique to assess the transcriptome, we list these genes as vTArgets, but have indicated their conflicting status. We list 13 vTArgets for KSHV, with ORF39 (glycoprotein M) as a potential fourteenth target. ORF39 was transcriptionally dependent on the vTAs; however, vTA binding status was indeterminate as the peak could have corresponded to the promoter of ORF39 or ORF40 [34].
Table 1. vTA-dependent gene targets (vTArgets).
When possible, HSV-1 gene homologs are listed in parentheses underneath the gene. Genes that express capsid proteins, glycoproteins, or tegument proteins are shaded as grey, blue, and green, respectively. CMV vTArgets were identified by integrating vTA-dependence (UL87 and UL79-dTAG/Control PRO-Seq ratio ≤0.2; ref. [123]) with vTBP occupancy (UL87 PIC > 100; TBP/UL87 PIC ratio ≤ 0.3; ref. [124]) for transcription start regions upstream of annotated CDS for the CMV strain TB40/E assembly KF297339.1. EBV vTArgets were determined by vTA-dependence (BDLF4 knockout/Control CAGE-Seq ratio ≤0.2; ref. [125]); viral genes with an asterisk were not reported as sensitive to vTA (BGLF3) knockdown by McKenzie et al. 2016 [45]. KSHV vTArgets were based on vTA-dependence (ORF24 knockout RNA-Seq) and vTA occupancy (ORF34 ChIP-Seq) as detailed in Nandakumar & Glaunsinger 2019 [34].
| CMV | EBV | KSHV | |||
|---|---|---|---|---|---|
| Gene | Function | Gene | Function | Gene | Function |
| UL86 (UL19) | Major capsid protein | BcLF1 (UL19) | Major capsid protein | ORF25 (UL19) | Major capsid protein |
| UL85 (UL18) | Triplex capsid protein | BFRF3 (UL35) | Hexon capsid protein | ORF65 (UL35) | Hexon capsid protein |
| UL46 (UL38) | Triplex capsid protein | BDLF1 (UL18) | Triplex capsid protein | ORF26 (UL18) | Triplex capsid protein |
| UL93 (UL17) | Capsid vertex | BORF1 (UL38) | Triplex capsid protein | ORF19 (UL25) | Capsid vertex |
| UL55 (UL27 or gB) | Glycoprotein | BGLF1 (UL17) | Capsid vertex | ORF17.5 | Internal scaffold protein |
| UL75 (UL22 or gH) | Glycoprotein | BBRF1 (UL6) | Portal protein | ORF8 (UL27 or gB) | Glycoprotein |
| UL115 (UL1 or gL) | Glycoprotein | BALF4 (UL27 or gB) | Glycoprotein | ORF53 (UL49.5 or gN) | Glycoprotein |
| UL100 (UL10 or gM | Glycoprotein | BXLF2* (UL22 or gH) | Glycoprotein | ORF27 | Glycoprotein |
| UL73 (UL49.5 or gN) | Glycoprotein | BZLF2 | Glycoprotein | K8.1 | Glycoprotein |
| RL10 | Glycoprotein | BLLF1 | Glycoprotein | ORF33 (UL16) | Secondary envelopment |
| UL74 | Glycoprotein | BILF2 | Glycoprotein | ORF38 (UL11) | Secondary envelopment |
| UL94 (UL16) | Secondary envelopment | BGLF2 (UL16) | Secondary envelopment | ORF45 | Immune evasion |
| UL99 (UL11) | Secondary envelopment | BOLF1* (UL37) | Secondary envelopment | ORF52 | Immune evasion |
| UL47 (UL37) | Secondary envelopment & trafficking | BPLF1* (UL36) | Secondary envelopment & trafficking | ||
| UL48 (UL36) | Secondary envelopment & trafficking | BNRF1 | Immune evasion | ||
| UL25 | Tegument formation | BNRF2 | Virion maturation and release | ||
| UL96 | Virion maturation and release | BVRF2 (UL26) | Scaffold protease | ||
| UL32 | Virion maturation and release | BFRF0.5* | Terminase subunit | ||
| UL76 (UL24) | Perturbs DNA Damage response | BFLF1* (UL32) | Packaging | ||
| UL97 (US3, UL13) | Protein kinase | BFRF1 | Capsid nuclear egress | ||
| UL45 (UL39) | R1 Ribonucleotide reductase | BXRF1 | Unknown | ||
| UL35 | Immune evasion | ||||
| UL82 | Immune evasion | ||||
| UL88 | Immune evasion | ||||
| UL50 (UL34) | Egress | ||||
| UL53 (UL31) | Egress | ||||
| UL80 (UL26.5) | Scaffold protease | ||||
| UL80.5 (UL26.5) | Scaffold protease | ||||
| UL116 | Glycoprotein chaperone | ||||
| UL119 | Decoy Fc receptor | ||||
| UL121 | Predicted membrane protein | ||||
| UL13 | Perturbs cellular respiration | ||||
| US27 | GPCR | ||||
| RL9A | Unknown | ||||
| UL14 | Unknown | ||||
| UL17 | Unknown | ||||
| UL19 | Unknown | ||||
| UL30A | Unknown | ||||
| UL41A | Unknown | ||||
| UL44, intragenic | Unknown | ||||
| UL49, intragenic | Unknown | ||||
| US17 | Unknown | ||||
All of the vTArgets identified for CMV and EBV were genome replication-dependent [51, 52]. To date, there are no papers globally assessing DNA replication-dependence of the KSHV transcriptome. Without this we cannot assess which of the vTArgets would classify as L1 or L2; however, in terms of their temporal expression, 12 of the 13 genes are expressed at late stages [40]. The majority of vTArgets are capsid, tegument and glycoproteins necessary for final stages of the lytic life cycle (Table 1). The major capsid protein, triplex protein-2, glycoprotein B, and secondary envelopment protein (HSV-1 UL16 homolog) were vTArgets identified for all three viruses. This functional grouping underscores the importance of a temporally coordinated cascade and suggests these commonly regulated proteins may be limiting for production of infectious progeny.
2.3. Promoter architecture of vTA-dependent genes
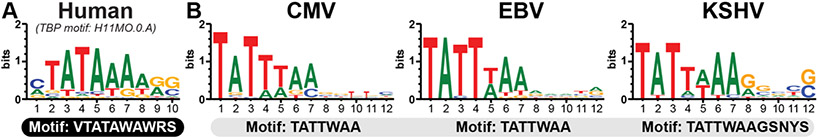
In 1998, Serio et al. first reported the existence of a functionally distinct TATA-box promoter that was required for EBV late gene transcription [53]. They discovered that the TATTAAA promoter element within the canonical late gene BcLF1 was essential for late gene kinetics. This TATA-box motif differed from motifs present in early genes, as it required a thymine at the fourth position [53-55]. Efficient BcLF1 gene expression was independent of sequences 5' to the TATT-box, in contrast to early gene promoters which possess a canonical TATA box motif and are dependent on 5' enhancer elements for efficient transcription. A similar TATT promoter motif required for properly coordinated late gene transcription was identified in CMV, KSHV, and murine gammaherpesvirus-68 (MHV68) [35, 56, 57]. These early studies focused on representative canonical examples of the class, such as CMV intragenic UL44, EBV BcLF1, and KSHV K8.1.
NGS techniques have extended the list of vTA-dependent genes, enabling generation of a consensus element within late gene promoters [34, 51, 52, 58]. In CMV and EBV, this motif is TATTWAA and is extended to TATTWAAGSNYS for KSHV (Figure 2, Table 2). Ultimately, the minimal promoter element required for vTA-dependent late gene activation in the beta/gammaherpesviruses is strikingly conserved and consists of a modified TATA-binding motif of TATTWAA located approximately 20-30 nucleotides upstream of the TSS.
Figure 2. vTArget promoter architecture.
A) HOmo sapiens COmprehensive MOdel COllection (hocomoco v.11) motif for human TBP binding. B) WebLogo sequence consensus plots for TATA-box elements from vTArgets for CMV (n=42 genes), EBV, (n=21 genes) and KSHV (n=13 genes).
Table 2. TATT motifs in vTA-dependent gene promoters.
Features are annotated relative to CMV strain TB40/E assembly KF297339.1, EBV strain B95-8 (V01555.2), or KSHV strain JSC-1 (GQ994935.1). Transcription start sites (TSS) or transcription start regions (TSR) are based on [123-126].
