Summary
Drug platforms that enable the directed delivery of therapeutics to sites of disease to maximize efficacy and limit off-target effects are needed. Here, we report the development of PROT3EcT, a suite of commensal Escherichia coli engineered to secrete proteins directly into their surroundings. These bacteria consist of three modular components: a modified bacterial protein secretion system, the associated regulatable transcriptional activator, and a secreted therapeutic payload. PROT3EcT secrete functional single-domain antibodies, nanobodies (Nbs), and stably colonize and maintain an active secretion system within the intestines of mice. Furthermore, a single prophylactic dose of a variant of PROT3EcT that secretes a tumor necrosis factor alpha (TNFα) neutralizing Nb is sufficient to ablate proinflammatory TNF levels and prevent the development of injury and inflammation in a chemically-induced model of colitis. This work lays the foundation for developing PROT3EcT as a platform for potential treatment of gastrointestinal-based diseases.
Graphical Abstract

eTOC
Lynch et al. describe the development of PROT3EcT, a suite of E. coli outfitted with a modified bacterial secretion system that enables the secretion of therapeutic payloads into their surroundings. Pretreating with a TNFα-neutralizing nanobody-secreting PROT3EcT prevents colitis in a preclinical IBD model, providing proof-of-concept for further platform development.
Introduction
Microbe-based therapeutics are emerging as a platform for the treatment of a variety of diseases, particularly those with etiologies linked to the gut1,2. Efforts are underway to identify cocktails of beneficial natural isolates, while synthetic biology-based approaches are ongoing to engineer microbes with additional therapeutic capabilities, including the targeted deposition of therapeutic payloads to sites of disease3–5. Due to their ease of production, administration, and natural capacity to synthesize and deliver complex biologics, engineered microorganisms hold enormous potential as affordable alternatives to traditional biologic therapies3–6. Microbes outfitted to deliver high-specificity payloads to sites of disease provide a platform for developing interventions with improved efficacy and limited off-target effects.
Escherichia coli Nissle 1917 (EcN), a probiotic with GRAS (generally recognized as safe) status7, is gaining traction as a chassis for synthetic biology6. EcN has inherent antibacterial and anti-inflammatory activities and is genetically tractable and has been used for over a century for the treatment of a variety of intestinal diseases, including inflammatory bowel disease6. A variety of strategies are being pursued to enhance EcN’s therapeutic potential. For example, variants with enhanced metabolic capabilities are being investigated for the removal of toxic intermediates associated with metabolic diseases8–10.
Efforts are also underway to engineer EcN and other commensal bacteria to deliver therapeutic payloads to sites of disease. In the case of Gram-positive bacteria, the native general secretion (Sec) and twin-arginine translocation (Tat) pathways have been repurposed to secrete therapeutic agents into their surroundings. For example, Lactococcus that secrete IL-10 or anti-TNF single-domain antibodies (also known as nanobodies or VHH) has been demonstrated to ameliorate gut inflammation in animal models11,12. However, for Gram-negative bacteria, the engineering of these conserved secretion systems is limited as they primarily function to deliver proteins into the periplasmic component of their outer envelope (for review, see13). Given the difficulty of engineering Gram-negative bacteria to secrete proteins into their surroundings, work has primarily focused on developing E. coli variants programmed to lyse and release intracellular cargo14–16 or to display proteins of therapeutic potential on their outer surface via fusion to outer membrane adhesins17 or Curli18,19.
Numerous Gram-negative bacterial pathogens utilize complex nanomachines, including type III secretions systems (T3SSs), to transport bacterial proteins referred to as effectors directly into the cytosol of host cells. The fully assembled type III secretion machines (apparatuses) (T3SAs) are embedded within the outer envelope of the bacterium with a needle-like extension that docks onto and forms pores in host cell membranes. We and others have previously established that the T3SAs of genetically related Gram-negative pathogens, including Shigella flexneri and enteropathogenic E. coli, are functional when introduced into laboratory strains of E. coli20–24.
Here, we report the development of the PROT3EcT (PRObiotic Type 3 secretion E. coli Therapeutic) platform, a suite of E. coli engineered to express a Shigella T3SA modified to secrete proteins into its surroundings rather than directly into eukaryotic cells. PROT3EcT can secrete a variety of heterologous proteins which have been outfitted with an N-terminal type III secretion sequence. PROT3EcT are modular in design and composed of three genetic elements: (1) the operons encoding the modified T3SA, (2) their master transcriptional regulator (VirB), and (3) a therapeutic payload. PROT3EcT-4, a variant engineered to maintain all elements in the absence of antibiotic selection, is unimpaired for growth in vitro or in the intestines of mice. As proof-of-concept of the therapeutic potential of PROT3EcT, we provide evidence that TNF-PROT3EcT, a variant that secretes an anti-TNFα Nb, is as effective as systemically administered anti-TNFα monoclonal antibody in suppressing the development of inflammation in a chemically induced preclinical model of inflammatory bowel disease. Together, these studies provide the foundation for the continued development of PROT3EcT as a versatile therapeutic platform.
Results
Development of PROT3EcT
The genes that encode the ~20 components that form the Shigella T3SA are contained within the ipa, mxi, and spa operons located adjacent to each other on a large virulence plasmid25,26. The mxi and spa operons encode all the structural components needed to form the T3SA. The ipa operon includes those that form the tip complex that holds the machine in an OFF configuration and, upon contact, forms a pore complex in the host cell membrane, which enables the injection of proteins directly into the cytosol of targeted mammalian cells27–29. We previously established a recombineering-based platform to transfer large regions of this virulence plasmid into engineered synthetic loci on the E. coli chromosome20–22,30. Using this platform, we developed laboratory strains of E. coli that encode the ipa, mxi, and spa operons, which we established can inject heterologous proteins into mammalian cells23.
With the goal of developing E. coli that secrete proteins into their surroundings, we compared the secretory activity of DH10b E. coli that contain the ipa, mxi, and spa operons vs. the mxi, and spa operons. In each case, the operons were inserted at the same chromosomal locus and a low-copy number plasmid that expresses VirB, the transcription factor that controls the expression of all three operons (Figure S1A), under the control of the IPTG (isopropyl β-d-1-thiogalactopyranoside)-inducible Ptrc promoter, was introduced. The resulting strains are referred to here as mT3Ec_Ipa-Mxi-Spa (Figure S1A) and mT3Ec_Mxi-Spa (Figure S1B).
When grown under conditions that promote expression of the operons and exposed to Congo red, a dye that triggers secretion in the absence of host cells31, mT3Ec_Ipa-Mxi-Spa and mT3Ec_Mxi-Spa, secreted similar levels of Ptrc-regulated OspC2 (a native Shigella type III secreted effector), demonstrating that the ipa operon plays no role in secretory activity (Figure 1A). GroEL, a highly abundant cytosolic protein, and reporter of host cell lysis, was not detected in the supernatant fractions (Figure 1A), providing evidence that proteins were secreted via the T3SA. In the absence of Congo red, OspC2 was abundantly secreted by mT3Ec_Mxi-Spa, but not mT3Ec_Ipa-Mxi-Spa, demonstrating that mT3Ec_Mxi-Spa constitutively secretes proteins into its surroundings without the need of host cell contact (Figure 1B). When we examined all proteins present in the supernatant of mT3Ec_Mxi-Spa, OspC2 was the most abundant protein, thus demonstrating that the introduction of the mxi-spa operons and VirB was sufficient to outfit DH10b E. coli with a robust Ptrc-regulated secretion system (Figure 1C).
Figure 1. E. coli engineered with a modified T3SA efficiently secrete proteins into their surroundings.

(A, B, C, E, F) Secretion assays of designated strains engineered to secrete OspC2-FLAG. Supernatant (TCA precipitated) (S) and whole-cell pellet lysate (P) fractions were obtained 30-minutes (A, B, E), 6-hours (C), or at times indicated (F) post-transfer to new media. Unless otherwise indicated in (A), IPTG was present to induce expression of VirB and OspC2. Congo red (CR) was added, when noted. Immunoblots labeled with anti-FLAG or anti-GroEL antibodies (A, B, E, F) or a Coomassie blue-stained gel (C) are shown. (D) Schematic of PROT3EcT-1 that expresses plasmid-encoded Ptrc VirB and Ptrc OspC2-FLAG. (G) Gentamicin protection assay to assess invasion of bacteria into intestinal epithelial cells (HCT8). Each dot represents a biological replicate, and the horizontal bar indicates the mean. Data were analyzed using one-way ANOVA with Tukey’s post-hoc test; for all strain comparisons to Shigella *** P < 0.0001. (H) Translocation assay to assess the injection of OspC2-FLAG into cervical epithelial cells (HeLa). The soluble and insoluble (bacteria-containing) fractions were separated and immunoblotted with anti-FLAG and anti-ß-actin. Data in each panel are representative of at least 2 independent experiments. See also Figure S1.
We next investigated whether similar modifications to two non-pathogenic human E. coli isolates, E. coli Nissle 1917 (EcN) and E. coli HS, would equip them with a functional protein secretion system. First, we developed PROT3EcT-1, EcN engineered with the mxi-spa operons at the analogous chromosomal locus as mT3Ec_Mxi-Spa. PROT3EcT-1 (Figure 1D) secreted OspC2, albeit at somewhat lower levels than mT3Ec_Mxi-Spa (Figure 1E). In each case, secretion was VirB-dependent (Figures 1A and 1E), indicating that the secretory activity of PROT3EcT-1 is dependent on the expression and assembly of a functional T3SA. Similar modifications to the E. coli HS led to the generation of PROT3EcT-2, which secreted OspC2 at levels equivalent to that of mT3Ec_Mxi-Spa (Figure S1C).
While our initial studies examined the amount of protein secreted over 30 minutes, we found that when grown in media that support their growth, over a 6-hour period, mT3Ec_Mxi-Spa and PROT3EcT-1 accumulated OspC2 in their secreted fractions (Figure 1F), demonstrating that, when induced, they continually secrete protein into their surroundings.
Shigella are intracellular pathogens that rely on their T3SS and its secreted proteins to invade non-phagocytic epithelial cells. Given that bacteria engineered with the mxi-spa operons lack effectors and components of the tip complex, they are not expected to invade host cells or inject proteins into host cells. To confirm, first, we compared the ability of Shigella, PROT3EcT-1, mT3Ec_Mx-Spa, DH10b E. coli, and EcN to invade HCT8 cells, a mammalian cell line of intestinal origin. As expected, Shigella but none of the other strains were protected from gentamicin, an antibiotic that kills extracellular but not intracellular bacteria (Figure 1G). Second, we compared the levels of OspC2 in the soluble cytosolic and insoluble bacterial containing fractions of mammalian HeLa cells exposed to WT Shigella, mT3Ec_Mx-Spa and PROT3EcT-1 (Figure 1H). As expected, OspC2 was only observed in fractions of cells infected with WT Shigella. Together these studies demonstrate that mT3Ec_Mx-Spa and PROT3EcT-1 do not invade or inject proteins into mammalian cells.
PROT3EcT can be engineered to secrete heterologous proteins
We next investigated whether PROT3EcT-1 recognizes heterologous proteins as secreted substrates. In prior studies, we had found that the fusion of the first 50 residues of multiple type III effectors fused to the N-termini of heterologous proteins was sufficient to generate secreted variants23. These residues encode the elements that define type III secretion sequences (SSs)32. As previously observed with mT3Ec_Ipa-Mxi-Spa, we found that PROT3EcT-1 recognized similarly modified heterologous proteins, including MyoD and Mef2c, two mammalian transcription factors, TALE, a transcription activator-like effector, and 60 and 70 kDa fragments of APC, a mammalian tumor suppressor protein (Figure S1D).
Nbs, the ~15kDa variable domains of heavy chain-only antibodies, are ideal substrates for our bacterial secretion system as they are small stable proteins that generally exhibit strong antigen-binding affinity. Monomeric Nbs were previously engineered to be recognized as secreted substrates of the T3SS of enteropathogenic E. coli33. Thus, we screened for modifications to a representative monomeric Nb that would enable its secretion by PROT3EcT. NbASC 34 was fused to the secretion sequences of nine Shigella effectors (OspC2, IpaH4.5, IpaH7.8, IpaH9.8, OspE, OspF, OspD3, VirA, and OspG)21,23. Variants fused to the OspC2 and OspG secretion sequences exhibited the highest level of secretion (Figure 2A). Fusion of the OspC2 sequence also enabled the secretion of monomeric NbPD-L1 35, NbCTLA4 36, NbNPI 37, and NbStx2 38 (Figure 2B) and single-chain heterodimeric and heterotrimeric NbStx2 (Figure 2C). As observed with full-length OspC2 (Figure 1C), heterodimeric SSOspC2-NbStx2 was the most abundant protein present in the supernatants of PROT3EcT-1 (Figure 2D) and exhibited binding and neutralizing activity against Shiga toxin, its target antigen (Figures 2E and 2F). This was an important finding as it demonstrated that the Nb, which like other type III secreted substrates is unfolded when loaded into the secretion apparatus, once secreted correctly refolds.
Figure 2. PROT3EcT can be engineered to secrete nanobodies.

