SUMMARY
The aryl-hydrocarbon receptor (AHR) is a ligand-activated transcription factor that buoys intestinal immune responses. AHR induces its own negative regulator, the AHR repressor (AHRR). Here we show that AHRR is vital to sustain intestinal intraepithelial lymphocytes (IEL). AHRR deficiency reduced IEL representation in a cell-intrinsic fashion. Single-cell RNA-sequencing revealed an oxidative stress profile in Ahrr−/− IEL. AHRR deficiency unleashed AHR-induced expression of CYP1A1, a monoxygenase that generates reactive-oxygen species, increasing redox imbalance, lipid peroxidation and ferroptosis in Ahrr−/− IEL. Dietary supplementation with selenium or Vitamin-E to restore redox homeostasis rescued Ahrr−/− IEL. Loss of IEL in Ahrr−/− mice caused susceptibility to Clostridium difficile infection and dextran sodium sulfate-induced colitis. Inflamed tissue of inflammatory-bowel disease patients showed reduced Ahrr expression that may contribute to disease. We conclude that AHR signaling must be tightly regulated to prevent oxidative stress and ferroptosis of IEL and preserve intestinal immune responses.
In Brief
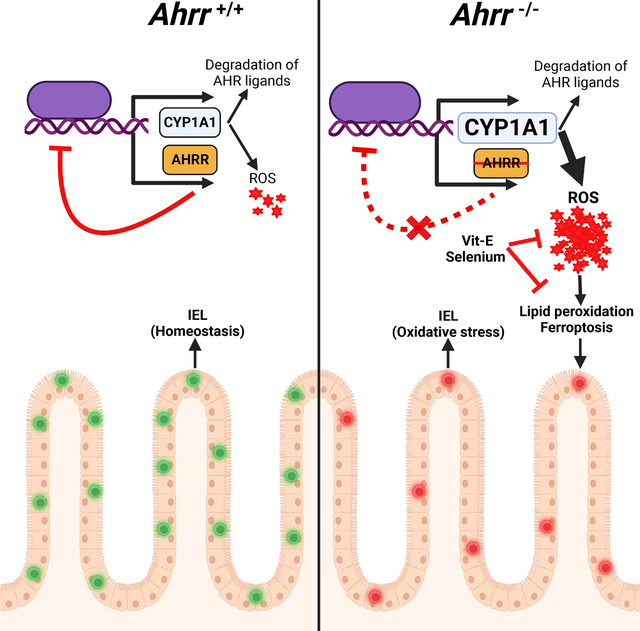
Intestinal IEL homeostasis relies on AHR, a ligand-induced transcription factor that is tightly controlled through feedback circuitries. Panda et al. demonstrated that lack of AHR repressor (AHRR) unleashes the AHR-induced monooxygenase CYP1A1, which causes oxidative stress and ferroptosis of IELs. This loss of IELs can be reversed by dietary anti-oxidants.
Graphical Abstract
INTRODUCTION
Intestinal intraepithelial lymphocytes (IEL) encompass natural TCRαβ+ CD8αα+ T cells and TCRγδ+ CD8αα+ T cells as well as induced TCRαβ+ CD4+ and TCRαβ+ CD8αβ+ T cells interspersed within intestinal epithelial cells (IEC)1. Due to their strategic presence at the interphase between the luminal environment and the intestinal barrier, IEL constantly sense fluctuating environmental components, such as commensal bacteria, pathobionts, pathogens, and dietary components and contribute to maintain the integrity of the intestinal epithelium in the steady state2,3. Moreover, IEL mediate immune responses against various pathogens4,5 and have protective effects in models of food intolerance and chronic inflammatory bowel disease (IBD)6. Mice deficient in IEL are susceptible to both dextran sodium sulfate (DSS)- and T cell-induced colitis7–9.
The aryl-hydrocarbon receptor (AHR) is a member of basic bHLH-PAS family of transcription factors that promotes gene programs devoted to metabolism of drugs, lipid metabolism, and circadian rhythm. AHR is activated by exogenous and endogenous ligands. Exogenous ligands include environmental toxins, such as 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)10,11, dietary derived compounds, such as indole-3-carbinol and its acidic condensation product indolo [3,2-b]carbazole, as well as catabolites of nutritional tryptophan generated by the microbiota11. Endogenous ligands include tryptophan metabolites such as kynurenine12. Upon ligand binding, AHR translocates into the nucleus and forms a complex with the AHR nuclear translocator (ARNT) that binds to dioxin response element (DRE)-containing genes, inducing their transcription13. One of the major effector molecules induced by AHR is the cytochrome P450 family 1 member A1 (CYP1A1), a member of the cytochrome p450 family of monooxygenases14. CYP1A1 oxidizes polycyclic aromatic hydrocarbons, often activating their carcinogenic properties15. Additionally, it metabolizes polyunsaturated long-chain fatty acids generating several classes of oxygenated metabolites that function as lipid mediators16. In the gastrointestinal system, the AHR pathway sustains healthy epithelium, along with the immune system and enteric nervous system. AHR is required for development, maintenance and function of IEL2,17, as well as the capacity of IEC to maintain barrier functions18. AHR is also necessary for the development and function of immune cell types in intestinal lamina propria (LP), including T helper-17 (Th17)19, regulatory T (Treg)20, T regulatory 1 (Tr1) cells21, group 3 innate lymphoid cells (ILC3)22–24 and group 2 ILC (ILC2)25, as well as enteric neurons26. Downstream of AHR, CYP1A1 also contributes to intestinal homeostasis by metabolizing AHR ligands27. Dysregulated CYP1A1 enzymatic activity in IEC degrades dietary and microbial derived AHR ligands, thereby thwarting AHR activation in ILC3 and Th17 cells and undermining their maintenance27.
In addition to CYP1A1, AHR drives the expression of an AHR repressor (AHRR), which contains both DRE-binding and ARNT-interacting domains but lacks ligand binding and transcription activation domains. Thus, AHRR competes with AHR for ARNT and DRE binding sites, preventing ligand-induced transcriptional activation of genes such as CYP1A128,29. Although AHR is required to maintain intestinal immunity, increased activation of AHR in Ahrr−/− mice is paradoxically deleterious. Ahrr−/− mice exhibit susceptibility to dextran sulfate sodium (DSS)-induced colitis and reduction of colonic γδ T cells30. Why AHRR deficiency affects γδ T cells and, in general, intestinal immunity is unclear. In this study, we found that AHRR deficiency has a broad impact on all IEL, reducing the number of both natural and induced IEL. The deficit of IEL was cell-intrinsic, since IEL were reduced a) after adoptive transfer of Ahrr−/− T cells into Rag1−/− mice, b) in bone marrow (BM) chimeras created with Ahrr−/− BM donors, and c) in mice with a conditional deletion of Ahrr gene in T cells. Single-cell RNA-sequencing (scRNA seq) of IEL revealed that AHRR deficiency induced a transcriptional signature indicative of cell stress and exposure to reactive oxygen species (ROS). We further noted that AHRR deficiency increased the expression and activity of CYP1A1, which produces ROS as a byproduct of its enzymatic activity31. We validated more intracellular ROS in Ahrr−/− IEL than in WT IEL, which was associated with increased lipid peroxidation and ferroptosis. Dietary supplementation with selenium, an essential element known to contribute to redox homeostasis32, or Vitamin E (Vit-E), which inhibits ROS, rescued Ahrr−/− IEL. The defect of IEL in mice with total or T cell restricted deletion of Ahrr increased susceptibility to infection by Clostridium difficile (C. difficile) as well as DSS-induced colitis. Taken together, these data reveal that dysregulation of the AHR-CYP1A1 axis due to AHRR deficiency causes excessive ROS generation and ferroptosis in IEL, affecting their maintenance and intestinal resistance to pathogens. Overall, IEL emerge as a T cell subset highly vulnerable to oxidative stress.
RESULTS
Ahrr−/− mice exhibit broad defects in IEL
Since IEL depend on AHR for their maintenance2, express AHRR30 and AHRR negatively regulates AHR, we hypothesized that AHRR deficiency may enhance the presence of IEL in the gut. Paradoxically, a marked reduction of intraepithelial T cells in the small intestine was evident in Ahrr−/− mice in comparison to wild-type (WT) controls (Figure 1A, B), whereas T cells in LP, mesenteric lymph nodes and spleen remain unaltered numerically (Figure 1C–E). Further, we analyzed various subsets of IEL (Figure S1A) and observed that γδ T (TCR γδ+ CD8αα+) cells, TCRαβ+ CD8αα+, TCRαβ+ CD8αβ+, and TCRαβ+ CD4+ IEL were reduced in the small intestine of Ahrr−/− mice in comparison to WT mice (Figure 1F–J). Similarly, total IEL as well as γδ T cells and TCRαβ+ CD4+ IEL were less abundant in the large intestinal epithelium of Ahrr−/− mice than WT mice (Figure S1B–G). Staining of CD8α in the ileal tissues confirmed the reduction of IEL in Ahrr−/− mice (Figure 1K, L). The receptor CD160 is predominantly expressed by CD8αα+ IEL and triggers IEL functions3. Consistent with the CD8αα+ IEL deficit, the frequency of CD160+ CD8αα+ IEL was reduced in Ahrr−/− mice (Figure 1M). IEL also include a subset of intraepithelial CD4+ T cells that downregulate the CD4 lineage transcription factor ThPOK, upregulate the CD8 lineage transcription factor Runx3, and express the CD8 cytolytic program33. The conversion of CD4+ T cells into CD4+CD8αα+ IEL (which we refer to double positive - DP) depends on AHR17. We found that these DP IEL were much less abundant in Ahrr−/− mice than in WT mice as assessed by staining for both CD4+CD8αα+ cells (Figure 1N, O) and ThPOKloCD8αα+ cells (Figure 1P, Q). Since there is regional variation in the distribution of IEL from the proximal to distal small intestine34, we ascertained whether the impact of Ahrr deletion extended along the small intestine or was limited to a specific segment. A progressive decrease of IEL numbers from the proximal to distal intestinal segments was evident in WT mice, with the exception of DP IEL that peaked in the distal intestine. AHRR-deficiency correlated with significantly reduced IEL numbers in the proximal and distal segments, along with a trend towards fewer IEL in the intermediate segment (Figure S1H–N). Thus, AHRR-deficiency affects IEL throughout the small intestine. Further, we examined whether Ahrr deletion affects IEL cytokine production. IEL of WT and Ahrr−/− mice were isolated from the small intestine, stimulated with immobilized anti-CD3 antibody overnight and analyzed for intracellular content of IFN-γ by flow cytometry. The percentage of IFN-γ+ cells was comparable between WT and Ahrr−/− IEL (Figure S1O–S), suggesting that AHRR deficiency does not impact the IEL cytokine production on a per cell basis. To investigate whether Ahrr gene dosage impacts IEL, we compared IEL subsets in WT and haplo-insufficient Ahrr+/− mice. Total CD45+ IEL, TCRαβ+ CD8αα+ IEL, and DP-IEL were reduced in Ahrr+/− mice, although other subsets were comparable (Figure S1T–Z), suggesting a partial effect of Ahrr gene dosage on IEL.
Figure 1. Ahrr−/− mice have reduced numbers of IEL.