| Virus | Gene | TSS or TSR Position | TATA Motif | Distance from TSS |
|---|---|---|---|---|
| CMV | RL10 | KF297339.1:8841-8861_+ | TGTTGAAGGGTA | −7 |
| RL9A | KF297339.1:8872-8892_− | TATTAAAACACC | −18 | |
| UL13 | KF297339.1:20004-20024_+ | TATTTGAAGCCA | −17 | |
| UL14 | KF297339.1:21658-21678_+ | TTTTTAAGGAGC | −17 | |
| UL17 | KF297339.1:23632-23652_+ | TGTTTACGCTGC | −19 | |
| UL19 | KF297339.1:25469-25489_+ | TACTTTACCACC | −18 | |
| UL25 | KF297339.1:30785-30805_+ | TATATAACGTCC | −18 | |
| UL30A | KF297339.1:38586-38606_− | TATATAAACCGC | −22 | |
| UL32 | KF297339.1:43861-43881_− | TATTAAACTACC | −17 | |
| UL35 | KF297339.1:46819-46839_+ | TATTTAACGTCA | −17 | |
| UL41A | KF297339.1:55215-55235_− | TATATTAGGGAT | −18 | |
| intragenic UL44 | KF297339.1:58066-58086_+ | TGTTAAAAGTGA | −18 | |
| UL45 | KF297339.1:61503-61523_− | TATTTAATCATC | −18 | |
| UL46 | KF297339.1:62149-62169_− | TATTTAACGCCT | −10 | |
| UL47 | KF297339.1:62046-62066_+ | TATTTACCTCGC | −20 | |
| UL48 | KF297339.1:64978-64998_+ | TATTAAACTGGT | −18 | |
| intragenic UL49 | KF297339.1:72579-72599_− | TATTATCGGGAC | −15 | |
| UL50 | KF297339.1:75058-75078_+ | TCTTTTCCCTCC | −18 | |
| UL53 | KF297339.1:77367-77387_+ | TATATAAGTCGA | −20 | |
| UL55 | KF297339.1:85559-85579_− | TATATAATGAAA | −17 | |
| UL73 | KF297339.1:107367-107387_+ | TATTAACGTAGG | −16 | |
| UL74 | KF297339.1:109384-109404_− | TATTTACGAGGT | −18 | |
| UL75 | KF297339.1:111925-111945_− | TATTAAATAGAC | −21 | |
| UL76 | KF297339.1:112000-112020_+ | TATTTAATACGA | −19 | |
| UL80 | KF297339.1:116776-116796_+ | TATTAGATGATT | −21 | |
| UL80.5 | KF297339.1:117687-117707_+ | TATTAAAGCGGC | −18 | |
| UL82 | KF297339.1:120871-120891_− | TATTTGCGACAG | −24 | |
| UL85 | KF297339.1:125807-125827_− | TATTTAACTCGT | −18 | |
| UL86 | KF297339.1:130007-130027_− | TATTAGAGCGTC | −21 | |
| UL88 | KF297339.1:132407-132427_+ | TATTTGAGGCGG | −21 | |
| UL93 | KF297339.1:136210-136230_+ | TATTTACTTCAC | −21 | |
| UL94 | KF297339.1:137671-137691_+ | TATTAAGGCGCT | −17 | |
| UL96 | KF297339.1:141130-141150_+ | TATATGACCTAC | −19 | |
| UL97 | KF297339.1:141950-141970_+ | TCTTTAACCGAG | −18 | |
| UL99 | KF297339.1:145819-145839_+ | TATATAAAGAAG | −20 | |
| UL100 | KF297339.1:148031-148051_− | TATTTAACGACC | −18 | |
| UL115 | KF297339.1:166243-166263_− | TATATAACGTTT | −18 | |
| UL116 | KF297339.1:167184-167204_− | TATTTAATATCA | −24 | |
| UL119 | KF297339.1:169712-169732_− | TATTAACGCCTC | −17 | |
| UL121 | KF297339.1:170930-170950_− | TATTAACGTGTG | −17 | |
| US17 | KF297339.1:214822-214842_− | TATTTACGAGAC | −17 | |
| US27 | KF297339.1:225900-225920_+ | TATTTTATGGAG | −20 | |
| EBV | BNRF1 | V01555.2:1709-1729_+ | TATTAAATTTTA | 7 |
| BFRF0.5 | V01555.2:58113-58119_+ | TATTTTATCAGG | −22 | |
| BFLF1 | V01555.2:58534-58539_− | TATTAAAAACCC | −17 | |
| BFRF1 | V01555.2:58860-58867_+ | TATTATAAAACA | −20 | |
| BFRF3 | V01555.2:61372-61381_+ | TATTTAACTTTG | −17 | |
| BPLF1 | V01555.2:72156-72163_− | TATTAAAACATA | −17 | |
| BORF1 | V01555.2:75047-75054_+ | TATTTAAAAAAT | −19 | |
| BOLF1 | V01555.2:75281-75294_− | TATTTAGACGCC | −16 | |
| BLRF2 | V01555.2:88894-88898_+ | TATTTAAAAGGG | −20 | |
| BLLF1 | V01555.2:92158-92163_− | TATTAAAGAGGA | −17 | |
| BZLF2 | V01555.2:102126-102129_− | TATTAATAAATA | −19 | |
| BBRF1 | V01555.2:113906-113915_+ | TATTTATGTGGC | −17 | |
| BGLF2 | V01555.2:126893-126902_− | TATTAAAGGCCA | −15 | |
| BGLF1 | V01555.2:128398-128404_− | TATTTAAATCCA | −16 | |
| BDLF1 | V01555.2:133316-133323_− | TATTAAAGTTTG | −17 | |
| BcLF1 | V01555.2:137669-137683_− | TATTAAACCGGG | 15 | |
| BXLF2 | V01555.2:143257-143282_− | TACTTAAGGAAG | +6 | |
| BXRF1 | V01555.2:144610-144618_+ | TATTAGATGTCA | −17 | |
| BVRF2 | V01555.2:147752-147758_+ | TATTTATCACGG | −20 | |
| BILF2 | V01555.2:150530-150544_− | TATTTAGGCCTG | −15 | |
| BALF4 | V01555.2:159332-159340_− | TATTTAAGGATC | −18 | |
| KSHV | ORF8 | GQ994935.1:8656_+ | TATTTAAAGACC | −18 |
| ORF17.5 | GQ994935.1:31536_− | TATTTAAAGGCC | −17 | |
| ORF19 | Unknown | TATTTAAGAATC | N/A | |
| ORF25 | GQ994935.1:42378_+ | TATAAAAGGGTG | −18 | |
| ORF26 | GQ994935.1:46724_+ | TATTAAAGCTCG | −18 | |
| ORF27 | Unknown | TATTTAATCTTG | N/A | |
| ORF33 | GQ994935.1:52553_+ | TATTAAAGGCCG | −19 | |
| ORF38 | GQ994935.1:58186_+ | TATTAAAGCCCG | −18 | |
| ORF45 | GQ994935.1:68952_− | TTTCATCATCAG | −19 | |
| ORF52 | GQ994935.1:77063_− | TATTTAAAGCTG | −17 | |
| ORF53 | GQ994935.1:77513_− | TATATAAGAGGC | −17 | |
| ORF65 | GQ994935.1:112340_− | TATTAAAGCACC | −19 | |
| K8.1 | GQ994935.1:75716_+ | TATTAAAGGGAC | −21 |
3. Six viral transcriptional activators (“vTAs”) are required for late gene transcription
Accumulating evidence in the 2000’s and early 2010’s suggested that there were several viral proteins essential for late gene expression in the beta/gammaherpesviruses. Early deletion and transposon-insertion screens in HCMV and MHV68 revealed that six proteins are similarly essential for viral replication [59-61]. These six proteins are uniquely conserved across the beta/gammaherpesviruses (i.e., there are no homologs in the alphaherpesviruses). Given their unclear mechanistic role in late gene transcription, we favor the term “viral transcriptional activators” or “vTAs” for these six proteins.
Many groups have demonstrated that the vTAs are essential genes and are important for late gene expression across the model beta/gammaherpesviruses (HCMV, MCMV, EBV, MHV68, and KSHV). Phenotypic analysis of vTA deletion viruses reveals that (1) the vTAs are essential for virion production (because of the loss of late gene expression), (2) the vTAs are expressed prior to viral DNA replication (i.e., with early or leaky late kinetics), (3) loss of a vTA does not generally have a significant effect on viral DNA replication, and (4) the vTAs are recruited to viral replication compartments within the infected nucleus.
Studying the vTAs in the context of the virus is not without challenges, as several of the vTA genes overlap with other essential coding genes in the viral genome, preventing the addition of epitope tags. Additionally, in KSHV, vTA deletion has mild but measurable effects on DNA replication and expression of almost all viral genes, including the viral genome replication machinery itself [34, 47, 48]. Carefully disentangling viral genome replication and copy number effect from specific loss of vTA function has thus been nontrivial.
The vTA proteins have also been studied extensively outside the context of a viral infection through transient transfection and immunoprecipitation or functional assays. This has allowed for detailed probing of pairwise protein-protein interactions and the facile introduction of mutations of interest. However, transfection may result in non-biological overexpression and localization. An important tool to study vTA complex function outside of viral infection is the late gene promoter-driven luciferase reporter assay [8]. Transfection of all six vTAs with this reporter plasmid allows for late gene promoter activation and vTA binding [34] and similarly allows for faster screening of vTA variants [8, 47, 62]. However, an important caveat to consider is that this assay is performed in the absence of viral genome replication and formation of replication compartments. Below, we describe the experimental evidence for the function of the vTAs and highlight their unique properties.
3.1. ORF24/BcRF1/UL87, a viral mimic of host TATA-binding protein (“vTBP”)
The careful work done mapping the TATT-motif of late gene promoters in EBV and KSHV suggested that a protein with affinity for a modified TATA sequence may be involved in late gene transcription. A bioinformatics homology search found that the beta- and gammaherpesviruses encode a protein containing a domain with distant homology to cellular TATA-binding protein (TBP) (Figure 3A) [10]. Despite very low overall sequence identity, key DNA-binding residues (asparagine residues in the center of the saddle and phenylalanine residues important for inducing the kink in bound DNA) are conserved between TBP and vTBP. Shortly after, the MHV68 homolog (ORF24) was found to be essential for transcription of several late genes [63]. Similarly, UL87 was found to be essential in HCMV for transcription of late genes [64].
Figure 3. vTA complex organization.
A) Known domain features of the KSHV vTAs. B) Known protein-protein interactions in the KSHV (left) or MCMV (right) vTA complexes based on references [67, 73, 85].
A major breakthrough came in 2012, when the Manet group showed that the EBV homolog BcRF1 is required for late gene transcription. They showed that purified BcRF1 protein is capable of binding to a late gene promoter in vitro and is dependent on the aforementioned asparagine residues predicted to be important for DNA binding in TBP [10, 65]. KSHV and MHV68 ORF24 were also found to be able to bind to TATT-containing DNA probes in vitro [9, 57]. Thus, the beta/gammaherpesviruses encode a viral TBP-like protein, or “vTBP” essential for late gene transcription.
What determines the specificity of vTBP binding to late gene promoters? In vitro binding assays with purified BcRF1 and ORF24 give conflicting answers about the specificity of TATT vs. TATA promoter recognition. BcRF1 is capable of binding to both a late gene promoter and early gene promoter, although it is unable to bind a probe lacking an A/T-rich region [65]. In contrast, KSHV and MHV68 ORF24 are incapable of binding an early gene promoter and were sensitive to mutations in the late gene promoter [9, 57]. Whether vTBP homologs have different DNA binding properties – and how sequence specific promoter binding is ultimately achieved – remains to be determined. Cellular TBP is capable of binding many A/T-rich promoter sequence variants in vitro and forms nearly identical TBP-DNA structures, including binding to a TATTAAAG sequence (the consensus vTBP sequence) [66]. Given its putative structural similarly to TBP, it remains unclear how vTBP accomplishes such stringent promoter recognition.
3.2. vTBP, unlike cellular TBP, directly interacts with Pol II
Early work suggested that one of the vTAs in EBV could recruit Pol II [8]. A study of the KSHV-human interactome where KSHV ORFs were epitope tagged, transfected into HEK293T cells, and subjected to AP-MS revealed that ORF24, but none of the other vTAs, interacts with Pol II in the absence of other viral factors [9]. ORF24 copurified with the majority of Pol II subunits, and this effect was reproduced in AP-MS of MHV68 ORF24 and HCMV UL87 from transfected cells [9]. This observation was surprising, given that cellular TBP does not stably interact with Pol II. When ORF24 was isolated from KSHV-infected cells, Pol II was also identified as an interactor, showing that the presence of other viral factors, including the other vTAs, does not prevent its association with Pol II. During mouse CMV (MCMV) infection, epitope tagging of vTAs M79 and M92 followed by IP-MS also identified Pol II subunits Rpb1, Rpb2, and Rpb3 as interactors. This enrichment of Pol II subunits likely occurred via interactions between M79 and M92 with M95, which in turn interacts with M87 (the MCMV vTBP homolog) [67].