Secretion assays of (A) NbASC fused to designated N-terminal type III secretion signals and a C-terminal HA-tag, (B) NbASC, -NbPD-L1, -NbCTLA-4 and -NbNP1 fused to the N-terminal OspC2 secretion signal (SSOspC2) and C-terminal HA-tag, (C) monomeric (1x), heterodimeric (2x) and heterotrimeric (3x) NbStx2, fused to an N-terminal SSOspC2and a C-terminal 3xFLAG tag, (D) NbStx2 dimer modified with an N-terminal SSOspC2 (SSOspC2-Nb2x) and C-terminal HA tag. (A, B, C) were obtained at 6-hours. Immunoblots labeled with anti-GroEL as well as anti-HA (A, B) or anti-FLAG (C) antibodies or (D) a Coomassie blue-stained gel are shown. (E) Stx2 ELISA. (F) Stx2 Vero killing assay. For (E-F), each dot represents an individual biological replicate. Data in each panel are representative of at least 2 independent experiments. See also Figure S1.
Development of constitutively active Nb-secreting PROT3EcT
Before studying the behavior of PROT3EcT in mice, we sought to generate a constitutively active variant that maintains all its genetically engineered components in the absence of antibiotic selection. First, to enable constitutive expression of the secretion system, we replaced the inducible Ptrc promoter of VirB, its master transcriptional regulator, with several predicted to be constitutively active in the gut, e.g., Shigella PvirF39, E. coli PompC40, and two synthetic promoters, PJ23115 and PJ2311941. PROT3EcT-1 variants that carried plasmids that express VirB under the control of each of these promoters secreted equivalent levels of Nbs (Figure S1E). Thus, we introduced the PJ23119-virB expression cassette into the chromosome of PROT3EcT-1 to generate PROT3EcT-3 (Figures S1F–G).
Next, to enable constitutive Nb expression, we replaced its inducible Ptrc promoter with the constitutive PJ23108 promoter (Figure S1H). In addition, to maintain flexibility in the introduction of expression circuits and to enable higher levels of payload expression, we developed a means to maintain plasmids in PROT3EcT in the absence of antibiotic pressure. Based upon the work from Hwang and colleagues42, we developed PROT3EcT-4, a derivative that lacks alr and dadX (Figure 3A). These genes encode EcN’s two alanine racemases that convert L- to D-alanine, an amino acid essential for cell wall biosynthesis that is very limited in the mammalian gastrointestinal tract43. In parallel, we inserted alr onto the Nb-producing plasmid. PROT3EcT-3 and PROT3EcT-4 that carry this plasmid secreted similar levels of Nbs. PROT3EcT-3 but not PROT3EcT-4 lost its ability to express and secrete Nbs in the absence of antibiotic selection (Figures S1I–J). Remarkably, despite expressing a constitutively active secretion system, NbStx2-secreting PROT3EcT-4 (Stx2-PROT3EcT) exhibited essentially identical growth patterns when grown in parallel (Figure 3B) or in competition (Figure S1K) with EcN, indicating that these modifications do not add a significant metabolic burden.
Figure 3. PROT3EcT stably colonizes the gastrointestinal tract of mice but does not affect the gut microbiota.
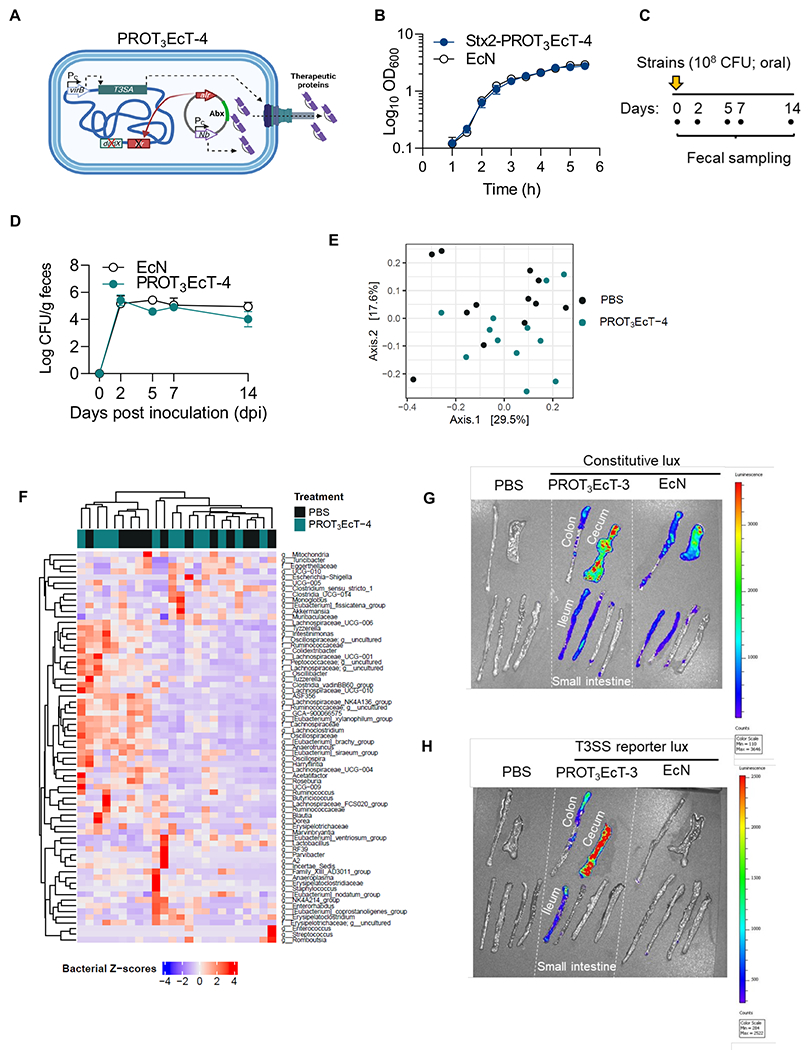
(A) Schematic of PROT3EcT-4, which expresses VirB via a constitutive promoter (Pc) from a chromosomal locus. The Nb is expressed via a constitutive promoter from a plasmid maintained via auxotrophic selection. (B) Growth curves of wild-type EcN and Stx2-PROT3EcT grown in parallel. Data are presented as the mean ± SEM. (C) Study design schematic. (D) Shed bacterial titers. Data are presented as the mean ± SEM, each with 4 mice per group, and reflect at least 2 independent experiments. (E) Principal Coordinate Analysis (PCoA) plot showing Bray-Curtis dissimilarity for 16S rRNA gene amplicon fecal surveys from sham-treated (PBS) (n=12) and PROT3EcT-treated (n=12) mice, collected on day 14 post-treatment. (F) A hierarchically clustered heatmap of the z-score abundance of bacterial family/genus (f = family, g = genus) within each sample (rows) from the same samples as in (E). Color codes indicate treatment conditions (PBS vs. PROT3EcT). (G-H) Bioluminescent imaging of intestinal explants isolated from individual mice 8 dpi inoculated orally with designated strains transformed with (D) a constitutive bioluminescent reporter (pMM543) or (E) a T3SS-dependent reporter system (pMxiE-lux+ and pNG162-IpgC). Starting 1 day before they were administered bacteria to ensure plasmid maintenance, the mice began receiving kanamycin and spectinomycin in their drinking water. See also Figures S1–S2 and Tables S1A–B.
PROT3EcT colonizes and maintains an active T3SA but does not perturb the gut microbiota of mice
Our initial in vivo experiments focused on investigating the ability of Nb-secreting PROT3EcT-4 to colonize the mouse gastrointestinal tract. We monitored the titers of bacteria shed in the feces of C57BL/6 mice orally inoculated with EcN or Stx2-PROT3EcT (Figure 3C). After administering a single dose of ~108 colony forming units (CFU), we detected ~105 CFU/g/day of each strain for at least 14 days (Figures 3D and S2A). The mice appeared well and demonstrated no evidence of significant weight loss throughout this time (Figure S2B).
To investigate whether the bacteria isolated from feces maintained secretory activity, we monitored the secretion of 39-40 isolates from a total of 4 mice on days 2, 5, and 14. Each of the 158 Stx2-PROT3EcT colonies examined secreted Nbs at levels equivalent to the inoculation strain (Figures S2C). These findings demonstrate that within the gastrointestinal tract of mice, in the absence of antibiotic selection, PROT3EcT-4 maintains a functional secretion system and the alr-Nb-expressing plasmid.
We also compared the fecal microbiome composition of C57BL/6 mice 14 days post-inoculation with PBS or ~108CFU of PROT3EcT-4 by conducting 16S rRNA gene amplicon surveys. At that time, ~105CFU/gm/day of PROT3EcT-4 were found in the feces of mice that received bacteria (Figure S2D). We observed no community-level changes in the gut microbiota composition of PROT3EcT-4 and sham-treated mice as assessed by Bray-Curtis dissimilarity of the reads (Figure 3E, Table S1A). Similarly, hierarchical clustering at the genus-level (Figure 3F) and differential abundance testing with MaAsLin2 (Table S1B) showed no significant taxonomic changes of the gut microbiota of mice that received PROT3EcT-4 versus PBS.
We examined the biogeography of PROT3EcT-3 and EcN within the intestines of mice inoculated with variants that constitutively express the luciferase-producing luxCDABE operon44, which exhibited equivalent luciferase activity when grown in vitro (Figures S2E–F). Eight days post-oral inoculation of the mice, the two strains showed similar patterns of luciferase expression in explanted sections of their ileum, cecum, and proximal colon, which had feces removed before imagining (Figure 3G).
To confirm that the modified T3SA present in PROT3EcT is actively transcribed within the intestines of mice, we developed a luxCDABE-based reporter that is only activated when the mxi-spa operons, which encode the modified T3SA, are transcribed (Figure S2G). Variants of PROT3EcT-3, but not EcN, that carry this reporter demonstrated luciferase production when grown in culture (Figures S2H–I) and within the explanted cecum, proximal colon, and ileum of inoculated mice (Figure 3H), demonstrating that the secretion system is expressed within the intestines of mice and does not perturb EcN colonization. For all the luciferase-based studies, we treated the mice with antibiotics to ensure maintenance of plasmids that encode the luxCDABE operon.
PROT3EcT can be engineered to secrete functional SSOspC2-NbTNF
To establish the use of PROT3EcT as a therapeutic platform, we focused efforts on investigating its efficacy in the treatment of inflammatory bowel diseases (IBD). The etiologies of ulcerative colitis and Crohn’s disease, collectively termed IBD, are complex and thought to be driven by host genetic, environmental, and microbiota factors. Yet, both diseases exhibit chronic inflammation accompanied by increased levels of pro-inflammatory cytokines45. Monoclonal antibodies (mAb) that target the pro-inflammatory cytokine TNFα, e.g., infliximab and adalimumab, are highly efficacious in controlling severe disease and in improving the quality of life of patients with IBD46. However, given the systemic administration of these therapeutics, patients receiving these agents are immunosuppressed and at increased risk of developing life-threatening infections and lymphoma47.
We hypothesized that the targeted delivery of anti-TNFα Nbs via PROT3EcT to the intestines would reduce intestinal inflammation. To investigate this possibility, we isolated Nbs from alpacas immunized with recombinant mouse (m)TNFα, including one (JTT-B10) that bound with high affinity (EC50 0.1 nM) and neutralized mTNFα (IC50 0.1 nM) (Table S2 and Figure S3A–C). We generated monomeric and homodimeric variants of this Nb (NbmTNF) engineered with an SSOspC2. The homodimer (SSOspC2-Nb2xmTNF) was secreted much more efficiently than the monomer (Figure 4A) and was detected at ~0.5 μg/mL in the supernatant of an 18-hour culture (Figure S3D). PROT3EcT-secreted SSOspC2-Nb2xmTNF was as effective as E. coli-purified homodimeric Nb in blocking mTNFα-induced death of mouse L929 cells (Figure 4B). Using a similar approach, we also isolated an alpaca-derived human-specific anti-TNFα Nb that binds with high affinity (0.5 nM) (Figure S3E) and blocks human TNFα-induced death of mouse L929 cells (Figure S3F). When engineered with an N-terminal SSOspC2, functional homodimers of this Nb (SSOspC2-Nb2xhTNF) are secreted by PROT3EcT (Figures S3G–H).
Figure 4. TNF-PROT3EcT secretes fully functional anti-TNF Nbs and colonizes mice but does not affect the gut microbiota.

(A) Secretion assays of PROT3EcT-1 engineered with monomeric (1x) or homodimeric (2x) NbTNF fused to an N-terminal OspC2ss and a C-terminal FLAG. Supernatant (TCA precipitated) (S) and whole-cell pellet lysate (P) fractions were obtained at 6-hours. Immunoblots labeled with an anti-FLAG antibody are shown. (B) Viability of L929 cells following incubation with 0.2 ng/ml of murine TNFα plus either supernatant (Sup) from designated strains or purified homodimeric NbTNF. Data are presented as the mean ± SEM (C) Shed bacterial titers. Each dot represents an individual mouse, and the horizontal bar indicates the mean. (D) PCoA plot showing Bray-Curtis dissimilarity for 16S rRNA gene amplicon fecal surveys from PROT3EcT (n=7) and TNF-PROT3EcT-treated (n=8) mice. (E) A hierarchically clustered heatmap of the z-score abundance of bacterial family/genus (f = family, g = genus) within each sample (rows) from the same samples as in (D). Color codes indicate treatment group (PROT3EcT vs. TNF-PROT3EcT) and caging information. Data in each panel are representative of at least 2 independent experiments. See also Figures S3–S5 and Tables S2 and S3A–B.