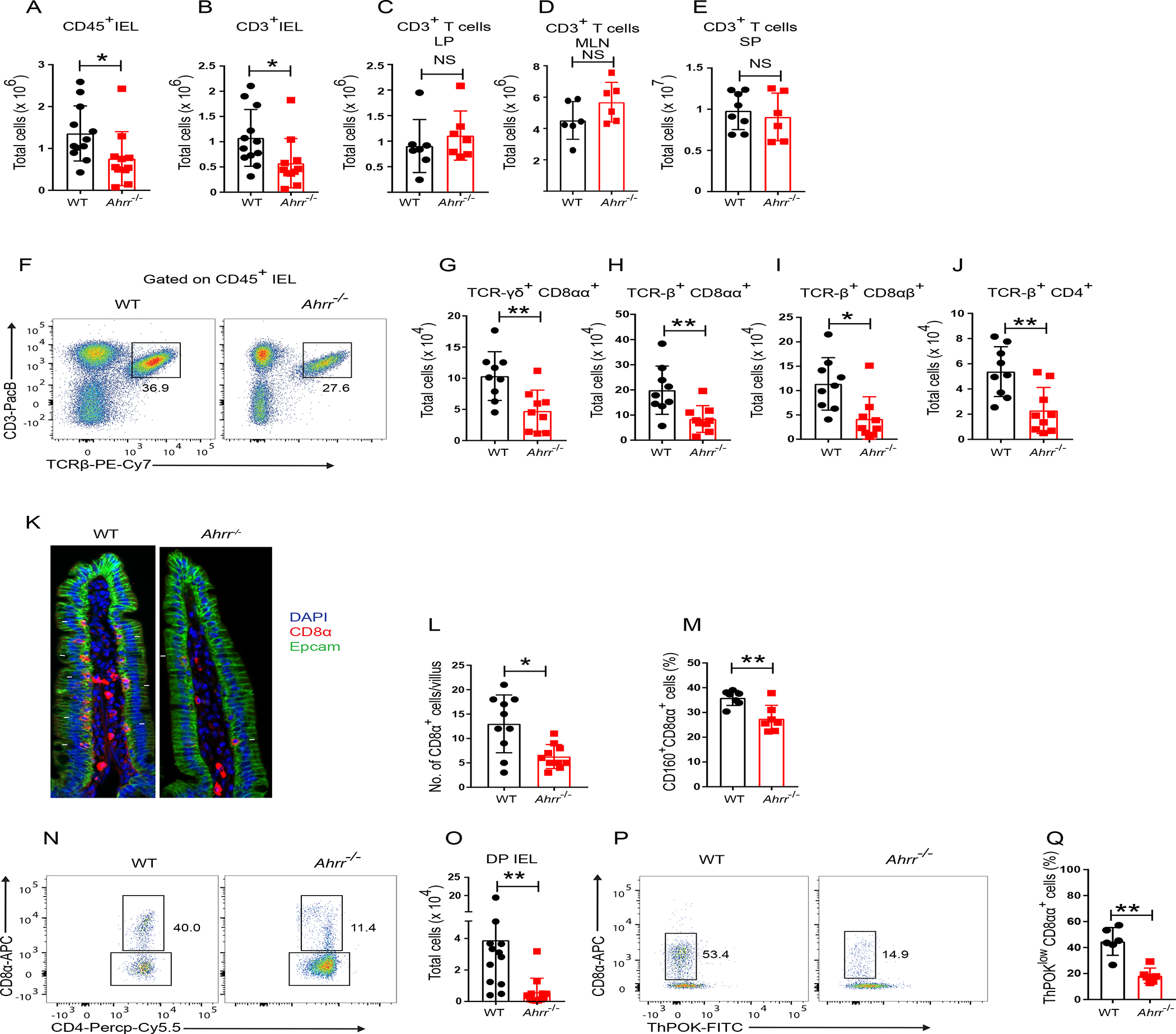
(A) Numbers of CD45+ IEL in the small intestine of WT and Ahrr−/− mice. (B-E) Numbers of T cells in small intestinal epithelium (B), small intestinal lamina propria (C), mesenteric lymph nodes (D) and spleen (E) of WT and Ahrr−/− mice. (F) Representative flow cytometry plots depicting frequency of TCR-β+ IEL in WT and Ahrr−/− mice. (G-J) IEL populations in small intestinal epithelium of WT and Ahrr−/− mice, including TCR-γδ+ CD8αα+ (G), TCR-β+ CD8αα+ (H), TCR-β+ CD8αβ+ (I), and TCR-β+ CD4+ (J). (K) Immunofluorescence staining of CD8α in the small intestine of WT and Ahrr−/− mice. (l) Quantification of CD8α+ cells per villus of WT and Ahrr−/− mice. (M) Frequency of CD160+ TCR-β+ CD8αα+ IELs in WT and Ahrr−/− mice. (N, O) Representative flow cytometry plots (N) and numbers of DP IEL (O) in WT and Ahrr−/− mice (gated on CD45+, CD3+, TCR γδ−, TCRαβ+, CD8β− CD4+ IEL). (P and Q) Representative flow cytometry plots plots (P) and frequency of ThPOKlow CD8αα+ cells (Q) in WT and Ahrr−/− mice. Each dot represents an individual mouse. Data are pool or representative of 2–3 individual experiments. Statistical significance was determined by Mann-Whitney test. *P<0.05, **P<0.01. Please also see Figures S1 and S2.
We further examined the impact of AHRR deficiency on LP lymphocytes which express AHRR30 and depend on AHR for development and/or function like IEL10. Although they express AHRR, ILC3, Th17 and Treg cells in the small intestine lamina propria were similarly represented in WT and Ahrr−/− mice (Figure S2A–I). Moreover, production of IL-22 by ILC3 in response to IL-23 was comparable in WT and Ahrr−/− mice (Figure S2J–L). Ahrr expression in IEL was significantly higher than in lamina propria T cells (Figure S2M), which may in part be the reason why IEL are more vulnerable to AHRR deficiency. The liver does not express AHRR30. Accordingly, while mice lacking AHR have hepatic steatosis and reduced body weight35,36, AHRR deficiency had no impact on liver or body weight (Figure S2N–R). We conclude that Ahrr deletion causes a selective reduction of IEL in the steady state. Since AHRR deficiency affected more profoundly small intestine than large intestine IEL, we focused on small intestine IEL for further analysis.
IEL defect in Ahrr−/− mice is cell intrinsic
Since AHRR is expressed not only in IEL, but also in intestinal dendritic cells (DC), ILCs and T cells30, we sought to determine whether the requirement of AHRR for IEL is cell-intrinsic or -extrinsic. To address this question, we co-transferred splenic T cells from WT (CD45.1) and Ahrr−/− (CD45.2) mice or WT (CD45.1) and Ahrr+/+(CD45.2) mice at a 1:1 ratio into Rag1−/− recipient mice (Figure 2A). After 8 weeks, T cells from both genotypes had reconstituted the spleen in equal proportions (Figure S3A–C). TCRαβ+ T cells were also detected in the epithelium of the small intestine, as previously reported37. The frequency of WT and AHRR sufficient IEL in the epithelium of reconstituted mice were comparable (Figure S3D). However, Ahrr−/− T cells were outcompeted by WT T cells in this location: the frequencies and numbers of WT TCRαβ+ CD8αα+, TCRαβ+ CD8αβ+, TCRαβ+ CD4 and DP IEL were significantly higher than their Ahrr−/− counterparts (Figure 2B–F, Figure S3E–I), indicating that the impact of AHRR on IEL differentiation or maintenance is cell-intrinsic. Whether the defect of γδ T cells in Ahrr−/− is also cell intrinsic could not be determined in this experiment, as we could not recover a sizeable population of γδ T cells from the intestine of adoptively transferred Rag1−/− mice.
Figure 2. Loss of IEL in Ahrr−/− mice is cell intrinsic.
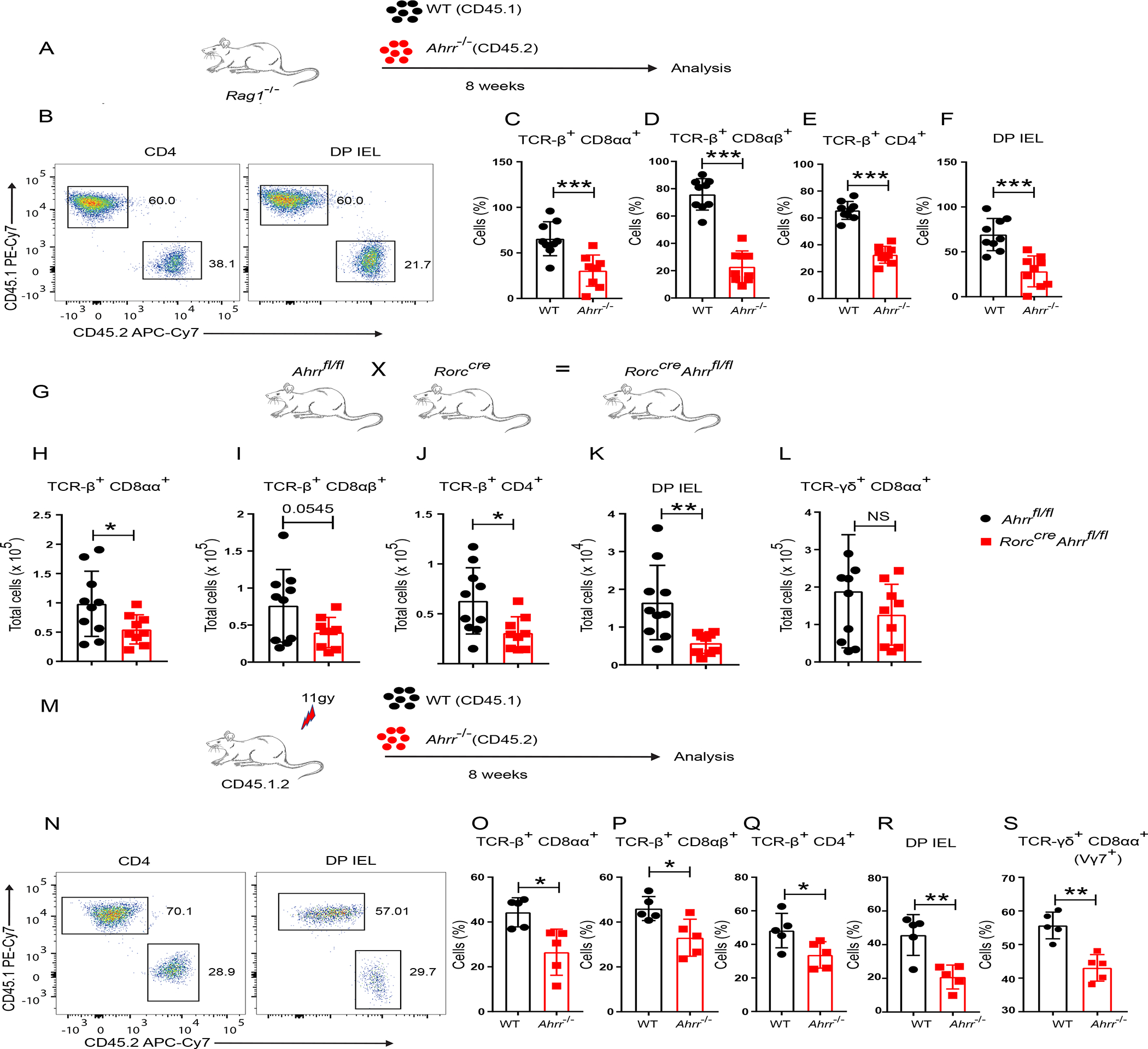
(A) Schematic of the experiment. Purified splenic T cells from WT (CD45.1) and Ahrr−/− (CD45.2) were transferred to Rag1−/− mice in 1:1 ratio and IEL were analyzed after 8 weeks. (B) Representative flow cytometry plots plots showing CD4+ T cells and DP IEL from small intestine of reconstituted mice. (C-F) Frequency of WT and Ahrr−/− TCR-β+ CD8αα+ (C), TCR-β+ CD8αβ+ (D), TCR-β+ CD4+ (E), and DP IEL (F), in the small intestine IEL of Rag1−/− mice after reconstitution. (G) Rorccre Ahrrfl/fl mice were generated by mating Rorccre with Ahrrfl/fl mice. (H-L) Cell numbers of TCR-β+ CD8αα+ (H), TCR-β+ CD8αβ+ (I), TCR-β+ CD4+ (J), DP IEL (K), and TCR-γδ+ CD8αα+ (L), in Ahrrfl/fl and Rorccre Ahrrfl/fl mice. (M) Schematic of the experiment: IEL from the small intestine of chimeric mice reconstituted with bone marrow cells from WT (CD45.1) and Ahrr−/− (CD45.2) mice in 1:1 ratio. (N) Representative flow cytometry plots showing CD4+ T cells and DP IEL from small intestine of reconstituted mice. o-s, Frequencies of WT and Ahrr−/− TCR-β+ CD8αα+ (O); TCR-β+ CD8 αβ+ (P), TCR-β+ CD4+ (Q), DP IEL (R), and γδ (vγ7+) IEL (S), in small intestinal epithelium of bone marrow chimeric mice. Each dot represents an individual mouse. Data are pool or representative of 2 individual experiments. Statistical significance was determined by Mann-Whitney test. *P<0.05, **P<0.01, ***P<0.001. Please also see Figure S3.