Although it was clear from the transfection-based approaches that additional viral factors are not required for the vTBP-Pol II interaction, it was unclear if additional cellular factors could be bridging the interaction or if the interaction was direct. A conserved leucine-rich motif in the N-terminal domain of KSHV ORF24 was identified as necessary for the interaction with Pol II (Figure 3A) [9]. This motif was also found to be important for vTBP-Pol II interactions in other homologs (MHV68 ORF24, BcRF1, and UL87), suggesting that the N-terminal domain functions similarly across the beta/gammaherpesvirus vTBPs [68]. A minimal N-terminal domain of vTBP was identified and shown to be necessary and sufficient for interaction with Pol II in a highly purified system, demonstrating that the interaction is direct. Using negative stain EM and in vitro binding assays, it was revealed that ORF24 specifically engages the largest subunit of Pol II, Rpb1.
Rpb1 contains a repetitive carboxy-terminal domain (CTD) consisting of 52 heptapeptide repeats, where consensus repeats have a sequence of Y1S2P3T4S5P6S7. Post-translational modifications of the CTD, particularly phosphorylation of serine 2 and serine 5, are known to be important for progression through the transcription cycle [69]. Intriguingly, ORF24 preferentially interacts with hypophosphorylated Rpb1 over phospho-S2 or phospho-S5 Rpb1, suggesting that ORF24 may have a role in vPIC complex formation [9]. In vitro binding experiments using purified ORF24 found that it binds to unphosphorylated CTD repeats, further suggesting that ORF24 is important for recruitment of Pol II to viral late gene PICs (vPICs) [68]. Recent work with DFF-ChIP sequencing of UL87 in HCMV similarly suggests it forms a viral PIC [51].
The dual ability of vTBP to specify late gene promoters through its TBP-like domain and recruit an RNA polymerase through its N-terminal domain make it conceptually akin to a bacterial sigma factor; in canonical eukaryotic cellular transcription, these activities are carried out by distinct general transcription factors. Surprisingly, despite the ability of vTBP to bind vTArget sequences and interact with Pol II, five other vTAs are still required for late gene transcription.
3.3. ORF34/BGLF3/UL95
ORF34 and its homologs are essential for late gene transcription in the beta/gammaherpesviruses [64, 70-72]. ORF34 is thought to act as the scaffold connecting vTBP to the other vTAs (Figure 3B). KSHV ORF34 interacts with vTBP (ORF24), ORF18, ORF31, and ORF66 [47, 62, 70, 73]. ORF24 interacts with ORF34 through a domain in between its N-terminal Pol II recruitment domain and its TBP-like domain [73]. Although this region is poorly conserved across the beta/gammaherpesvirus vTBPs, mutation of one of the conserved residues (R328 in KSHV ORF24) is sufficient to disrupt the interaction with ORF34 and thereby prevent late gene transcription [73]. The C-terminal region of ORF34 is important for interaction with ORF24, while the central region of ORF34 is important for interactions with ORF18, ORF31, and ORF66 [70].
ORF34 has also been reported to bind several host factors. MHV68 ORF34 was found to interact with PCBP1 in a yeast two-hybrid assay [74]. PCBP1 specifically inhibited late gene transcription, suggesting that it may have a potential negative regulatory role in late gene transcription through its interaction with ORF34. ORF34 has also been reported to bind and regulate the stability of cellular hypoxia-inducing factors HIF-1α and HIF-2α [75, 76], although downregulation of HIF-1α and upregulation of HIF-2α during KSHV have recently been attributed to vCyclin and ORF57 respectively [77, 78]. Whether ORF34 and its homologs – or any of the vTAs – have roles beyond late gene transcription remains to be investigated.
3.4. ORF18/BVLF1/UL79 and ORF30/BDLF3.5/UL91
Although ORF31 was the first vTA identified as essential for viral replication and for expression of several late viral proteins [79], ORF18 was the first vTA shown to be directly important for late gene mRNA levels and late promoter activity [80]. Soon after, ORF30 was found to be important for late gene transcription and promoter activity, as well as recruitment of Pol II to late gene promoters in MHV68 [71]. Homologs of ORF18 and ORF30 were similarly identified as essential for late gene expression in HCMV, MCMV, and EBV [24, 48, 64, 81-83].
ORF18 is a mid-sized vTA while ORF30 is the smallest vTA, ranging from 8-14 kDa (Table 3). In KSHV, ORF18 interacts with ORF31, ORF34, ORF66, and ORF30, while ORF30 only interacts with ORF18 [9, 62] (Figure 3B). The ORF18-ORF30 interface has been extensively mapped. All of the residues in ORF18 that are conserved in its MHV68, EBV, HCMV, MCMV, and BHV4 homologs (25 residues) were mutated to alanine, and the effect on pairwise interaction with ORF30, ORF31, or ORF66 was measured [62]. The ORF18-ORF30 interaction was particularly sensitive to mutation, and key residues were identified. This work was replicated using a bimolecular fluorescence complementation approach to measure the ORF18-ORF30 interaction [84]. Single alanine mutations in ORF18 were sufficient to disrupt ORF18-ORF30 binding, and this disruption resulted in a loss of late gene transcription and infectious virion production [62]. Interaction with ORF18 is also critical for the stability of ORF30, as its half-life was drastically reduced in the absence of ORF18 [62]. The inverse dependence was also reported in the EBV homologs, where BVLF1 levels were improved by co-expression of BDLF3.5 [8].
Table 3. vTA homologs across the beta/gammaherpesviruses.
Length of the proteins, in amino acids (a.a.) were based on annotated ORFs in reference sequences MCMV: HE610455.1; HCMV: RefSeq GCF_000845245.1; KSHV: RefSeq GCF_000838265.1; EBV: RefSeq GCF_002402265.1; HHV7: NC_001716.2; HHV6B: KY316030.1; MHV68: NC_001826.2
| HCMV (HHV-5) |
MCMV | HHV-6A/B | HHV-7 | EBV (HHV-4) |
MHV68 | KSHV (HHV-8) |
Notable features |
|---|---|---|---|---|---|---|---|
| UL87 (941 a.a.) | M87 (925 a.a.) | U58 (772 a.a.) | U58 (775 a.a.) | BcRF1 (750 a.a.) | ORF24 (717 a.a.) | ORF24 (752 a.a.) | vTBP domain, Pol II-interaction domain |
| UL79 (295 a.a.) | M79 (258 a.a.) | U52 (258 a.a.) | U52 (254 a.a.) | BVLF1 (272 a.a.) | ORF18 (284 a.a.) | ORF18 (257 a.a.) | |
| UL91 (111 a.a.) | M91 (134 a.a.) | U62 (87 a.a.) | U62 (75 a.a.) | BDLF3.5 (77 a.a.) | ORF30 (80 a.a.) | ORF30 (77 a.a.) | Smallest vTA -only interacts with ORF18 |
| UL92 (201 a.a.) | M92 (230 a.a.) | U63 (218 a.a.) | U63 (211 a.a.) | BDLF4 (225 a.a.) | ORF31 (200 a.a.) | ORF31 (224 a.a.) | Contains cysteine-rich motifs |
| UL95 (533 a.a.) | M95 (414 a.a.) | U67 (350 a.a.) | U67 (346 a.a.) | BGLF3 (332 a.a.) | ORF34 (332 a.a.) | ORF34 (327 a.a.) | Scaffold protein – interacts with all but one of the vTAs; contains cysteine-rich motifs |
| UL49 (570 a.a.) | M49 (536 a.a.) | U33 (470 a.a.) | U33 (477 a.a.) | BFRF2 (591 a.a.) | ORF66 (409 a.a.) | ORF66 (429 a.a.) | Four cysteine-rich motifs |
The binding interface between these proteins was also investigated in MCMV, where residues in UL91 critical for the UL79-UL91 were identified, including several negatively charged residues conserved between HCMV and MCMV UL91 [85]. ORF30 and its homologs are generally poorly conserved across the beta/gammaherpesviruses. Interestingly, the first 71 residues of HCMV UL91 are sufficient for its function [82], which correspond to the residues best conserved across the beta/gammaherpesvirus homologs. The poorly conserved C-terminal region of MCMV UL91 does not contribute to the UL79-UL91 interaction [85]. MCMV UL91 also has an N-terminal extension relative to HCMV UL91, and this N-terminal domain also does not contribute to the UL79-UL91 interaction [85]. Thus, ORF30 homologs appear to have a minimal domain of ~70 residues that is sufficient for interaction with ORF18 and for its critical function. The presence of key residues in ORF18 and its homologs and a similar minimal domain of ORF30 and its homologs (despite low sequence homology) suggests that the protein-protein interface, and thus the structure of this subcomplex, may be conserved across the beta/gammaherpesviruses. Disrupting incorporation of this small vTA can result in a complete loss of late gene transcription, highlighting the highly specialized organization of the vTA complex.
The role of the vTAs putatively occurs in the nucleus, where late gene transcription occurs. Many studies have looked at the localization of vTAs, although few have studied the mechanism by which nuclear localization occurs. UL79 was predicted to have a nuclear localization signal (NLS) with a “PY” motif, and this sequence was found to be important for nuclear localization of UL79 in transfected cells [86]. Although the NLS is in a well-conserved region, the PY-NLS motif is not conserved in other beta/gammaherpesvirus homologs. How UL79 and the other vTAs are transported into the nucleus is unknown. The vTAs are generally small enough that they may passively diffuse through the nuclear pore, although it is unclear if vTBP would need to be actively transported into the nucleus based on its size [87]. The vTAs may occupy both compartments until they are assembled into a larger complex or assemble on viral DNA. How the vTA complex assembles in nuclear replication compartments and whether any of the vTAs have a role in the cytoplasm outside of late gene transcription remains to be determined. Relatedly, it is unclear if any of the vTAs are packaged into new virions or if they have a potential early role in de novo infection. ORF18 and ORF24 have been reported to be components of KSHV virions [88, 89], although UL79 was not reported to incorporated into HCMV virions [81]. ORF18 and ORF30 are required for transcription of very similar targets during KSHV reactivation and de novo infection, suggesting that vTA-dependent transcription may occur similarly during these two modes of replication and that their roles in the tegument may be limited or unrelated to vTA-mediated transcription [48].