TNF-PROT3EcT inhibits the development of disease in a mouse model of colitis
To investigate the utility of PROT3EcT as a live biotherapeutic for the treatment of intestinal inflammation, we chose a preclinical chemically induced mouse colitis model whereby a mixture of TNBS (2,4,6-trinitrobenzene sulfonic acid), a hapten, and ethanol, which disrupts the mucosal barrier, is rectally instilled into the colon. TNBS bound to colonic tissue proteins induces inflammation driven by pro-inflammatory cytokines, including TNFα48. For these studies, we used BALB/c mice, which are more susceptible to TNBS than C57BL/6 mice48,49. As previously reported50, mice treated intraperitoneally with a neutralizing anti-TNF monoclonal antibody (1 day prior and 2- and 4-days post-administration of TNBS) were protected from weight loss, colon shortening, and histologic evidence of colitis (Figure S4A–G).
Before testing the efficacy of Nb2xmTNF-secreting PROT3EcT-4 (TNF-PROT3EcT) in colitis suppression, we compared the composition of the fecal microbiota of BALB/c mice colonized with TNF-PROT3EcT or PROT3EcT-4. We carried out 16S rRNA gene amplicon surveys on the feces from mice 14-days post-inoculation with TNF-PROT3EcT and PROT3EcT-4, which like C57BL/6 mice continued to shed ~105 CFU/gm/day (Figure 4C). We observed no separation in the gut microbiota at the community level between TNF-PROT3EcT and PROT3EcT-4 treated mice, as assessed via Bray-Curtis dissimilarity of the reads (Figure 4D, Table S3A). Consistent with this ordination analysis, hierarchical clustering at the genus level (Figure 4E) and differential abundance testing using MaAsLin2 (Table S3B) showed no significant taxonomic changes that correlate with TNF-PROT3EcT versus PROT3EcT-4 administration. Thus, Nb2xmTNF secretion by PROT3EcT does not significantly affect gut microbiota community composition as assessed.
Next, to test the therapeutic efficacy of TNF-PROT3EcT, one day before as well as two and four days after they received TNBS, we orally gavaged mice with 108 CFU of TNF-PROT3EcT, PROT3EcT-4 or PBS (Figure 5A). All animals that received bacteria consistently shed ~105 CFU/g in their feces (Figures 5B and S4H). Treatment with TNF-PROT3EcT significantly reduced weight loss, blunted colon shortening, and decreased or completely abrogated epithelial injury and inflammation throughout the length of the colonic mucosa (i.e., the distal and proximal colon), including less polymorphonuclear and mononuclear cell infiltration (Figures 5C–F). In contrast, PROT3EcT-4 did not provide any protection as assessed by each of these metrics, demonstrating that the therapeutic efficacy afforded by TNF-PROT3EcT is due to the secreted SSOspC2-Nb2xmTNF and not EcN intrinsic.
Figure 5. TNF-PROT3EcT inhibits the development of TNBS-induced colitis.
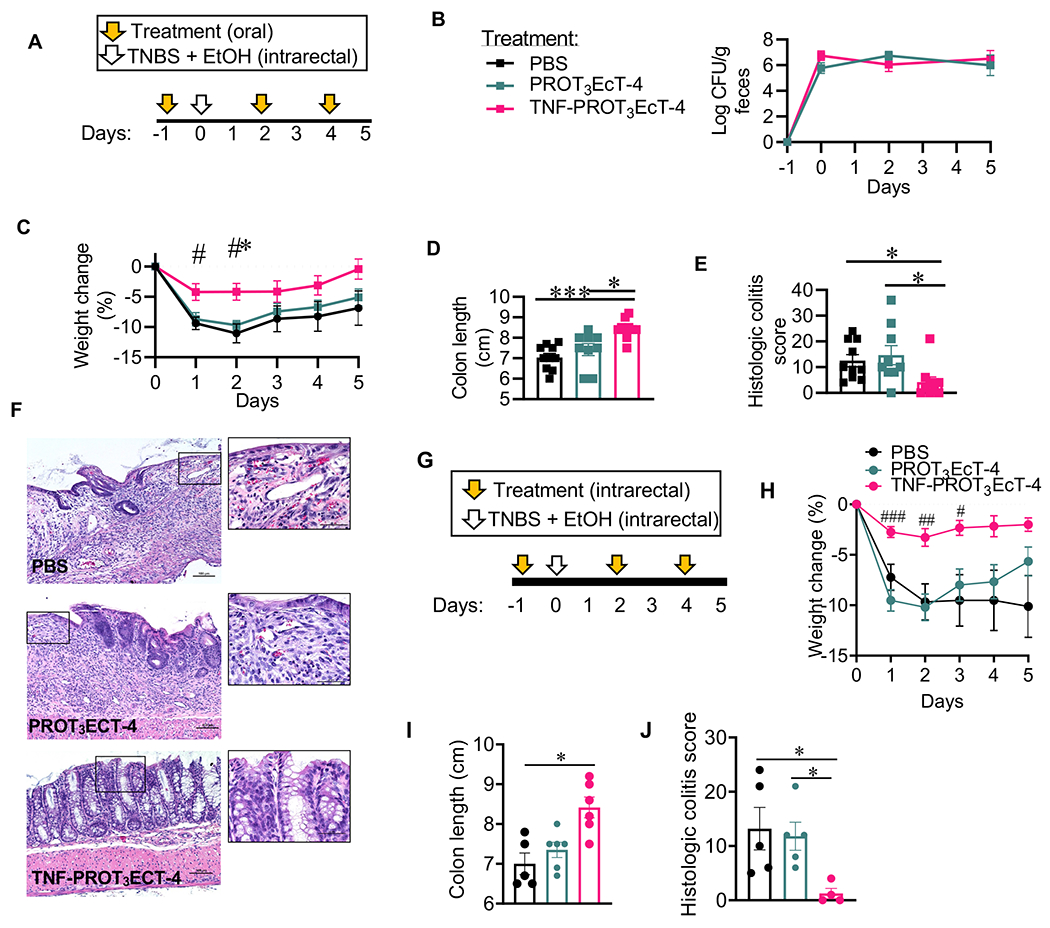
(A, G) Study design schematic. (B) Shed bacterial titers. (C, H) Body weight change (%). In (C), *denotes comparison to PBS group, P=0.0118; # denotes comparison to PROT3EcT-4; day 1, P=0.0238; day 2, P=0.0122. In (H), # denotes comparison to PROT3EcT-4, day 1, P=0.0003, day 2, P=0.0018, day 3, P=0.02. (D, I) Colon length. In (D), *, P=0.0219; ***, P=0.0004. In (I), *, P=0.0123. (E, J) Histologic colitis scores. In (E), vs. PBS *, P=0.0231; vs. PROT3EcT-4 *, P=0.0141. In (J), vs. PBS, P= 0.0406 and vs. PROT3EcT-4, P=0.0331. (F) Representative colon histology sections stained with hematoxylin and eosin. Scale bars = 100 μm. Data reflect at least 2 independent experiments, each with 3-5 mice per group, and are presented as mean ± SEM (B-C, H) or individual values ± SEM (D-E, I-J). Data were analyzed with a two-way ANOVA with Tukey’s post hoc test (B-C, H) or a Kruskal-Wallis test with Dunn’s multiple correction test (D-E, I-J). TNBS = 2,4,6-Trinitrobenzenesulfonic acid. EtOH = ethanol. See also Figures S4 and S6–7.
Given that enemas are commonly used for drug delivery for patients with IBD, we also investigated the efficacy of intrarectally delivered TNF-PROT3EcT in limiting TNBS-induced colitis using the same dosing strategy as described above (Figure 5G). As with oral delivery, intrarectally delivered TNF-PROT3EcT, but not PROT3EcT-4, ameliorated weight loss, colon shortening, and colitis (Figures 5H–J). Both strains were shed at similar levels in feces (Figure S4I).
To evaluate the effectiveness of TNF-PROT3EcT in another colitis model, we chose dextran sodium sulfate (DSS)-induced colitis in which DSS administration disrupts the colonic epithelium leading to mucosal injury and inflammation51. However, we observed that neither the administration of anti-TNF mAb nor TNF-PROT3EcT via an oral or intrarectal route offered protection against the development of colitis as assessed by weight loss, colon shortening and histological examination of the colon (Figure S5A–N). Thus, further substantiating the specificity of TNF-PROT3EcT in blocking TNFα-driven inflammation.
SSOspC2-Nb2xmTNF secretion but not E. coli colonization is required for protection against TNBS-induced colitis
Therapeutic strains that cannot compete for and establish a replicative niche (i.e., non-colonizing) within the colon may be useful if administered repeatedly to patients. To assess whether treatment with similarly engineered DH10b E. coli also suppresses colonic inflammation, we developed T3EcT, a variant of mT3Ec-Mxi-Spa engineered with the chromosomally encoded PJ23119 VirB gene cassette, and a variant of T3EcT that secretes SSOspC2-Nb2xmTNF, TNF-T3EcT. After establishing that TNF-T3EcT constitutively secreted SSOspC2-Nb2xmTNF (Figure S6A), TNF-T3EcT and T3EcT were administered orally or intrarectally to mice using the strategy outlined above (Figures S6B–C). The levels of each strain shed in stool remained low (between zero and 103 CFU/g) and, unlike PROT3EcT, T3EcT and TNF-T3EcT were cleared from >50% of the mice (Figures S6D–E). However, while orally administered TNF-T3EcT provided no protection (Figures S6F), rectally delivered TNF-T3EcT, but not T3EcT, suppressed colitis (Figures S6G), likely reflecting repeated transient delivery of SSOspC2-Nb2xmTNF into the colon.
To address whether bacterial secreted Nbs were restricted to the gut, we measured Nb levels in the colonic contents, colon tissue homogenates, and serum of mice treated with each strain across all the TNBS experiments. To measure SSOspC2-Nb2xmTNF, we used a direct ELISA, which can also detect the anti-TNF mAb. In mice administered the anti-TNF mAb via an intraperitoneal route, we detected mAb in the serum of 50% of the mice (Figure S7A). By contrast, levels of serum SSOspC2-Nb2xmTNF were below the level of detection in all mice orally inoculated with TNF-PROT3EcT and only detectable in 20% of mice treated with TNF-PROT3EcT or TNF-T3EcT via enema. We did not detect evidence of SSOspC2-Nb2xmTNF in colonic contents or homogenates (Figures S7B–C), likely due to their degradation via gut proteases.
A single oral dose of TNF-PROT3EcT is associated with TNFα suppression and inhibition of intestinal inflammation
Given that the TNF-PROT3EcT-treated mice exhibited minimal weight-loss post-administration of TNBS, we tested whether pretreatment with a single dose was therapeutically efficacious. Two days post-TNBS administration, mice pre-treated with a single oral dose of TNF-PROT3EcT or PROT3EcT (Figure 6A) shed similar levels of each strain in their feces (Figures 6B, S6H), and when tested, colonies of shed TNF-PROT3EcT retained the ability to secrete anti-TNF Nb (Figure S6I). Strikingly, mice treated TNF-PROT3EcT exhibited minimal evidence of weight loss, colon shortening, and colitis, whereas PROT3EcT-4 and the vehicle diluent (PBS) had no effect (Figures 6C–E). Similar protection was observed five days post-TNBS administration when mice were pre-treated with a single oral dose of TNF-PROT3EcT (Figures S6J–M).
Figure 6. A single prophylactic dose of TNF-PROT3EcT attenuates TNBS-induced colitis.

(A) Study design schematic. (B) Shed bacteria titers. (C) Body weight change (%). *denotes comparison to PBS, day 1, P=0.0002, day 2, P<0.0001; #denotes comparison to PROT3EcT-4, day 1, P=0.054, day 2, P<0.0001. (D) Colon length. *, P=0.0184; **, P=0.0029. (E) Histologic colitis scores. **, P=0.0045. (F-H) Colon homogenates were analyzed for the levels of TNFα (F) (***, P=0.0005, *, P=0.0433), IL-6 (G) (vs. PROT3EcT-4 *, P=0.0356; vs. PBS *, P=0.0322) and IL-10 (H) by ELISA. Data reflect 2 independent experiments, each with 4-5 mice per group, and are presented as individual values ± SEM (D-G) or mean ± SEM (B-C). Data were analyzed using a two-way ANOVA with Tukey’s post hoc test (B-C) or a Kruskal-Wallis test with Dunn’s multiple correction test (D-H). TNBS = 2,4,6-Trinitrobenzenesulfonic acid. EtOH = ethanol. See also Figure S6.
For the mice that were sacrificed two days post-administration of TNBS, a time point at which we reproducibly detected elevated pro-inflammatory cytokine levels within colonic tissue in controls, significantly lower levels of TNFα and IL-6 were detectable within the colonic tissue of mice pretreated with TNF-PROT3EcT but not PROT3EcT (Figures 6F–G), suggesting that the secreted anti-TNFα Nb sequesters its target and leads to a reduction in IL-6. While others have observed that TNF neutralization or EcN treatment can increase IL-10 production in the gut, we observed equivalent levels of IL-10, regardless of the intervention (Figure 6H).
