Abstract
Biodegradable bone adhesives represent a highly sought-after type of biomaterial which would enable replacement of traditional metallic devices for fixation of bone. However, these biomaterials should fulfil an extremely large number of requirements. As a consequence, bone-adhesive biomaterials which meet all of these requirements are not yet commercially available. Therefore, this comprehensive review provides an extensive overview of the development of bone adhesives from a translational perspective. First, the definition, classification, and chemistry of various types of bone adhesives are highlighted to provide a detailed overview of this emerging class of biomaterials. In this review we particularly focused studies which describe the use of materials that are capable of gluing two pieces of bone together within a time frame of minutes to days. Second, this review critically reflects on i) the experimental conditions of commonly employed adhesion tests to assess bone adhesion and ii) the current state-of-the-art regarding their preclinical and clinical applicability.
Keywords: Bone adhesive, Bone glue, Biomaterials, Orthopedic surgery, Bone fracture
Graphical abstract
Highlights
-
•
Biodegradable bone-adhesives can be effective alternatives for replacing conventional methods for fixation of bone fractures.
-
•
An adhesive which could provide bone-to-bone adhesion via formation of a load-bearing bond is not yet commercially available.
-
•
This review provides a comprehensive overview of the current state-of-the-art of bone adhesives.
1. Introduction
Bone tissue has a dynamic structure where old or damaged tissue is replaced by new bone tissue via a process called remodeling. Bone remodeling is a delicately regulated process where local and systemic factors influence bone formation and resorption performed by bone cells referred as to osteoblasts and osteoclasts, respectively [1]. Osteoblasts form bone tissue by producing proteins of the bone matrix and inducing mineralization. On the other hand, osteoclasts resorb bone tissue by solubilizing the mineral and digesting the bone matrix. The third type of bone cell, osteocytes, are derived from differentiation of osteoblasts and act as mechanosensors which are involved in the regulation of the bone remodeling process. Any imbalance between bone resorption and formation may lead to medical conditions which results in a fractured bone.
Numerous surgical procedures are performed each year to cure injuries or medical conditions which require fixation of fractured bones. The conventional method of fixation involves the use of metal plates, pins, screws etc. to stabilize the fracture [2]. However, these non-degradable devices need to be removed during a second surgery. In addition, metallic fixation devices are associated with a high risk of infection, damage to surrounding tissue, and refracture due to stress shielding. Finally, conventional methods are not effective for treatment of small bone fragments or periarticular fractures.
The application of a biodegradable bone adhesive would be a simple and effective alternative solution for fixation of bone fractures [3,4]. Application of such a biodegradable bone adhesive would eliminate the need for a second surgery to remove metallic fixation devices and reduce the risk of infection and other complications. In addition, self-hardening adhesives which can initially flow and penetrate onto the substrate surface and subsequently solidify automatically create a larger contact area between adhesive and substrate, thereby enhancing the amount of molecular interactions and improving the ultimate adhesive strength. The large contact area of glues, which strongly contrasts the smaller focal contacts of metallic pins, could also decrease the occurrence of refractures due to stress shielding. In such way, biodegradable bone adhesives would be much more effective as compared to the use of conventional metallic fixation devices for e.g. treatment of comminuted fractures.
To date, no generally accepted definition of a bone adhesive has been proposed. Herein, we define bone adhesives as follows:
Bone adhesives are biocompatible materials which provide bone-to-bone and/or bone-to-implant adhesion via formation of a robust and durable load-bearing bond within a reasonable timeframe under wet conditions and eventually degrade into nontoxic materials at a rate compatible with new bone formation.
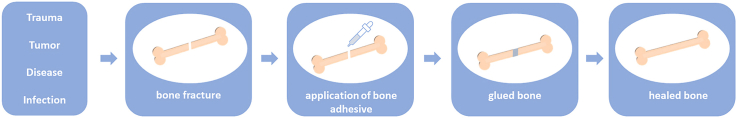
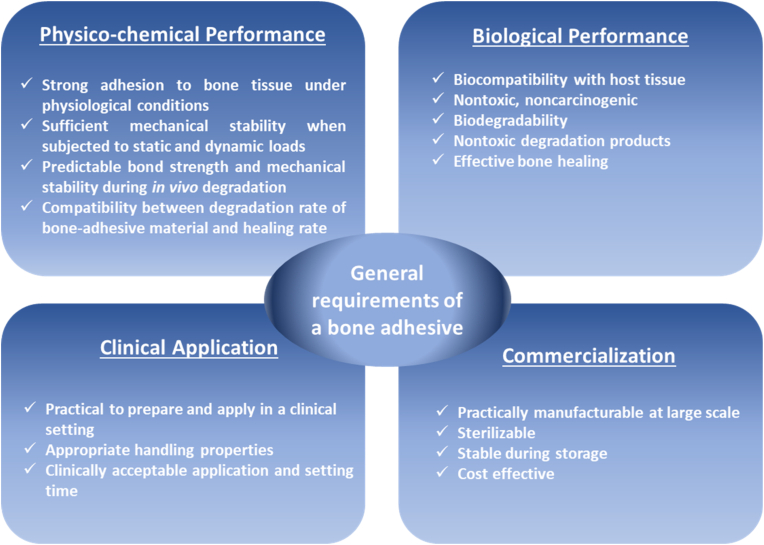
From the above definition, it is evident that bone adhesives should meet multiple requirements to function adequately in clinical practice (Fig. 1) [5]. First of all, the adhesive should exhibit strong adhesion to bone tissue in the presence of moist and blood. The minimum bond strength to bone for effective clinical practice was reported as 200 kPa by Weber and Chapman [6]. According to their experience, it was not possible to achieve effective bone fracture fixation with lower bond strength values. In addition, bone adhesives should maintain their bond strength for several weeks to months, and display sufficient mechanical stability when subjected to static and dynamic loads. The employed biomaterials and their degradation by-products should be nontoxic and biocompatible. The bond strength and mechanical stability should remain reliable and predictable during in vivo degradation of the adhesive, and the rate of degradation and bone healing should be compatible. Moreover, preparation and application of these adhesives to the surgical site should be facile within a clinically acceptable timeframe. Finally, the adhesive should be easily manufacturable and sterilizable, retain its functionality during storage, and be cost effective.
Fig. 1.
Application of a bone adhesive for bone fracture repair.
Currently, no commercially available product can be regarded as a “true” bone adhesive fulfilling all of the above requirements. Commercial bone cements and bone void fillers do not form strong chemical bonds with bone, but only adhere to bone by mechanical interlocking, which results in weak adhesion strength. In contrast, soft tissue adhesives are already commercially available, which do adhere to soft tissue by formation of such strong chemical bonds. Unfortunately, these soft tissue adhesives are much weaker than the strength required for fixation of bone, since soft tissues experience much lower stresses than bone. This discrepancy emphasizes the main challenge for the development of effective bone adhesives, i.e. to achieve a combination of high adhesive and cohesive strength without compromising crucial requirements such as degradability and biocompatibility. Bone adhesive materials offer significant promise for bone fracture repair, but maintaining effective adhesive strength under wet conditions is one of the main challenges to overcome. Other major hurdles include the lack of standardized tests for performance evaluation, upscaling of production, and the complicated market approval process. Although several formulations have previously reached clinical testing phases, none of them has met the large number of requirements associated with effective and safe clinical usage of bone adhesives. However, there has been a tremendous effort on development of bone adhesive materials in the past years and recent progress in biomaterials science has created new hope for translation of novel bone adhesives into clinical practice [[7], [8], [9], [10]].
The main aim of this comprehensive review is to provide an overview of the state of the art regarding bone adhesive biomaterials from a chemical perspective. In this study, we focused on short-term bone adhesion based on instantaneous formation of molecular bonds between living bone and bone adhesives capable to achieve direct bone adhesion after surgical application. In other words, we included publications which describe use of materials that can glue two pieces of bone together, either ex vivo or in vivo, within a time frame of minutes to days. This fundamentally differs from long-term bone-bonding behavior observed after weeks to months of in vivo implantation of biomaterials in osseous skeletal sites. In such cases, long-term bone-bonding behavior is achieved via implantation of bone substitutes, bone fillers or bone tissue engineering scaffolds which primarily aim to aid bone healing and induce bone formation. These latter cases of in vivo bone bonding behavior were out of the scope of the current review.