What is the function of ORF18 and its homologs? The first potential mechanistic role came from a study that immunoprecipitated UL79 from HCMV-infected cells and observed an enrichment of Pol II subunits [90]. This result is consistent with vTA complex formation and Pol II recruitment via vTBP. However, UL79 was also found to interact with Pol II in the absence of viral factors [90]. Unlike vTBP, UL79 immunoprecipitated phospho-S2 Rpb1 (Ser2p), although it is unclear if it selectively enriched for this CTD modification. As Ser2 phosphorylation is an indicator of transcription elongation, it was proposed that UL79 could act as a late gene transcription elongation factor. Additionally, loss of UL79 resulted in an increased occupancy of Pol II at viral loci and reduced the elongation rate on viral genes, suggestive of an elongation defect in the absence of UL79. UL79 did not appear to be important for promoter specification, as it did not specifically ChIP to late gene promoters. These data collectively suggest that UL79 is involved not in pre-initiation, but rather in transcription elongation. In contrast, ORF18 has not been reported to interact with Pol II directly [62]. Additionally, ORF30 – which is connected to the vTA complex solely through its interaction with ORF18 – is required for vTA complex formation at promoters, suggesting that the whole complex (including ORF18) must be present [47]. Whether ORF18 and UL79 have distinct functions in KSHV vs. HCMV late gene transcription remains to be determined, as does the question of whether the vTAs have functions in transcription beyond promoter selection.
3.5. ORF31/BDLF4/UL92
The second smallest vTA, ORF31, was identified very early on as a factor essential for late gene expression in MHV68 [79]. The homologs in HCMV, MCMV, and EBV were similarly found to be essential [91-93]. ORF31 and its homologs have an N-terminal cysteine-rich region containing 6 conserved cysteines and 1 conserved histidine, followed by a poorly conserved interior region and a well-conserved C-terminal charged region containing conserved lysine, arginine, and aspartic acid residues. All three regions are essential for function of ORF31 [46] (Figure 3A).
UL92 can be complemented by Rh127, its close homolog from RhCMV (90% identity) [92] and by M92, its homolog from MCMV (50% identity) [91]. Despite even lower sequence identity (38% identity), KSHV ORF31 could complement loss of MHV68 ORF31 [79]. ORF31 may be less sensitive to variation than other vTAs like vTBP. In a KSHV late gene transcription reporter assay, KSHV ORF24 cannot be exchanged for MHV68 ORF24 (40% identity) [68]. As with the other vTAs, determining which interactions are maintained or lost when mutations are made or homologs are exchanged will facilitate mapping of interaction surfaces.
In KSHV, ORF31 interacts with ORF34, ORF18, and ORF66, although anecdotally (and in a pairwise split luciferase assay), interactions between ORF31 and the other vTAs are weak [46, 47, 62, 73]. In MCMV, M92 has most recently been reported to only interact with vTBP (M87) [85], suggestive of a distinct organization within the complex (Figure 3B). However, M92 was previously shown to interact with M79 during MCMV infection [91] and coevolution analysis of the MCMV vTAs suggests that M92 is connected to M95 and M49 [67], suggesting a complex organization similar to the KSHV vTAs. In EBV, immunoprecipitation of BDLF4 can enrich for the other known vTAs [93], but the pairwise interactions within the complex are unclear.
Phosphorylation of EBV BDLF4 by cellular S-like-phase cyclin-dependent kinases affects its stability and prevents its proteasomal degradation [94]. BDLF4 is phosphorylated at residue T91, and mutation of this residue to alanine reduces BDLF4 expression, ultimately affecting late gene expression and infectious virion production. This residue is very poorly conserved across beta/gammaherpesviral homologs of BDLF4 and it is unknown if they are also post-translationally regulated by phosphorylation.
3.6. ORF66/BFRF2/UL49
The last vTA to be identified as essential late gene transcription was EBV BFRF2 [8]. This protein is the second largest vTA after vTBP and has a well-conserved cysteine-rich C-terminal domain. Four pairs of bidentate cysteines (CX2-6C) are perfectly conserved across all beta/gammaherpesviral homologs (Figure 3A). As zinc finger motifs were originally identified in a transcription factor [95] and are common in DNA-binding proteins, it was hypothesized that this vTA could be important for promoter binding. Two groups independently found that these cysteine residues are essential [47, 96]. Interestingly, these cysteine residues were critical for interaction with ORF34, but not ORF31 or ORF18, suggesting that the zinc finger-like motifs may be important for protein-protein interactions in the ORF34-ORF66 interface rather than promoter binding. Truncation and mutation analysis of the MCMV homolog, M49, revealed that its C-terminal domain was similarly required for interaction with M95, the homolog of ORF34 [97]. It is now clear that ORF66 is an essential component of the vTA complex in the beta/gammaherpesviruses and that it makes critical protein-protein contacts with ORF34. Whether ORF66 contains true zinc-binding domains, and whether it contacts promoter DNA in the context of the vPIC, remains to be determined.
4. The vTAs form a complex
The six vTAs make a number of protein-protein interactions that allow formation of a vTA complex. For nomenclature, we suggest that the term “vTA complex” be used when referring to the complex of the six vTAs while “vPIC” should refer to the full preinitiation complex assembled at vTA-dependent late gene promoters (which may or may not contain additional viral or host GTFs). When plasmids for all six vTAs are co-transfected into HEK293T cells lacking the viral genome, enriching for any one of the vTAs will pull out the remaining vTAs, suggesting that the vTA complex is stable in the absence of other viral factors and late gene promoters [8, 9, 47, 62, 85].
Pairwise mapping of the KSHV vTAs using a split luciferase system generated a map of protein-protein interactions that has been substantiated by pairwise IP-western experiments [9, 47, 62] (Figure 3B). In KSHV, vTBP is the only vTA to interact with Pol II, and vTBP is recruited to the complex by ORF34. ORF34 acts as a scaffold and interacts with ORF18, ORF31, and ORF66. ORF30 only interacts with ORF18. The overarching network of protein-protein interactions does seem to be largely conserved, particularly the role of ORF34/BGLF3/UL95 as a scaffold that connects vTBP to the rest of the vTAs. It also appears to be generally true that ORF30/BDLF3.5/UL91 only interacts with ORF18/BVLF1/UL79.
It remains to be determined whether the connectivity of the complex, and the pairwise protein-protein interactions, are identical in other beta/gammaherpesviruses, particularly for homologs of ORF31 (see section 3.4) (Figure 3B). It is likely that the presence of other interacting vTAs, the presence of other host or viral factors, and the immunoprecipitation conditions can modify observed affinities within the complex. Post-translational modifications during infection may also change the observed interactions. Extensive work detailed in the previous sections has helped identify potential protein-protein interaction surfaces by pinpointing key regions or residues. Ultimately, structural information for the individual vTAs and for the complex as a whole will be necessary to identify critical and targetable protein-protein interaction surfaces and develop therapeutics to disrupt these interactions. The stoichiometry of the vTAs within this complex also remains to be determined. If the complex contains a single copy of each vTA, it should weigh approximately 250 kDa. The size of the complex varies across the beta/gammaherpesviruses, where the MHV68 vTA complex has ~75% of the amino acid content of the HCMV vTA complex (Table 3). Whether the vTA complex has a conserved structural core across the beta/gammaherpesviruses, whether the same vTA-vTA interactions are maintained, and if vTA complexes from different viruses have unique functions or properties remains to be determined.
4.1. A viral preinitiation complex assembles at vTArget promoters
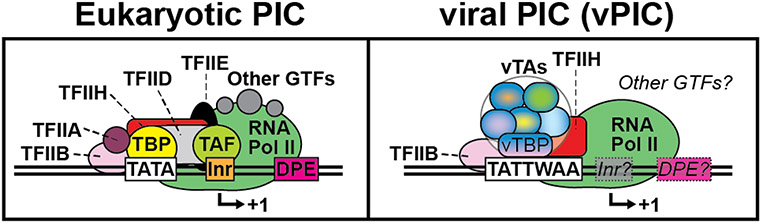
While the vTA complex can form in the absence of newly replicated viral DNA, during viral lytic replication, the vTA complex specifically binds to late gene promoters. ChIP of epitope-tagged vTAs has been useful to map the occupancy of various factors at select DNA regions. Using ChIP-PCR, ORF24 was found to bind to a late gene promoter during viral infection, suggesting that its TBP-like domain could be recognizing late gene promoters in vivo [9, 46]. ORF34, ORF31, and ORF66 were also found to ChIP to late gene promoters, consistent with their ability to interact with ORF24 directly or indirectly within the vTA complex [34, 46, 47, 70, 96]. Thus, it is likely that in KSHV, the entire vTA complex assembles at late gene promoters. Interestingly, ORF24 was unable to bind to late gene promoters in viruses that lacked either ORF30 or ORF66, suggesting that the entire vTA complex must be present for vTArget recognition [47]. Although ORF24 may have intrinsic sequence-specific binding ability in vitro, in the context of lytic replication, it requires formation of the vTA complex to recognize late gene promoters. These data, along with the observation the vTBP recruits hypophosphorylated Pol II [9, 68], support the model that the vTA complex assembles on late gene promoters into a vPIC. What other cellular or viral factors are in a vPIC (Figure 4)? Typical cellular PICs include the general transcription factors TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, and Pol II [98, 99] (Figure 4). TFIID is composed of cellular TBP and the TBP-associated factors (TAFs), and serves to broaden TBP’s binding specificity beyond the ~15% of cellular promoters that possess a TATA sequence [99].
Figure 4. Canonical and vTA-modified preinitiation complex (PIC).
Generally, eukaryotic PICs include the general transcription factors TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, and Pol II. TBP and associated TAFs convey promoter specificity, discriminating on the basis of cis-elements including the TATA-box and Initiator (Inr) element. For late gene viral PICs (vPICs), vTBP in complex with other vTAs binds at a TATTWAA motif 20-30 bp upstream of the TSS. vTA binding occurs instead of host TBP (and likely other members of the TFIID complex). The full composition of the vPIC has yet to be determined, although recent evidence supports the presence of the canonical GTF’s, TFIIB and TFIIH.