To corroborate the cell-intrinsic function of AHRR in TCRαβ+ IEL, we crossed Ahrrfl/fl mice with Rorccre mice (Figure 2G), such that the Ahrr gene is deleted in all T cells as well as ILC3. TCRαβ+ CD8αα+, TCRαβ+ CD8αβ+, TCRαβ+ CD4+ and DP IEL were significantly reduced in Rorccre Ahrrfl/fl mice in comparison to Ahrrfl/fl mice, corroborating the cell-intrinsic requirement for AHRR in TCRαβ+ IEL development or maintenance (Figure 2H–K). No marked differences were observed in total γδ T cells (Figure 2L). To further confirm the cell-intrinsic function of AHRR, we performed a competitive BM chimera experiment. Lethally irradiated CD45.1.2 mice were reconstituted with BM cells from WT (CD45.1) and Ahrr−/− (CD45.2) mice or, as a control, with WT (CD45.1) and Ahrr+/+ (CD45.2) mice; all were examined for IEL reconstitution after 8 weeks (Figure 2M). Again, we observed that the frequencies and numbers of Ahrr−/− TCRαβ+ CD8αα+, TCRαβ+ CD8αβ+, TCRαβ+ CD4, and DP IEL were significantly lower than their WT IEL counterparts (Figure 2N–R, Figure S3J–N), whereas the frequencies of Ahrr+/+ CD45.2 IEL and WT IEL were comparable in the control BM chimeras (Figure S3O). Analysis of γδ T cells revealed that Ahrr deletion was associated with underrepresentation of Vγ7+δ T cells (Figure 2S, Figure S3P), which are the gut-resident γδ T cell subset2, whereas circulating γδ T cells were unaffected (Figure S3Q). Altogether, these data corroborate the intrinsic requirement of AHRR for IEL maintenance.
IEL development and homing are AHRR-independent
We asked how AHRR defect impacts intestinal IEL numbers. First, we investigated IEL development and/or migration in Ahrr−/− mice. TCRαβ+ natural IEL develop from CD4−CD8−NK1.1−TCRαβ+CD5+ thymic IEL progenitors (IELp)38,39, which migrate into the gut and acquire CD8αα expression upon reaching the IL-15-rich intestinal environment. WT and Ahrr−/− mice had similar distributions of various CD4- and CD8-expressing thymocyte populations (Figure S4A) and the frequency of thymic IELp was comparable in WT and Ahrr−/− mice (Figure S4B, C), indicating that AHRR deficiency does not affect IEL development in thymus. Since differentiation of IEL in the gut requires IL-15 signaling through CD122 (IL2Rβ), we examined expression of CD122 on IELp. Expression of CD122 on the surface of IELp was comparable in WT and Ahrr−/− mice (Figure S4D). Moreover, molecules that promote gut homing and tissue retention, such as CCR9, CD103 and CD6937, were equally expressed in IEL from WT and Ahrr−/− mice (Figure S4E–H). Altogether, these data suggest that AHRR deficiency does not affect thymic development or expression of gut homing molecules.
The transcriptional profile of Ahrr−/− IEL reveals an oxidative stress response
To further investigate the mechanism by which Ahrr deficiency affects IEL numbers in the small intestine, we sorted IEL from WT and Ahrr−/− mice and analyzed them by droplet-based 3’ single cell RNA sequencing (10X Genomics). Through unsupervised clustering, we identified 14 IEL clusters visualized by uniform manifold and projection (UMAP) (Figure 3A). Based on the expression of Cd8a, Cd8b1, and Cd4, we identified CD8αβ IEL (Cluster 3), CD4+ IEL (Clusters 10 and 12), and CD8αα IEL (Clusters 0–2 and 4–8) (Figure 3B). TCRγδ+ IEL clustered together with TCRαβ IEL. Among CD8αα IEL, clusters 0 and 5 expressed genes indicative of effector function, such as Tyrobp, Gzma, and Gzmb (Figure 3C). Cluster 1 had a profile similar to those of clusters 0 and 5 but was uniquely enriched for expression of Gzmk. Clusters 7 and 8 were characterized by expression of various NK cell receptors such as Klre1, Klra1, Klra5, Klra6, and Klra7 (Figure 3B, C). Cluster 4 was distinguished by expression of the chemokines Ccl3 and Ccl4. Cluster 9 encompassed proliferating IEL, whereas cluster 13 included Ifng-producing IEL. Cluster 2 was marked by expression of the master transcriptional regulators Tcf7 and Id3 and was depleted for the effector molecules Tyrobp and Gzma (Figure 3B, C), a profile similar to that of long-lived memory precursor T cells40. Gene Set Enrichment (GSE) analysis corroborated that cluster 2 was enriched for a memory/stem cell signature (Figure S5A). Pseudo time trajectory analysis placed Tcf7-expressing IEL at one end of a differentiation hierarchy terminating in several possible effector fates, corroborating a stem-like feature for this population (Figure S5B–D). Further inspection of genes expressed in this cluster revealed that Ahr features were abundant in this population relative to other IEL populations, highlighting a potential role for AHR signaling in the function or maintenance of this cell subset (Figure S5E).
Figure 3. scRNAseq of WT and Ahrr−/− IEL.
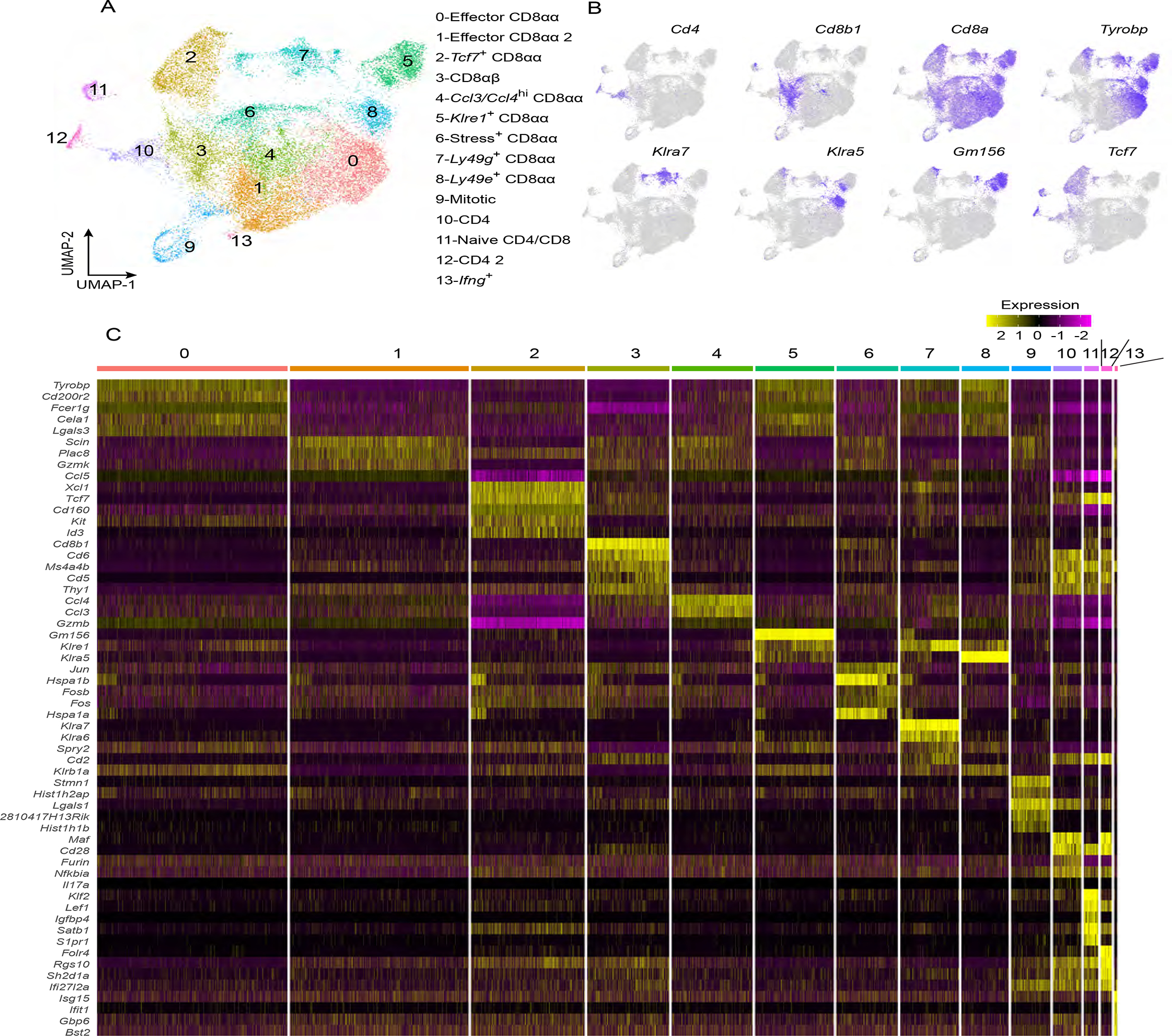
IEL (CD45+ CD3+ CD19−) from both WT and Ahrr−/− mice were subjected to single cell RNA sequencing analysis. (A) UMAP plot depicting various populations of IEL. (B) Feature plots depicting expression of Cd4, Cd8b, Cd8a, Tyrobp, Klra7, Klra5, Gm156 and Tcf7. (C) Heatmap displaying the top 5 characteristic genes for each cluster of the UMAP in A. Please also see Figure S5.
Comparison of IEL clusters between WT and Ahrr−/− mice revealed a selective expansion of cluster 6 expressing a stress response signature (Dnajb1, Hspa1a, Hspa1b, Hsph1) in Ahrr−/− mice (Figure 4A–C), whereas the abundance of all other clusters and their transcriptional profiles remained comparable (Figure S5F, G). These results suggested that the number of IEL experiencing oxidative stress was higher in Ahrr−/− than WT mice. We confirmed the enhanced expression of the stress response genes Hspa1a, Hsph1 and Dnajb1 in CD8αα+ IEL by RT-PCR (Figure 4D). One common cause of cell stress is the production of ROS and both HSPA1A and DNAJB1 are considered as markers of oxidative stress41. Thus, we examined Ahrr−/− IEL for signs of ROS exposure. Indeed, Ahrr−/− IEL more highly expressed genes indicating a response to ROS than did WT IEL (Figure 4E). These genes included Glutathione peroxidase 1 (Gpx1), which catalyzes the reduction of lipid peroxides in a glutathione-dependent reaction42, as well as Uba52, which is a ubiquitin gene induced by oxidative stress43. Thus, scRNAseq suggested that IEL require AHRR to prevent excessive ROS.
Figure 4. Ahrr deficiency induces oxidative stress in IEL.