Discussion
Here we describe the development of PROT3EcT, a suite of E. coli engineered for the in-situ delivery of high-specificity protein payloads to sites of disease. Using synthetic biology-based approaches, we have engineered both laboratory and non-pathogenic human E. coli isolates with a modified T3SA which can secrete proteins in a regulated or constitutive manner. Furthermore, we have developed PROT3EcT-3 and PROT3EcT-4, variants outfitted with a constitutively active secretion system that is maintained in the absence of antibiotic selection, which, when constitutively secreting Nb, exhibits in vitro and in vivo growth rates and colonization profiles equivalent to native EcN.
In terms of its therapeutic potential, orally or rectally administered TNF-PROT3EcT, a variant that constitutively secretes anti-mTNF Nbs, was as efficacious as systemically administered anti-TNF mAb in limiting the development of TNBS-induced colitis in mice. Although PROT3EcT, like EcN, appeared to poorly colonize the distal colon, orally administered TNF-PROT3EcT, but not PROT3EcT, suppressed inflammation throughout the colon. These observations suggest that low levels of TNF-PROT3EcT present in the distal colon or descending anti-TNF Nb secreted above are sufficient to block the development of downstream TNFα-mediated colitis. While we were unable to detect Nb within the intestines of mice, presumably due to their degradation via gut proteases, we provide evidence that the T3SA of PROT3EcT is active within the intestines of mice and TNF-PROT3EcT, but not PROT3EcT, significantly ablates its target molecule, TNFα.
Future studies will focus on the ability of TNF-PROT3EcT to suppress inflammation post the induction of colitis, i.e., as a treatment intervention. Nevertheless, our observation that TNF-PROT3EcT was as efficacious as systematically administered anti-TNFα monoclonal antibodies in suppressing the development of colitis suggests that it has the potential to serve in a similar capacity, i.e., as maintenance therapy in patients with IBD.
Other groups have also engineered microbes to treat gut inflammation, the most closely related being variants of Lactococcus lactis that secrete IL-10, an anti-inflammatory cytokine, or an anti-TNF Nb11,12. A native secretion system of this Gram-positive bacterium was repurposed for the secretion of these therapeutic payloads. However, the strain of L. lactis used does not colonize the intestines of humans or mice52,53, perhaps accounting for why its engineered variants only moderately suppressed inflammation when administered daily to mice. In this regard, although our studies focused on different preclinical models of colitis, it is interesting that we observed that pre-treatment with just a single oral dose of colonizing TNF-PROT3EcT, but not orally administered poorly colonizing TNF-T3EcT, significantly ameliorated colonic inflammation and injury. These observations suggest an advantage to treating with colonizing bacteria, particularly when using variants that secrete therapeutic agents.
The modular design of PROT3EcT is such that it can be adapted to secrete different payloads as well as to respond to environmental cues. For example, by altering the conditions that induce expression of VirB, PROT3EcT’s T3SA could be endowed with an ‘on switch’ triggered by specific signals of the gut’s inflammatory milieu, e.g., reactive nitrogen species44,54–56. In terms of therapeutic payloads, we demonstrate the versatility of PROT3EcT to secrete different Nbs, including Nbs that inhibit the activity of bacterial toxins (Nbstx2), immune checkpoint molecules (NbPD-L1 and NbCTLA-4), as well as other heterologous proteins. While the Nbs we studied were each derived from immunized alpacas, synthetic yeast- and bacterial-based Nb libraries are also available that can be screened rapidly for Nbs with desired properties57,58. By altering its route of administration, PROT3EcT can be expanded for the deposition of therapeutics not only to the gastrointestinal tract but also to solid tumors, as EcN home to and colonize a variety of solid tumors when administered intravenously, at least in mice59.
In studies similar to ours, a type I secretion system (T1SS), native to the Gram-negative plant pathogen Erwinia chrysanthemi, was introduced into EcN and engineered to secrete epidermal growth factor into the gut60. They also observed evidence of suppression of gut inflammation in a mouse model of colitis. In the case of T1SSs, proteins of interest are engineered with a carboxy-terminal rather than an N-terminal type III secretion signal sequence. In future studies, it will be interesting to compare the behavior of E. coli engineered with Type I vs. modified Type III secretion systems, particularly as it has been previously demonstrated that Nbs remain active when secreted via this platform from laboratory strains of E. coli61.
Given its inherent anti-inflammatory and anti-microbial properties, EcN is GRAS and has been used for over a century to treat various intestinal diseases and is available over the counter in some countries. However, EcN contains an operon that mediates the synthesis of colibactin, a genotoxin capable of promoting the formation of DNA crosslinks62. Other colibactin-producing E. coli promote the development of colorectal cancer (CRC) in mouse models63 and induce mutational signatures found in human CRC64. Whether this will turn out to be an issue that limits the use of EcN-based therapeutics in humans remains to be discovered. However, EcN mutants deficient in colibactin biosynthesis are not impaired in their ability to colonize the intestines of at least mice65,66 or primates67. In future studies, we intend to test the ability of colibactin-deficient EcN-based TNF-PROT3EcT to suppress intestinal inflammation.
Here, we report that two colibactin-negative strains, E. coli HS and DH10ß, like EcN, can also be engineered with a functional modified type III secretion system. While the anti-TNF Nb-secreting, E. coli DH10ß based, TNF-T3EcT, was unable to colonize the intestines, daily dosing of rectally administered TNF-T3EcT suppressed TNBS-induced inflammation as effectively as EcN-based TNF-PROT3EcT. Additionally, while EcN has been shown to colonize, albeit variably, healthy human subjects for up to 2 weeks, the inflammatory milieu of the IBD gut will likely favor colonization of engineered E. coli strains68. Furthermore, other commensal E. coli isolates, including HS, which we study herein, or patient-specific isolates69,70 could serve as more optimal chassis for the PROT3EcT platform. Regardless, before testing any of these modified strains in humans, they must be equipped with means to control biocontainment. Some strategies being developed include incorporating circuit-activated kill switches, i.e., those turned on at colder environmental temperatures when bacteria are excreted or auxotrophies that limit environmental growth (for review, see71).
In summary, we describe the development and characterization of PROT3EcT, programmable E. coli engineered for the delivery of therapeutic payloads to the gastrointestinal tract. While the presented studies support the further development of PROT3EcT for the treatment of IBD, its modularity permits its rapid adaptation into a therapeutic platform for a broad range of diseases.
Limitations of the study
Although our study provides proof of concept for the use of PROT3EcT as a strategy for the delivery of therapeutics to the gut, further preclinical studies are required to demonstrate its efficacy as a therapeutic for IBD. While we have demonstrated that TNF-PROT3EcT is efficacious in limiting TNBS-driven colitis model; it remains to be determined whether this intervention can ameliorate pre-existing colitis or is efficacious in chronic pre-clinical models of colitis.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for other reagents may be directed to and will be fulfilled by the Lead Contact, Cammie F Lesser (clesser@mgh.harvard.edu).
Materials Availability
Engineered strains and plasmids used in this study are available from the lead contact upon request with a completed Materials Transfer Agreement. The anti-hTNF Nb sequence is restricted pending patent application filing and approval.
Data and Code Availability
Illumina sequences obtained in the present study were deposited in the Sequence Read Archives (SRA) NCBI database under accession number PRJNA913459 and are currently available. Original western blot images have been deposited at Mendeley and are publicly available as of the date of publication and are currently available at DOI: 10.17632/wwzxwcwj62.1.
No original code was generated for these studies.
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains
Unless otherwise noted in the experimental method details, E. coli strains were grown in luria broth (LB: 10g/L tryptone, 5g/L yeast extract, 10g/L NaCl) and Shigella flexneri in tryptic soy broth at 37°C with aeration on a roller or on solid media (15% agar). When appropriate, antibiotics (100 μg/ml spectinomycin, 100 μg/ml ampicillin, 50 μg/ml kanamycin, 12.5 μg/ml tetracycline ), Congo Red (10 μM), D-alanine (50 μg/mL), and/or IPTG (1 mM) were added. Bacterial strains are summarized in Table S4. Bacterial strains were grown from a glycerol stock (10% w/v glycerol) by streaking onto solid media. After 18 h of growth, single colonies were picked and inoculated into liquid cultures. Construction strategies and transformation protocols are described in method details.
Cell lines
All cell lines were cultured in a 5% CO2 incubator at 37°C. HeLa cells were maintained in Dulbeco’s Modified Eagle Medium (c) (Thermo Fisher Scientific #11965), and HCT8 and L929 cells in GlutaMAX-supplemented RPMI 1640 (Thermo Fisher Scientific #61870127). In each case, the media was supplemented with 10% heat-inactivated fetal bovine serum (FBS, R&D systems #S11150), 100 IU/mL penicillin, and 100 μg/mL streptomycin) (Life Technologies #15140). Antibiotics were removed prior to conducting infections.
Mice
All mice were purchased at 6 weeks of age from The Jackson Laboratory and upon arrival allowed to acclimate for at least one week prior to the start of an experiment. Mice were housed in microisolator cages under specific pathogen-free conditions in the barrier facility of Harvard T.H. Chan School of Public Health and had access to food (irradiated PicoLab® Mouse Diet 20, 5058) and water (autoclaved reverse osmosis) ad libitum. A 12:12 hour light/dark cycle was used. Female C57BL/6 mice were used for colonization studies (Figure 3C and 3D) and female BALB/c mice were used for the TNBS colitis models (Figures 5 and 6) and female C57BL/6 mice were used for the DSS studies (Figure S5). For these studies, the mice were randomly grouped in cages at a maximum of five animals per cage. For the in vivo luciferase imaging assays, kanamycin (1 g/L) and spectinomycin (2 g/L) (Figures 3G and 3H) was administered to mice via their drinking water. For the microbiome studies (Figures 3 and 4), female and male mice were used, and mice were housed n=2 per cage to minimize cage effects. No blinding was performed, except for histopathology scoring. Animal experiments were approved and carried out in accordance with Harvard Medical School’s Standing Committee on Animals and the National Institutes of Health guidelines for animal use and care.
METHOD DETAILS
Plasmids
Plasmids are summarized in Table S4. Sequences of DNA inserts and oligos are summarized in Table S5A–B. Strains transformed with temperature-sensitive plasmids pCP20, pKD46 and pTKRED were maintained at 30°C and cured by incubation 42°C. All gateway entry clone inserts were sequence verified. Synthetic DNA fragments were purchased from Integrated DNA Technologies (IDT), Twist Bioscience, or Genewiz.
Plasmid construction
APC expression plasmids:
DNA fragments composed of APC (amino acids 1342 to 1887) or (1342 to 1982) fused to an OspC2ss were generated using SOEing PCR with oligomers (P1/P2 and P3/P4 or P3/P5) to generate attB1- SSOspC2-APC60-attB2 and SSOspC2-APC70-attB2, respectively. The resulting fragment was introduced in pDONR221 followed by pDSW206-ccdB-FLAG via BP and LR reaction to generate pDSW206- SSOspC2-APC60and pDSW206-SSOspC2-APC70.
NbPD-L1, NbCTLA-4, NbASC and NbNP1 Gateway compatible destination vectors:
Gateway compatible destination vectors that enable the in-frame introduction of sequences upstream of NbPD-L1, NbCTLA-4, NbASC and NbNP1 were generated by using Gibson cloning to join (1) PCR amplified Nb-HA fragments from pHEN6 Nb52 [anti-IAV NP]/Nb KV 022 [anti-IAV NP]/Nb PD-L1 B3/Nb CTLA-4 H11/Nb ASC with oligomers (P6/P7) and (2) pDSW206-ccdB-MyoD NS with oligomers (P8/P9). Each Nb-containing fragment was introduced into the pDSW206-based vectors via Gibson cloning. The resulting clones were sequence verified. The resulting plasmids are referred to as pDSW206-ccdB-Nb* (* = name of Nb present in construct).
NbpD-L1, NbCTLA-4, NbASC and NbNP1 expression plasmids.
A variety of type III secretion signal sequences were introduced into pDSW206-ccdB-NbASC via LR reaction using pENTR-secretion sequence entry clones. An OspC2 secretion signal (OspC2ss) was introduced into pDSW206-ccdB-NbPD-L1, pDSW206-ccdB-NbCTLA4 or pDSW206-ccdB-NbNP1 via a LR reaction with pENTR-OspC2ss.
Alr plasmids.
A DNA fragment containing alr and its native promoter with flanking attB1 and attB2 sites PCR-amplified from EcN using oligomers (P10/P11) was introduced into pDONR221 via a BP reaction followed by pCMD136-ccdB-FLAG via an LR reaction. The resulting plasmid, pCMD136-alr, was used as a template for nested PCR with oligomers (P12/P13, P12/P14 and P15/P16) to generate a DNA fragment (BspHI-Palr-alr-T7t-AseI) that was introduced via traditional cloning into pDSW206 digested with NcoI/NdeI to create pCGP-alr.
NbSTX2 expression plasmids.