Recent progress in development of bone adhesives are covered from a translational perspective by highlighting basic research on chemical formulations of various types of bone adhesives, followed by evaluation of their preclinical and clinical performance. Since standardized testing of bone adhesion has not yet been established, we have also included the experimental conditions of commonly employed bone adhesion tests in terms of test type, test substrate (type of bone), adhesion/storage conditions before testing, and the obtained maximum adhesion strength values. Preclinical and clinical applications reported in the literature are also summarized and critically discussed to provide an extensive overview. Finally, the main challenges during translation of bone adhesives from bench to bedside and future research trends regarding the development of bone adhesives are summarized.
2. Chemical composition of bone adhesives
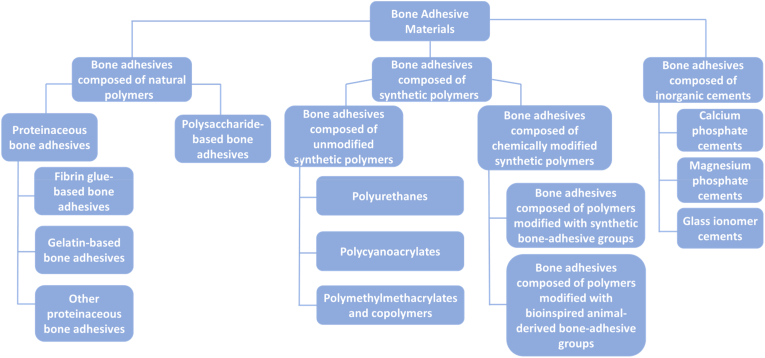
The adhesive materials discussed in this review are divided into three main categories, i.e. i) natural polymers, ii) synthetic polymers and iii) inorganic cements. These main categories and subcategories of these adhesive materials are shown in Fig. 2.
Fig. 2.
Chemical composition of bone adhesives.
Natural polymers, sometimes referred to as biopolymers, are derived from living organisms such as plants or animals. Natural polymers have been extensively explored for use in medical applications as bone adhesives, sealants or hemostats. Adhesives composed of natural polymers generally exhibit superior biocompatibility, degradability, and non-immunogenicity as compared to synthetic adhesives [11]. However, they suffer from low mechanical strength. Generally, natural polymers used for medical adhesives can be categorized as either proteinaceous materials (e.g. fibrin glue, gelatin-based materials) or polysaccharide-based materials (e.g. chitosan, starch, dextran, alginate). Bone adhesives composed of proteinaceous biomaterials have already showed promising performance levels in preclinical studies and have been clinically tested for fixation of bone fractures. On the other hand, bone adhesives made of polysaccharide-based materials are still in early stages of research and development.
Synthetic polymers are man-made polymers prepared via chemical reactions. These polymers enable versatile and tailored design by chemical functionalization via various synthesis strategies. They generally exhibit high mechanical strength as compared to natural polymers, which render them more suitable for load-bearing applications. However, their biocompatibility is usually inferior to natural polymers, and they often generate heat during polymerization which may negatively affect surrounding tissues. Herein, synthetic polymers used as adhesives for bone reconstructive surgery are sub-divided into two main categories, i.e. i) unmodified synthetic polymers (polyurethanes, polycyanoacrylates, polymethylmethacrylates), and ii) synthetic polymers modified with bone-adhesive functional groups (see Table 1 for specific details on their bone adhesion strength as well as (pre)clinical status).
Table 1.
Bone adhesion tests, preclinical and clinical applications of bone adhesive materials.
| Composition of Adhesive Material | Bone Adhesion Tests |
Preclinical Studies | Clinical Studies | |||
|---|---|---|---|---|---|---|
| Type of Test | Test Substrate | Adhesion/Storage Conditions | Adhesion Strength (kPa) | |||
| Fibrin glue | pull-off | bovine cancellous bone | R.T.1, 10 min, held in compression | 5–17 [6] | • Fixation of osteochondral fractures in a dog radial head and femoral condyle model [12,13] • Fixation of osteochondral fractures in a rabbit knee model [14] • Fixation of osteochondral fractures in a rabbit lateral condyle model [15] |
• Fixation of osteochondral fractures involving proximal interphalangeal joint [16] • Fixation of fractures of intercondylar distal humerus [17] • Stabilization of bone fragments in comminuted nasal fractures [18] • Stabilization of bone fragments in inferior orbital wall fractures [19,20] • Stabilization of bone fragments in anterior wall of the maxillary sinus fractures [21,22] • Fixation of displaced comminuted fractures of the head of the radius [23] • Fixation of displaced fractures of the humeral capitulum [24] • Fixation of various chondral and osteochondral fragments in lateral femoral condyle, medial surface of patella, talar trochlea, head of radius, capitellum of humerus, MCP joint [25] • Fixation of large osteochondral fractures of the patella [26] • Fixation of osteochondral talar fractures [27] • Reattachment of the delaminated chondral surface to the subchondral bone beneath it [28] |
| pull-off | porcine cortical bone | R.T., 1–2 min, held firmly between paper towels soaked in PBS/R.T., 1 h | 11 [29] | |||
| Gelatin/resorcinol/formaldehyde (GRF) | pull-off | porcine bone | R.T., 1–2 min, held firmly between paper towels soaked in PBS/R.T., 1 h | 200 [29] | – | – |
| Carbodiimide crosslinked gelatin and alginate loaded with hydroxyapatite (HA) and tricalcium phosphate (TCP) | pull-off | cortical parts of bovine femurs | 37 °C, 30 min, 100% humidity, under load | 26–71 [30] | – | – |
| Tyrosinase modified soybean protein | lap shear | porcine bone | R.T., 20 min, fixed with rubber band/25 °C, 15 min, in calcium chloride followed by 25 °C, 48 h in incubator | 400 [31] | – | – |
| Covalently (glutaraldehyde) and/or ionically (CaCO3/hydroxyapatite)-crosslinked chitosan-based hydrogels | pull-off | cancellous bovine humerus bone | R.T., 24 h/no storage or 37 °C, 5 min, in water | 72–260 [32] | – | – |
| Glutaraldehyde crosslinked chitosan | pull-off | cancellous bovine humerus bone | R.T., 24 h or 37 °C, 24 h, in water |
Dry 200 ± 60 [33] wet 24 ± 3.6 [33] | – | – |
| lap shear | dry 220 ± 90 [33] wet 31 ± 7 [33] |
– | – | |||
| Catechol−chitosan/zeolitic imidazolate framework-8 nanoparticles | pull-off | bovine cortical bone | Pressured, 37 °C, overnight, in PBS | 310–770 [34] | • Stabilization of bone graft material in a rat cranial model [34] | – |
| DOPA-functional two component system based on chitosan and dextran or starch | tear-off | bovine cortical bone | 37 °C, 3 h, in physiologic saline solution under constant pressure | 180–410 [35] | – | – |
| Oxidized dextran-gelatin network/amine-modified mesoporous bioactive glass nanoparticles | pull-off | porcine femur bone | 37 °C, 10 min/37 °C, overnight, in PBS | 127 ± 10 [36] | • Fixation of a comminuted radius fracture in a rabbit model [36] | – |
| lap shear | 108 ± 8 [36] | |||||
| Ostamer (trihydroxy resin/diisocyanate polyurethane) | – | – | – | – | • Fixation of defects in dog radius and femur models [37] | • Acute fractures of the tibia, non-unions and pathologic fractures of the femur [37] |
| Various polyurethane- based adhesive formulations | pull-off | bovine rib | no information | 1340 ± 106 [38] | • Fixation of bones in a frog hind limb tarsus model [39] • Reunion of a bone fragments in a dog tibia model [40] • Fixation of osteotomies in a rabbit ilium model [41] |
– |
| pull-off | porcine rib | 37 °C, 24 h, wrapped in phosphate buffered saline-soaked gauze | 690–1250 [41] | |||
| Kryptonite (castor oil derived polyurethane/calcium carbonate) | – | – | – | – | – | • Augmentation conventional wire cerclage in primary sternal closure [[42], [43], [44]] • Late sternal re-entry after use of Kryptonite [45] • Fixation of vertebral fractures [46] • Sealing of retrograde cavities [47] • Midface reconstruction [48] |