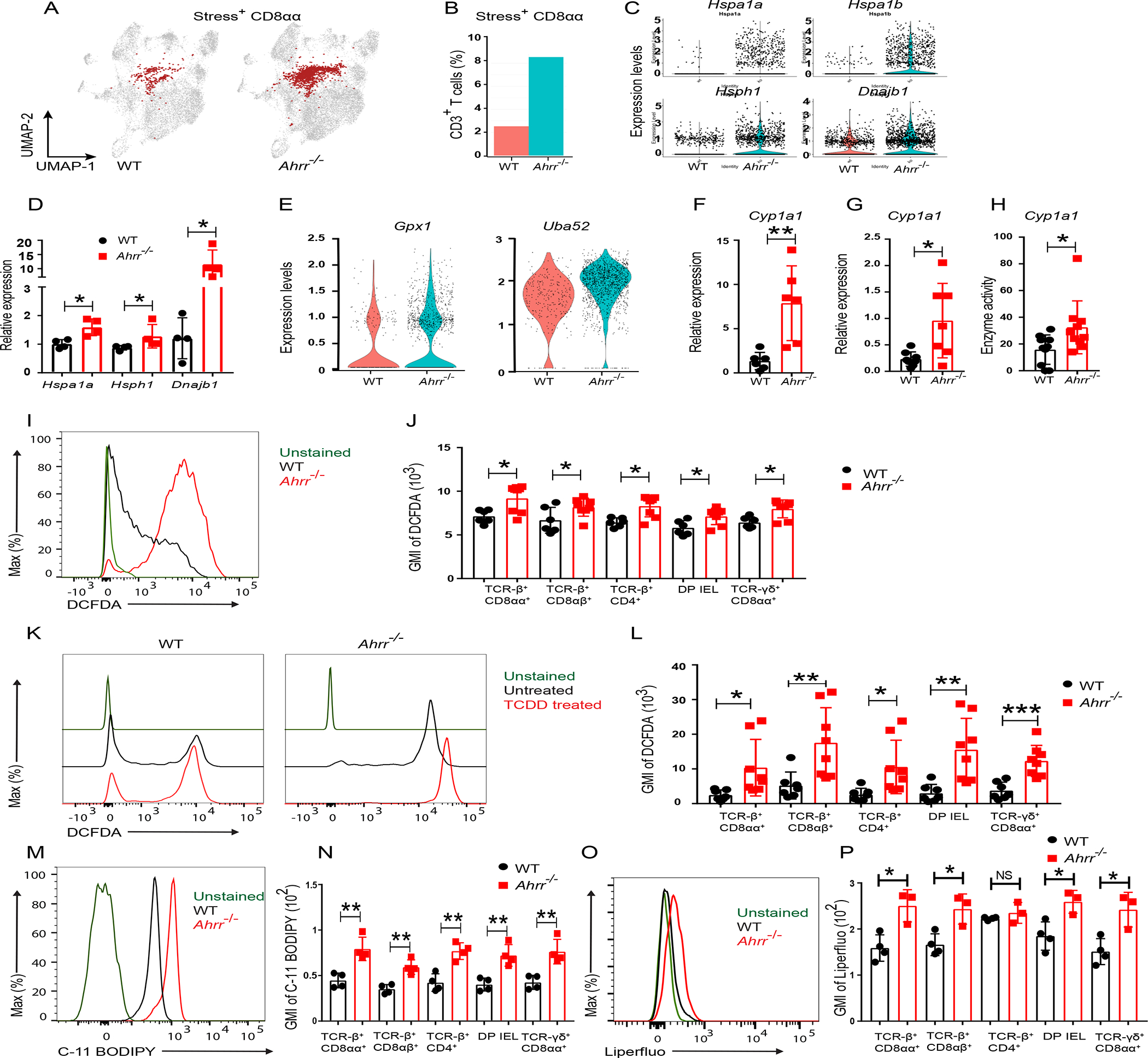
(A) UMAP plot of stress response+ CD8αα+ IEL and (B) frequency of stress response+ CD8αα out of CD3+ T cells in WT and Ahrr−/− mice. (C) Differential expression of Hspa1a, Hspa1b, Hsph1 and Dnajb1 transcripts in stress response+ CD8αα+ from WT and Ahrr−/− mice. (D) Q-PCR analysis of stress response genes in CD8αα+ IEL from WT and Ahrr−/− mice. (E) Violin plot depicting the expression of Gpx1 and Uba52 in stress response+ CD8αα+ IEL from WT and Ahrr−/− mice. (F) CYP1A1 expression in ileal tissues of WT and Ahrr−/− mice analyzed by qPCR. (G) Relative expression of CYP1A1 in IEL from WT and Ahrr−/− mice. (H) CYP1A1 enzymatic activity in IEL from WT and Ahrr−/− mice upon stimulation with TCDD. (I, J) DCFDA staining of IEL from WT and Ahrr−/− mice: representative flow cytometry plots histogram of TCR-β+ CD8αα+ cells (I) and GMI of DCFDA in different IEL subsets (J). (K, L) DCFDA staining of WT and Ahrr−/− IEL upon TCDD stimulation: representative flow cytometry plots histograms TCR-β+ CD8αα+ cells (K) and quantification in different IEL subsets (L). (M, N) C-11 BODIPY staining of WT and Ahrr−/− IEL: representative histograms of TCR-β+ CD8αα+ cells (M) and quantification in different IEL subsets (N). Liperflou staining of WT and Ahrr−/− IEL: representative histograms of TCR-β+ CD8αα+ cells (O) and quantification in different IEL subsets (P). Data are pool or representative of 2 individual experiments. Statistical significance was determined by Mann-Whitney test. *P<0.05, **P<0.01, ***P<0.001. Please also see Figure S6.
AHRR deficiency causes lipid peroxidation and ferroptosis of IEL
It has been shown that enzymes of the CYP family produce ROS when metabolizing their substrates31,44. Moreover, CYPs metabolize arachidonic acid into lipid metabolites that increase NADPH oxidase activity resulting in further ROS production45–47. Therefore, we speculated that the oxidative stress response in Ahrr−/− IELs may be due to dysregulated induction of the AHR-CYP1A1 axis, leading to increased CYP1A1 enzymatic activity and production of ROS. Supporting this hypothesis, Cyp1a1 mRNA was more expressed in ileal tissue (Figure 4F) and IEL (Figure 4G) of Ahrr−/− mice than in matched tissue and IEL of WT mice. Higher expression was paralleled by increased CYP1A1 enzymatic activity (Figure 4H). We next tested ROS production in IEL. Incubation of different IEL subsets with a substrate indicative of ROS showed that Ahrr−/− IEL produced more ROS than did WT IEL (Figure 4I, J). Moreover, treatment with TCDD, a potent AHR ligand and substrate of CYP1A1, induced more cellular ROS in Ahrr−/− IEL than in WT IEL (Figure 4K, L). Because ROS has been shown to cause lipid peroxidation and ferroptosis, we further examined whether AHRR deficiency is associated with ferroptosis. To test this, we stained IEL of WT and Ahrr−/− mice with C-11 BODIPY, a marker of lipid peroxidation. Ahrr−/− IEL evinced a marked increase in lipid peroxidation compared to WT IEL (Figure 4M, N). This finding was further confirmed by both flow cytometry and microscopy using Liperflou, a lipid soluble fluorescent probe that specifically interacts with lipid peroxides and is widely considered a marker of ferroptosis48 (Figure 4O, P and Figure S6A,B). To directly validate the role of CYP1A1 in inducing ferroptosis, we transduced a T cell line with a CYP1A1-encoding retrovirus and stained cells with C-11 BODIPY. CYP1A1 transduced cells showed increased lipid peroxidation compared to vector transduced cells (Figures S6C). Moreover, inhibition of CYP1A1 by Rhapontigenin49 reduced TCDD-induced lipid peroxidation in Ahrr−/− IEL (Figure S6D–H). We conclude that Ahrr deficiency affects IEL maintenance by causing lipid peroxidation and ferroptosis.
Dietary selenium supplementation rescues IEL loss in Ahrr−/− mice
Selenium and selenoproteins have a wide range of cellular functions including the maintenance of redox homeostasis and buffering ROS production, thereby limiting lipid peroxidation and ferroptosis32. Thus, we tested whether dietary supplementation of selenium could rescue IEL loss in Ahrr−/− mice. WT and Ahrr−/− mice were analyzed for lipid peroxidation of IEL by C11-BODIPY staining after adding selenium to the drinking water for 4 weeks (Figure 5A). While mice were on a normal dietary regimen, all subsets of Ahrr−/− IEL exhibited higher lipid peroxidation than did WT IEL; however, after selenium supplementation, peroxidation in Ahrr−/− IEL was reduced to amounts similar to those of WT IEL (Figure 5B–E). In parallel, selenium supplementation augmented the numbers of all Ahrr−/− IEL subsets, including TCRαβ+ CD8αα+, TCRαβ+ CD8αβ+, TCRαβ+ CD4+ IEL, DP IEL and γδ T cells, attaining values comparable to those of WT mice (Figure 5F–M). These data corroborate that loss of IEL in Ahrr−/− mice is caused by increased ROS generation and consequent ferroptosis.
Figure 5. Dietary selenium supplementation rescues IEL loss in Ahrr−/− mice.
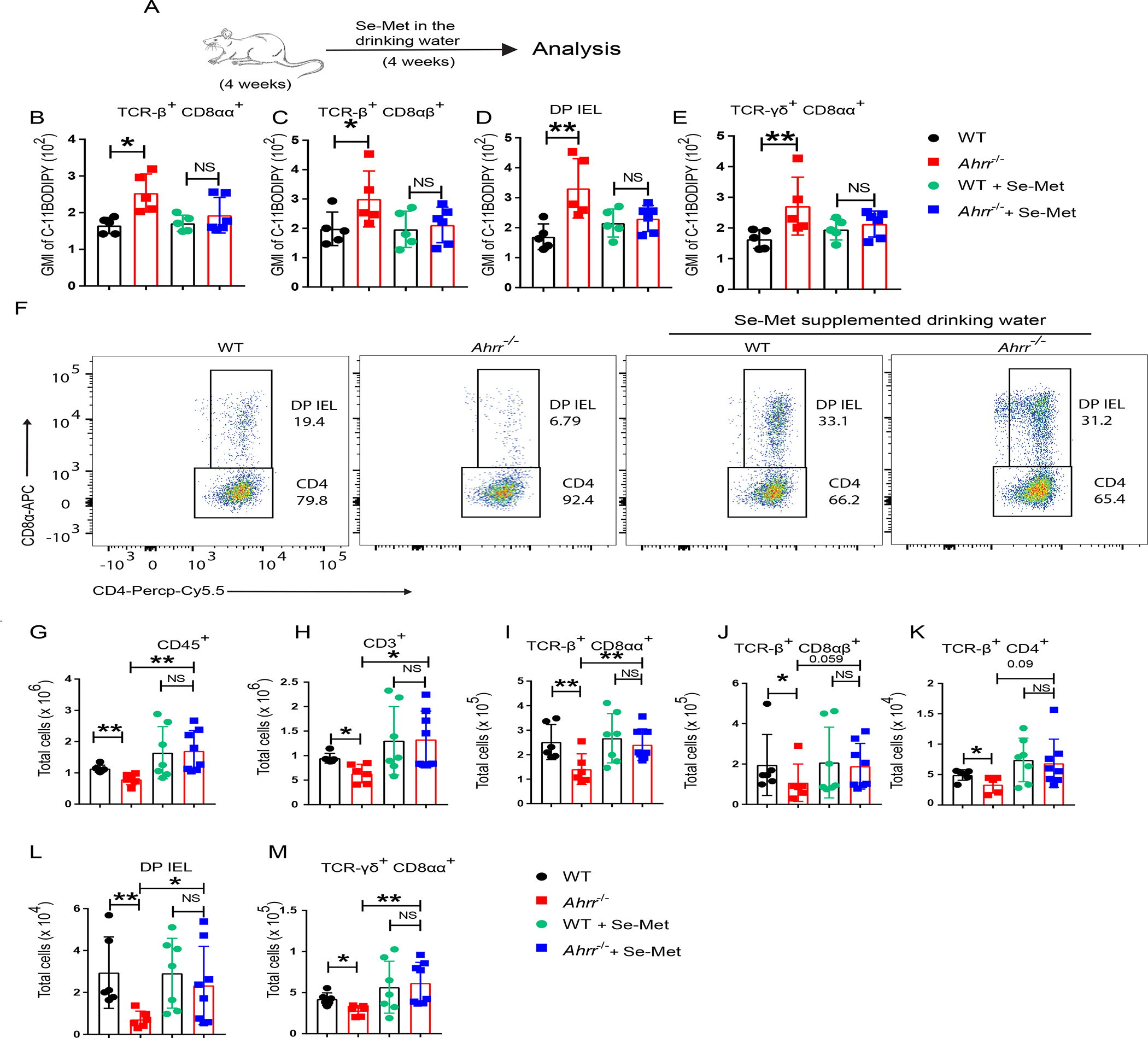
(A) WT and Ahrr−/− mice were treated with selenium supplemented drinking water (2mg/l) for 4 weeks and then IELs were stained for C-11 BODIPY. (B-E) GMI of C-11 BODIPY in different IEL populations: TCR-β+ CD8αα+ (B); TCR-β+ CD8 αβ+ (C); DP IEL (D); and γδ IEL (E). (F) Representative flow cytometry plots plots showing the DP IEL in WT and Ahrr−/− mice with and without dietary selenium supplementation. (G-M) Numbers of small intestinal CD45+ IEL (G), T cells in small intestinal epithelium (H); TCR-β+ CD8αα+ IEL (I), TCR-β+ CD8αβ+ IEL (J); TCR-β+ CD4+ IEL (K); DP-IEL (L); and TCR-γδ+ CD8αα+ IEL (M), of WT and Ahrr−/− mice with and without selenium supplementation. Each dot represents an individual mouse. Data are pool or representative of 2 individual experiments. Statistical significance was determined by Mann-Whitney test. *P<0.05, **P<0.01, ***P<0.001.
Inhibition of ROS by dietary Vit-E supplementation restores IEL in Ahrr−/− mice
Since Vit-E is a lipid soluble anti-oxidant known to inhibit ferroptosis and lipid peroxidation 32,50, we tested whether Vit-E could rescue IEL loss in Ahrr−/− mice. WT and Ahrr−/− mice were fed a Vit-E rich diet or normal chow immediately after weaning for 5 weeks (Figure 6A). Lipid peroxidation in IEL was analyzed by C-11 BODIPY staining. Ahrr−/− IEL showed more lipid peroxidation than WT IEL subjected when fed normal chow. However, Vit-E supplementation reduced lipid peroxidation of Ahrr−/− IEL to the extent of WT IEL (Figure 6B–E). In addition, WT and Ahrr−/− mice fed a Vit-E supplemented diet showed comparable IEL abundance, while Ahrr−/− mice had lower IEL numbers than WT mice in normal dietary regimen (Figure 6F–M). Collectively, these data demonstrate that Ahrr deficiency causes IEL loss by inducing ferroptosis.