A synthetic DNA fragment [attB1-OspC2ss-three NbStx2 (JFG-H6, JFD-A5 and JGH-G1)-E-tag-attB2] was introduced into pDONR221 via a BP reaction followed by pDSW206-ccdB-3xFLAG via an LR reaction to create pDSW206- SSOspC2-Nb3xStx2. Heterodimeric (JFG-H6, JFD-A5) and monomeric (JFG-H6) DNA fragments PCR amplified from pDSW206- SSOspC2-Nb3xStx2using oligomers (P17/P18 and P17/P19) were introduced into pDONR221 via a BP reaction followed by pDSW206-ccdB-3xFLAG via LR reactions to create pDSW206- SSOspC2-Nb2xStx2and pDSW206- SSOspC2-Nb3xStx2.
To replace the IPTG-inducible Ptrc promoter and the lacIq repressor in pDSW206- SSOspC2-Nb2xStx2 with a constitutive promoter, two complementary oligos (P20/P21) were annealed to create a BBa_J23115 promoter (Anderson Collection) with cohesive SphI and SacI ends. The dsDNA fragment was cloned into SphI/SacI digested pDSW206- SSOspC2-Nb2xStx2to create pDSW206-J23115- SSOspC2-Nb2xStx2. The (PJ23115- SSOspC2-Nb2xStx2) fragment in this plasmid was PCR amplified (P22/P23) and introduced into KpnI/XbaI-digested pCGP-alr to create pCGP-alr-PJ23115- SSOspC2-Nb2xStx2. Two complementary oligos (P19/P20) were annealed to create a BBa_J23018 (Anderson Collection) promoter with cohesive SacI ends. The dsDNA fragment was cloned into SacI-digested pCGP-alr-PJ23115- SSOspC2-Nb2xStx2 replacing PJ23115 to create pCGP-alr-PJ23108- SSOspC2-Nb2xStx2.
NbTNF expression plasmids.
Synthetic DNA fragments [attB1–SSOspC2-NbmTNF-attB2] and [attB1- SSOspC2-Nb2xhTNF-attB2] were introduced into pDONR221 via BPs reaction followed by pDSW206-ccdB-FLAG via LR reactions to create pDSW206- SSOspC2-NbmTNF and pDSW206- SSOspC2-Nb2xhTNF. A DNA fragment composed of homodimer NbmTNF fused to an OspC2ss was generated using SOEing PCR with oligomers (P17/P27 and P26/P23) to generate attB1- SSOspC2-Nb2xmTNF-attB2. The resulting fragment was introduced in pDONR221 followed by pDSW206-ccdB-FLAG via BP and LR reaction to generate pDSW206- SSOspC2-Nb2xmTNF and pDSW206-alr-PJ23115- SSOspC2-Nb2xhTNF were constructed as previously described for NbStx2.
VirB expression plasmids.
Entry clones that contain virB under the control of various promoters were obtained via SOEing PCR using two synthetic DNA fragments and oligomers (P28/P29). One synthetic DNA fragment contained a promoter flanked by an upstream attB site and downstream by 40 bp of homology to virB. The second DNA fragment contained the open reading frame of virB codon-optimized for expression in E. coli with an upstream RBS and a downstream stop codon followed by an attB site. The RBS Calculator tool version 1.182, with organism option as E. coli str. K-12 substr. MG1655 was used to choose the RBS. The resulting DNA fragments were introduced into pDONR221 via a BP reaction followed by pCMD136-ccdB-FLAG via an LR reaction. pTKIP-PJ23119-virB was generated by PCR amplifying PJ23119-virB from pCGP-PJ23119-virB-Nb with oligomers (P30/P31) that add a 5′ KpnI site and a 3′ rrnB-homology region. Using pCMD136 as a template, the rrnB terminator was amplified with a 5′ virB-homology region and a 3′ HindIII site using oligomers (P32/P33). The two fragments were fused together by SOEing PCR using oligomers (P30/P33). The product was digested with KpnI and HindIII and ligated into the polylinker of pTKIP-hph.
MxiE-Luciferase expression plasmid.
A DNA fragment that contains a MxiE-promoter was PCR amplified from pTSAR1Ud2.4s2 using oligomers (P34/P35) that add flanking 5′ XhoI and 3′ KpnI restriction sites and an RBS. The digested PCR product was ligated into XhoI/KpnI pMM534 to generate pMxiE-lux.
Strain construction
PROT3EcT-1 and PROT3EcT-2.
A synthetic 1.3 kb landing pad insertion site was introduced into the atg/gid loci of EcN and E. coli HS to generate EcN-LPatp/gid and EcHS-LPatp/gid using the lambda red recombination system and the pTKRED helper plasmid. The landing pad fragment was PCR amplified from pTKIP-tefA with oligos (P36/P37) to introduce homology to the atg/gid locus and integration was confirmed by PCR with oligo pairs (P38/P39 and P40/P41). The pmT3SA plasmid which contains the 20 kb mxi-spa operons flanked by LP and SceI sequences was introduced into EcN-LPatp/gid and EcHS-LPatp/gid via triparental mating: donor (DH10ß/pT3SA), helper HB101 (pRK2073) and recipient (EcN- or EcHS-LPatp/gid/pKD46). pKD46-cured EcN- and HS-LPatp/gid containing pT3SA were transformed with pTKRED and the landing pad recombination system was used to generate PROT3EcT-1 and PROT3EcT-2. KAN resistant/TET susceptible transformants were screened for proper integration junctions by PCR with oligo pairs (P38/P42 and P40/P43).
PROT3EcT-3.
PROT3EcT-1 was modified with a landing pad at its yieN/trkB locus to create PROT3EcT-1-LPyie/trk. By PCR, the landing pad with appropriate homology regions was amplified with oligos (P44/P45) and its integration was confirmed with P46/P39 and P47/P41. PROT3EcT-1-LPyie/trk was transformed with pTKred and pTKIP-PJ23119-virB and the landing pad platform was used to introduce the VirB expression construct into the chromosome. Integration was confirmed by PCR with oligos P48/P33 and P49/P32.
PROT3EcT-4.
After first resolving the KANR marker previously used to introduce the mxi-spa operons into PROT3EcT-1 using the FLP recombinase, the lambda red recombination system74 was used to sequentially delete aIr and dadX from PROT3EcT-3 using oligomers (P50/P51 and P52/P53). The KANR was removed from the aIr locus, before proceeding to delete dadX. Deletions were confirmed by PCR with oligomers (P54/P55 and P56/P57, respectively).
Gentamicin protection assays
HCT8 cells were seeded in 96-well plates (4×104 cells per well) for 18 h prior to exposure to bacteria. Bacteria grown overnight with aeration at 37°C were back-diluted and subcultured for one hour before the addition of IPTG (1 mM). One hour later, the HCT8 cells were washed and then infected at an MOI of 100. After 30 min, cells were washed 3 times and gentamicin (50 μg/mL) was added, and 30 minutes later, cells were washed 6 times then lysed with 1% triton X-100 in PBS. Bacteria were plated and enumerated. Percentage of internalized bacteria was determined by calculating the ratio of gentamicin-resistant bacteria to the initial inoculum.
Translocation assays
Translocation assays were performed as previously described22 with some modifications. HeLa cells were seeded in 6-well plates (3x105 cells per well) for 18 h prior to exposure to bacteria. Bacteria grown overnight with aeration at 37°C were back-diluted and subcultured for one hour before the addition of IPTG (1 mM). One hour later, the cells were washed 3 times then infected at an MOI of 100. After 1 h, gentamicin (50 μg/mL) was added, and 2 h later, cells were washed 6 times then lysed with RIPA buffer (25 mM Tris, pH 8, 150 mM NaCl, 0.1% SDS, 1% NP-40 plus cOmpleteTM cocktail protease inhibitors. The soluble and insoluble (bacterial-containing) fractions were separated by centrifugation before the addition of protein loading dye (final concentration: 10% glycerol, 50 mM Tris/HCl pH 6.8, 2% SDS, 0.02% bromophenol blue, 1% beta-mercaptoethanol). 60 μL of each fraction was loaded onto 12% SDS-PAGE gel for analysis. Proteins were transferred to nitrocellulose membranes and immunoblotted with mouse anti-FLAG (1:10,000) and anti-Beta actin (1:10,000). Unprocessed immunoblots can be found at DOI: 10.17632/wwzxwcwj62.1.
Liquid secretion assays
Liquid Secretion assays were performed as previously described3 with some modifications. Overnight cultures of E. coli grown in LB (Luria broth) were back diluted 1:50. Once cultures reached OD600 of 1.2-1.5, the bacteria were pelleted and resuspended in 2 mL of fresh media or PBS and incubated for the times indicated. IPTG (1 mM) was added when studying Ptrc regulated virB and/or nb. When indicated, bacteria were exposed to 10 μM Congo red. After designated periods of time, total cell and supernatant fractions were separated by centrifugation at 20,000g for 2 min. The cell pellet was taken as the whole cell lysate fraction. The supernatant fraction was subjected to a second centrifugation step to remove any remaining bacteria. To account for differences in bacterial titers, for each set of experiments, the volume of protein loading dye used to resuspend each sample was normalized by the OD600 reading of the slowest growing culture. For the time course assays, samples were not normalized. The pellet was resuspended in 100 μL or more protein loading dye, depending on the OD600. Proteins in the supernatant were precipitated with trichloroacetic acid (TCA) (10% v/v) and resuspended in 50 μL or more protein loading dye, depending on the OD600. Proteins resuspended in loading dye were incubated at 100°C for 15 min. Ten microliters of TCA-precipitated supernatant samples (20%) and five microliters of the pellet (5%) were loaded onto a 12% SDS-PAGE gel for analysis (i.e., the ratio of supernatant to pellet samples loaded was 3:2). In a typical experiment for an OD600 of 1.5, this equates to loading an OD600 of approximately 0.075 or 7.5x106 bacteria cell lysates (OD600/CFU conversion of 1*10^8), for pellet samples. Proteins were transferred to nitrocellulose membranes and immunoblotted with mouse anti-FLAG (1:10,000), mouse anti-HA (1:1000) or rabbit anti-GroEL (1:100,000). Unprocessed immunoblots can be found at DOI: 10.17632/wwzxwcwj62.1. When stated, SDS-PAGE gels were stained with GelCode™ Blue Stain Reagent, per the manufacturer’s instructions.
Plasmid retention assay
Bacterial cultures were back diluted daily for 7 days into LB media containing no antibiotics. Each day cultures were sampled and plated on LB media to quantify total bacteria and LB/ampicillin plates to quantify the percentage of bacteria that retained the plasmid Nb-expressing plasmid.
Stx2 binding assay
Bacterial supernatants were assessed for their ability to bind to Stx2 (0157) using an ELISA, as previously described 83. Briefly, 1 μg of Stx2 was coated onto high bind plates. After overnight incubation, the plates were washed in PBS-Tween 20 (0.05%) and blocked for 1 h using 4% milk/PBS-Tween 20 (0.05%) then serial dilutions of bacterial supernatants were added in a total volume of 100 μL. Following a 1 h incubation, the plates were washed, and HRP/anti-FLAG antibody added. After a 1 h incubation the plates were washed and developed using TMB substrate solution. After a 20 min incubation, the reaction was stopped using 1N HCL, and the plates read at 450 nm.
Vero cell cytotoxicity assay
Bacterial supernatants were assessed for their ability to neutralize Stx2 (0157) using an Stx2-induced cytotoxicity assay in Vero cells, as previously described84. Briefly, Vero cells were seeded on 96-well plates (5 × 104 cells/well). After overnight incubation, the culture medium was replaced with serial dilutions of bacterial supernatants prepared in RPMI media containing a final concentration of Stx2 (2 ng/mL), a dose known to kill more than 90% of the cells under the conditions used. After 48h, plates were developed by crystal violet staining and the absorbance at 590 nm was read.
Bacterial growth assays
Overnight cultures of E. coli strains grown in LB were back diluted 1:100, normalizing to the slowest growth culture. At the time points indicated, OD600 readings were recorded. For the in vitro competition assay, overnight cultures of E. coli strains grown in LB were diluted 1:100 and added at 1:1 ratio into fresh media. At the time points indicated, serial dilutions were plated on LB agar containing antibiotic selective for each of the strain and after 18 h at 37°C, CFUs were counted.
Solid plate secretion assay
Solid plate secretion assays were performed using a BMC3-BC pinning robot (S&P Robotics) as previously described. Briefly, single colonies grown overnight in 96 well plates were quad spotted onto a solid agar21. After overnight growth, a 384-pin tool is used to transfer equivalent amounts of bacteria to a media-containing plate over which a nitrocellulose membrane was immediately laid. After 6 h, the membrane was removed, washed to remove adherent bacteria, and immunoblotted for protein of interest. All incubations were carried out at 37°C
Fecal shedding assay.
Fecal pellets were collected and weighed. A 10x volume of PBS was added and the samples homogenized by pipetting and mashing using wide mouth pipette tips before being serially diluted and plated on MacConkey agar plates with antibiotics. After overnight incubation at 37°C colonies were counted and the total number of CFU estimated. On days where bacterial inoculations were performed, fecal pellets were sampled prior to inoculation.
In vitro luciferase monitoring
In a 96-well white plate, 1:100 dilutions of overnight bacterial cultures were incubated at 37°C with shaking for 5 h. Readings were performed on a SpectraMax i3x Multi-Mode Microplate Detection Platform (Molecular devices).