| N-butyl-2-cyanoacrylate (Histoacryl) | pull-off | compact femoral or tibial bovine bone | R.T., 3 min under pressure/R.T. or 37 °C, 24 h, in water | 12100 [49] | – | – |
| Isobutyl-2-cyanoacrylate (Bucrylat) | 7900 [49] | |||||
| Ethyl-2-cyanoacrylate | 7400 [49] | |||||
| N-butyl-2-cyanoacrylate | pull-off | human cadaver parietal bone | 37 °C, 10–20 min, under humid conditions | 2180 ± 700 [50] | • Fixation of mandibular angulus fracture in an ovine model [51] • Fixation of osteotomies in the craniofacial skeleton of a miniature pig craniofacial model [52] • Fixation of osteotomies using bone grafts in rabbit thoracic limb model [53] • Fixation of fractures in a rat segmental tibia model [54] • Fixation of displaced zygomatic bone fractures in a rabbit model [55] • Fixation of the craniofacial skeleton in a rabbit model [56] • Fixation of osteotomized cranial bone fragments in a pig model [57] • Fixation of mandibular osteotomies in a rabbit model [58] • Fixation of onlay cortical bone block grafts on mandibles in a rabbit model [59] • Fixation of osteotomies in cadaver parietal bone segments in a sheep model [60] |
• Fixation of mandibular fractures [61] • Fixation of a talar osteochondral fracture [62] • Stabilization of bone flaps in the skull [63] • Adjunct to interosseous wiring and miniplate fixation in facial bone fractures (comminuted fractures of the orbital roof, frontal sinus, maxillary anterior wall and a skull defect) [64] • Fixation of fractures in the orbital-maxillo-zygomatic complex [65] |
| lap shear | 1970 ± 220 [50] | |||||
| 2-octyl-2-cyanoacrylate | pull-off | human cadaver parietal bone | 37 °C, 10–20 min, under humid conditions | 1080 ± 220 [50] | • Autogenous bone graft fixation of calvaria in a rat model [66] • Fixation of osteochondral plugs in a rat hind leg model [67] |
– |
| lap shear | 1080 ± 140 [50] |
|||||
| Ethyl-2-cyanoacrylate (Super Bonder®) | lap shear | pig jaw samples | R.T., 20–30 s, under compression/R.T., 60 s | 1160 ± 430 [68] | – | – |
| N-butyl-2-cyanoacrylate (Histoacryl®) | 1220 ± 500 [68] | |||||
| PMMA | pull-off | bovine cancellous bone | R.T., 10 min, held in compression | 1010 [6] | – | – |
| PMMA | pull-off | bovine femoral or tibial bone | R.T., manually pressed | 350 [69] | – | – |
| Various lactide-methacrylate systems | – | – | – | – | • Augmentation of K-wires in stabilization of monocondylar osteotomy of the distal femur in a rabbit model [70] • Stabilization of osteotomies of the metaphyseal ulna in a ovine model [71] • Augmentation of screws in the stabilization of lateral tibial condyle in an ovine model [72] |
– |
| Lactide-methacrylate systems reinforced with inorganic particles | lap shear | bovine bone | 25 °C, 1–10 min | 3100–13900 [73] | • Reimplantation of proximal tibia and distal femur in a rabbit model [74] | – |
| 25 °C, 1–10 min/37 °C, 24 h, SBF | 300–7700 [73] | |||||
| Crosslinked polypropylene fumarate (PPF)/HEMA/nanobioactive glass particles | lap shear | sheep rib trabecular bone | R.T., 2 min/37 °C, 1 d, wrapped in PBS-soaked gauze | 6000 - 9000 [75] | – | – |
| 4-META/MMA/TBB cement | pull-off | human femur -metal adherend | R.T., 30 min/37 °C, 24 h, in water | > 7000 [76] | • Stabilization of Ti implant-tibial bone interface in a dog model [77] • Stabilization of stainless-steel implant- femoral bone interface in a rabbit model [78] |
– |
| Clearfill New Bond (Bis-GMA, HEMA, MDP, hydrophobic monomer, initiator) | pull-off | porcine mandible bone | Photopolymerized for 60 s | 6390 [79] | – | – |
| Adper PLP/Relyx, Optibond/Maxcem, AdheSE/Multilink, G-Bond/GCem | lap shear | bond porcine mandibular bone | Cured according to the manufacturers' specifications/R.T., 15 min | 2100–4830 [80] | – | – |
| Alendronate-functional poly (2-oxazoline) dual crosslinked hydrogel | pull-off | porcine bone | R.T., 2 min, pre-wetted with a PBS-soaked gauze | dry 116 wet 47 [81] | – | – |
| Interpenetrating network of PEGDMA matrix/isocyanate functional star-shaped prepolymers/biodegradable ceramic fillers | lap shear | bovine femur | 1 h/37 °C, 1 d or 7 d, PBS | 170–250 [82] | – | – |
| DOPA and l-lysine containing biomimetic copolypeptides | lap shear | porcine bone | 25 °C, 12 h or 37 °C, 0.5 h followed by 37 °C, 12 h, in water | dry 295 wet 155 [83] |
– | – |
| DOPA-functional polypeptide−pluronic−polypeptide triblock copolymers | pull-off | porcine femur bone | 2 h–24 h | 50–290 [84] | • Fixation of tibial osteotomy in a rat model [84] | – |
| DOPA-functional polypeptides | pull-off | porcine bone | 24 h | 600 [85] | • Fixation of tibial osteotomy in a rat model [85] | – |
| DOPA-functional hyperbranched polypeptides | pull-off | porcine femur bone | 24 h | 790 [86] | • Fixation of tibial osteotomy in a rat model [86] | – |
| DOPA-functional chitosan-graft-polypeptides | pull-off | porcine femur bone | 24 h, 37 °C, in PBS | 640 [87] | • Fixation of tibial osteotomy in a rat model [87] | – |
| Lap shear | Aluminium adherend | 3000 [87] | ||||
| DOPA and citric acid functional PEG/hydroxyapatite | lap shear | chicken bone | R.T., 2 h, wet | 110 [88] | • Fixation of comminuted radial fracture in a rabbit model [88] | – |
| R.T., 48 h, dry | 740 [88] | |||||
| Catechol-functional methacrylate/N-vinylcaprolactam copolymer-based hydrogels | pull-off | chicken bone plates | 60 °C, 2 min/5 h or 18 h | 5 h 776 ± 87 18 h 696 ± 91 [89] | – | – |
| Adipic dihydrazide-modified poly (l-glutamic acid)/catechol and aldehyde dual modified alginate | – | porcine bone | 37 °C, 24 h in 0.01 M PBS | Not quantified. Survived flushing for 5 min under the water [90] | – | – |
| Catechol-functional hyperbranched PEG-based coacervates | lap shear | bone (not specified) | R.T., 12 h, in water | 270 [91] | – | – |
| Synthetic polyelectrolytes condensed into liquid complex coacervatesSynthetic polyelectrolytes condensed into liquid complex coacervates | lap shear | Wet bovine cortical bone | 37 °C, 24 h, 100% humidity | 100 [92] | – | – |
| Complex coacervates made from amine modified gelatin/polyphosphodopamide/Ca2+ or Mg2+ | lap shear | wet aluminium adherend | 37 °C, 24 h, in water | 760 [93] | • Fixation of cranial fractures in a rat model [94] | – |
| Phosphoserine-valine poly (ester urea) copolymers | lap shear | cortical bovine bone | R.T, 1 h & 75 °C, 24 h/kept in PBS-soaked gauze, 37 °C, 24 h & at 95% humidity, 2 h | 400 ± 200 [95] | – | – |
| aluminium adherend |
R.T, 1 h followed by 75 °C, 24 h | 1170 ± 190 [95] | ||||
| Biomimetic polypeptide | pull-off | bovine bone | 3 days at 23 °C | 696 [96] | – | – |
| Phosphoserine modified tetracalcium phosphate cement | lap shear | cortical & cancellous porcine bone | R.T., 10 min/kept in PBS, 37 °C, 48 h | > 1000 [97] | • Fixation of critical size distal femur defects in a rabbit model [97] • Stabilization of dental implants in oversized osteotomies in a canine model [98] • Cranial bone flap fixation in an ovine model [99] |
– |
| porous and polished titanium | R.T., 10 min/kept in PBS, 37 °C, 48 h | > 3000 [97] | ||||
| Phosphoserine modified α-tricalcium phosphate cement | lap shear | cortical bovine bone | in water, 37 °C, 24 h |
4000 [100] | • Development of a preclinical murine distal femoral bone model for evaluation of adhesives in osseous and osteochondral tissue reconstruction [101] • Subcutaneous implantation in a rat model to assess in vivo safety [102] |