In vPICs, vTBP replaces TBP at late gene promoters, suggesting that TFIID is not used in its canonical fashion in late gene transcription [9, 51]. Analysis of vTA-dependent gene promoters identified only the modified TATT element as a significantly enriched promoter element (Figure 2) [34, 52, 58]. Notably, downstream initiator (Inr) elements cannot be identified in vTArget promoters, suggesting that TFIID may not bind. This is in contrast to alphaherpesviruses, where a downstream Inr element is required for efficient late gene transcription [3, 100]. Recent work using DFF-ChIP-seq in HCMV revealed that vPICs likely have many properties that differ from typical PICs [51]. Normal TBP-containing PICs contact DNA both upstream and downstream of the TSS. The vPIC occupies less real estate, binding only upstream of the TSS. This fundamental difference in size could be due to a loss of TFIID, consistent with the replacement of TBP with vTBP. Thus, it seems likely that replacing TBP with vTBP at late gene promoters also removes the requirement for the rest of TFIID. Given their ability to form a complex containing vTBP, do the other vTAs function as viral TAFs?
The role of other GTFs is also unknown. In cellular transcription, additional interactions between TBP and TFIIA and TFIIB are required for promoter binding and stabilization of the PIC. B recognition elements (BREs) also appear to be missing in vTArget promoters, suggesting that TFIIB may not be involved in promoter selection. However, ChIP-PCR of TFIIB in KSHV suggests that it binds both early gene and late gene promoters [9]. XPB, a component of TFIIH, is required for transcription of some late genes in EBV, although it is unclear if vTA-dependent genes are uniquely or universally dependent on TFIIH [101]. Do the other vTAs functionally mimic or replace other general transcription factors? Identifying which GTFs are lost or maintained at late gene promoters will suggest potential functional roles for the vTAs and will reveal key mechanistic differences in host and viral PIC formation and transcription initiation.
5. Other viral factors implicated in late gene transcription
There may be other viral factors beyond the six vTAs and components of the lytic DNA replication machinery that contribute to late gene transcription. Recently, a CRISPR/Cas9 screen using guides that tile across the viral genome was performed on a KSHV cell line containing a fluorescent reporter driven by a model late gene promoter [102]. This screen successfully identified the vTAs and viral DNA replication machinery as top hits. The screen also identified ORF46 as a strong hit. Follow-up experiments suggested that the DNA-binding activity, but not catalytic activity of ORF46, is required for viral DNA replication in KSHV, analogous to its homolog in EBV, BKRF3 [103, 104]. This reporter-based screening approach highlights that in KSHV, no viral factors beyond the vTAs and DNA replication machinery are significant contributors to late gene transcription in vitro. However, this approach could be used in other herpesviruses to identify potential accessory factors or factors that have mild but measurable effects on late gene transcription. Below, we discuss other viral factors that have been implicated in late gene transcription.
5.1. ORF23/BTRF1/UL88
In addition to the six vTAs, there is a seventh gene that is conserved across the beta/gammaherpesviruses but missing in the alphaherpesviruses. However, early screens for lytic replication in HCMV and MHV68 showed that ORF23 and UL88 were not essential, unlike the other six vTAs [59-61]. It was later shown independently that MHV68 ORF23 is not required for replication in cell culture or in vivo [105]. The EBV homolog, BTRF1, was also found to be dispensable [24]. However, ORF23 was shown to interact with ORF34 [70]. Whether ORF23 and its homologs are incorporated into vTA complexes and if they play an important role in late gene transcription in specific biological contexts remains to be determined.
5.2. BGLF4 (ORF36/UL97)
Herpesviruses encode a conserved serine/threonine protein kinase [106]. In EBV, the protein kinase BGLF4 was shown to be essential for late gene transcription through knockdown and complementation experiments [72]. Mass spectrometry of the vTA BGLF3 (homolog of ORF34/UL95) revealed that it is phosphorylated by BGLF4 [107]. Mutation of BGLF3 T42 to alanine – but not to serine, which can also be phosphorylated by BGLF4 – reduced interactions between BGLF3 and the vTAs BVLF1 and BFRF2. The effect of the BGLF4(T42A) mutation on late gene transcription was partially rescued by overexpression of BVLF1 and BFRF2, suggesting that phosphorylation of BGLF3(T42) by BGLF4 promotes vTA complex assembly.
The HCMV homolog of the kinase, UL97, is not thought to be important for late gene transcription [108]; however, UL97 was recently identified as an interaction partner of UL87, UL95, and UL79 by mass spectrometry [109]. It is unclear if the KSHV protein kinase ORF36 is required for phosphorylation of ORF34 and whether phosphorylation promotes interaction of an ORF34-ORF18-ORF66 subcomplex. A threonine or serine at the same position as T42 in BGLF3 is conserved across the β/γ-HV homologs. However, while ORF36 deletion does have an effect on virion production and affects the levels of some late genes in KSHV, it is unclear whether it has a direct role in late gene transcription [110]. It is possible that vTA complexes in KSHV and other herpesviruses are less sensitive to the phosphorylation state, that KSHV vTA complex formation is primarily driven by other interactions, or that BGLF3 homologs in other viruses are modified by other viral or cellular proteins. More broadly, the post-translational modification (PTM) state of the vTAs during viral replication remains largely uncharacterized. PTMs, including phosphorylation, ubiquitination, and sumoylation, are critical for regulation of cellular transcription factors [111], and these PTMs may change the localization, stability, or function of the vTAs.
5.3. EBV SM protein (also called BMLF1, EB2, Mta); homologous to KSHV ORF57 and HCMV UL69
The EBV SM protein (also referred to as Mta, for mRNA transcript accumulation) is an RNA-binding protein linked to a number of post-transcriptional regulatory processes including RNA stability, splicing, export, and translation [112-115]. Analysis of an SM-knockout virus revealed that a subset of late genes are strongly affected by loss of SM [116]. It was suggested that SM could act at a transcriptional level, rather than post-transcriptional level, through association with XPB [101]. While the group of SM-dependent genes is largely expressed at a late temporal stage, it includes genes that are thought to be leaky late (BRRF2, BBRF3, BLFR1, and BLRF2) and vPIC-independent late genes (BCRF1, BDLF2, BDLF3) [45, 52, 116]. Thus, SM is unlikely to be uniquely involved in vTA-mediated late gene transcription, although it has important functions for expression of late genes. KSHV ORF57 (Mta) is similarly thought to act as a multifunctional post-transcriptional regulator [117]. While ORF57 is essential for infectious virion production and does have an effect on viral gene expression, it affects a subset of both early and late genes [118]. Similarly, UL69 is important for lytic replication and post-transcriptionally regulates viral gene expression by promoting nuclear export of unspliced mRNA in HCMV [119, 120].
5.4. ORF10
KSHV ORF10 was identified as an interactor of the Rae1-Nup98 mRNA export pathway that blocks nuclear export of select mRNAs [121, 122]. Loss of ORF10 resulted in a reduction of viral late gene transcripts without a defect in viral DNA replication [122]. However, protein levels of the vTA ORF31 were severely reduced in the ORF10 deletion virus, and transcript levels of several of the vTAs (ORF31, ORF30, and ORF66) were attenuated to a similar degree as late genes. Thus, ORF10 may indirectly support late gene transcription by promoting the expression of vTAs or other factors required for late gene expression.
6. Conclusions
In this review, we have highlighted key features of vTA-dependent late gene transcription in the beta/gammaherpesviruses. vTA-dependent late gene promoters – vTArgets – contain a TATTWAA-motif and are bereft of other common motifs found in cellular promoters. Six vTAs, one of which functions as a viral TBP mimic, are required for late gene transcription, and have largely conserved properties and protein-protein interactions. By integrating information and highlighting similarities in phenotype across multiple viruses, we have sought to underscore the common mechanisms that may underlie vTA function. However, there may be additional functions of the vTAs in transcription or other processes during viral infection, and some of these differences may be virus-specific. We have attempted to highlight open questions by focusing on the mechanism of vTArget promoter selection and vTA function throughout this review. Many additional open questions remain about how vTA-mediated transcription fits into the bigger picture of lytic viral replication. Late gene transcription does not happen in isolation and may be coupled to or competing with other nuclear processes. Does the same viral genome undergo replication, transcription, and packaging at the same time, or does the virus coordinate which templates undergo which processes? How do the biophysical properties of replication compartments affect these processes? Ultimately, understanding vTA-mediated late gene transcription in the beta/gammaherpesviruses in molecular and mechanistic detail will unveil new facets of gene regulation and may point towards new therapeutic strategies.
Acknowledgements
We appreciate the work on late gene transcription performed in the last twenty years and apologize to our colleagues for any work we have unintentionally overlooked. We thank Chloe McCollum, Divya Nandakumar, and John Zinder for discussion and comments on the manuscript. S.E.D. is supported by funds from the National Institutes of Health, Division of Intramural Research National Cancer Institute (ZIA BC011176). A.L.D. is The Rhee Family Dale F. Frey Breakthrough Scientist of the Damon Runyon Cancer Research Foundation (DFS-52-22).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Allison Didychuk has received funding as the Rhee Family Dale F. Frey Breakthrough Scientist of the Damon Runyon Cancer Research Foundation (DFS-52-22). The authors have no conflicts of interest to declare.
