Abstract
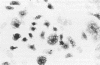
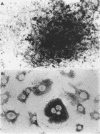
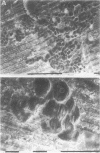
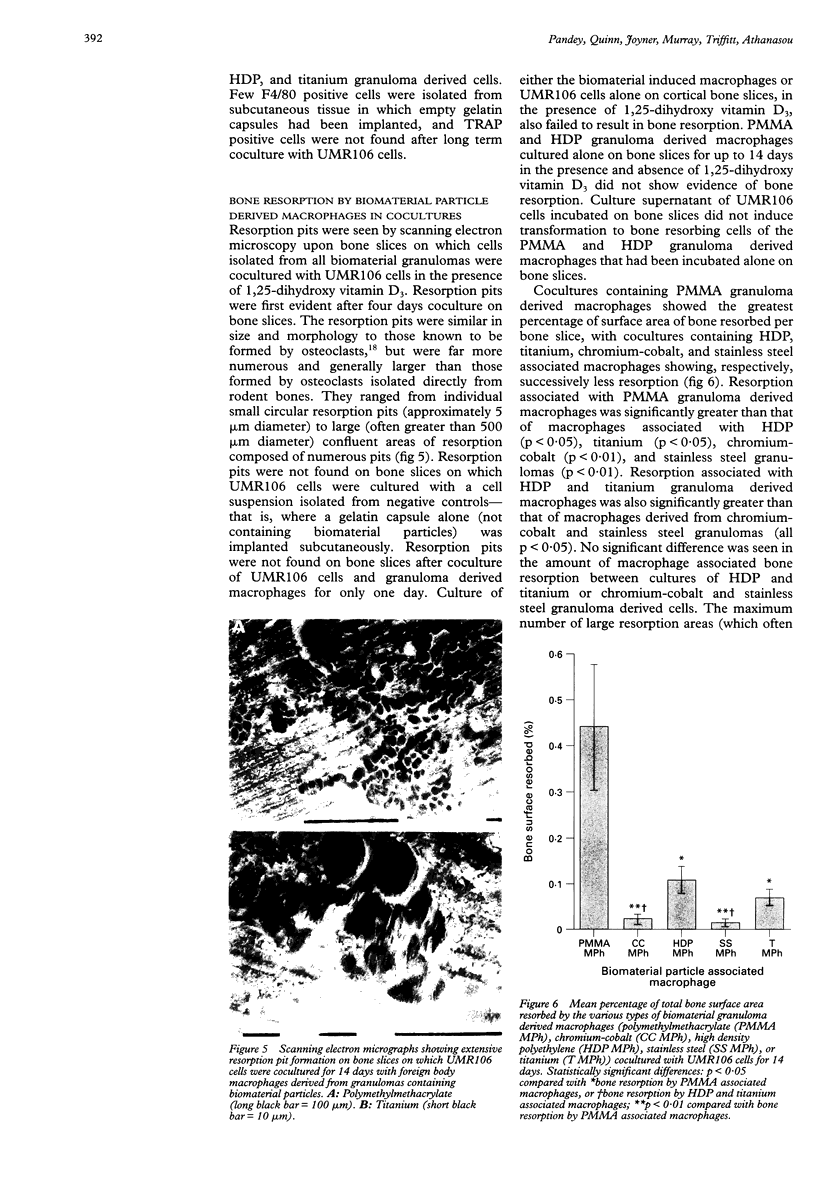
OBJECTIVE: To study the pathogenesis of aseptic loosening: in particular, to determine whether macrophages responding to particles of biomaterials commonly used in arthroplasty surgery for arthritis are capable of differentiating into osteoclastic bone resorbing cells, and the cellular and hormonal conditions required for this to occur. METHODS: Biomaterial particles (polymethylmethacrylate, high density polyethylene, titanium, chromium-cobalt, stainless steel) were implanted subcutaneously into mice. Macrophages were isolated from the foreign body granulomas that resulted, cultured on bone slices and coverslips, and assessed for both cytochemical and functional evidence of osteoclast differentiation. RESULTS: Tartrate resistant acid phosphatase (TRAP) negative macrophages isolated from granulomas containing particles of all types of biomaterial composition were capable of differentiating into TRAP positive cells capable of extensive lacunar bone resorption (assessed by scanning electron microscopy). The presence of both UMR106 rat osteoblast-like cells and 1,25-dihydroxy vitamin D3 was necessary for this to occur. CONCLUSION: All implant materials produce wear particles that are the focus of a heavy foreign body macrophage response in the fibrous membrane between a loose implant component and the host bone undergoing resorption. These findings underline the importance of biomaterial wear particle generation and the macrophage response to different types of biomaterial wear particles in the pathogenesis of aseptic loosening.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Amstutz H. C., Campbell P., Kossovsky N., Clarke I. C. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992 Mar;(276):7–18. [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J. M. Human tumour-associated macrophages are capable of bone resorption. Br J Cancer. 1992 Apr;65(4):523–526. doi: 10.1038/bjc.1992.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasou N. A., Wells C. A., Quinn J., Ferguson D. P., Heryet A., McGee J. O. The origin and nature of stromal osteoclast-like multinucleated giant cells in breast carcinoma: implications for tumour osteolysis and macrophage biology. Br J Cancer. 1989 Apr;59(4):491–498. doi: 10.1038/bjc.1989.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bullough P. G., DiCarlo E. F., Hansraj K. K., Neves M. C. Pathologic studies of total joint replacement. Orthop Clin North Am. 1988 Jul;19(3):611–625. [PubMed] [Google Scholar]
- Chambers T. J., Revell P. A., Fuller K., Athanasou N. A. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984 Mar;66:383–399. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- Gelb H., Schumacher H. R., Cuckler J., Ducheyne P., Baker D. G. In vivo inflammatory response to polymethylmethacrylate particulate debris: effect of size, morphology, and surface area. J Orthop Res. 1994 Jan;12(1):83–92. doi: 10.1002/jor.1100120111. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Jacobs J. J., Molnár G., Shanbhag A. S., Valyon M., Galante J. O. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res. 1993 Sep;8(9):1071–1079. doi: 10.1002/jbmr.5650080907. [DOI] [PubMed] [Google Scholar]
- Harris W. H., Schiller A. L., Scholler J. M., Freiberg R. A., Scott R. Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg Am. 1976 Jul;58(5):612–618. [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Haynes D. R., Rogers S. D., Hay S., Pearcy M. J., Howie D. W. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J Bone Joint Surg Am. 1993 Jun;75(6):825–834. doi: 10.2106/00004623-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Jasty M. J., Floyd W. E., 3rd, Schiller A. L., Goldring S. R., Harris W. H. Localized osteolysis in stable, non-septic total hip replacement. J Bone Joint Surg Am. 1986 Jul;68(6):912–919. [PubMed] [Google Scholar]