Figure 6. Dietary supplementation with Vit-E restores IEL in Ahrr−/− mice.
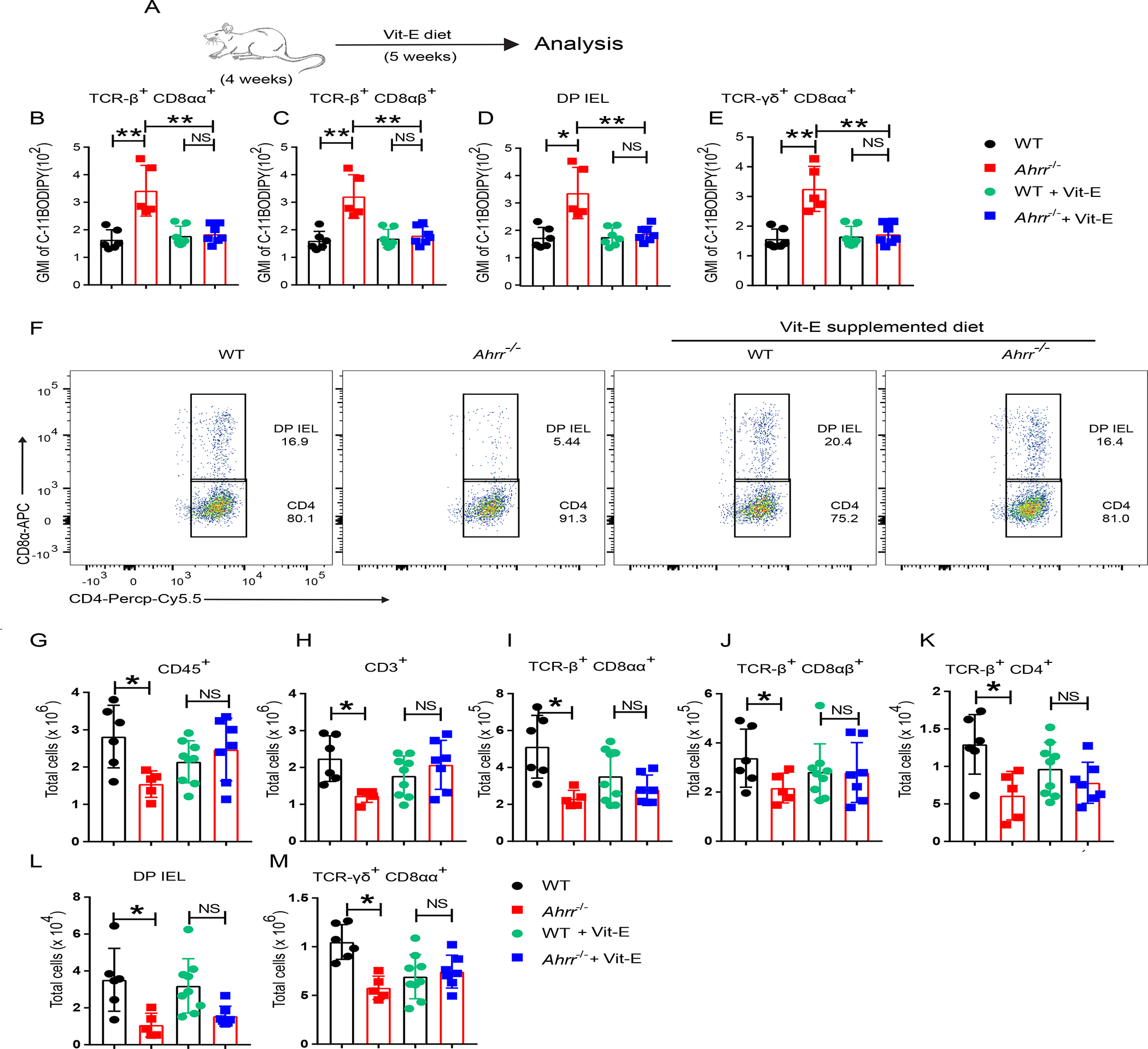
(A) WT and Ahrr−/− mice were fed a Vit-E rich diet for 5 weeks; IELs were stained for C-11 BODIPY. (B-E) GMI of C-11 BODIPY in different IEL populations: TCR-β+ CD8αα+ (B); TCR-β+ CD8 αβ+ (C); DP IEL (D); and γδ IEL (E). (F) Representative flow cytometry plots plots showing DP IEL in WT and Ahrr−/− mice with and without dietary Vit-E supplementation. (G-M) Numbers of small intestinal CD45+ IEL (G), T cells in the small intestinal epithelium (H); TCR-β+ CD8αα+ IEL (I); TCR-β+ CD8αβ+ IEL (J); TCR-β+ CD4+ IEL (K); DP-IEL (L); and TCR-γδ+ CD8αα+ IEL (M), in WT and Ahrr−/− mice with and without Vit-E supplementation. Each dot represents an individual mouse. Data are pool or representative of 2 individual experiments. Statistical significance was determined by Mann-Whitney test. *P<0.05, **P<0.01, ***P<0.001.
Ahrr deficiency increases susceptibility to intestinal pathology
We sought to determine the functional impact of the diminished IEL associated with AHRRdeficiency. Analysis of various inflammatory markers in the ileal tissue in the steady state, such as Tnfa, Ifng and Il6, divulged similar amounts in Ahrr−/− and WT mice, indicating that lack of Ahrr does not cause spontaneous tissue inflammation (Figure S7A). We next explored the impact of reduced IEL in Ahrr−/− mice in the model of C. difficile infection (Figure 7A). Both Ahrr−/− and WT mice were treated with antibiotics to induce dysbiosis followed by oral infection with C. difficile. Evaluation of clinical score and body weight demonstrated that Ahrr−/− mice were more susceptible to C. difficile infection than WT mice (Figure 7B, C). Similarly, RorccreAhrrfl/fl mice, which carry a deletion of Ahrr in T cells, were more susceptible to C. difficile infection than Ahrrfl/fl mice, corroborating that AHRR deficiency has a T cell intrinsic impact (Figure 7D, E). Since IEL promote anti-bacterial responses by releasing inflammatory cytokines and inducing expression of antimicrobial proteins by epithelial cells3, we measured the mRNA expression of Ifng, Tnfa, Il6 and the antimicrobial peptide genes RegIIIg and RegIIIb in the intestinal tissues upon C. difficile infection. Ahrr−/− mice expressed much less mRNA from these genes in both colonic and ileal tissues than did WT mice (Figure 7F–I). Because it was previously shown that Ahrr−/− mice are more susceptible to DSS-induced colitis30, we sought to determine whether this was due to a defect of IEL. We compared the severity of DSS-induced colitis in WT mice, Ahrr−/− mice and Ahrr−/− mice that had been reconstituted with WT IEL 3 days prior to DSS treatment. Analysis of weight loss and colon retraction demonstrated that adoptive transfer of IEL protected Ahrr−/− mice from colitis (Figure 7J, K). Altogether, these results demonstrate that IEL reduction in Ahrr−/− mice is functionally impactful, increasing their susceptibility to intestinal pathology.
Figure 7. Ahrr deficiency augments susceptibility to intestinal pathology.
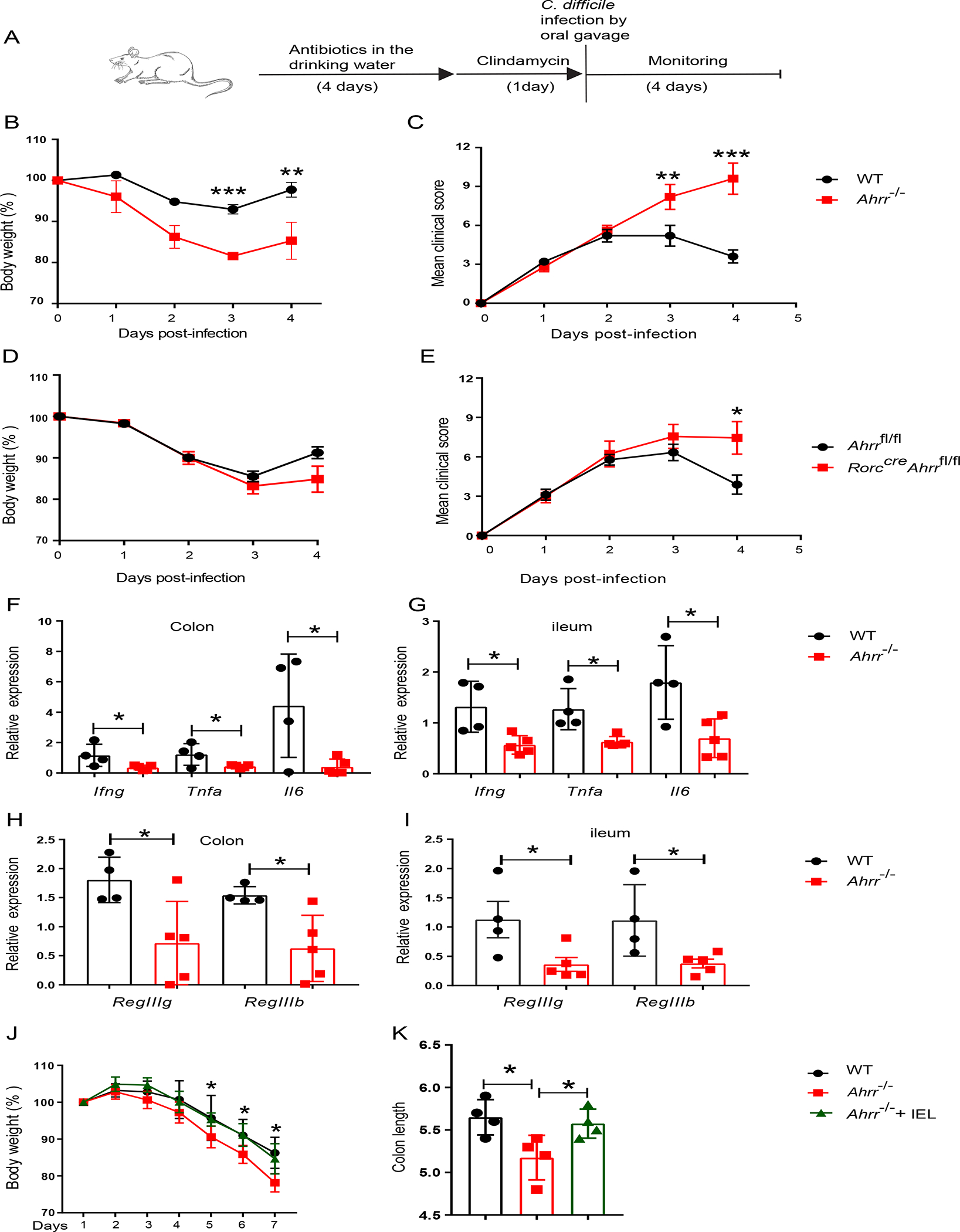
(A) Schematic of C. difficile infection in WT and Ahrr−/− mice or Ahrrfl/fl and Rorccre Ahrrfl/fl mice. (B) Body weight and c, clinical score in WT and Ahrr−/− mice. (D) Body weight and (E), clinical score in Ahrrfl/fl and Rorccre Ahrrfl/fl mice. (F-I) Expression of Ifng, Tnfa, Il6, RegIIIg and RegIIIb in colonic and ileal tissues of WT and Ahrr−/− mice upon infection with C. difficile. (J, K) Ahrr−/− mice were reconstituted with WT IEL and, after 3 days, were challenged with 3% DSS for 7 days. (J) % of body weight variation and (K) colon length at day 7 in WT, Ahrr−/− and Ahrr−/− mice reconstituted with WT IEL. Each dot represents an individual mouse. Data are pooled or representative of 2 individual experiments. Statistical significance was determined by Mann-Whitney test. *P<0.05, **P<0.01, ***P<0.001. Please also see Figure S7.