References
- 1.Liu Z, Carmichael GG. Polyoma virus early-late switch: regulation of late RNA accumulation by DNA replication. Proc Natl Acad Sci U S A. 1993;90(18):8494–8. Epub 1993/09/15. doi: 10.1073/pnas.90.18.8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo F, Mertz JE. Simian virus 40 late gene expression is regulated by members of the steroid/thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1995;92(19):8586–90. Epub 1995/09/12. doi: 10.1073/pnas.92.19.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dremel SE, DeLuca NA. Genome replication affects transcription factor binding mediating the cascade of herpes simplex virus transcription. Proc Natl Acad Sci U S A. 2019;116(9):3734–9. Epub 2019/02/28. doi: 10.1073/pnas.1818463116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiduschek EP, Kassavetis GA. Transcription of the T4 late genes. Virol J. 2010;7:288. Epub 2010/10/30. doi: 10.1186/1743-422X-7-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guarino LA, Xu B, Jin J, Dong W. A virus-encoded RNA polymerase purified from baculovirus-infected cells. J Virol. 1998;72(10):7985–91. Epub 1998/09/12. doi: 10.1128/JVI.72.10.7985-7991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keck JG, Baldick CJ Jr., Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell. 1990;61(5):801–9. Epub 1990/06/01. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- 7.Kassavetis GA, Elliott T, Rabussay DP, Geiduschek EP. Initiation of transcription at phage T4 late promoters with purified RNA polymerase. Cell. 1983;33(3):887–97. Epub 1983/07/01. doi: 10.1016/0092-8674(83)90031-4. [DOI] [PubMed] [Google Scholar]
- 8.Aubry V, Mure F, Mariame B, Deschamps T, Wyrwicz LS, Manet E, et al. Epstein-Barr virus late gene transcription depends on the assembly of a virus-specific preinitiation complex. J Virol. 2014;88(21):12825–38. Epub 2014/08/29. doi: 10.1128/JVI.02139-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis ZH, Verschueren E, Jang GM, Kleffman K, Johnson JR, Park J, et al. Global mapping of herpesvirus-host protein complexes reveals a transcription strategy for late genes. Mol Cell. 2015;57(2):349–60. Epub 2014/12/30. doi: 10.1016/j.molcel.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyrwicz LS, Rychlewski L. Identification of Herpes TATT-binding protein. Antiviral Res. 2007;75(2):167–72. Epub 2007/04/03. doi: 10.1016/j.antiviral.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Maruri-Avidal L, Sisler J, Stuart CA, Moss B. Cascade regulation of vaccinia virus gene expression is modulated by multistage promoters. Virology. 2013;447(1-2):213–20. Epub 2013/11/12. doi: 10.1016/j.virol.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5(1):9. Epub 2019/02/02. doi: 10.1038/s41572-019-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6-19, 2003-2010. PLoS One. 2013;8(5):e64921. Epub 2013/05/30. doi: 10.1371/journal.pone.0064921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034. Epub 2019/02/02. doi: 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 15.James C, Harfouche M, Welton NJ, Turner KM, Abu-Raddad LJ, Gottlieb SL, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315–29. Epub 2020/06/10. doi: 10.2471/BLT.19.237149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akpan US, Pillarisetty LS. Congenital Cytomegalovirus Infection. StatPearls. Treasure Island (FL)2022. [PubMed] [Google Scholar]
- 17.Krug LT, Pellett PE. Roseolovirus molecular biology: recent advances. Curr Opin Virol. 2014;9:170–7. Epub 2014/12/02. doi: 10.1016/j.coviro.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen KW, Wang L, Menke JR, Damania B. Cancers associated with human gammaherpesviruses. FEBS J. 2021. Epub 2021/09/19. doi: 10.1111/febs.16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. Epub 2022/01/14. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 20.Caragliano E, Bonazza S, Frascaroli G, Tang J, Soh TK, Grunewald K, et al. Human cytomegalovirus forms phase-separated compartments at viral genomes to facilitate viral replication. Cell Rep. 2022;38(10):110469. Epub 2022/03/10. doi: 10.1016/j.celrep.2022.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caragliano E, Brune W, Bosse JB. Herpesvirus Replication Compartments: Dynamic Biomolecular Condensates? Viruses. 2022;14(5). Epub 2022/05/29. doi: 10.3390/v14050960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Fu W, Swaminathan S. Continuous DNA replication is required for late gene transcription and maintenance of replication compartments in gammaherpesviruses. PLoS Pathog. 2018;14(5):e1007070. Epub 2018/05/31. doi: 10.1371/journal.ppat.1007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McSwiggen DT, Hansen AS, Teves SS, Marie-Nelly H, Hao Y, Heckert AB, et al. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. Elife. 2019;8. Epub 2019/05/01. doi: 10.7554/eLife.47098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djavadian R, Chiu YF, Johannsen E. An Epstein-Barr Virus-Encoded Protein Complex Requires an Origin of Lytic Replication In Cis to Mediate Late Gene Transcription. PLoS Pathog. 2016;12(6):e1005718. Epub 2016/06/28. doi: 10.1371/journal.ppat.1005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruffat H, Marchione R, Manet E. Herpesvirus Late Gene Expression: A Viral-Specific Pre-initiation Complex Is Key. Front Microbiol. 2016;7:869. Epub 2016/07/05. doi: 10.3389/fmicb.2016.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dremel SE, DeLuca NA. Herpes simplex viral nucleoprotein creates a competitive transcriptional environment facilitating robust viral transcription and host shut off. Elife. 2019;8. Epub 2019/10/23. doi: 10.7554/eLife.51109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dembowski JA, DeLuca NA. Temporal Viral Genome-Protein Interactions Define Distinct Stages of Productive Herpesviral Infection. mBio. 2018;9(4). Epub 2018/07/19. doi: 10.1128/mBio.01182-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preston CM. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979;29(1):275–84. Epub 1979/01/01. doi: 10.1128/JVI.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson RJ, Clements JB. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980;285(5763):329–30. Epub 1980/05/29. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- 30.DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56(2):558–70. Epub 1985/11/01. doi: 10.1128/JVI.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixon RA, Schaffer PA. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980;36(1):189–203. Epub 1980/10/01. doi: 10.1128/JVI.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dembowski JA, Dremel SE, DeLuca NA. Replication-Coupled Recruitment of Viral and Cellular Factors to Herpes Simplex Virus Type 1 Replication Forks for the Maintenance and Expression of Viral Genomes. PLoS Pathog. 2017;13(1):e1006166. Epub 2017/01/18. doi: 10.1371/journal.ppat.1006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heath JR, Dembowski JA. Fashionably late: Temporal regulation of HSV-1 late gene transcription. PLoS Pathog. 2022;18(6):e1010536. Epub 2022/06/17. doi: 10.1371/journal.ppat.1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandakumar D, Glaunsinger B. An integrative approach identifies direct targets of the late viral transcription complex and an expanded promoter recognition motif in Kaposi's sarcoma-associated herpesvirus. PLoS Pathog. 2019;15(5):e1007774. Epub 2019/05/17. doi: 10.1371/journal.ppat.1007774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang S, Yamanegi K, Zheng ZM. Requirement of a 12-base-pair TATT-containing sequence and viral lytic DNA replication in activation of the Kaposi's sarcoma-associated herpesvirus K8.1 late promoter. J Virol. 2004;78(5):2609–14. Epub 2004/02/14. doi: 10.1128/jvi.78.5.2609-2614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravorty A, Sugden B, Johannsen EC. An Epigenetic Journey: Epstein-Barr Virus Transcribes Chromatinized and Subsequently Unchromatinized Templates during Its Lytic Cycle. J Virol. 2019;93(8). Epub 2019/02/01. doi: 10.1128/JVI.02247-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14(1):8–19. Epub 1974/07/01. doi: 10.1128/JVI.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975;72(4):1276–80. Epub 1975/04/01. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones PC, Roizman B. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J Virol. 1979;31(2):299–314. Epub 1979/08/01. doi: 10.1128/JVI.31.2.299-314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arias C, Weisburd B, Stern-Ginossar N, Mercier A, Madrid AS, Bellare P, et al. KSHV 2.0: a comprehensive annotation of the Kaposi's sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features. PLoS Pathog. 2014;10(1):e1003847. Epub 2014/01/24. doi: 10.1371/journal.ppat.1003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Grady T, Wang X, Honer Zu Bentrup K, Baddoo M, Concha M, Flemington EK. Global transcript structure resolution of high gene density genomes through multi-platform data integration. Nucleic Acids Res. 2016;44(18):e145. Epub 2016/07/14. doi: 10.1093/nar/gkw629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozman B, Nachshon A, Levi Samia R, Lavi M, Schwartz M, Stern-Ginossar N. Temporal dynamics of HCMV gene expression in lytic and latent infections. Cell Rep. 2022;39(2):110653. Epub 2022/04/14. doi: 10.1016/j.celrep.2022.110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatherer D, Seirafian S, Cunningham C, Holton M, Dargan DJ, Baluchova K, et al. High-resolution human cytomegalovirus transcriptome. Proc Natl Acad Sci U S A. 2011;108(49):19755–60. Epub 2011/11/24. doi: 10.1073/pnas.1115861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Ball CB, Collins G, Hu Q, Luse DS, Price DH, et al. Human cytomegalovirus IE2 drives transcription initiation from a select subset of late infection viral promoters by host RNA polymerase II. PLoS Pathog. 2020;16(4):e1008402. Epub 2020/04/07. doi: 10.1371/journal.ppat.1008402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenzie J, Lopez-Giraldez F, Delecluse HJ, Walsh A, El-Guindy A. The Epstein-Barr Virus Immunoevasins BCRF1 and BPLF1 Are Expressed by a Mechanism Independent of the Canonical Late Pre-initiation Complex. PLoS Pathog. 2016;12(11):e1006008. Epub 2016/11/18. doi: 10.1371/journal.ppat.1006008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brulois K, Wong LY, Lee HR, Sivadas P, Ensser A, Feng P, et al. Association of Kaposi's Sarcoma-Associated Herpesvirus ORF31 with ORF34 and ORF24 Is Critical for Late Gene Expression. J Virol. 2015;89(11):6148–54. Epub 2015/03/27. doi: 10.1128/JVI.00272-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Didychuk AL, Castaneda AF, Kushnir LO, Huang CJ, Glaunsinger BA. Conserved CxnC Motifs in Kaposi's Sarcoma-Associated Herpesvirus ORF66 Are Required for Viral Late Gene Expression and Are Essential for Its Interaction with ORF34. J Virol. 