– |
| Modified magnesium phosphate cement: farringtonite/phytic acid | lap shear | bovine cortical bone | R.T., 10 min | 1220 ± 410 [103] | – | – |
| R.T., 10 min/37 °C, 7 d, PBS at | 810 ± 120 [103] | |||||
| Osteocrete: magnesium phosphate cement | – | – | – | – | • Tendon-to-bone healing in anterior cruciate ligament reconstruction in a rabbit model [104] • Fixation of Y-shaped osteotomy of the second and fourth metatarsal bones in a horse model [105] • Stabilization of bone-screw interfaces in the third metacarpal and third metatarsal bones in a horse model [106] • Stabilization of dental implants in a dog model [107] • Treatment of critical-sized skull defects and cementing bone flaps in rabbit models [108] • Tendon-to-bone healing in a dog model [109] |
– |
| Aluminium-free glass ionomer cements | – | – | – | – | • Fixation and stabilization of sternum, distal radius and percutaneous upper extremities in human cadaveric models [110,[111], [112], [113], [114]] | – |
Finally, inorganic cements are self-curing materials which are used to fill cavities or provide fixation of bones. These cements are more brittle than polymers, and their interaction with bone mainly relies on mechanical interlocking. Inorganic cements mainly consist of calcium and magnesium phosphates.
The current status including the advantages and limitations of each category of bone-adhesive materials are listed in Table 2.
Table 2.
Current status of bone adhesive materials.
| Bone-Adhesive Material | Current Status | ||
|---|---|---|---|
| Bone adhesives composed of natural polymers | Proteinaceous bone adhesives | Fibrin-glue based bone adhesives |
|
| Gelatin-based bone adhesives |
|
||
| Other proteinaceous bone adhesives |
|
||
| Polysaccharide-based bone adhesives | Chitosan- based bone adhesives Starch- based bone adhesives Dextran- based bone adhesives |
|
|
| Bone adhesives composed of synthetic polymers | Bone adhesives composed of unmodified synthetic polymers | Polyurethanes |
|
| Polycyanoacrylates |
|
||
| Polymethyl methacrylates and copolymers |
|
||
| Bone adhesives composed of chemically modified synthetic polymers | Bone adhesives composed of polymers modified with synthetic bone adhesive groups |
|
|
| Bone adhesives composed of polymers modified with bioinspired animal-derived bone adhesive groups |
|
||
| Bone adhesives composed of inorganic cements | Calcium phosphate cements |
|
|
| Magnesium phosphate cements |
|
||
| Glass ionomer cements |
|
||
2.1. Bone adhesives composed of natural polymers
2.1.1. Proteinaceous bone adhesives
2.1.1.1. Fibrin glue-based bone adhesives
Fibrin glue is a natural material which mimics the final step of the blood coagulation cascade. The first simple version of fibrin glue was introduced in the 1940s [115]. Fibrin glue obtained a CE mark in 1982, and FDA approval was granted in 1998. Fibrin glue is a two-component system where the first component contains fibrinogen and factor XIII, and the other component contains thrombin, calcium and an antifibrinolytic agent (aprotinin or tranexamic acid). When these two components are mixed, thrombin cleaves fibrinogen into soluble fibrin monomers. The fibrin monomers form unstable physical crosslinks via hydrogen bonding. Meanwhile, thrombin activates factor XIII in the presence of calcium. The activated factor XIII then facilitates covalent crosslinking of the previously formed physical crosslink to obtain an insoluble and stable clot [3]. The time required for the formation of the clot depends on the concentration of proteins and stabilizing agents [116]. In order to achieve stabilization of the clot, mechanical stress should be avoided after clot formation during a few minutes [117]. After clotting, the fibrin glue is resorbed in the body within a few days [118].
The cohesive strength of fibrin glue is relatively weak as compared to synthetic adhesives [5]. However, the use of fibrin glue is well tolerated in the body due to its excellent biocompatibility and biodegradability. Fibrin glue has a triple function since it can be surgically applied as an adhesive, sealant and/or hemostat [119]. The concentration of the constituents of fibrin glue should be tailored according to the intended application [120,121]. It has been demonstrated that the adhesion strength of fibrin glue depends on various parameters such as the composition, the substrate, and the presence of water, fat or collagen at the adhesion site [122]. Hence, the surface onto which the fibrin glue is to be applied must be dry in order to achieve proper adhesion. Various commercial fibrin glues are sold under different brand names, which differ in composition and sterilization method used during fabrication.
The bone adhesive property of fibrin glue was investigated by Arbes et al. who applied the adhesive in the clinic for cancellous bone grafting [123]. The adhesion strength of fibrin glue to bovine cancellous bone has been reported in the range between 5 and 17 kPa whereas the adhesion strength to porcine cortical bone has been reported as 11 kPa [6,29]. This relatively low strength of fibrin glue to bone renders this biomaterial incompatible with clinical scenarios involving significant tensile loads. Therefore, fibrin glue can only be used when mechanical force disturbing and dislocating fragments are absent [124]. Fibrin glue has been claimed to promote neovascularization and hence support bone repair [117]. To this end, fibrin glue should be applied as a thin layer to avoid disturbance of vascular ingrowth, since thick layers of fibrin glue may act as a physical barrier which retards cell migration and wound healing [16]. Fibrin glue displayed successful results for repair of osteochondral fractures in several preclinical models [[12], [13], [14], [15]]. Based on the above-mentioned features of fibrin glue, the main clinical application areas of fibrin glue are fixation of i) comminuted bone fractures, ii) bone grafts, iii) articular fractures and iv) osteochondral fractures.
Fibrin glue is mainly used to reduce and secure comminuted bone fragments in areas which are not exposed to mechanical stress and for fixation of autologous bone grafts or alloplasts in non-load-bearing applications. In such cases fibrin glue is a more convenient option than the use of interosseous wires and metallic plates for internal fixation. Occasionally, fibrin glue is used as an adjuvant method to align and stabilize small bone fragments after larger bone fragments were fixated using conventional methods [21,17]. To this end, fibrin glue is mostly used in craniofacial and maxillofacial surgery and was shown to successfully allow for reconstruction of mandibular discontinuity defects, fixation of comminuted nasal fractures and reconstruction of inferior orbital walls [[18], [19], [20], [22]]. Another common application area of fibrin glue involves fixation of articular fractures [23,24]. Application of fibrin glue was reported to establish bony union of chondral and osteochondral fragments in various injury sites such as the patella, talar knee, ankle joint, elbow or hand [21,17,[25], [26], [27], [28]]. According to patient follow-ups performed via CT scans and clinical evaluations, fibrin glue provided accurate fixation and satisfactory surgical outcome in most of the abovementioned cases. However, its usage is limited to non-load-bearing applications due to its low cohesive strength.