Ahrr is expressed in human intestinal γδ T cells, but less abundantly in IBD
We asked whether AHRR may impact human intestinal T cells. To define organ and cellular distribution of human AHRR, we examined the recently published Cross-Tissue Immune Cell Atlas (CTICA)51, which contains scRNA-seq data for all known immune cell types in adult humans from a variety of different organs. Across all organs, Ahrr was mainly expressed by tissue resident memory γδ T cells and intestinal macrophages (Figure S7B). Because γδ T cells can be found in a variety of different organs, we then plotted the expression of Ahrr by all cells grouped by the organ of origin (Figure S7C). Among all organs, caecum, sigmoid colon, ileum, transverse colon, jejunal epithelium and jejunal lamina propria contained cells with the highest expression of Ahrr. To further characterize the cells that express Ahrr in these organs, we plotted the average expression of Ahrr by every cell in each organ (Figure S7D). We found that tissue resident memory γδ T cells expressing abundant Ahrr were mainly found in the transverse colon, sigmoid colon, ileum, and caecum. Ahrr expression by intestinal γδ T cells was confirmed in a scRNA-seq database generated as part of a human environmental enteropathy study52 (Figure S7E). To see whether AHRR expression is affected in IBD, we examined a collection of surgical specimens from CD patients that we recently characterized for frequency and function of IEL53. Each specimen was divided into macroscopically visibly inflamed and non-inflamed tissue by a pathologist. Less Ahrr expression was detected in inflamed tissue from CD patients than in non-inflamed and control tissue (Figure S7F).This observation was consistent with a reduced frequency of γδ T cells we previously reported in the same samples53. Thus, AHRR expression inversely correlates with intestinal inflammation, suggesting a possible contribution to pathogenesis that needs to be further explored.
DISCUSSION
The AHR provides a fundamental mechanism by which the epithelium, immune system and nervous system of the intestine sense and respond to various exogenous and endogenous ligands that access the gastrointestinal tract. AHR induces its own negative regulator, AHRR. This study demonstrated that this negative feedback loop was essential for IEL maintenance. AHRR deficiency selectively impaired intestinal IEL in a cell intrinsic fashion, whereas the T cell composition in spleen and MLN was unaffected. scRNA-seq unveiled expansion of cells exposed to oxidative stress among Ahrr−/− deficient IEL. AHRR deficiency augmented AHR-mediated induction of CYP1A1 enzymatic activity, resulting in excessive generation of ROS, which caused lipid peroxidation and ferroptosis in IEL. Conversely, dietary supplementation with anti-oxidative elements - selenium or Vit-E - rescued IEL loss in Ahrr−/− mice. Depletion of the IEL compartment in Ahrr−/− mice was functionally impactful, heightening susceptibility to intestinal pathology. We conclude that AHR signaling must be tightly regulated to protect IEL from ferroptosis and preserve intestinal immune responses.
Ferroptosis is a type of iron-dependent cell death; it is mainly driven by redox imbalance that leads to excessive iron-dependent lipid peroxidation of membrane phospholipids, especially those with polyunsaturated fatty acids, and ultimately to severe cell membrane dysfunction54. While ferroptosis has been extensively demonstrated in tumor cells, there is very limited information regarding its role in T cell biology. One study shows that ferroptosis regulates the homeostasis of follicular helper T (Tfh) cells in the germinal center and that attenuation of ferroptosis by glutathione peroxidase 4 is necessary for Tfh 32. Our study demonstrates that tight control of lipid peroxidation and ferroptosis by AHRR is necessary to sustain the maintenance of IEL and their capacity to control susceptibility to colitis. This result is consistent with the recent observation that IEL have lower amounts of cellular ROS than memory T cells to be able to be poised to activation while preserving their viability55.
Our study presents a mechanism by which dysregulated CYP1A1 affects the intestinal immune system, i.e. by inducing excessive ROS production and lipid peroxidation in IEL. Substrate oxidation by NADPH-dependent CYP involves a six-step reaction during which heme-thiolate iron fluctuates between ferric and ferrous forms, interacts with oxygen, and oxidizes the substrate31,44. However, the transfer of oxygen to a substrate is not tight, leading to uncoupling and formation of ROS. In addition, CYPs metabolize arachidonic acid to produce 20-hydroxyeicosatetraenoic acid, which increases NADPH oxidase activity resulting in further ROS production45,46. In addition to our study, the role of CYPs in production of ROS and cell death has been corroborated in experimental models of heart and liver injury56–59.
Notably, the dysregulation of AHR-CYP1A1 in Ahrr−/− mice selectively affected IEL, while it had no impact on ILC3 or Th17 cells, even though all of these cells express AHRR. Why are IEL so sensitive to AHRR deficiency? The higher expression of Ahrr in IEL than lamina propria T cells may in part explain this phenomenon. Moreover, because of their proximity to the intestinal lumen, IELs plausibly have abundant access to AHR ligands to degrade, resulting in substantial production of ROS, whereas ILC3 and Th17 cells may be less exposed to exogenous AHR ligands, resulting in relatively weak activation of the AHR-CYP1A1 axis and modest production of ROS byproducts. The expansion of stressed cells was the fundamental difference between Ahrr−/− and WT IEL; no other changes in transcriptional profiles were detected. Hence, our data imply that oxidative stress may be the major cause of IEL reduction and that IEL are particularly sensitive to ROS.
IBD has been associated with alterations in intestinal AHR ligands, such as tryptophan, indole acetic acid (IAA) and kynurenine60,61, as well as changes in enzymes involved in their generation, such as indoleamine 2,3-dioxygenase (IDO)62. The AHR downstream target CYP1A1 has been found to increase in inflamed tissues of ulcerative colitis and Crohn’s disease patients compared to control tissues63; moreover, a gain of function mutation in CYP1A1 is associated with UC64. Altogether these observations suggest that dysregulated AHR signaling may be deleterious in IBD pathogenesis. Our analysis of human databases and tissues shows that Ahrr is highly expressed in human intestinal γδ T cells and that pediatric CD is associated with a concomitant reduction of Ahrr expression and γδ T cells. Given the role of AHRR in balancing AHR signaling, reduced expression of AHRR may contribute to IBD pathogenesis by facilitating generation of ROS and ferroptosis in human IEL. Future research will establish whether reduced AHRR expression in IBD is a primary event or secondary to dysregulated AHR signaling associated with IBD pathogenesis.
Limitations of the study
In a previous report, germline deletion of Ahrr was associated not only with reduced colonic γδ T cells in the steady state, but also with expansion of intestinal DC during DSS-induced colitis. Alteration of the DC compartment correlated with increased production of IL-1β and expansion of lamina propria Th17 cells, which lowered the Th1/Th17 cell ratio30. Furthermore, AHRR deficiency was associated with increased IL-1β production by bone-marrow derived macrophages in vitro. Thus, it will be important to evaluate the impact of AHRR on lamina propria DC and macrophages, particularly their production of inflammatory cytokines and capacity to polarize intestinal T cells in mice with selective deletions of Ahrr in DC and macrophages in the context of IBD pathology.
STAR Methods
Resource Availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Marco Colonna (mcolonna@wustl.edu) upon reasonable request.
Materials availability
This study did not generate any new unique reagents.
Data and code availability
Single-cell RNA-seq data have been deposited at GEO having accession number-GSE199960.
EXPERIMENTAL MODELS
Mice
Ahrrfl/fl (Ahrr tm1c/tm1c) mice and Ahrr−/− (Ahrrtm1b/tm1b) mice were generated in our laboratory from Ahrrtm1a sperm (from ES clone Ahrr H01) purchased from Eucomm. Rorccre/+ mice were kindly provided by Dr. Alexei Tumanov. Rorccre, Ahrrfl/fl mice were generated in our animal facility. CD45.1 mice were purchased from Jackson lab. CD45.1/2 mice were generated in our animal facility. WT and Ahrr−/− pregnant females were co-housed 1.5–2 weeks before delivery and the pups share the same litter till weaning. After weaning co-housing of WT and Ahrr−/− mice were ensured. The animal studies reported in this manuscript were conducted in accordance with guidelines of Washington University animal studies committee. 8–10-week-old mice were used unless otherwise specified.
C. difficile infection
C. difficile infection was described in a previous study65.
METHOD DETAILS
Cell culture
Primary murine cells were cultured in complete RPMI-1640 medium supplemented with 10% BCS, kanamycin, sodium pyruvate, glutamine and nonessential amino acids with and without treatment as mentioned in the figure legends.
Isolation of IEL and lamina propria lymphocytes (LPL)
IEL and LPL were isolated from small intestinal epithelium and lamina propria respectively. Small intestines were flushed with HBSS to remove the fecal content. Peyer’s patches were removed followed by longitudinal opening and cut into 1cm pieces. Then, the pieces were washed by gently agitating for 20 minutes followed by vertexing. The flow through was collected and the cycle repeated once again. The flow through was subjected to DTT treatment and Percoll 40–70% gradient separation for isolation of IEL. For LPL isolation, the pieces were rinsed properly with HBSS and then proceeded to digestion with complete RPMI supplemented with collagenase-IV. The undigested tissue chunks were removed by passing through 100-micron mess and then the digests were subjected to 40%–70% Percoll gradient centrifugation for isolation of LPL.
Flow cytometry plots analysis
Cell suspensions were incubated with FC block for 10–15 minutes followed by incubation with cocktail of antibodies for detection of ILC, IEL and T cells. To exclude dead cells the cell suspension was stained with live/dead fixable cell stain kit. For staining of intracellular proteins, cells were fixed and permeabilized using either BD Bioscience fixation/permeabilization Kit (for cytokines) or eBioscience FOXP3 staining kit (for transcription factors) based on the requirement. To identify different IEL populations, IEL were stained with anti-CD45-APC-Cy7, anti-CD3 PACB, anti-CD4-Percp-Cy5.5, anti-CD8α-APC, anti-CD8β-FITC, anti-TCR-γδ-PE. Live, singlet, lymphocyte sized CD45+, CD3−CD5−CD19−, RORγT+ (ILC3), GATA3high (ILC2) and RORγT− GATA3int/− as (ILC1 + NK). Identification of different IEL based on different markers described in Figure S1.
Intracellular cytokine analysis
LPL were stimulated with IL-23 for a total of 4 hrs with brefeldin-A for last 3 hours. IEL were stimulated with plastic coated anti-CD3 antibody overnight and incubated with brefeldin-A for the last 3 hours. Then the cells were stained for intracellular cytokines as previously described 37. Briefly, first the surface staining was performed followed by fixation. Then the cells were permeabilized and stained for intracellular cytokines.
Adoptive transfer of splenic T cells
Splenic T cells from C57BL/6 (CD45.1), WT (CD45.2) and Ahrr−/− (CD45.2) mice were isolated using Pan T Cells Isolation Kit (Miltenyi Biotech) according to manufacturer’s instruction. Both CD45.1 and CD45.2 cells were mixed together in 1:1 ratio. Rag1−/− mice were injected with 106 cells intravenously. Frequencies of CD45.1 vs CD45.2 in different subsets of IEL were analyzed after 8 weeks in the small intestine of recipient mice.
Bone marrow chimera
C57BL/6 (CD45.1/2) mice were lethally irradiated with two cycles of 550 Rads and then were injected with 1×106 bone marrow cells from C57BL/6 (CD45.1) along with either WT (CD45.2) or Ahrr−/− (CD45.2) in 1:1 ratio. Frequency of different subsets of IEL in the recipient mice were analyzed after 8 weeks.
Q-RT PCR
RNA from Ileal/colonic tissues or sorted cells were isolated using Qiagen RNA easy micro RNA Kit and cDNA synthesized using Quanta bio cDNA synthesis Kit. RT PCR was performed using SYBR green master mix and appropriate primer sets and data were normalized with GAPDH.
ROS detection
2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) is a chemically reduced form of fluorescein used as an indicator for ROS. Cytosolic ROS was detected by staining the IEL with CM-H2DCFDA (Thermo Fisher) in PBS for 30 min followed by incubation with RPMI media for 1 hr at 37°C. The cells were washed two times with PBS. Surface staining of different IEL markers were performed and analyzed by flow cytometry plots.
C-11 BODIPY and Liperflou staining
IEL from both WT and Ahrr−/− mice were surface stained for different markers. Then the cells were incubated with C-11 BODIPY (Thermo Fisher) or Liperflou (Dojindo Labs) for 30 mins at 37°C followed by washing 2 times with PBS and analyzed by flow cytometry plots.
Retroviral transduction
Cyp1a1 was cloned from the Horizon discovery mammalian gene collection plasmid clone 40129955. The amplicon was then cloned into a MSCV-IRES-Thy1.1 over expression plasmid by Gibson assembly. The control plasmid was empty MSCV-IRES-Thy1.1. Retroviral vectors were transfected into Plat-E cells pseudo typed with the pantropic envelop protein pCL-10A1. Two days after transfection, viral supernatants were collected and separated from cell debris by centrifugation. Jurkat cells were infected with viral supernatants with 2 μg/ml polybrene by ‘spin infection’ at 700 RCF for 60 min. On day 3, the cells were harvested and analyzed for Thy1.1 expression to evaluate transduction efficiency and further analyzed for lipid peroxidation by C-11 BODIPY staining.
C. difficile infection model
Both WT and Ahrr−/− mice were infected with C. difficile as previously described 65. Briefly, mice were administered with antibiotics supplemented drinking water for 4 days followed by clindamycin for 1 day. Then, mice were orally gavaged with C. difficile and monitored for clinical manifestations and body weight variation.