2020;94(2). Epub 2019/10/04. doi: 10.1128/JVI.01299-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong D, Wu NC, Xie Y, Feng J, Tong L, Brulois KF, et al. Kaposi's sarcoma-associated herpesvirus ORF18 and ORF30 are essential for late gene expression during lytic replication. J Virol. 2014;88(19):11369–82. Epub 2014/07/25. doi: 10.1128/JVI.00793-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ersing I, Nobre L, Wang LW, Soday L, Ma Y, Paulo JA, et al. A Temporal Proteomic Map of Epstein-Barr Virus Lytic Replication in B Cells. Cell Rep. 2017;19(7):1479–93. Epub 2017/05/18. doi: 10.1016/j.celrep.2017.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabaev I, Williamson JC, Crozier TWM, Schulz TF, Lehner PJ. Quantitative Proteomics Analysis of Lytic KSHV Infection in Human Endothelial Cells Reveals Targets of Viral Immune Modulation. Cell Rep. 2020;33(2):108249. Epub 2020/10/15. doi: 10.1016/j.celrep.2020.108249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spector BM, Parida M, Li M, Ball CB, Meier JL, Luse DS, et al. Differences in RNA polymerase II complexes and their interactions with surrounding chromatin on human and cytomegalovirus genomes. Nat Commun. 2022;13(1):2006. Epub 2022/04/16. doi: 10.1038/s41467-022-29739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Djavadian R, Hayes M, Johannsen E. CAGE-seq analysis of Epstein-Barr virus lytic gene transcription: 3 kinetic classes from 2 mechanisms. PLoS Pathog. 2018;14(6):e1007114. Epub 2018/06/05. doi: 10.1371/journal.ppat.1007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serio TR, Cahill N, Prout ME, Miller G. A functionally distinct TATA box required for late progression through the Epstein-Barr virus life cycle. J Virol. 1998;72(10):8338–43. Epub 1998/09/12. doi: 10.1128/JVI.72.10.8338-8343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amon W, Binne UK, Bryant H, Jenkins PJ, Karstegl CE, Farrell PJ. Lytic cycle gene regulation of Epstein-Barr virus. J Virol. 2004;78(24):13460–9. Epub 2004/11/27. doi: 10.1128/JVI.78.24.13460-13469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang PJ, Chang YS, Liu ST. Characterization of the BcLF1 promoter in Epstein-Barr virus. J Gen Virol. 1998;79 ( Pt 8):2003–6. Epub 1998/08/26. doi: 10.1099/0022-1317-79-8-2003. [DOI] [PubMed] [Google Scholar]
- 56.Isomura H, Stinski MF, Kudoh A, Murata T, Nakayama S, Sato Y, et al. Noncanonical TATA sequence in the UL44 late promoter of human cytomegalovirus is required for the accumulation of late viral transcripts. J Virol. 2008;82(4):1638–46. Epub 2007/12/07. doi: 10.1128/JVI.01917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong-Ho E, Wu TT, Davis ZH, Zhang B, Huang J, Gong H, et al. Unconventional sequence requirement for viral late gene core promoters of murine gammaherpesvirus 68. J Virol. 2014;88(6):3411–22. Epub 2014/01/10. doi: 10.1128/JVI.01374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Hu Q, Collins G, Parida M, Ball CB, Price DH, et al. Cytomegalovirus late transcription factor target sequence diversity orchestrates viral early to late transcription. PLoS Pathog. 2021;17(8):e1009796. Epub 2021/08/03. doi: 10.1371/journal.ppat.1009796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, et al. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A. 2003;100(24):14223–8. Epub 2003/11/19. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song MJ, Hwang S, Wong WH, Wu TT, Lee S, Liao HI, et al. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc Natl Acad Sci U S A. 2005;102(10):3805–10. Epub 2005/03/02. doi: 10.1073/pnas.0404521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu D, Silva MC, Shenk T. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc Natl Acad Sci U S A. 2003;100(21):12396–401. Epub 2003/10/02. doi: 10.1073/pnas.1635160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castaneda AF, Glaunsinger BA. The Interaction between ORF18 and ORF30 Is Required for Late Gene Expression in Kaposi's Sarcoma-Associated Herpesvirus. J Virol. 2019;93(1). Epub 2018/10/12. doi: 10.1128/JVI.01488-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong E, Wu TT, Reyes N, Deng H, Sun R. Murine gammaherpesvirus 68 open reading frame 24 is required for late gene expression after DNA replication. J Virol. 2007;81(12):6761–4. Epub 2007/03/30. doi: 10.1128/JVI.02726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isomura H, Stinski MF, Murata T, Yamashita Y, Kanda T, Toyokuni S, et al. The human cytomegalovirus gene products essential for late viral gene expression assemble into prereplication complexes before viral DNA replication. J Virol. 2011;85(13):6629–44. Epub 2011/04/22. doi: 10.1128/JVI.00384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruffat H, Kadjouf F, Mariame B, Manet E. The Epstein-Barr virus BcRF1 gene product is a TBP-like protein with an essential role in late gene expression. J Virol. 2012;86(11):6023–32. Epub 2012/03/30. doi: 10.1128/JVI.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patikoglou GA, Kim JL, Sun L, Yang SH, Kodadek T, Burley SK. TATA element recognition by the TATA box-binding protein has been conserved throughout evolution. Genes Dev. 1999;13(24):3217–30. Epub 2000/01/05. doi: 10.1101/gad.13.24.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chapa TJ, Du Y, Sun R, Yu D, French AR. Proteomic and phylogenetic coevolution analyses of pM79 and pM92 identify interactions with RNA polymerase II and delineate the murine cytomegalovirus late transcription complex. J Gen Virol. 2017;98(2):242–50. Epub 2016/12/08. doi: 10.1099/jgv.0.000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castaneda AF, Didychuk AL, Louder RK, McCollum CO, Davis ZH, Nogales E, et al. The gammaherpesviral TATA-box-binding protein directly interacts with the CTD of host RNA Pol II to direct late gene transcription. PLoS Pathog. 2020;16(9):e1008843. Epub 2020/09/05. doi: 10.1371/journal.ppat.1008843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaborowska J, Egloff S, Murphy S. The pol II CTD: new twists in the tail. Nat Struct Mol Biol. 2016;23(9):771–7. Epub 2016/09/09. doi: 10.1038/nsmb.3285. [DOI] [PubMed] [Google Scholar]
- 70.Nishimura M, Watanabe T, Yagi S, Yamanaka T, Fujimuro M. Kaposi's sarcoma-associated herpesvirus ORF34 is essential for late gene expression and virus production. Sci Rep. 2017;7(1):329. Epub 2017/03/25. doi: 10.1038/s41598-017-00401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu TT, Park T, Kim H, Tran T, Tong L, Martinez-Guzman D, et al. ORF30 and ORF34 are essential for expression of late genes in murine gammaherpesvirus 68. J Virol. 2009;83(5):2265–73. Epub 2008/12/19. doi: 10.1128/JVI.01785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Guindy A, Lopez-Giraldez F, Delecluse HJ, McKenzie J, Miller G. A locus encompassing the Epstein-Barr virus bglf4 kinase regulates expression of genes encoding viral structural proteins. PLoS Pathog. 2014;10(8):e1004307. Epub 2014/08/29. doi: 10.1371/journal.ppat.1004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis ZH, Hesser CR, Park J, Glaunsinger BA. Interaction between ORF24 and ORF34 in the Kaposi's Sarcoma-Associated Herpesvirus Late Gene Transcription Factor Complex Is Essential for Viral Late Gene Expression. J Virol. 2016;90(1):599–604. Epub 2015/10/16. doi: 10.1128/JVI.02157-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S, Salwinski L, Zhang C, Chu D, Sampankanpanich C, Reyes NA, et al. An integrated approach to elucidate the intra-viral and viral-cellular protein interaction networks of a gamma-herpesvirus. PLoS Pathog. 2011;7(10):e1002297. Epub 2011/10/27. doi: 10.1371/journal.ppat.1002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haque M, Kousoulas KG. The Kaposi's Sarcoma-Associated Herpesvirus ORF34 Protein Interacts and Stabilizes HIF-2alpha via Binding to the HIF-2alpha bHLH and PAS Domains. J Virol. 2019;93(17). Epub 2019/06/14. doi: 10.1128/JVI.00764-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haque M, Kousoulas KG. The Kaposi's sarcoma-associated herpesvirus ORF34 protein binds to HIF-1alpha and causes its degradation via the proteasome pathway. J Virol. 2013;87(4):2164–73. Epub 2012/12/12. doi: 10.1128/JVI.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumar Singh R, Pei Y, Bose D, Lamplugh ZL, Sun K, Yuan Y, et al. KSHV-encoded vCyclin can modulate HIF1alpha levels to promote DNA replication in hypoxia. Elife. 2021;10. Epub 2021/07/20. doi: 10.7554/eLife.57436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendez-Solis O, Bendjennat M, Naipauer J, Theodoridis PR, Ho JJD, Verdun RE, et al. Kaposi's sarcoma herpesvirus activates the hypoxia response to usurp HIF2alpha-dependent translation initiation for replication and oncogenesis. Cell Rep. 2021;37(13):110144. Epub 2021/12/30. doi: 10.1016/j.celrep.2021.110144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jia Q, Wu TT, Liao HI, Chernishof V, Sun R. Murine gammaherpesvirus 68 open reading frame 31 is required for viral replication. J Virol. 2004;78(12):6610–20. Epub 2004/05/28. doi: 10.1128/JVI.78.12.6610-6620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arumugaswami V, Wu TT, Martinez-Guzman D, Jia Q, Deng H, Reyes N, et al. ORF18 is a transfactor that is essential for late gene transcription of a gammaherpesvirus. J Virol. 2006;80(19):9730–40. Epub 2006/09/16. doi: 10.1128/JVI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perng YC, Qian Z, Fehr AR, Xuan B, Yu D. The human cytomegalovirus gene UL79 is required for the accumulation of late viral transcripts. J Virol. 2011;85(10):4841–52. Epub 2011/03/04. doi: 10.1128/JVI.02344-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omoto S, Mocarski ES. Cytomegalovirus UL91 is essential for transcription of viral true late (gamma2) genes. J Virol. 2013;87(15):8651–64. Epub 2013/05/31. doi: 10.1128/JVI.01052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chapa TJ, Johnson LS, Affolter C, Valentine MC, Fehr AR, Yokoyama WM, et al. Murine cytomegalovirus protein pM79 is a key regulator for viral late transcription. J Virol. 2013;87(16):9135–47. Epub 2013/06/14. doi: 10.1128/JVI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maeda Y, Watanabe T, Izumi T, Kuriyama K, Ohno S, Fujimuro M. Biomolecular Fluorescence Complementation Profiling and Artificial Intelligence Structure Prediction of the Kaposi's Sarcoma-Associated Herpesvirus ORF18 and ORF30 Interaction. Int J Mol Sci. 2022;23(17). Epub 2022/09/10. doi: 10.3390/ijms23179647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan D, Han T, Tang S, Xu W, Bao Q, Sun Y, et al. Murine Cytomegalovirus Protein pM91 Interacts with pM79 and Is Critical for Viral Late Gene Expression. J Virol. 2018;92(18). Epub 2018/07/13. doi: 10.1128/JVI.00675-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang L, Li M, Cai M, Xing J, Wang S, Zheng C. A PY-nuclear localization signal is required for nuclear accumulation of HCMV UL79 protein. Med Microbiol Immunol. 2012;201(3):381–7. Epub 2012/05/26. doi: 10.1007/s00430-012-0243-4. [DOI] [PubMed] [Google Scholar]