2.1.1.2. Gelatin-based bone adhesives
Gelatin is obtained through partial hydrolysis of collagen, which is the major structural protein in connective tissues. It can be extracted from body parts such as skin, cartilage or bones. The use of gelatin has been reported for fabrication of scaffolds for hard tissue regeneration and it is a valuable material for development of bone adhesives as well [[125], [126], [127]]. Crosslinking of gelatin with resorcinol and formaldehyde forms the gelatin-resorcinol-formaldehyde (GRF) adhesive, which allows for adhesion to and sealing of tissues [5]. Its adhesion mechanism is based on formation of crosslinks between formaldehyde and the amines present in the biological tissues. The in vitro adhesion strength of GRF adhesive to bone was measured as 200 kPa [29]. The resulting gelatin-resorcinol-formaldehyde/glutaraldehyde (GRFG) systems are referred to as “French adhesive”, where gelatin provides elasticity and degradability whereas formaldehyde and glutaraldehyde improve adhesion strength and in vivo stability, respectively [128]. Although GRFG adhesives exhibit adhesion to biological tissues, the toxicity of aldehydes has raised concerns on the safety of this adhesive for clinical use [129]. Furthermore, mild tissue necrosis and local tissue inflammation were observed in histological studies [120]. In-depth studies are required to thoroughly evaluate the long-term toxicity associated with GRF and GRFG adhesives.
To improve the adhesive strength of gelatin-based formulations to bone, gelatin and alginate have been crosslinked using carbodiimide and loaded with bioactive fillers such as hydroxyapatite or tricalcium phosphate [30]. Loading of 0.25% w/v hydroxyapatite considerably increased the adhesive strength to bovine femurs.
2.1.1.3. Other proteinaceous bone adhesives
Various proteinaceous materials including soybean proteins, protein-based materials secreted by Australian frogs, and crosslinked bovine serum albumin have been investigated for bone adhesion applications. After extraction of soybean protein, tyrosine residues can be converted into l-DOPA by tyrosinase to render these residues adhesive. The catechol moiety present in l-DOPA is known to facilitate adhesion via several mechanisms including hydrogen bonding, formation of complexes with multivalent metals, and π-π and π-cation interactions. This l-DOPA modified soy bean protein showed a maximum adhesive strength of around 400 kPa to porcine bone [31].
Australian frog Notaden bennetti secretes a sticky and elastic protein-based material from its dermal glands which is rich in Gly, Pro and Glu/Gln residues [130]. This adhesive compound can function under moist conditions and spontaneously adhere to various surfaces. The biocompatibility of frog adhesive has been demonstrated by in vitro and in vivo studies [131]. The potential of frog adhesive to bond biological tissues was explored by an ex vivo study performed on a longitudinal tear in sheep menisci using tear propagation method [132]. In this study, frog glue showed superior mechanical properties compared to other biological adhesives such as fibrin glue and gelatin. The effect of frog glue on three different types of rotator cuff repair techniques was investigated using cadaveric sheep infraspinatus tendon [133]. The presence of frog adhesive at the tendon-bone-suture interface enhanced the ultimate load and total energy required to failure in all repair techniques.
A commercial protein-aldehyde system consisting of bovine serum albumin crosslinked by glutaraldehyde is sold under the brand name BioGlue (6). The aldehyde moieties present in this system covalently bond/crosslink the proteins of the adhesive as well as the amines in proteins present at the application site to form a flexible seal [118]. Shear strength values of dissected periosteum re-fixated to bone using BioGlue were comparable to the native periosteum-bone bond. The authors suggest that this product may provide for an alternative fixation method in endoscopic brow-lifting operations [134].
2.1.2. Polysaccharide-based bone adhesives
Compared to proteinaceous bone adhesives, polysaccharide-based bone adhesives are still in early stages of research and development. Among different types of polysaccharides, chitosan has gained considerable interest. Consequently, this natural polymer has been investigated in several studies either alone or in blends with other polysaccharides such as starch and dextran. To improve the cohesive and adhesive strength of these formulations, chitosan has been crosslinked, mixed with inorganic particles or functionalized with catechol.
Covalently and ionically crosslinked chitosan-based hydrogels (using e.g. glutaraldehyde, calcium carbonate and/or hydroxyapatite) showed adhesive strengths up to 260 kPa to cancellous bovine bone under dry conditions [32]. However, the adhesive and cohesive strengths as well as fracture toughness of these chitosan-based adhesive formulations were compromised under wet conditions [33].
Catechol-functional chitosan hydrogels were modified with zeolitic imidazolate framework-8 (ZIF-8) nanoparticles, since release of Zn2+ ions is known to promote osteogenesis, angiogenesis, and antibacterial efficacy. Bone regeneration was improved in a rat cranial defect model where this adhesive was used for stabilizing bone graft material. In addition, ZIF-8 nanoparticles served as a structural support and increased the adhesive strength of the multifunctional adhesive to bovine cortical bone up to 770 kPa [34].
An l-DOPA-functional two-component polysaccharide system based on chitosan and dextran or starch has been investigated as a bone adhesive. The aldehyde moieties on starch or dextran react with the amines present in chitosan and in bone via the formation of a Schiff's base. The adhesive strength of these formulations were in the range 180–410 kPa depending on the specific composition. Interestingly, the conjugation of l-DOPA did not improve the bonding ability of the adhesive, which however was not explained by the authors [35].
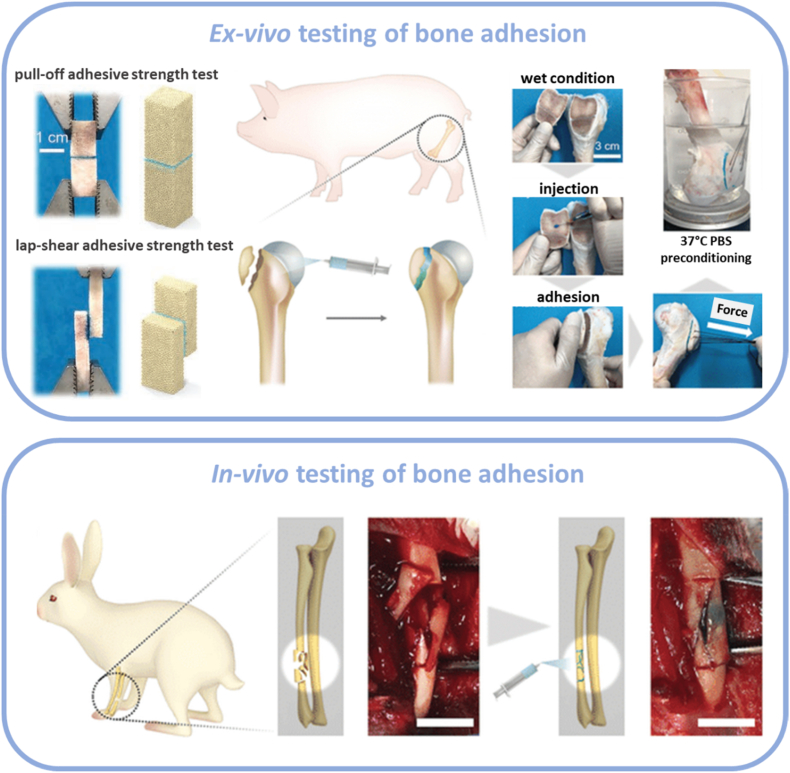
An oxidized dextran-gelatin network based on a Schiff's base reaction between aldehyde groups on dextran and amine groups on gelatin molecule was reinforced by amine-modified mesoporous bioactive glass nanoparticles aiming to form a flexible and injectable organic-inorganic structure stailized by reversible dynamic covalent bonds. This formulation showed strong adhesion to ex vivo porcine bone samples and improved fracture healing in a rabbit radius fracture model via induction of bone regeneration during the degradation of the adhesive (Fig. 3) [36].
Fig. 3.
Ex-vivo and in vivo testing of bone adhesives. Reproduced with permission [36] Copyright 2021, The John Wiley & Sons.