In vivo luminescence assays
To image luciferase-expressing bacteria in the GI tract, mice pre-treated for 1 day with kanamycin (1 g/L) and spectinomycin (2 g/L) in their drinking water were orally gavaged with 108 CFU of PROT3EcT-3 or EcN harboring the constitutive luciferase or MxiE reporter plasmids. After sacrificing the mice, the cecum, colon, and small intestine were harvested, the contents gently removed, and the tissues placed on a black mat for imaging using an IVIS Spectrum CT. Tissues were imaged using a luminescence filter, with field of view (FOV) = D (22.2 cm), fstop = 1 and large binning. Data was analyzed using Living Image Software 4.3.1.
Discovery and characterization of Nbs targeting murine and human TNFα.
Two alpacas were each immunized once with 200 μg murine (m)TNF-α in CpG/alum adjuvant, followed by four boosters with 100 μg mTNFα in alum adjuvant only. Two different alpacas were similarly immunized with human TNFα. Construction of the Nb display phage libraries from both alpaca pairs and panning and screening of the libraries were done as previously described83. Initial panning for anti-mTNFα Nb was performed with the target immobilized by coating to MaxiSorp Nunc-lmmuno tubes. Because the yield was low, panning was repeated with mTNFα bound to JTT-B10 Nb, a Nb obtained in the initial screen. This second panning yielded 20 unique mTNFα-binding Nb families. The anti-hTNF Nb was isolated by panning a 6His-tagged hTNFα immobilized using an anti-His-tag mAb. The coding sequences of representative members of each anti-TNFα family were introduced into a pET32b expression vector and expressed as thioredoxin, 6His, E-tag fusion proteins in E. coli Rosetta-gami 2 (DE3) pLacI as fusions to thioredoxin and to 6His to facilitate purification using standard Ni-IMAC chromatography methods, and a carboxyl-terminal E-tag for detection. Based on ELISA83, 11 unique anti-mouse and 1 unique anti-human TNF Nbs with 10 nM or better apparent affinities were selected for further analyses (Table S2).
A competition study was conducted to determine whether any of the other unique Nb bound to epitopes not recognized by JTT-B10 by performing replicate dilution ELISAs in which the only variation was that one set of ELISAs contained 20 μg/ml of the JTT-B10 Nb protein as a competitor in which the E-tag detection tag was replaced with a myc tag. The study identified three Nb families that bind to epitopes not competed by JTT-B10 (Table S2).
Quantification of PROT3EcT secreted NbmTNF
500 μL of bacterial culture supernatants were concentrated using Amicon® Ultra 0.5 mL Centrifugal Filters per the manufacturer’s protocol with modifications. Filters containing bacterial supernatants were centrifuged for 20 min at 2,500 g. Loading dye was added to the supernatant and 15 uL of this mixture was incubated at 100°C for 15 min before being run on a 12% SDS-PAGE gel. A purified Nb2xTNF at a known concentration was serially diluted in loading dye, boiled, and run alongside the concentrated bacterial supernatant samples. Gels were probed with GelCodeBlue Reagent and densitometry was performed using ImageJ to generate a standard curve of the Nb2xTNF standards from which the concentration of SSOspC2-Nb2xmTNF in bacterial supernatants was interpolated.
L929 cell cytotoxicity assay
Murine and human NbsTNF and bacterial supernatants were assessed for their ability to neutralize mTNFα or hTNFα using a TNFα-induced cytotoxicity assay in L929 cells, as previously described 85. Briefly, 100 μl/well of murine fibroblast L929 cells seeded in 96-well plates (5 × 104 cells/well). After overnight incubation, the culture medium was replaced with serial dilutions of bacterial supernatants or purified Nb prepared in RPMI media containing a final concentration of 1.0 μg/ml actinomycin D and 4 ng/mL murine or human TNF-α. Plates were then incubated at 37 °C for 24 h after which an MTT assay was performed as per the manufacturer’s instructions.
TNBS and DSS mouse models of colitis and treatment protocols
Time points and doses for all treatments and administrations are indicated in the Figures and text. TNBS was diluted to 20 mg/mL in ethanol (50% v/v) and 100 μl administered via enema by inserting a 3.5 French catheter 4 cm into the colon. DSS (3% w/v) was provided ad libitum in the drinking water for 5 days at the time points indicated in the Figures. Bacterial strains were prepared as described and administered via oral gavage or enema. Anti-TNF mAb was administered intraperitoneally (i.p.). Mice were euthanized by CO2 overdose. Upon sacrifice, blood was harvested by cardiac bleed, the GI tracts excised, and colon lengths measured. Blood was collected into serum separator tubes, spun for 5 min at 5000 rpm and serum stored at −20°C. The colon was cut longitudinally, the contents were removed, and the tissue dissected. Half of the tissue was fixed using 4% paraformaldehyde (PFA) overnight at 4°C for histology. The other half was homogenized in 1 mL of PBS containing 1x HALT protease inhibitor cocktail before being centrifuged for 10 min at 20,000 g and the supernatant stored at −20°C for later analysis by ELISA.
16S rRNA gene amplicon sequencing of stool
16S rRNA gene amplicon sequencing was performed on stool isolated from mice using protocols adapted from the Earth Microbiome Project, as previously described86. From extracted fecal DNA, the 16S rRNA V4 region was amplified by PCR with a single-indexing approach and indexing barcodes as listed in Table S5B. Sequencing was performed on a MiSeq instrument (Illumina, San Diego, CA) using 1x150bp paired-end protocol (Mid Output: 10M clusters) by the Molecular Biology Core Facilities (MBCF) at Dana-Farber Cancer Institute (DFCI). The quality of the reads was checked using FastQC (v0.11.5). Read pairs were imported to the QIIME2 environment (version 2021.2), where they were joined, denoised, and checked for chimeras using the DADA2 plug-in in prior to taxonomic assignment. Taxonomic assignment of each amplicon sequence variant (ASV) was performed using a pre-trained Naive Bayes classifier Tables S1A and S3A with the SILVA database (version 138.1). Differential abundance analysis of all ASVs was performed using MaAslin2 ASVs found in less than 10% of samples were not included in the testing. Each ASV was modeled as a function of treatment and sex (categorical variables), with caging as a random effect. ASVs with a corrected q-value of less than 0.2 are considered significant. Raw sequences were deposited in NCBI SRA databank, Bioproject PRJNA913459.
Cytokine and Nb ELISAs
The concentrations of mouse TNFα, IL-10, IL-6 were quantified by ELISA per the manufacturer’s instructions. The anti-Nb ELISA was performed as previously described85.
Histology
PFA-fixed colon tissue was transferred to 70% ethanol before processing by routine paraffin embedding, sectioning and H&E staining by the DF/HCC Rodent Histopathology Core. A pathologist (J.N.G.), blinded to experimental parameters, determined colitis scores. Each of the following four histologic parameters were scored as absent (0), mild (1), moderate (2), or severe (3): mononuclear cell infiltration, polymorphonuclear cell infiltration, epithelial hyperplasia, and epithelial injury. The scores for the parameters were summed to generate the cumulative histologic colitis score87. The cumulative histologic colitis score was then multiplied by an extent score, indicating the proportion (%) of colon involved by colitis: (1) < 10%; (2) 10%–25%; (3) 25%–50%; (4) > 50%. Images were captured at 10× or 40× magnification with a Nikon Eclipse NI-U and NSI-Element Basic Research software (Nikon).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using GraphPad Prism v.8.3.0 or MaAslin2. Details of statistical analysis and sample size are provided in the figure legends. No samples were excluded from any experiments performed in this study unless in the case of technical failure. Experimenters, with the exception of the pathologist, were not blinded to experimental conditions.
Supplementary Material
Table S2 mTNF Nb affinities (related to Figure 3)
Table S1 16S rRNA gene amplicon fecal survey data and analysis from C57BL/6 mice (related to Figure 3)
Table S3 16S rRNA gene amplicon fecal survey data and analysis from BALB/c mice (related to Figure 4)
Table S4 Plasmids and strains (related to STAR Methods)
Table S5 DNA and oligo sequences (related to STAR Methods)
KEY RESOURCES TABLE
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Anti-GroEL antibody, rabbit | Sigma | Cat#G6532 |
| Anti-FLAG, mouse (clone M2) | Sigma | Cat#F1804 |
| Anti-HA.11, mouse (clone 16B12) | Biolegend | Cat#901501 |
| Anti-Beta actin, mouse (clone AC-15) | Abcam | Cat#ab6276 |
| Anti-rabbit antibody | Jackson ImmunoResearch Laboratories | Cat#111-035-144 |
| Anti-mouse antibody | Jackson ImmunoResearch Laboratories | Cat#115-035-003 |
| Anti-TNFα, mouse (clone TN3-19.12) | BioxCell | Cat#BE0244 |
| Anti-his-tag mAb | Genescript | Cat#A00186 |
| Chemicals, Peptides and Recombinant Proteins | ||
| Congo red | Sigma Aldrich | Cat#C6277 |
| Recombinant Mouse TNFα | Biolegend | Cat#575206 |
| Recombinant Human TNFα | Biolegend | Cat#570106 |
| PicoLab® Mouse Diet 20 | Constant Nutrition | Cat#5058 |
| CpG/alum adjuvant | InvivoGen | Cat#2395 |
| Stx2 (0157) | Tufts University | N/A |
| His-tagged hTNFα | Genescript | Cat#TNA-H5228 |
| TNBS | Sigma | Cat#92822 |
| DSS | MP Biomedicals | Cat#160110 |
| Ambion UltraPure Buffer Saturated Phenol | Thermo Scientific | Cat#15513039 |
| Commercial assays | ||
| Mouse TNFα ELISA | Biolegend | Cat#430915 |
| Mouse IL-10 ELISA | Biolegend | Cat#431414 |
| Mouse IL-6 ELISA | Biolegend | Cat#431316 |
| Gibson Cloning Kit | New England Biolabs | Cat#E5510S |
| MTT assay | Trevigen | Cat#4890-25-K |
| TMB substrate solution | Biolegend | Cat#421501 |
| Amicon® Ultra 0.5 mL Centrifugal Filters | MerckMillipore | Cat# UFC5010BK |
| Deposited data | ||
| 16S rRNA gene amplicon sequencing. ASVs with a corrected q-value of < 0.2 were considered significant and deposited. | This paper | SRA BioProject: PRJNA913459 |
| Experimental Models: | ||
| Cell Lines | ||
| L929 | Gift of Marcia Goldberg | N/A |
| HCT8 | Gift of Marcia Goldberg | N/A |
| HeLa CCL2 | American Type Culture Collection (ATCC) | N/A |
| Mice | ||
| C57BL/6 | Jackson Labs | IMSR_JAX:000664 |
| BALB/c | Jackson Labs | IMSR_JAX:000651 |
| Bacterial Strains (see Table S4 for additional details) | ||
| Shigella flexneri | Gift of Marcia Goldberg | |
| E. coli DH10ß | Life Technologies | N/A |
| EcN | Mutaflor, Canada | Cat#18290015 |
| EcHS | Gift of Mark Goulian | N/A |
| E. coli HB101 | Gift of Stephen Lory | N/A |
| mT3Ec_Ipa-Mxi-Spa | Reeves et al.23 | N/A |
| mT3Ec_Mxi-Spa | Ernst et al.21 | N/A |
| mT3Ec_Mxi-Spa-VirBINT | This study | N/A |
| Stx2-mT3Ec_Mxi-Spa-VirBINT | This study | N/A |
| TNF-mT3Ec_Mxi-Spa-VirBINT | This study | N/A |
| EcN-LPatp/gid | This study | N/A |
| EcHS- LPatp/gid | This study | N/A |
| PROT3EcT-1 | This study | N/A |
| PROT3EcT-2 | This study | N/A |
| PROT3EcT-1- LPyie/trk | This study | N/A |
| PROT3EcT-3 | This study | N/A |
| PROT3EcT-4 | This study | N/A |
| Stx2-PROT3EcT-4 | This study | N/A |
| TNF-PROT3EcT-4 | This study | N/A |
| Oligonucleotides (see Table S5B) | ||
| Recombinant DNA (see Table S4 for additional details) | ||
| pDSW206-ccdB-MyoD NS | Reeves et al.23 | N/A |
| pDSW206-OspC2-FLAG | Schmitz et al.72 | N/A |
| pRK2073 | Leong et al.73 | N/A |
| pKD46 | Datsenko and Wanner74 | N/A |
| pCMD136-ccdB-FLAG | Mou et al.22 | N/A |
| pDSW206 | Weiss et al.75 | N/A |
| pDSW206-ccdB-FLAG | Schmitz et al.72 | N/A |
| pTKIP-hph | Kuhlman and Cox76 | N/A |
| pTKred | Kuhlman and Cox76 | Addgene #41066 |
| pTKLP-tetA | Tas et al.77 | Addgene #41062 |
| pMM534 | Mimee et al.44 | Addgene #71325 |
| pTSAR1Ud2.4s | Campbell-Valois et al.78 | Addgene #112530 |
| pCP20 | Datsenko and Wanner 74 | N/A |
| pKD4 | Datsenko and Wanner 74 | N/A |
| pMixE-lux | This study | N/A |
| pNG162-IpgC | This study | N/A |
| pDONR221 | Invitrogen | Cat#1253601 |
| pCGP-alr | This study | N/A |
| pmT3SA | Ernst et al.21 | N/A |
| pmT3SS | Reeves et al.23 | N/A |
| pNG162-virB | Reeves et al.23 | N/A |
| pCMD136-PompC-virB | This study | N/A |
| pCMD136-PvirF-virB | This study | N/A |
| pCMD136-PJ23119-virB | This study | N/A |
| pCMD136-PJ23115-virB | This study | N/A |
| pCGP-PJ23119-virB-Nb | This study | N/A |
| pTKIP-PJ23119-virB | This study | N/A |
| pENTR-OspC2ss-APC60 | This study | N/A |
| pENTR-OspC2ss-APC70 | This study | N/A |
| pDSW206-OspC2ss-APC60 | This study | N/A |
| pDSW206-OspC2ss-APC70 | This study | N/A |
| pDSW206-OspGss-MyoD | Reeves et al.23 | N/A |
| pDSW206-OspGss-Mef2C | Reeves et al.23 | N/A |
| pDSW206-OspCss-TALE | Reeves et al.23 | N/A |
| pENTR-OspC2ss | Reeves et al.23 | N/A |
| pENTR-IpaH4.5ss | Reeves et al.23 | N/A |
| pENTR-IpaH7.8ss | Reeves et al.23 | N/A |
| pENTR-IpaH9.8ss | Reeves et al.23 | N/A |
| pENTR-OspEss | Reeves et al.23 | N/A |
| pENTR-OspFss | Reeves et al.23 | N/A |
| pENTR-OspD3ss | Reeves et al.23 | N/A |
| pENTR-VirAss | Reeves et al.23 | N/A |
| pENTR-OspGss | Reeves et al.23 | N/A |
| pDSW206-ccdB-NbASC | Reeves et al.23 | N/A |
| pDSW206-ccdB-NbPD-L1 | This study | N/A |
| pDSW206-ccdB-NbCTLA4 | This study | N/A |
| pDSW206-ccdB-NbNP1 | This study | N/A |
| pDSW206-ccdB-NbASC | This study | N/A |
| pDSW206-OspC2ss-3xNbStx2 | This study | N/A |
| pDSW206-OspC2ss-2xNbStx2 | This study | N/A |
| pDSW206-OspC2ss-NbStx2 | This study | N/A |
| pDSW206-PJ23115-OspC2ss-2xNbStx2 | This study | N/A |
| pCGP-alr-PJ23115-OspC2ss-2xNbStx2 | This study | N/A |
| pCGP-alr-PJ23108-OspC2ss-2xNbStx2 | This study | N/A |
| pENTR-OspC2ss-NbmTNF | This study | N/A |
| pDSW206-OspC2ss-NbmTNF | This study | N/A |
| pENTR-OspC2ss-NbmTNF | This study | N/A |
| pDSW206-OspC2ss-2xNbmTNF | This study | N/A |
| pDSW206-PJ23115-OspC2-2xNbmTNF | This study | N/A |
| pCGP-alr-PJ23115-OspC2-2xNbmTNF | This study | N/A |
| pENTR-OspC2ss-2xNbhTNF | This study | N/A |
| pDSW206-OspC2ss-2xNbhTNF | This study | N/A |
| Software and Algorithms | ||
| GraphPad Prism 9 | GraphPad Software | https://www.graphpad.com/ |
| BioRender | BioRender Company | https://biorender.com/ |
| ImageJ | NIH | https://imagej.nih.gov/ |
| QIIME2 | Bolyen et al.79 | https://qiime2.org/ |
| DADA2 | Callahan et al.80 | https://benjjneb.github.io/dada2/ |
| MaAsLin 2 | Mallick et al.81 | https://github.com/biobakery/Maaslin2 |
| R | version 4.2.1 | N/A |
Highlights.