- Jasty M., Jiranek W., Harris W. H. Acrylic fragmentation in total hip replacements and its biological consequences. Clin Orthop Relat Res. 1992 Dec;(285):116–128. [PubMed] [Google Scholar]
- Jiranek W. A., Machado M., Jasty M., Jevsevar D., Wolfe H. J., Goldring S. R., Goldberg M. J., Harris W. H. Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am. 1993 Jun;75(6):863–879. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- Johanson N. A., Bullough P. G., Wilson P. D., Jr, Salvati E. A., Ranawat C. S. The microscopic anatomy of the bone-cement interface in failed total hip arthroplasties. Clin Orthop Relat Res. 1987 May;(218):123–135. [PubMed] [Google Scholar]
- Jones L. C., Hungerford D. S. Cement disease. Clin Orthop Relat Res. 1987 Dec;(225):192–206. [PubMed] [Google Scholar]
- Lennox D. W., Schofield B. H., McDonald D. F., Riley L. H., Jr A histologic comparison of aseptic loosening of cemented, press-fit, and biologic ingrowth prostheses. Clin Orthop Relat Res. 1987 Dec;(225):171–191. [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. 1,25-Dihydroxyvitamin D3 stimulates rat osteoblastic cells to release a soluble factor that increases osteoclastic bone resorption. J Clin Invest. 1987 Aug;80(2):425–429. doi: 10.1172/JCI113089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982 May;34(3):285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Murray D. W., Rushton N. Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg Br. 1990 Nov;72(6):988–992. doi: 10.1302/0301-620X.72B6.2246303. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Alcorn D., Michelangeli V. P., Kemp B. E., Ryan G. B., Martin T. J. Functional properties of hormonally responsive cultured normal and malignant rat osteoblastic cells. Endocrinology. 1981 Jan;108(1):213–219. doi: 10.1210/endo-108-1-213. [DOI] [PubMed] [Google Scholar]
- Pazzaglia U. E., Pringle J. A. The role of macrophages and giant cells in loosening of joint replacement. Arch Orthop Trauma Surg. 1988;107(1):20–26. doi: 10.1007/BF00463520. [DOI] [PubMed] [Google Scholar]
- Quinn J. M., McGee J. O., Athanasou N. A. Cellular and hormonal factors influencing monocyte differentiation to osteoclastic bone-resorbing cells. Endocrinology. 1994 Jun;134(6):2416–2423. doi: 10.1210/endo.134.6.8194468. [DOI] [PubMed] [Google Scholar]
- Quinn J., Joyner C., Triffitt J. T., Athanasou N. A. Polymethylmethacrylate-induced inflammatory macrophages resorb bone. J Bone Joint Surg Br. 1992 Sep;74(5):652–658. doi: 10.1302/0301-620X.74B5.1527108. [DOI] [PubMed] [Google Scholar]
- Revell P. A. Tissue reactions to joint prostheses and the products of wear and corrosion. Curr Top Pathol. 1982;71:73–101. doi: 10.1007/978-3-642-68382-4_3. [DOI] [PubMed] [Google Scholar]
- Santavirta S., Hoikka V., Eskola A., Konttinen Y. T., Paavilainen T., Tallroth K. Aggressive granulomatous lesions in cementless total hip arthroplasty. J Bone Joint Surg Br. 1990 Nov;72(6):980–984. doi: 10.1302/0301-620X.72B6.2246301. [DOI] [PubMed] [Google Scholar]
- Santavirta S., Konttinen Y. T., Bergroth V., Eskola A., Tallroth K., Lindholm T. S. Aggressive granulomatous lesions associated with hip arthroplasty. Immunopathological studies. J Bone Joint Surg Am. 1990 Feb;72(2):252–258. [PubMed] [Google Scholar]
- Spector W. G., Lykke A. W. The cellular evolution of inflammatory granulomata. J Pathol Bacteriol. 1966 Jul;92(1):163–167. doi: 10.1002/path.1700920117. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yamana H., Yoshiki S., Roodman G. D., Mundy G. R., Jones S. J., Boyde A., Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988 Apr;122(4):1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S. L., Stewart C. C., Kahn A. J. Rodent peritoneal macrophages as bone resorbing cells. Calcif Tissue Int. 1979 Jul 3;27(3):255–261. doi: 10.1007/BF02441194. [DOI] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T. J., Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]