DSS induced colitis
Both WT and Ahrr−/− mice were administered with 3% DSS (MP Biomedicals) in the drinking water for 7 days. Body weight and colitis associated clinical parameters were monitored every day. 8th day the mice were sacked, and colon length was monitored.
Single cell RNA sequencing
IEL (CD45+, CD3+, CD19−) from two WT and two Ahrr−/− 8-week-old male mice were sorted using BD FACS ARIA-II. Sorted cells were sequenced using the 10x Genomics platform. Cell Ranger pipeline was used to process chromium single-cell RNA-seq output to align reads and generate gene–cell expression matrices (https://support.10xgenomics.com/single-cell-gene-expression/software/overview/welcome). Briefly, short sequencing reads were aligned to the mm10 reference genome and Ensembl transcriptome by STAR. The uniquely aligned reads were used to quantify gene expression for all Ensembl genes. We filtered out low-quality cells from the dataset if the number of UMI was fewer than 1000 or genes detected was fewer than 500. Cells with low complexity (< 0.8), as defined by the fraction of genes over UMI, were filtered out. We also excluded those cells with a high percentage of mitochondrion reads (>20%). In addition, all genes that were not detected in at least 10 of all the single cells were discarded. After quality control, data from two mice in each group were pooled together; total 10,613 WT and 14,882 Ahrr−/− IEL were subjected to final analysis. Data analyses were performed using the R software package Seurat (http://satijalab.org/seurat/) 66. Data were scaled and transformed and variable genes identified using the SC Transform function 67, and linear regression was performed to remove unwanted variation due to cell quality (% mitochondrial reads. Data from both genotypes were then integrated using the top 3000 variable features and principal component analysis was performed using these variable genes, and the first 40 principal components (PCs) were used to perform UMAP to embed the dataset into two dimensions. Next, the first 40 PCs were used to construct a shared nearest-neighbor graph (SNN; Find Neighbors) and this SNN was used to cluster the dataset (Find Clusters (resolution = 0.4)) using a graph-based modularity-optimization algorithm of the Louvain method for community detection. Despite sorting on CD3+ cells, minor clusters of contaminating B cells and myeloid cells were identified and removed for downstream analysis. After filtering out contaminating cell types, PCA, UMAP, and clustering analysis were redone using the same parameters as above. Cellular identity was determined by finding DE genes for each cluster using Seurat’s implementation of the Wilcoxon rank-sum test (Find Markers) and comparing those markers to known cell type-specific genes from previous datasets. Differential gene analysis comparing IEL from different genotypes was performed using the R package MAST68.
Statistical analysis
Two experimental groups were compared by Mann-Whitney U test. Statistical analysis was performed using GraphPad prism software (version 7). P<0.05 was considered statistically significant. * P<0.05, ** P<0.01, *** p< 0.001.
Supplementary Material
Key resources table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-mouse CD45-APC-Cy7 | Biolegend | Cat#103116; RRID: AB_312981 |
| anti- mouse CD45-AF700 | Biolegend | Cat#103128; RRID: AB_493715 |
| anti- mouse CD45.1-PE-Cy7 | Biolegend | Cat#110730; RRID: AB_1134168 |
| anti- mouse CD45.2-APC-Cy7 | Biolegend | Cat#109824; RRID: AB_830789 |
| anti- mouse CD3 PACB | Biolegend | Cat#100334; RRID: AB_2028475 |
| anti- mouse CD3 PE/Cy7 | Biolegend | Cat#100320; RRID: AB_312685 |
| anti- mouse CD3 APC/Cy7 | Biolegend | Cat#100222; RRID: AB_2242784 |
| anti- mouse CD4-PerCP-Cy5.5 | Biolegend | Cat#100434; RRID: AB_893324 |
| anti- mouse CD4- PE/Cy7 | Biolegend | Cat# 100422; RRID: AB_312707 |
| anti-CD8α-APC | Biolegend | Cat#100712; RRID: AB_ 312751 |
| anti-CD8β-FITC | Biolegend | Cat#126606; RRID: AB_ 961295 |
| anti-CD8β-PerCP-Cy5.5 | Biolegend | Cat#126610; RRID: AB_ 2260149 |
| anti-TCR-γδ-PE | Biolegend | Cat#118108; RRID: AB_ 313832 |
| anti-TCR-γδ-BV421 | Biolegend | Cat#118119; RRID: AB_ 2562566 |
| anti-CD3-PerCP-Cy5.5 | Biolegend | Cat#100328; RRID: AB_ 893318 |
| anti-CD5-PerCP-Cy5.5 | Biolegend | Cat#100624; RRID: AB_ 2563433 |
| anti-CD19-PerCP-Cy5.5 | Biolegend | Cat#115534; RRID: AB_ 2072925 |
| anti-RORγT-APC | Invitrogen | Cat#17-6988-82; RRID: AB_10609207 |
| anti-RORγT-PE | Invitrogen | Cat#12-6988-82; RRID: AB_1834470 |
| anti-GATA3-AF488 | BD | Cat#560077; RRID: AB_1645303 |
| anti-EOMES-PE | Invitrogen | Cat#12-4875-82; RRID: AB_1603275 |
| anti-FoxP3-AF647 | Biolegend | Cat#124608; RRID: AB_ 1089115 |
| anti-CD69-BV605 | Biolegend | Cat#104529; RRID: AB_ 11203710 |
| anti-CCR9- PE/Cy7 | Biolegend | Cat#128712; RRID: AB_ 10933082 |
| anti-CD103-Biotin | Invitrogen | Cat#13-1031-85; RRID: AB_ 466553 |
| anti-NKp46-Biotin | Biolegend | Cat# 137616; RRID: AB_ 11218796 |
| anti-CCR6-BV421 | Biolegend | Cat# 129818; RRID: AB_ 11219003 |
| anti-CD90.2-APC | Biolegend | Cat# 129818; RRID: AB_ 313183 |
| anti-IL-17A-FITC | Biolegend | Cat# 506908; RRID: AB_ 536010 |
| anti-IL-22-PE | Invitrogen | Cat# 12-7221-82; RRID: AB_ 10597428 |
| anti-IFN-γ-PE | Biolegend | Cat# 505808; RRID: AB_ 315402 |
| Biological samples | ||
| Intestinal tissue from healthy and IBD patients | Washington University in St. Louis | N/A |
| Bacteria | ||
| C. difficile | Colonna Lab | VPI 10463 |
| Chemicals, peptides, and recombinant proteins | ||
| Mouse IL-23 | R&D | Cat#1887-ML |
| Mouse IL-2 supernatant | Colonna Lab | N/A |
| Mouse IL-4 supernatant | Colonna Lab | N/A |
| Collagenase from Clostridium histolyticum | Sigma | Cat#C5138 |
| Critical commercial assays | ||
| Foxp3 transcription factor staining Buffer set | eBioscience | Cat#00-5523-00 |
| BD Cytofix/Cytoperm Plus | BD Biosciences | Cat#555028 |
| LS Columns | Miltenyi Biotec | Cat#130-042-401 |
| CD45 MicroBeads, mouse | Miltenyi Biotec | Cat#130-052-301 |
| Pan T Cell Isolation Kit II, mouse | Miltenyi Biotec | Cat#130-095-130 |
| RNeasy Plus Micro Kit | QIAGEN | Cat#74034 |
| Deposited data | ||
| Single cell RNAseq data | This paper | GSE199960 |
| Experimental models: Organisms/strains | ||
| Mouse: Ahrrfl/fl, Ahrr−/− | This study | N/A |
| Mouse: Rorccre | Tumanov lab | N/A |
| Mouse:CD45.1 | Jackson lab | JAX:002014 |
| Mouse: CD45.1/2 | This study | N/A |
| Oligonucleotides | Sequence | |
| Reg-IIIb-Fwd | Integrated DNA Technologies | TCCCAGGCTTATGGCTCCTA |
| Reg-IIIb-Rev | Integrated DNA Technologies | GCAGGCCAGTTCTGCATCA |
| Reg-IIIg-Fwd | Integrated DNA Technologies | CATCAACTGGGAGACGAATCC |
| Reg-IIIg-Rev | Integrated DNA Technologies | CAGAAATCCTGAGGCTCTTGACA |
| Ahrr-Fwd | Integrated DNA Technologies | CTGCCCCGGGATCAAAGATG |
| Ahrr-Rev | Integrated DNA Technologies | CTCTGTATTGAGGCGGTCCC |
| Cyp1a1- Fwd | Integrated DNA Technologies | GACATTTGAGAAGGGCCACAT |
| Cyp1a1-Rev | Integrated DNA Technologies | TCCTGGATCTTTCTCTGTACCC |
| Il6- Fwd | Integrated DNA Technologies | GAGGATACCACTCCCAACAGACC |
| Il6- Rev | Integrated DNA Technologies | AAGTGCATCATCGTTGTTCATACA |
| Ifng- Fwd | Integrated DNA Technologies | AGGAACTGGCAAAAGGATGGT |
| Ifng- Rev | Integrated DNA Technologies | ACCTGTGGGTTGTTGACCTC |
| Tnfa- Fwd | Integrated DNA Technologies | CATCTTCTCAAAATTCGAGTGACAA |
| Tnfα- Rev | Integrated DNA Technologies | TGGGAGTAGACAAGGTACAACCC |
| Hsph1 -Fwd | Integrated DNA Technologies | TGCAGCACTATGCCAAGATTG |
| Hsph1 -Rev | Integrated DNA Technologies | TTCTCAACCTTCTTCATTTCTGATTC |
| Dnajb1-Fwd | Integrated DNA Technologies | TTCGACCGCTATGGAGAGGAA |
| Dnajb1-Rev | Integrated DNA Technologies | CACCGAAGAACTCAGCAAACA |
| Hspa1a-Fwd | Integrated DNA Technologies | ATGGACAAGGCGCAGATCC |
| Hspa1a-Rev | Integrated DNA Technologies | CTCCGACTTGTCCCCCAT |
| Software and algorithms | ||
| FlowJo | Tree Star | https://www.flowjo.com/ |
| Prism | Graphpad | https://www.graphpad.com/ |
| Endnote | Endnote | https://endnote.com/ |
| Seurat | R | https://satijalab.org/seurat/ |
| Biorender | Biorender | https://biorender.com/ |
Highlights.
Intestinal IELs decline in the absence of Ahrr in a cell-intrinsic fashion.
Lack of Ahrr fuels CYP1A1 oxygenase activity, lipid peroxidation, and ferroptosis.
Restoration of redox homeostasis by dietary anti-oxidants rescues IEL numbers.
Fewer IELs, due to an Ahrr defect, provoke intestinal infection and inflammation.
ACKNOWLEDGEMENTS
The work was supported by NIH (R01 DE025884, R01 AI134236, and R01 AI134035) to Marco Colonna. The authors would like to thank Jennifer K. Bando and Wei-Le Wang for helpful discussions.