2.2. Bone adhesives composed of synthetic polymers
2.2.1. Bone adhesives composed of unmodified synthetic polymers
2.2.1.1. Polyurethanes
Isocyanate groups exhibit high affinity towards nucleophiles such as amines and hydroxy groups, which are abundantly present in biological tissues, rendering polyurethanes attractive candidates as bioadhesives [135]. Moreover, the mechanical properties and degradability of polyurethanes can be easily tailored by selection of the building blocks, i.e. polyisocyanates and polyols. Mixing of these components at the surgical site leads to liberation of carbon dioxide and results in a foam-like rigid but porous structure. Polyurethanes are considered as biocompatible, although their degradation products can be toxic and limit their applications as a biomaterial [136].
In the late 1950s, a polyurethane-based adhesive referred to as “Ostamer”, was developed and investigated for bone fixation for several indications including acute fracture of the tibia, non-unions and pathologic fractures of the femur [37]. However, later studies found that adhesion was poor, resulting in complications such as infection and tissue necrosis [118]. Polyurethane-based adhesives fabricated from a polycaprolactone-based polyol showed nearly a two-fold increase in adhesion to primed bone compared to the conventional methylmethacrylate-based bone cement [39]. Biocompatibility was demonstrated by in vitro cell culture tests and in vivo frog hind limb tarsus bone model. However, immunological response in the vicinity of the application site stressed the necessity of further elaborate in vivo studies.
Several studies explored the effect of reinforcement of polyurethane-based adhesives using calcium phosphate particles. A series of non-elastomeric polyurethane adhesives containing calcium phosphate particles showed rapid bonding to calcified tissues under wet conditions [137]. These adhesives demonstrated successful re-union of a bone fragment to dog tibias [40]. β-Tricalcium phosphate-reinforced polyurethane adhesives exhibited a maximum adhesive strength of 1340 ± 106 kPa to moist bovine rib bone [38]. Elsewhere, the wet bonding strength of a β -tricalcium phosphate reinforced polyurethane adhesive formulation to porcine rib bone was reported in the range 690–1250 kPa depending on the composition [41]. In vivo studies using adhesive formulation demonstrated enhancement of bone ingrowth in a rabbit model.
Castor oil was modified with isophorone diisocyanate to obtain a urethane-based prepolymer adhesive containing free isocyanate groups capable of forming urea bonds with amine groups present in biological tissues [138]. The binding ability of the adhesive was demonstrated by using gelatin sheets as a model substrate. Later, a castor oil-derived polyurethane bone adhesive containing calcium carbonate was developed and named as “Kryptonite”. When used as an augment to conventional wire cerclage, it provided rapid sternal fixation and prevented postoperative sternal displacement under physiologic loading conditions on a human cadaveric model [139]. More than thirty patients recovered without any adverse side effects after augmented primary sternal closure [42]. A single centre, pilot, randomized clinical trial performed with a total of 55 patients which underwent augmented closure of the sternum indicated the safety and effectiveness of this adhesive [43]. Reduced postoperative pain and accelerated sternal healing was confirmed by other studies [44]. The material was found to be stable over several years and late sternal re-entry was demonstrated to be feasible and safe [45]. In addition, successful outcomes were reported for vertebroplasty and in dentistry for sealing retrograde cavities [46,47]. Later, a study reported unpredictable swelling during the postoperative period [140]. Due to complications arising from reduction in strength and stiffness of the material at body temperature, the product was withdrawn from the market [141]. Meanwhile, severe infectious complications were reported after use for midface reconstruction [48].
2.2.1.2. Polycyanoacrylates
Cyanoacrylates were already widely used for industrial non-medical applications prior to their introduction as medical adhesives in the 1970s [142]. Cyanoacrylate adhesives polymerize within seconds via anionic polymerization in the presence of nucleophiles such as water or amines without any external trigger such as catalyst, heat or pressure [143]. The adhesion mechanism relies on the formation of covalent bonds between the acrylates and the amine groups of biological tissues and/or blood. Mechanical interlocking also contributes to enhancement of the adhesive strength. Polycyanoacrylates have very strong adhesion capability, however they are associated with several major drawbacks including i) rapid heat release at the application site due to the highly exothermic polymerization, ii) formation of a brittle and stiff adhesive, and iii) accumulation of toxic degradation products formaldehyde and cyanoacetate in the surrounding tissues.
The length of the alkyl chain directly influences the properties of the cyanoacrylate adhesives [120]. Cyanoacrylates with shorter alkyl chains are highly reactive and cause rapid heat release during the reaction. Thus, they are associated with increased risk of inflammation, tissue necrosis and infection. With increasing length of alkyl groups, adhesives become more flexible, less toxic, and more biocompatible [144]. Cyanoacrylates that were tested for medical applications include - with increasing alkyl chain length - ethyl, butyl and octyl 2-cyanoacrylates.
The bonding strength between different cyanoacrylates and bovine bone has been investigated by various researchers to evaluate the performance of cyanoacrylates as bone adhesives. The initial bonding strength between different alkyl cyanoacrylates and acid-treated, surface-roughened bones after 24 h immersion in water at room temperature was reported to vary from 9600 to 11200 kPa [49]. However, these values decreased after 3–6 weeks due to the degradation of the adhesives in aqueous media. Two studies comparing the performance of cyanoacrylates to conventional fixation methods reported contrasting results. Bone bonding strengths of cyanoacrylates to human cadaver parietal bone were reported to be higher than that for the plate and screws [50]. However, bond strength of titanium screws to pig jaw samples was higher than the tested cyanoacrylate adhesives [68].
In the 1960s, ethyl 2-cyanoacrylate adhesive named “Cyacrin” was reported to cause non-union and infection when used for bone fixation. Later, a new adhesive named “Biobond”, which contains ethyl 2-cyanoacrylate, polyisocyanate and nitrile rubber, was developed [118]. Although initial studies were promising, it was not used further due to toxicity issues [145].
N-butyl-2-cyanoacrylates, “Histoacryl” and “Histoacryl Blue”, were started to be used in skin closure applications in Europe and Canada in the 1980s and were subsequently approved by the FDA. Many preclinical studies demonstrated successful application of N-butyl-2-cyanoacrylate adhesive for bone bonding applications. These studies include fixation of sheep mandibular angulus fracture, osteotomies in the craniofacial skeleton, osteotomies using bone grafts in thoracic limbs of rabbits, fractures in segmental rat tibia, displaced zygomatic bone fractures and the cranial skeleton in rabbits, as well as osteotomized cranial bone fragments of pigs [[51], [52], [53], [54], [55], [56], [57]]. However, studies with less favourable results have also been reported for fixation of osteotomies in rabbit mandibles and sheep cadaver parietal bone segments [[58], [59], [60]]. Isobutyl 2-cyanoacrylate was reported to interfere with healing mechanism when used for osseous repair of rat tibiae [146]. N-butyl-2-cyanoacrylate adhesives gave successful surgical outcome in several clinical studies including fixation of skull, mandible and talar osteochondral fractures when used alone or as an adjunct to interosseous wires, plates and screws in treatment of facial bone fractures including comminuted fractures of the orbital roof, frontal sinus, maxillary anterior wall and skull [[61], [62], [63], [64], [65]].
A 2-octyl-2-cyanoacrylate adhesive “Dermabond”, was approved by FDA for topical skin approximation in 1998 and has been investigated for a variety of surgical applications since then. However, there are only a small number of reports evaluating its potential for bone fixation [147]. 2-Octyl-2-cyanoacrylate caused non-union and moderate to severe inflammation when used for autogenous bone graft fixation in rat calvaria [66]. Recently, it was found to inhibit bridging bone formation in an animal study conducted with rats, supporting the results of the abovementioned study [67]. In spite the long alkyl chain length, which is generally associated with favourable biological performance, bone adhesion studies with 2-octyl-2-cyanoacrylates showed unsatisfactory outcome.
Although alkyl cyanoacrylates have been widely investigated for bone fixation applications, the reported results are often contradictory. Consequently, the use of cyanoacrylates for bone fracture fixation still remains controversary, especially due to concerns regarding biocompatibility [5,[148], [149], [150], [151]].