Engineering of PROT3EcT, E. coli outfitted with a programmable protein secretion system
PROT3EcT secrete functional nanobodies into their surroundings
Pretreating with Anti-TNFα secreting PROT3EcT limits colitis in a preclinical IBD model
Acknowledgements
We thank Dr. Pam Silver for critically reading the manuscript; Drs. Gökhan S. Hotami§ligil and Karen Inouye at the Harvard T. H. Chan School of Public Health for their assistance with the IVIS experiments; Dr. Markus Brown for assistance in the construction of APC clones and Sue Chapman for assistance in the L929/TNF assay; and The Molecular Biology Core Facilities at the Dana-Farber Cancer Institute for performing the amplicon sequencing. Some graphics were created with BioRender.com. Supported by NIH grants (AI064285, DK113599), a Kenneth Rainin Foundation grant and the Brit d’Arbeloff Research Scholar award to C.F.L, Massachusetts General Hospital Executive Committee on Research Fund for Medical Discovery Postdoctoral Fellowship Awards to C.G.P and A.Z.R., and an Endeavour Australia Research Fellowship and a Crohn’s & Colitis Foundation Research Fellow Award, award number 654758, to J.P.L and a contract from Vicero, Inc. The authors thank members of the Lesser, Garrett, Leong, Barczak and Goldberg labs for helpful discussions and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
C.F.L is on the scientific advisory board (SAB). J.P.L is an employee at AdAlta Ltd and A.Z.R is an employee and has equity at Synlogic Therapeutics. W.S.G is on the SABs of Kintai Therapeutics, SanaRx, Evelo Biosciences and Tenza. F.I.S. is a cofounder and shareholder of Odyssey Therapeutics and a shareholder of IFM Therapeutics. C.B.S is a scientific advisor, and V.K. is the CEO and co-founder of Vicero, Inc. PROT3EcT is the subject of US Patent No. 9,951,340 and US Patent 10,702,559, both filed by Massachusetts General Hospital.
Inclusion and Diversity
We support inclusive, diverse, and equitable conduct of research.
References
- 1.Basso PJ, Câmara NOS, and Sales-Campos H (2019). Microbial-Based Therapies in the Treatment of Inflammatory Bowel Disease – An Overview of Human Studies. Frontiers in Pharmacology 9. 10.3389/fphar.2018.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durack J, and Lynch SV (2019). The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med 216, 20–40. 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubillos-Ruiz A, Guo T, Sokolovska A, Miller PF, Collins JJ, Lu TK, and Lora JM (2021). Engineering living therapeutics with synthetic biology. Nat Rev Drug Discov 20, 941–960. 10.1038/S41573-021-00285-3. [DOI] [PubMed] [Google Scholar]
- 4.McNerney MP, Doiron KE, Ng TL, Chang TZ, and Silver PA (2021). Theranostic cells: emerging clinical applications of synthetic biology. Nature Reviews Genetics 22, 730–746. 10.1038/s41576-021-00383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riglar DT, and Silver PA (2018). Engineering bacteria for diagnostic and therapeutic applications. Nat Rev Microbiol 16, 214–225. 10.1038/nrmicro.2017.172. [DOI] [PubMed] [Google Scholar]
- 6.Lynch JP, Goers L, and Lesser CF (2022). Emerging strategies for engineering Escherichia coli Nissle 1917-based therapeutics. Trends Pharmacol Sci 43, 772–786. 10.1016/j.tips.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenborn U, and Schulze J (2009). The non-pathogenic Escherichia coli strain Nissle 1917 – features of a versatile probiotic. Microbial Ecology in Health and Disease 21, 122–158. 10.3109/08910600903444267. [DOI] [Google Scholar]
- 8.Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM, Kotula JW, Antipov E, Dagon Y, Denney WS, et al. (2019). An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med 11, eaau7975. 10.1126/scitranslmed.aau7975. [DOI] [PubMed] [Google Scholar]
- 9.Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, Millet YA, Anderson CL, Li N, Fisher AB, West KA, et al. (2018). Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol 36, 857–864. 10.1038/nbt.4222. [DOI] [PubMed] [Google Scholar]
- 10.Crook N, Ferreiro A, Gasparrini AJ, Pesesky MW, Gibson MK, Wang B, Sun X, Condiotte Z, Dobrowolski S, Peterson D, and Dantas G (2019). Adaptive Strategies of the Candidate Probiotic E. coli Nissle in the Mammalian Gut. Cell Host Microbe 25, 499–512 e498. 10.1016/j.chom.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, and Remaut E (2000). Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science (New York, N.Y.) 289, 1352–1355. 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 12.Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, Van Huysse J, Demetter P, Steidler L, Remaut E, et al. (2010). Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal immunology 3, 49–56. 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 13.Dalbey RE, and Kuhn A (2012). Protein Traffic in Gram-negative bacteria – how exported and secreted proteins find their way. FEMS Microbiology Reviews 36, 1023–1045. 10.1111/j.1574-6976.2012.00327.x. [DOI] [PubMed] [Google Scholar]
- 14.Danino T, Prindle A, Kwong GA, Skalak M, Li H, Allen K, Hasty J, and Bhatia SN (2015). Programmable probiotics for detection of cancer in urine. Sci Transl Med 7, 289ra284. 10.1126/scitranslmed.aaa3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Din MO, Danino T, Prindle A, Skalak M, Selimkhanov J, Allen K, Julio E, Atolia E, Tsimring LS, Bhatia SN, and Hasty J (2016). Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85. 10.1038/nature18930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurbatri CR, Lia I, Vincent R, Coker C, Castro S, Treuting PM, Hinchliffe TE, Arpaia N, and Danino T (2020). Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med 12. 10.1126/scitranslmed.aax0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piñero-Lambea C, Bodelón G, Fernández-Periáñez R, Cuesta AM, Álvarez-Vallina L, and Fernández LÁ (2015). Programming Controlled Adhesion of E. coli to Target Surfaces, Cells, and Tumors with Synthetic Adhesins. ACS Synthetic Biology 4, 463–473. 10.1021/sb500252a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Praveschotinunt P, Duraj-Thatte AM, Gelfat I, Bahl F, Chou DB, and Joshi NS (2019). Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat Commun 10, 5580. 10.1038/s41467-019-13336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfat I, Aqeel Y, Tremblay JM, Jaskiewicz JJ, Shrestha A, Lee JN, Hu S, Qian X, Magoun L, Sheoran A, et al. (2022). Single domain antibodies against enteric pathogen virulence factors are active as curli fiber fusions on probiotic E. coli Nissle 1917. PLoS Pathog 18, e1010713. 10.1371/journal.ppat.1010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du J, Reeves AZ, Klein JA, Twedt DJ, Knodler LA, and Lesser CF (2016). The type III secretion system apparatus determines the intracellular niche of bacterial pathogens. Proc Natl Acad Sci U S A 113, 4794–4799. 10.1073/pnas.1520699113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst NH, Reeves AZ, Ramseyer JE, and Lesser CF (2018). High-Throughput Screening of Type III Secretion Determinants Reveals a Major Chaperone-Independent Pathway. MBio 9. 10.1128/mBio.01050-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mou X, Souter S, Du J, Reeves AZ, and Lesser CF (2018). Synthetic bottom-up approach reveals the complex interplay of Shigella effectors in regulation of epithelial cell death. Proc Natl Acad Sci U S A 115, 6452–6457. 10.1073/pnas.1801310115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves AZ, Spears WE, Du J, Tan KY, Wagers AJ, and Lesser CF (2015). Engineering Escherichia coli into a protein delivery system for mammalian cells. ACS Synth Biol 4, 644–654. 10.1021/acssynbio.5b00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruano-Gallego D, Álvarez B, and Fernández L (2015). Engineering the Controlled Assembly of Filamentous Injectisomes in E. coli K-12 for Protein Translocation into Mammalian Cells. ACS Synth Biol 4, 1030–1041. 10.1021/acssynbio.5b00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchrieser C, Glaser P, Rusniok C, Nedjari H, D’Hauteville H, Kunst F, Sansonetti P, and Parsot C (2000). The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol Microbiol 38, 760–771. [DOI] [PubMed] [Google Scholar]
- 26.Venkatesan MM, Goldberg MB, Rose DJ, Grotbeck EJ, Burland V, and Blattner FR (2001). Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect Immun 69, 3271–3285. 10.1128/IAI.69.5.3271-3285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasakawa C, Adler B, Tobe T, Okada N, Nagai S, Komatsu K, and Yoshikawa M (1989). Functional organization and nucleotide sequence of virulence Region-2 on the large virulence plasmid in Shigella flexneri 2a. Molecular Microbiology 3, 1191–1201. 10.1111/j.1365-2958.1989. [DOI] [PubMed] [Google Scholar]
- 28.Ménard R, Sansonetti P, Parsot C, and Vasselon T (1994). Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79, 515–525. 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 29.Ménard R, Prévost MC, Gounon P, Sansonetti P, and Dehio C (1996). The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proceedings of the National Academy of Sciences 93, 1254–1258. 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves AZ, and Lesser CF (2016). Transfer of Large Contiguous DNA Fragments onto a Low Copy Plasmid or into the Bacterial Chromosome. Bio Protoc 6. 10.21769/BioProtoc.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahrani FK, Sansonetti PJ, and Parsot C (1997). Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun 65, 4005–4010. 10.1128/iai.65.10.4005-4010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa SC, Schmitz AM, Jahufar FF, Boyd JD, Cho MY, Glicksman MA, and Lesser CF (2012). A new means to identify type 3 secreted effectors: functionally interchangeable class IB chaperones recognize a conserved sequence. mBio 3. 10.1128/mBio.00243-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanco-Toribio A, Muyldermans S, Frankel G, and Fernández LÁ (2010). Direct Injection of Functional Single-Domain Antibodies from E. coli into Human Cells. PLOS ONE 5, e15227. 10.1371/journal.pone.0015227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt FI, Lu A, Chen JW, Ruan J, Tang C, Wu H, and Ploegh HL (2016). A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. J Exp Med 213, 771–790. 10.1084/jem.20151790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram JR, Dougan M, Rashidian M, Knoll M, Keliher EJ, Garrett S, Garforth S, Blomberg OS, Espinosa C, Bhan A, et al. (2017). PD-L1 is an activation-independent marker of brown adipocytes. Nat Commun 8, 647. 10.1038/s41467-017-00799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingram JR, Blomberg OS, Rashidian M, Ali L, Garforth S, Fedorov E, Fedorov AA, Bonanno JB, Le Gall C, Crowley S, et al. (2018). Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc Natl Acad Sci U S A 115, 3912–3917. 10.1073/pnas.1801524115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashour J, Schmidt FI, Hanke L, Cragnolini J, Cavallari M, Altenburg A, Brewer R, Ingram J, Shoemaker C, and Ploegh HL (2015). Intracellular expression of camelid single-domain antibodies specific for influenza virus nucleoprotein uncovers distinct features of its nuclear localization. J Virol 89, 2792–2800. 10.1128/jvi.02693-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremblay JM, Mukherjee J, Leysath CE, Debatis M, Ofori K, Baldwin K, Boucher C, Peters R, Beamer G, Sheoran A, et al. (2013). A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2. Infect Immun 81, 4592–4603. 10.1128/iai.01033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulissi U, Fabbretti A, Sette M, Giuliodori AM, and Spurio R (2014). Time-resolved assembly of a nucleoprotein complex between Shigella flexneri virF promoter and its transcriptional repressor H-NS. Nucleic Acids Res 42, 13039–13050. 10.1093/nar/gku1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morin CE, and Kaper JB (2009). Use of stabilized luciferase-expressing plasmids to examine in vivo-induced promoters in the Vibrio cholerae vaccine strain CVD 103-HgR. FEMS Immunol Med Microbiol 57, 69–79. 10.1111/j.1574-695X.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho CL, Tan HQ, Chua KJ, Kang A, Lim KH, Ling KL, Yew WS, Lee YS, Thiery JP, and Chang MW (2018). Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat Biomed Eng 2, 27–37. 10.1038/s41551-017-0181-y. [DOI] [PubMed] [Google Scholar]