Footnotes
DECLARATION OF INTERESTS
Marco Colonna receives research support from Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cheroutre H, Lambolez F, and Mucida D (2011). The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11, 445–456. nri3007 [pii] 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, and Veldhoen M (2011). Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640. S0092–8674(11)01136–6 [pii] 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Shui JW, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, and Kronenberg M (2012). HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature 488, 222–225. nature11242 [pii] 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, and Lefrancois L (2001). Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol 166, 3402–3409. 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 5.Van Kaer L, Algood HMS, Singh K, Parekh VV, Greer MJ, Piazuelo MB, Weitkamp JH, Matta P, Chaturvedi R, Wilson KT, and Olivares-Villagomez D (2014). CD8alphaalpha(+) innate-type lymphocytes in the intestinal epithelium mediate mucosal immunity. Immunity 41, 451–464. S1074–7613(14)00304–5 [pii] 10.1016/j.immuni.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sujino T, London M, Hoytema van Konijnenburg DP, Rendon T, Buch T, Silva HM, Lafaille JJ, Reis BS, and Mucida D (2016). Tissue adaptation of regulatory and intraepithelial CD4(+) T cells controls gut inflammation. Science 352, 1581–1586. science.aaf3892 [pii] 10.1126/science.aaf3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klose CS, Blatz K, d’Hargues Y, Hernandez PP, Kofoed-Nielsen M, Ripka JF, Ebert K, Arnold SJ, Diefenbach A, Palmer E, and Tanriver Y (2014). The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8alphaalpha(+) intraepithelial lymphocyte development. Immunity 41, 230–243. S1074–7613(14)00274-X [pii] 10.1016/j.immuni.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Das G, Augustine MM, Das J, Bottomly K, Ray P, and Ray A (2003). An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A 100, 5324–5329. 10.1073/pnas.08310371000831037100 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Gong T, Tao W, Lin B, Li C, Zheng X, Zhu S, Jiang W, and Zhou R (2019). Commensal viruses maintain intestinal intraepithelial lymphocytes via noncanonical RIG-I signaling. Nat Immunol 20, 1681–1691. 10.1038/s41590-019-0513-z10.1038/s41590-019-0513-z [pii]. [DOI] [PubMed] [Google Scholar]
- 10.Cervantes-Barragan L, and Colonna M (2018). AHR signaling in the development and function of intestinal immune cells and beyond. Semin Immunopathol 40, 371–377. 10.1007/s00281-018-0694-910.1007/s00281-018-0694-9 [pii]. [DOI] [PubMed] [Google Scholar]
- 11.Rothhammer V, and Quintana FJ (2019). The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 19, 184–197. 10.1038/s41577-019-0125-810.1038/s41577-019-0125-8 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, and Perdew GH (2010). Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 115, 89–97. 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockinger B, Di Meglio P, Gialitakis M, and Duarte JH (2014). The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 32, 403–432. 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 14.Beedanagari SR, Bebenek I, Bui P, and Hankinson O (2009). Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci 110, 61–67. 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mescher M, and Haarmann-Stemmann T (2018). Modulation of CYP1A1 metabolism: From adverse health effects to chemoprevention and therapeutic options. Pharmacol Ther 187, 71–87. 10.1016/j.pharmthera.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Lucas D, Goulitquer S, Marienhagen J, Fer M, Dreano Y, Schwaneberg U, Amet Y, and Corcos L (2010). Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450. J Lipid Res 51, 1125–1133. 10.1194/jlr.M003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, et al. (2017). Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science 357, 806–810. science.aah5825 [pii] 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metidji A, Omenetti S, Crotta S, Li Y, Nye E, Ross E, Li V, Maradana MR, Schiering C, and Stockinger B (2018). The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity 49, 353–362 e355. S1074–7613(18)30330–3 [pii] 10.1016/j.immuni.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, and Kishimoto T (2008). Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A 105, 9721–9726. 0804231105 [pii] 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, and Weiner HL (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71. nature06880 [pii] 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 21.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, and Kuchroo VK (2010). The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol 11, 854–861. ni.1912 [pii] 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, and Zhou L (2012). The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104. S1074–7613(11)00505-X [pii] 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, and Diefenbach A (2011). Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565. 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, and Colonna M (2011). AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 13, 144–151. ni.2187 [pii] 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Bostick JW, Ye J, Qiu J, Zhang B, Urban JF Jr., Avram D, and Zhou L (2018). Aryl Hydrocarbon Receptor Signaling Cell Intrinsically Inhibits Intestinal Group 2 Innate Lymphoid Cell Function. Immunity 49, 915–928 e915. S1074–7613(18)30430–8 [pii] 10.1016/j.immuni.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obata Y, Castano A, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Aguero MG, Yilmaz B, Lopes R, Huseynova A, et al. (2020). Neuronal programming by microbiota regulates intestinal physiology. Nature 578, 284–289. 10.1038/s41586-020-1975-810.1038/s41586-020-1975-8 [pii]. [DOI] [PubMed] [Google Scholar]
- 27.Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, and Stockinger B (2017). Feedback control of AHR signalling regulates intestinal immunity. Nature 542, 242–245. nature21080 [pii] 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haarmann-Stemmann T, Bothe H, Kohli A, Sydlik U, Abel J, and Fritsche E (2007). Analysis of the transcriptional regulation and molecular function of the aryl hydrocarbon receptor repressor in human cell lines. Drug Metab Dispos 35, 2262–2269. dmd.107.016253 [pii] 10.1124/dmd.107.016253. [DOI] [PubMed] [Google Scholar]
- 29.Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K, and Fujii-Kuriyama Y (2001). Structure and expression of the Ah receptor repressor gene. J Biol Chem 276, 33101–33110. 10.1074/jbc.M011497200M011497200 [pii]. [DOI] [PubMed] [Google Scholar]
- 30.Brandstatter O, Schanz O, Vorac J, Konig J, Mori T, Maruyama T, Korkowski M, Haarmann-Stemmann T, von Smolinski D, Schultze JL, et al. (2016). Balancing intestinal and systemic inflammation through cell type-specific expression of the aryl hydrocarbon receptor repressor. Sci Rep 6, 26091. srep26091 [pii] 10.1038/srep26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veith A, and Moorthy B (2018). Role of Cytochrome P450s in the Generation and Metabolism of Reactive Oxygen Species. Curr Opin Toxicol 7, 44–51. 10.1016/j.cotox.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J, Wei Y, Wan Y, Wang N, Zhou M, et al. (2021). Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol 22, 1127–1139. 10.1038/s41590-021-00996-0. [DOI] [PubMed] [Google Scholar]
- 33.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, and Mucida D (2013). Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat Immunol 14, 271–280. ni.2518 [pii] 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayassi T, and Jabri B (2018). Human intraepithelial lymphocytes. Mucosal Immunol 11, 1281–1289. 10.1038/s41385-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, and Bradfield CA (2005). The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol 67, 714–720. mol.104.008888 [pii] 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- 36.Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, Hanberg A, and Poellinger L (2002). A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci U S A 99, 9990–9995. 10.1073/pnas.152706299152706299 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortez VS, Cervantes-Barragan L, Song C, Gilfillan S, McDonald KG, Tussiwand R, Edelson BT, Murakami Y, Murphy KM, Newberry RD, et al. (2014). CRTAM controls residency of gut CD4+CD8+ T cells in the steady state and maintenance of gut CD4+ Th17 during parasitic infection. J Exp Med 211, 623–633. jem.20130904 [pii] 10.1084/jem.20130904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, and Cheroutre H (2006). Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity 25, 631–641. S1074–7613(06)00429–8 [pii] 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Golec DP, Hoeppli RE, Henao Caviedes LM, McCann J, Levings MK, and Baldwin TA (2017). Thymic progenitors of TCRalphabeta(+) CD8alphaalpha intestinal intraepithelial lymphocytes require RasGRP1 for development. J Exp Med 214, 2421–2435. jem.20170844 [pii] 10.1084/jem.20170844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, and Xue HH (2010). Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity 33, 229–240. S1074–7613(10)00285–2 [pii] 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett MJ, Alones V, Wang KX, Phan L, and Swerdlow RH (2004). Mitochondria-derived oxidative stress induces a heat shock protein response. J Neurosci Res 78, 420–429. 10.1002/jnr.20249. [DOI] [PubMed] [Google Scholar]
- 42.Lei XG, Cheng WH, and McClung JP (2007). Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr 27, 41–61. 10.1146/annurev.nutr.27.061406.093716. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes R, Ramalho J, and Pereira P (2006). Oxidative stress upregulates ubiquitin proteasome pathway in retinal endothelial cells. Mol Vis 12, 1526–1535. [PubMed] [Google Scholar]
- 44.Hrycay EG, and Bandiera SM (2015). Monooxygenase, peroxidase and peroxygenase properties and reaction mechanisms of cytochrome P450 enzymes. Adv Exp Med Biol 851, 1–61. 10.1007/978-3-319-16009-2_1. [DOI] [PubMed] [Google Scholar]
- 45.Zeng Q, Han Y, Bao Y, Li W, Li X, Shen X, Wang X, Yao F, O’Rourke ST, and Sun C (2010). 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am J Physiol Heart Circ Physiol 299, H1109–1117. 10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han Y, Zhao H, Tang H, Li X, Tan J, Zeng Q, and Sun C (2013). 20-Hydroxyeicosatetraenoic acid mediates isolated heart ischemia/reperfusion injury by increasing NADPH oxidase-derived reactive oxygen species production. Circ J 77, 1807–1816. 10.1253/circj.cj-12-1211. [DOI] [PubMed] [Google Scholar]
- 47.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, Sandoval-Gomez G, Clish CB, Doench JG, and Schreiber SL (2020). Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 16, 302–309. 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13, 81–90. 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chun YJ, Ryu SY, Jeong TC, and Kim MY (2001). Mechanism-based inhibition of human cytochrome P450 1A1 by rhapontigenin. Drug Metab Dispos 29, 389–393. [PubMed] [Google Scholar]
- 50.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, and Kopf M (2015). T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med 212, 555–568. 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominguez Conde C, Xu C, Jarvis LB, Rainbow DB, Wells SB, Gomes T, Howlett SK, Suchanek O, Polanski K, King HW, et al. (2022). Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science 376, eabl5197. 10.1126/science.abl5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kummerlowe C, Wallach T, Mwakamui S, Hughes TK, Mulugeta N, Mudenda V, Besa E, Zyambo K, Fleming I, Vukovic M, et al. (2021). Single-cell profiling of environmental enteropathy reveals signatures of epithelial remodeling and immune activation in severe disease. bioRxiv, 2021.2004.2011.439202. 10.1101/2021.04.11.439202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaeger N, Gamini R, Cella M, Schettini JL, Bugatti M, Zhao S, Rosadini CV, Esaulova E, Di Luccia B, Kinnett B, et al. (2021). Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat Commun 12, 1921. 10.1038/s41467-021-22164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, et al. (2017). Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285. 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konjar S, Frising UC, Ferreira C, Hinterleitner R, Mayassi T, Zhang Q, Blankenhaus B, Haberman N, Loo Y, Guedes J, et al. (2018). Mitochondria maintain controlled activation state of epithelial-resident T lymphocytes. Sci Immunol 3. 10.1126/sciimmunol.aan2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, Wentworth P Jr., Yeager M, and Gottlieb RA (2004). Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci U S A 101, 1321–1326. 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaik IH, and Mehvar R (2010). Effects of cytochrome p450 inhibition by cimetidine on the warm hepatic ischemia-reperfusion injury in rats. J Surg Res 159, 680–688. 10.1016/j.jss.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Shaik IH, and Mehvar R (2010). Cytochrome P450 induction by phenobarbital exacerbates warm hepatic ischemia-reperfusion injury in rat livers. Free Radic Res 44, 441–453. 10.3109/10715761003610729. [DOI] [PubMed] [Google Scholar]
- 59.Ishihara Y, Sekine M, Hamaguchi A, Kobayashi Y, Harayama T, Nakazawa M, and Shimamoto N (2010). Effects of sulfaphenazole derivatives on cardiac ischemia-reperfusion injury: association of cytochrome P450 activity and infarct size. J Pharmacol Sci 113, 335–342. 10.1254/jphs.10103fp. [DOI] [PubMed] [Google Scholar]
- 60.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, et al. (2016). CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22, 598–605. 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge J, Rehman A, Tran F, Aden K, Hasler R, et al. (2017). Increased Tryptophan Metabolism Is Associated With Activity of Inflammatory Bowel Diseases. Gastroenterology 153, 1504–1516 e1502. 10.1053/j.gastro.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 62.Sofia MA, Ciorba MA, Meckel K, Lim CK, Guillemin GJ, Weber CR, Bissonnette M, and Pekow JR (2018). Tryptophan Metabolism through the Kynurenine Pathway is Associated with Endoscopic Inflammation in Ulcerative Colitis. Inflamm Bowel Dis 24, 1471–1480. 10.1093/ibd/izy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sen A, and Stark H (2019). Role of cytochrome P450 polymorphisms and functions in development of ulcerative colitis. World J Gastroenterol 25, 2846–2862. 10.3748/wjg.v25.i23.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buyukgoze O, Osmanoglu N, Arslan S, and Sen A (2013). Association of the CYP1A1*2A, GSTT1 null, GSTM1 null, mEPHX*3, and XRCC1–399 genetic polymorphisms with ulcerative colitis. Int J Colorectal Dis 28, 593–595. 10.1007/s00384-012-1507-6. [DOI] [PubMed] [Google Scholar]
- 65.Fachi JL, Secca C, Rodrigues PB, Mato FCP, Di Luccia B, Felipe JS, Pral LP, Rungue M, Rocha VM, Sato FT, et al. (2020). Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J Exp Med 217. 133544 [pii] 10.1084/jem.20190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler A, Hoffman P, Smibert P, Papalexi E, and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36, 411–420. nbt.4096 [pii] 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hafemeister C, and Satija R (2019). Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 20, 296. 10.1186/s13059-019-1874-110.1186/s13059-019-1874-1 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, Slichter CK, Miller HW, McElrath MJ, Prlic M, et al. (2015). MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol 16, 278. 10.1186/s13059-015-0844-510.1186/s13059-015-0844-5 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single-cell RNA-seq data have been deposited at GEO having accession number-GSE199960.