2.2.1.3. Polymethylmethacrylates and copolymers
Polymethylmethacrylate (PMMA), which is commonly referred to as acrylic bone cement, has been widely exploited in orthopedic surgery as a cement to fixate prosthetics or as a void filler for bone defects since the 1930s [118,152]. Currently, many PMMA-based products are commercially available for bone fixation in orthopedics and dentistry [153,154]. However, PMMA is essentially not a true bone adhesive, since its adhesion mechanism is not based on specific interactions with bone tissue at the molecular scale. Instead, it acts as a cement at the bone-implant interface by means of mechanical interlocking with bone (particularly cancellous bone) [155]. The low viscosity of bone cements prior to cement setting enhance the contact area between the implant and bone, thereby providing improved anchoring [156]. The bond strength to PMMA has been reported as 350 kPa and 1000 kPa for compact cortical bone and porous cancellous bone, respectively [5]. These values confirm that PMMA cement binds more efficiently to cancellous bone via mechanical interlocking. The main drawbacks of PMMA cements include the weak bone adhesion (especially under wet conditions), volume shrinkage, risk of monomer leakage, release of heat to surrounding tissues upon polymerization, and a poor compatibility to bone tissue [3]. Strategies to overcome these disadvantages of PMMA-based adhesives focused on i) surface modifications to improve chemical bonding, ii) incorporation of chemical groups to impart degradability, and iii) addition of inorganic particles to improve mechanical strength.
Different strategies including pre-treatment of bone and application of intermediate bonding materials have been exploited to enhance the adhesion of PMMA bone cements to bone surface. Three-to five-fold enhancement of the bonding strength of bone cement and composite resins to bone was observed when a liquid acrylic intermediary material was applied [69]. Acid etching of the bone surface was demonstrated to cause demineralization and to expose collagen fibers for subsequent infiltration by monomers, in a manner similar to pre-treatments performed in restorative dentistry [157]. The infiltrated monomers are subsequently light-cured to obtain a hybrid layer which can be regarded as a composite of collagen fibers and the adhesive resin, which increases the bond strength by nearly two orders of magnitude according to the measured work of fracture.
Since PMMA-based bone cements are generally not biodegradable, polylactide and its copolymers have been incorporated to methacrylate systems to impart biodegradability. Methacrylate end-capped poly (lactide-co-propylene glycol-co-lactide) triblock copolymers have been photopolymerized to produce biodegradable adhesives [158]. Later, these systems were reinforced with calcium phosphates particles [[159], [160], [161], [162]]. The mechanical efficacy of biodegradable alkylene bis(dilactoyl)-methacrylate bone adhesive was investigated using bovine vertebrae, human vertebrae and human femoral condyles [163]. The adhesive significantly improved the anchorage of screws in cancellous bone, and its performance was comparable to PMMA cement. Initial in vivo studies on rabbit and ovine models seemed to be promising [70,71]. However, extensive tissue response and interference with the skeletal repair process were observed in a long-term biocompatibility study, where the adhesive was used to augment screw fixation in an ovine model [72].
Biodegradable methacrylate-based bone adhesives were reinforced with inorganic particles to obtain composite materials with enhanced bone-adhesive properties. An adhesive system consisting of methacrylate end-capped polylactide macromers, β-tricalcium phosphate and a radical starter exhibited an adhesive strength of 3100–13900 kPa in dry state and 300–7700 kPa in humid state to bovine bone specimens [73]. β-Tricalcium phosphate served as a buffering substance for the release of acidic degradation products. No evidence of significant toxicity or inflammation was present after implantation into femur and tibia in a rabbit model [74]. A bone adhesive fabricated from polypropylene fumarate (PPF)/hydroxyethyl methacrylate (HEMA) reinforced with nanobioactive glass showed bone adhesion to wet sheep rib trabecular bone samples [75].
2.2.2. Bone adhesives composed of chemically modified synthetic polymers
A widely pursued strategy to improve the adhesion of polymers to the surface of bone entails modification of the polymer backbone with bone-adhesive functional groups [3]. These functional groups used for such modification can be either synthetic groups with known affinity to (in)organic components of bone, or bioinspired animal-derived groups which rely on adhesion mechanisms of natural organisms.
2.2.2.1. Bone adhesives composed of polymers modified with synthetic bone-adhesive groups
Many researchers have designed materials from polymers modified with synthetic functional groups having affinity to bone to render these materials bone-adhesive. For example, carboxylic acids and phosphorous-containing functional groups such as phosphoric, phosphonic or bisphosphonic acids are known for their strong interaction with calcium present in the mineral phase of bone. Amine groups present in collagen, which is the primary constituent of the organic phase of bone, can interact with a variety of molecules to form covalent bonds and promote adhesion.
4-methacryloyloxyethyl trimellitic anhydride (4-META) functionalized PMMA cements are capable of binding to calcium ions via the carboxylate ions formed by hydrolysis of the anhydride groups. This material is particularly of interest, since it displays enhanced bone adhesion due to infiltration of monomers and formation of a hybrid layer as discussed earlier [164]. Bond strength between bone and a metal adherend glued by 4-META/methylmethacrylate copolymer was measured higher than 7000 kPa [76]. The addition of hydroxyapatite increased the bond strength, stabilized the cement and demonstrated direct bone apposition in vivo at the Ti implant/bone interface [77]. Strong bonding to both bone and metal was observed even under in vivo weight-bearing conditions where metal prostheses were implanted into rabbit femora using this cement [78].
Acidic monomers in self-etching dental adhesive systems etch the tooth surface to increase the roughness and facilitate micromechanical bonding [165]. Self-etching adhesives used in dentistry may be easily adapted to bone fixation applications, since the chemical composition of dentine and bone are quite similar. Indeed, a methacryloyloxydecyl dihydrogen phosphate (MDP)-containing dental adhesive system (Clearfill New Bond) was successfully used to adhere two pieces of porcine mandible bone samples, resulting in a reported adhesive strength of 6390 kPa [79]. Bone adhesive strengths of porcine mandible fragments cemented with different self-etching adhesive and resin cement combinations were measured in the range between 2100 and 4830 kPa [80].
Recently, dual crosslinked bone-adhesive hydrogels based on poly(2-oxazoline)s were developed [81]. Alendronate-functional poly(2-oxazoline)s formed physical crosslinks with dissolved calcium ions in the formulation as well as providing adhesion to the surface of bone since alendronate is a bisphosphonate well known for its strong interactions with bone. In addition, amine- and N-hydroxysuccinimide-functional poly(2-oxazoline)s in the formulation formed a covalent network to improve the cohesive strength of the adhesive. The dual crosslinked bone-adhesive hydrogels showed strong adhesion to apatite-coated and amine-coated model surfaces which mimic the inorganic and organic components of bone, respectively. Adhesion strength to porcine rib was measured as 116 kPa which is 18-fold higher than the adhesion strength measured for the commercially available fibrin glue controls (TISSEEL).
Isocyanate groups can promote bone adhesion by forming covalent bonds with amine groups of collagen present in the organic phase of bone structure. An interpenetrating network consisting of a photocurable poly (ethylene glycol) dimethacrylate matrix, isocyanate functional star-shaped prepolymers, and biodegradable ceramic fillers showed a maximum adhesive strength of 250 kPa to bovine femur. Adhesion was preserved upon storage in PBS for 7 days, which demonstrated sustainable adhesion under wet conditions [82].
2.2.2.2. Bone adhesives composed of polymers modified with bioinspired animal-derived bone-adhesive groups
Inspired by adhesion mechanisms found in nature, several tissue-adhesive functional groups have been identified recently. Natural organisms produce adhesive proteins for various purposes such as defence, shelter or attachment. Marine mussels and barnacles use their byssus and secreted cement proteins to adhere to hard surfaces, while sandcastle worms and caddisfly larvae produce secretions to glue sand grains or shells and assemble themselves protective shelters [166,167]. Adhesive proteins produced by marine organisms are capable of facilitating robust adhesion to various surfaces under harsh aquatic conditions [168]. This valuable property can be mimicked to fabricate adhesives suitable for medical applications, which also require robust adhesion in the presence of body fluids [169]. However, the extraction of these adhesive proteins from organisms is usually not feasible at industrial scale. Therefore, alternative approaches focus on production of either synthetic or genetically engineered analogues of these adhesive proteins [170]. The amino acid sequences of the adhesive proteins are known to have certain repetitive motifs responsible for their unique function and properties [171]. Identification of the functional groups in these repetitive motifs responsible for bone adhesion have led to development of bioinspired synthetic polymers functionalized using these bone-adhesive moieties.