- 87.Wang R, Brattain MG. The maximal size of protein to diffuse through the nuclear pore is larger than 60kDa. FEBS Lett. 2007;581(17):3164–70. Epub 2007/06/26. doi: 10.1016/j.febslet.2007.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bechtel JT, Winant RC, Ganem D. Host and viral proteins in the virion of Kaposi's sarcoma-associated herpesvirus. J Virol. 2005;79(8):4952–64. Epub 2005/03/30. doi: 10.1128/JVI.79.8.4952-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nabiee R, Syed B, Ramirez Castano J, Lalani R, Totonchy JE. An Update of the Virion Proteome of Kaposi Sarcoma-Associated Herpesvirus. Viruses. 2020;12(12). Epub 2020/12/06. doi: 10.3390/v12121382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perng YC, Campbell JA, Lenschow DJ, Yu D. Human cytomegalovirus pUL79 is an elongation factor of RNA polymerase II for viral gene transcription. PLoS Pathog. 2014;10(8):e1004350. Epub 2014/08/29. doi: 10.1371/journal.ppat.1004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chapa TJ, Perng YC, French AR, Yu D. Murine cytomegalovirus protein pM92 is a conserved regulator of viral late gene expression. J Virol. 2014;88(1):131–42. Epub 2013/10/18. doi: 10.1128/JVI.02684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Omoto S, Mocarski ES. Transcription of true late (gamma2) cytomegalovirus genes requires UL92 function that is conserved among beta- and gammaherpesviruses. J Virol. 2014;88(1):120–30. Epub 2013/10/18. doi: 10.1128/JVI.02983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watanabe T, Narita Y, Yoshida M, Sato Y, Goshima F, Kimura H, et al. The Epstein-Barr Virus BDLF4 Gene Is Required for Efficient Expression of Viral Late Lytic Genes. J Virol. 2015;89(19):10120–4. Epub 2015/07/24. doi: 10.1128/JVI.01604-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato Y, Watanabe T, Suzuki C, Abe Y, Masud H, Inagaki T, et al. S-Like-Phase Cyclin-Dependent Kinases Stabilize the Epstein-Barr Virus BDLF4 Protein To Temporally Control Late Gene Transcription. J Virol. 2019;93(8). Epub 2019/02/01. doi: 10.1128/JVI.01707-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985;4(6):1609–14. Epub 1985/06/01. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watanabe T, Nishimura M, Izumi T, Kuriyama K, Iwaisako Y, Hosokawa K, et al. Kaposi's Sarcoma-Associated Herpesvirus ORF66 Is Essential for Late Gene Expression and Virus Production via Interaction with ORF34. J Virol. 2020;94(2). Epub 2019/11/07. doi: 10.1128/JVI.01300-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han T, Hao H, Sleman SS, Xuan B, Tang S, Yue N, et al. Murine Cytomegalovirus Protein pM49 Interacts with pM95 and Is Critical for Viral Late Gene Expression. J Virol. 2020;94(6). Epub 2020/01/04. doi: 10.1128/JVI.01956-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greber BJ, Nogales E. The Structures of Eukaryotic Transcription Pre-initiation Complexes and Their Functional Implications. Subcell Biochem. 2019;93:143–92. Epub 2020/01/16. doi: 10.1007/978-3-030-28151-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen X, Xu Y. Structural insights into assembly of transcription preinitiation complex. Curr Opin Struct Biol. 2022;75:102404. Epub 2022/06/15. doi: 10.1016/j.sbi.2022.102404. [DOI] [PubMed] [Google Scholar]
- 100.Kim DB, Zabierowski S, DeLuca NA. The initiator element in a herpes simplex virus type 1 late-gene promoter enhances activation by ICP4, resulting in abundant late-gene expression. J Virol. 2002;76(4):1548–58. Epub 2002/01/19. doi: 10.1128/jvi.76.4.1548-1558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verma D, Church TM, Swaminathan S. Epstein-Barr virus co-opts TFIIH component XPB to specifically activate essential viral lytic promoters. Proc Natl Acad Sci U S A. 2020;117(23):13044–55. Epub 2020/05/22. doi: 10.1073/pnas.2000625117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morgens DW, Nandakumar D, Didychuk AL, Yang KJ, Glaunsinger BA. A Two-tiered functional screen identifies herpesviral transcriptional modifiers and their essential domains. PLoS Pathog. 2022;18(1):e1010236. Epub 2022/01/19. doi: 10.1371/journal.ppat.1010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Earl C, Bagneris C, Zeman K, Cole A, Barrett T, Savva R. A structurally conserved motif in gamma-herpesvirus uracil-DNA glycosylases elicits duplex nucleotide-flipping. Nucleic Acids Res. 2018;46(8):4286–300. Epub 2018/03/30. doi: 10.1093/nar/gky217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su MT, Liu IH, Wu CW, Chang SM, Tsai CH, Yang PW, et al. Uracil DNA glycosylase BKRF3 contributes to Epstein-Barr virus DNA replication through physical interactions with proteins in viral DNA replication complex. J Virol. 2014;88(16):8883–99. Epub 2014/05/30. doi: 10.1128/JVI.00950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohno S, Steer B, Sattler C, Adler H. ORF23 of murine gammaherpesvirus 68 is non-essential for in vitro and in vivo infection. J Gen Virol. 2012;93(Pt 5):1076–80. Epub 2012/01/20. doi: 10.1099/vir.0.041129-0. [DOI] [PubMed] [Google Scholar]
- 106.Gershburg E, Pagano JS. Conserved herpesvirus protein kinases. Biochim Biophys Acta. 2008;1784(1):203–12. Epub 2007/09/21. doi: 10.1016/j.bbapap.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li J, Walsh A, Lam TT, Delecluse HJ, El-Guindy A. A single phosphoacceptor residue in BGLF3 is essential for transcription of Epstein-Barr virus late genes. PLoS Pathog. 2019;15(8):e1007980. Epub 2019/08/29. doi: 10.1371/journal.ppat.1007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krosky PM, Baek MC, Coen DM. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol. 2003;77(2):905–14. Epub 2002/12/28. doi: 10.1128/jvi.77.2.905-914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nobre LV, Nightingale K, Ravenhill BJ, Antrobus R, Soday L, Nichols J, et al. Human cytomegalovirus interactome analysis identifies degradation hubs, domain associations and viral protein functions. Elife. 2019;8. Epub 2019/12/25. doi: 10.7554/eLife.49894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Avey D, Tepper S, Pifer B, Bahga A, Williams H, Gillen J, et al. Discovery of a Coregulatory Interaction between Kaposi's Sarcoma-Associated Herpesvirus ORF45 and the Viral Protein Kinase ORF36. J Virol. 2016;90(13):5953–64. Epub 2016/04/22. doi: 10.1128/JVI.00516-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qian M, Yan F, Yuan T, Yang B, He Q, Zhu H. Targeting post-translational modification of transcription factors as cancer therapy. Drug Discov Today. 2020;25(8):1502–12. Epub 2020/06/17. doi: 10.1016/j.drudis.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Mure F, Panthu B, Zanella-Cleon I, Delolme F, Manet E, Ohlmann T, et al. Epstein-Barr Virus Protein EB2 Stimulates Translation Initiation of mRNAs through Direct Interactions with both Poly(A)-Binding Protein and Eukaryotic Initiation Factor 4G. J Virol. 2018;92(3). Epub 2017/11/17. doi: 10.1128/JVI.01917-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verma D, Bais S, Gaillard M, Swaminathan S. Epstein-Barr Virus SM protein utilizes cellular splicing factor SRp20 to mediate alternative splicing. J Virol. 2010;84(22):11781–9. Epub 2010/09/03. doi: 10.1128/JVI.01359-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han Z, Verma D, Hilscher C, Dittmer DP, Swaminathan S. General and target-specific RNA binding properties of Epstein-Barr virus SM posttranscriptional regulatory protein. J Virol. 2009;83(22):11635–44. Epub 2009/09/04. doi: 10.1128/JVI.01483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gruffat H, Batisse J, Pich D, Neuhierl B, Manet E, Hammerschmidt W, et al. Epstein-Barr virus mRNA export factor EB2 is essential for production of infectious virus. J Virol. 2002;76(19):9635–44. Epub 2002/09/05. doi: 10.1128/jvi.76.19.9635-9644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thompson J, Verma D, Li D, Mosbruger T, Swaminathan S. Identification and Characterization of the Physiological Gene Targets of the Essential Lytic Replicative Epstein-Barr Virus SM Protein. J Virol. 2016;90(3):1206–21. Epub 2015/11/13. doi: 10.1128/JVI.02393-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Majerciak V, Zheng ZM. KSHV ORF57, a protein of many faces. Viruses. 2015;7(2):604–33. Epub 2015/02/13. doi: 10.3390/v7020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verma D, Li DJ, Krueger B, Renne R, Swaminathan S. Identification of the physiological gene targets of the essential lytic replicative Kaposi's sarcoma-associated herpesvirus ORF57 protein. J Virol. 2015;89(3):1688–702. Epub 2014/11/21. doi: 10.1128/JVI.02663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Toth Z, Stamminger T. The human cytomegalovirus regulatory protein UL69 and its effect on mRNA export. Front Biosci. 2008;13:2939–49. Epub 2007/11/06. doi: 10.2741/2899. [DOI] [PubMed] [Google Scholar]
- 120.Hayashi ML, Blankenship C, Shenk T. Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc Natl Acad Sci U S A. 2000;97(6):2692–6. Epub 2000/03/08. doi: 10.1073/pnas.050587597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng H, Tian H, Wang Y, Zhang Q, Lin N, Liu S, et al. Molecular mechanism underlying selective inhibition of mRNA nuclear export by herpesvirus protein ORF10. Proc Natl Acad Sci U S A. 2020;117(43):26719–27. Epub 2020/10/10. doi: 10.1073/pnas.2007774117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gong D, Kim YH, Xiao Y, Du Y, Xie Y, Lee KK, et al. A Herpesvirus Protein Selectively Inhibits Cellular mRNA Nuclear Export. Cell Host Microbe. 2016;20(5):642–53. Epub 2016/11/11. doi: 10.1016/j.chom.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M, Hu Q, Collins G, Parida M, Ball CB, Price DH, et al. Cytomegalovirus late transcription factor target sequence diversity orchestrates viral early to late transcription. PLOS Pathogens. 2021;17(8):e1009796. doi: 10.1371/journal.ppat.1009796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spector BM, Parida M, Li M, Ball CB, Meier JL, Luse DS, et al. Differences in RNA polymerase II complexes and their interactions with surrounding chromatin on human and cytomegalovirus genomes. Nature Communications. 2022;13(1):2006. doi: 10.1038/s41467-022-29739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Djavadian R, Hayes M, Johannsen E. CAGE-seq analysis of Epstein-Barr virus lytic gene transcription: 3 kinetic classes from 2 mechanisms. PLOS Pathogens. 2018;14(6):e1007114. doi: 10.1371/journal.ppat.1007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arias C, Weisburd B, Stern-Ginossar N, Mercier A, Madrid AS, Bellare P, et al. KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features. PLOS Pathogens. 2014;10(1):e1003847. doi: 10.1371/journal.ppat.1003847. [DOI] [PMC free article] [PubMed] [Google Scholar]