- 42.Hwang IY, Koh E, Wong A, March JC, Bentley WE, Lee YS, and Chang MW (2017). Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat Commun 8, 15028. 10.1038/ncomms15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto M, Kunisawa A, Hattori T, Kawana S, Kitada Y, Tamada H, Kawano S, Hayakawa Y, Iida J, and Fukusaki E (2018). Free D-amino acids produced by commensal bacteria in the colonic lumen. Scientific Reports 8, 17915. 10.1038/s41598-018-36244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mimee M, Nadeau P, Hayward A, Carim S, Flanagan S, Jerger L, Collins J, McDonnell S, Swartwout R, Citorik RJ, et al. (2018). An ingestible bacterial-electronic system to monitor gastrointestinal health. Science (New York, N.Y.) 360, 915–918. 10.1126/science.aas9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham DB, and Xavier RJ (2020). Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 578, 527–539. 10.1038/s41586-020-2025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neurath MF (2017). Current and emerging therapeutic targets for IBD. Nature Reviews Gastroenterology & Hepatology 14, 269–278. 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 47.Sandborn WJ, and Loftus EV (2004). Balancing the risks and benefits of infliximab in the treatment of inflammatory bowel disease. Gut 53, 780–782. 10.1136/gut.2003.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, and Neurath MF (2017). Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 12, 1295–1309. 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 49.Scheiffele F, and Fuss IJ (2002). Induction of TNBS colitis in mice. Curr Protoc Immunol Chapter 15, Unit 15 19. 10.1002/0471142735.im1519s49. [DOI] [PubMed] [Google Scholar]
- 50.Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Büschenfelde KH, Strober W, and Kollias G (1997). Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol 27, 1743–1750. 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 51.Chassaing B, Aitken JD, Malleshappa M, and Vijay-Kumar M (2014). Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104, 15.25.11–15.25.14. 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimoto H, Nomura M, Kobayashi M, Mizumachi K, and Okamoto T (2003). Survival of lactococci during passage through mouse digestive tract. Can J Microbiol 49, 707–711. 10.1139/w03-092. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Derrien M, Levenez F, Brazeilles R, Ballal SA, Kim J, Degivry M-C, Quéré G, Garault P, van Hylckama Vlieg JET, et al. (2016). Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. The ISME Journal 10, 2235–2245. 10.1038/ismej.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKay R, Ghodasra M, Schardt J, Quan D, Pottash AE, Shang W, Jay SM, Payne GF, Chang MW, March JC, and Bentley WE (2018). A platform of genetically engineered bacteria as vehicles for localized delivery of therapeutics: Toward applications for Crohn’s disease. Bioeng Transl Med 3, 209–221. 10.1002/btm2.10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riglar DT, Giessen TW, Baym M, Kerns SJ, Niederhuber MJ, Bronson RT, Kotula JW, Gerber GK, Way JC, and Silver PA (2017). Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nature Biotechnology 35, 653–658. 10.1038/nbt.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aurand TC, and March JC (2016). Development of a synthetic receptor protein for sensing inflammatory mediators interferon-γ and tumor necrosis factor-α. Biotechnol Bioeng 113, 492–500. 10.1002/bit.25832. [DOI] [PubMed] [Google Scholar]
- 57.McMahon C, Baier AS, Pascolutti R, Wegrecki M, Zheng S, Ong JX, Erlandson SC, Hilger D, Rasmussen SGF, Ring AM, et al. (2018). Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nature Structural & Molecular Biology 25, 289–296. 10.1038/s41594-018-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmermann I, Egloff P, Hutter CA, Arnold FM, Stohler P, Bocquet N, Hug MN, Huber S, Siegrist M, Hetemann L, et al. (2018). Synthetic single domain antibodies for the conformational trapping of membrane proteins. Elife 7. 10.7554/eLife.34317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, and Szalay AA (2007). Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol 297, 151–162. 10.1016/j.ijmm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Yu M, Kim J, Ahn JH, and Moon Y (2019). Nononcogenic restoration of the intestinal barrier by E. coli–delivered human EGF. JCI Insight 4. 10.1172/jci.insight.125166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruano-Gallego D, Fraile S, Gutierrez C, and Fernandez LA (2019). Screening and purification of nanobodies from E. coli culture supernatants using the hemolysin secretion system. Microb Cell Fact 18, 47. 10.1186/s12934-019-1094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nougayrède J-P, Chagneau CV, Motta J-P, Bossuet-Greif N, Belloy M, Taieb F, Gratadoux J-J, Thomas M, Langella P, Oswald E, and Torres AG (2021). A Toxic Friend: Genotoxic and Mutagenic Activity of the Probiotic Strain Escherichia coli Nissle 1917. mSphere 6, e00624–00621. 10.1128/mSphere.00624-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science (New York, N.Y.) 338, 120–123. 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Fluber A, van Hoeck A, Wood HM, Nomburg J, Gurjao C, Manders F, Dalmasso G, Stege PB, et al. (2020). Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 580, 269–273. 10.1038/s41586-020-2080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olier M, Marcq I, Salvador-Cartier C, Secher T, Dobrindt U, Boury M, Bacquié V, Pénary M, Gaultier E, Nougayrède JP, et al. (2012). Genotoxicity of Escherichia coli Nissle 1917 strain cannot be dissociated from its probiotic activity. Gut microbes 3, 501–509. 10.4161/gmic.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massip C, Branchu P, Bossuet-Greif N, Chagneau CV, Gaillard D, Martin P, Boury M, Sécher T, Dubois D, Nougayrède J-P, and Oswald E (2019). Deciphering the interplay between the genotoxic and probiotic activities of Escherichia coli Nissle 1917. PLOS Pathogens 15, e1008029. 10.1371/journal.ppat.1008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalantari A, James MJ, Renaud LA, Perreault M, Monahan CE, McDonald MN, Hava DL, and Isabella VM (2023). Robust performance of a live bacterial therapeutic chassis lacking the colibactin gene cluster. PLoS One 18, e0280499. 10.1371/journal.pone.0280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller BM, and Bäumler AJ (2021). The Habitat Filters of Microbiota-Nourishing Immunity. Annual Review of Immunology 39, 1–18. 10.1146/annurev-immunol-101819-024945. [DOI] [PubMed] [Google Scholar]
- 69.Russell BJ, Brown SD, Siguenza N, Mai I, Saran AR, Lingaraju A, Maissy ES, Dantas Machado AC, Pinto AFM, Sanchez C, et al. (2022). Intestinal transgene delivery with native E. coli chassis allows persistent physiological changes. Cell 185, 3263–3277 e3215. 10.1016/j.cell.2022.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell BJ, Brown SD, Saran AR, Mai I, Lingaraju A, Siguenza N, Maissy E, Dantas Machado AC, Pinto AFM, Miyamoto Y, et al. (2021). Intestinal Transgene Delivery with Native E. coli Chassis Allows Persistent Physiological Changes. bioRxiv, 2021.2011.2011.468006. 10.1101/2021.11.11.468006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee JW, Chan CTY, Slomovic S, and Collins JJ (2018). Next-generation biocontainment systems for engineered organisms. Nat Chem Biol 14, 530–537. 10.1038/s41589-018-0056-x. [DOI] [PubMed] [Google Scholar]
- 72.Schmitz AM, Morrison MF, Agunwamba AO, Nibert ML, and Lesser CF (2009). Protein interaction platforms: visualization of interacting proteins in yeast. Nat Methods 6, 500–502. 10.1038/nmeth.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leong SA, Ditta GS, and Helinski DR (1982). Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. Journal of Biological Chemistry 257, 8724–8730. 10.1016/s0021-9258(18)34188-7. [DOI] [PubMed] [Google Scholar]
- 74.Datsenko KA, and Wanner BL (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss DS, Chen JC, Ghigo JM, Boyd D, and Beckwith J (1999). Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J Bacteriol 181, 508–520. 10.1128/JB.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhlman TE, and Cox EC (2010). Site-specific chromosomal integration of large synthetic constructs. Nucleic Acids Res 38, e92. 10.1093/nar/gkp1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tas H, Nguyen CT, Patel R, Kim NH, and Kuhlman TE (2015). An Integrated System for Precise Genome Modification in Escherichia coli. PLoS One 10, e0136963. 10.1371/journal.pone.0136963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campbell-Valois FX, Schnupf P, Nigro G, Sachse M, Sansonetti PJ, and Parsot C (2014). A fluorescent reporter reveals on/off regulation of the Shigella type III secretion apparatus during entry and cell-to-cell spread. Cell Host Microbe 15, 177–189. 10.1016/j.chom.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37, 852–857. 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, and Holmes SP (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13, 581–583. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, Tickle TL, Weingart G, Ren B, Schwager EH, et al. (2021). Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol 17, e1009442. 10.1371/journal.pcbi.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salis HM, Mirsky EA, and Voigt CA (2009). Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27, 946–950. 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaskiewicz JJ, Tremblay JM, Tzipori S, and Shoemaker CB (2021). Identification and characterization of a new 34 kDa MORN motif-containing sporozoite surface-exposed protein, Cp-P34, unique to Cryptosporidium. Int J Parasitol 51, 761–775. 10.1016/j.ijpara.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tremblay JM, Mukherjee J, Leysath CE, Debatis M, Ofori K, Baldwin K, Boucher C, Peters R, Beamer G, Sheoran A, et al. (2013). A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2. Infect Immun 81, 4592–4603. 10.1128/IAI.01033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baarsch MJ, Wannemuehler MJ, Molitor TW, and Murtaugh MP (1991). Detection of tumor necrosis factor alpha from porcine alveolar macrophages using an L929 fibroblast bioassay. J Immunol Methods 140, 15–22. 10.1016/0022-1759(91)90121-u. [DOI] [PubMed] [Google Scholar]
- 86.Lobel L, Cao YG, Fenn K, Glickman JN, and Garrett WS (2020). Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science (New York, N.Y.) 369, 1518–1524. 10.1126/science.abb3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, and Glimcher LH (2007). Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45. 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2 mTNF Nb affinities (related to Figure 3)
Table S1 16S rRNA gene amplicon fecal survey data and analysis from C57BL/6 mice (related to Figure 3)
Table S3 16S rRNA gene amplicon fecal survey data and analysis from BALB/c mice (related to Figure 4)
Table S4 Plasmids and strains (related to STAR Methods)
Table S5 DNA and oligo sequences (related to STAR Methods)
Data Availability Statement
Illumina sequences obtained in the present study were deposited in the Sequence Read Archives (SRA) NCBI database under accession number PRJNA913459 and are currently available. Original western blot images have been deposited at Mendeley and are publicly available as of the date of publication and are currently available at DOI: 10.17632/wwzxwcwj62.1.
No original code was generated for these studies.
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.