Blue mussels (Mytilus edulis) are able to robustly anchor themselves onto a wide range of substrates such as surfaces of rocks or ships underwater despite intertidal conditions [172,173]. Analysis of secreted mussel-adhesive proteins suggest that 3,4-dihydroxyphenyl-alanine (DOPA) residues mediate adhesion and crosslinking, although the exact mechanism behind this enhanced adhesion is not yet fully understood [174,175]. DOPA residues can form bidentate hydrogen bonds with various surfaces or form complexes with multivalent metals and metal ions (especially Fe3+) via the hydroxyl groups of the catechol moiety. The catechol moiety also contributes to the adhesive and/or cohesive strength by π-π and π-cation interactions. Furthermore, catechols can be oxidized into quinone groups, which trigger subsequent intermolecular covalent crosslinking. Inspired by the adhesion mechanism of blue mussels, several researchers have designed biomimetic polypeptides, polymers, hydrogels or supramolecular systems which contain DOPA or its analogues containing catechol for use as adhesive systems [175,176].
DOPA-functional biomimetic co-polypeptides, polypeptide−pluronic−polypeptide triblock copolymers, and thermo-responsive polypeptide-based gels were synthesized via ring-opening polymerization of N-carboxy-α-amino acid anhydrides [[83], [84], [85], [86]]. These materials tightly adhered to porcine bone, and in vivo tests have confirmed that these materials improved bone healing in a rat osteotomy gap model. Chitosan/polypeptide adhesives designed using N-carboxy anhydride chemistry showed accelerated bone fracture healing and hemostatic efficacy [87]. DOPA-functional polymer/hydroxyapatite composites have been explored as adhesives for bone fixation in two different studies. DOPA- and citric acid-functional PEG has been crosslinked with hydroxyapatite to form an injectable and bone-adhesive material which sets within a few minutes [88]. Released citrate was demonstrated to promote mineralization and to enhance osteoinduction. In addition, increased bone formation, neovascularization and bone ingrowth was demonstrated using a rabbit comminuted radial fracture model. A DOPA and/or sodium iodate containing injectable self-setting bone adhesive based on thermo-gelling PLA-PEG-PLA triblock copolymers and α-tricalcium phosphate has been shown to enable tight adherence to pig femurs [177].
Catechol, the moiety responsible for adhesion of DOPA as mentioned above, has also been widely used itself for modification of synthetic polymers to create bioinspired bone-adhesive materials. Catechol-functional methacrylate/N-vinylcaprolactam copolymer-based hydrogels adhered to chicken bone plates and showed thermo-responsive, antioxidant, and anti-inflammatory properties [89]. Adipic dihydrazide-modified poly (l-glutamic acid) and catechol/aldehyde dual modified alginate was crosslinked via Schiff base reaction to form injectable hydrogels [90]. Porcine bone defects of 1 cm diameter were filled with the adhesive hydrogel, immersed in 0.01 M PBS at 37 °C for 24 h and later flushed under water for 5 min. The adhesive hydrogel maintained its stability and did not detach from the bone surface. Catechol-functional PEG-based hyperbranched polymers formed coacervates upon contact with water to facilitate strong adhesion under water by holding two pieces of bone together even after immersion in water for 72 h [91]. A Fe3+ crosslinked matrix of catechol-modified hyperbranched poly(β-amino ester) reinforced with nanohydroxyapatite was proposed as an adhesive for sternal closure and showed good adhesion to PMMA sheets used as bone models [178]. Applying adhesives directly onto bone fracture surfaces has the drawback of interfering with the natural process of bone healing. To overcome this problem, fiber-reinforced adhesive patches (FRAP), which are applied circumferentially around the bone fracture, have been developed [179,180]. Owing to its catechol groups, application of a dopamine solution on wet bone as a FRAP primer almost tripled the adhesive strength [181]. Hence, a library of dopamine derivatives was synthesized and evaluated as FRAP primers for enhancing bone fracture stabilization [182]. Hetero-functional cross-linkable dendritic scaffolds post-functionalized either by DOPA or carboxyl groups improved the adhesive strength when used as a FRAP primer [183].
Sandcastle worms (Phragmatopoma californica) are reef-forming marine worms which construct a protective shelter by binding sand and pieces of seashells using a proteinaceous adhesive. The adhesive is composed of oppositely charged proteins which are complexed with magnesium and calcium ions [184]. The negatively charged proteins contain phosphoserine residues, while the positively charged proteins contain residues with amine sidechains. The water-immiscible proteinaceous fluidic adhesive cures under the seawater within 30 s after being secreted and forms a porous solid structure through a self-initiated covalent crosslinking mechanism [185]. This natural mechanism resembles the formation of complex coacervates and provides a valuable approach for the design of biomimetic underwater adhesives [186]. Inspired by sandcastle worms, various coacervates based on biomimetic polyelectrolytes have been designed for bone adhesion. Bovine cortical bone specimens bonded with complex coacervates made from polyelectrolytes containing phosphates, primary amines and catechols exhibited approximately 37% of the shear modulus and strength as compared to bones glued together using a commercially available cyanoacrylate adhesive as a control [92]. Complex coacervates made from amine-modified gelatin, polyphosphodopamide, and Ca2+ or Mg2+ showed no cytotoxicity and enhanced attachment, spreading and migration of mouse osteoblasts. The adhesive maintained three-dimensional bone alignment in a rat calvarial fracture model over a period of 12 weeks, while histological studies confirmed that these adhesives were gradually resorbed and replaced by newly formed bone, emphasizing the strong potential for clinical use in reconstruction of craniofacial fractures [93,94]. It was demonstrated that addition of human bone nanopowder to the above-mentioned complex coacervate formulations enhanced bonding strength to model surfaces [187].
Caddisflies are insects which can secrete an adhesive silk with underwater binding ability. It was found that caddisfly adhesive silk structure contains a (SX)4 pattern where S is serine (more than 60% is phosphorylated) and X is usually valine or isoleucine [188]. Hence, this protein structure has a negative charge which ensures strong interaction with divalent cations such as Ca2+ and Mg2+. Ca2+-crosslinked phosphoserine-valine poly (ester urea) copolymers inspired by caddisfly adhesive silk exhibited adhesion to cortical bovine bone and aluminium substrates [95].
Barnacles are a type of marine arthropod which secrete an adhesive cement containing different proteins that enable firm attachment to various surfaces in aqueous environments [189]. Polypeptides that mimic barnacle (Balanus balanoides or Balanus hameri) adhesive proteins were synthesized by ring opening polymerization of amino acid N-carboxyanhydride monomers and were shown to provide firm bone-to bone as well as bone-to-iron adhesion [96,190].
2.3. Bone adhesives composed of inorganic cements
2.3.1. Calcium phosphate cements
Since inorganic calcium phosphate bone cements (CPCs) are generally recognized as biocompatible and osteoconductive, these biomaterials are highly suitable for applications in orthopedics and dentistry [191]. Calcium phosphate based inorganic materials, particularly hydroxyapatite, have been widely used as components of bone scaffolds, bone graft substitutes, void fillers or bone cements [[192], [193], [194], [195]]. However, unlike acrylic cements which are much less viscous before setting, CPCs are highly viscous prior to setting, which impedes deep penetration of the cement into cavities and pores of bone [196]. CPCs do not adhere tightly to bone immediately after surgical application, since they initially interact with bone based on mechanical interlocking. Over time, an adhesive bond between the cement and bone is gradually formed, which however does not provide stabilization during and directly after surgical application [197]. Moreover, CPCs are brittle and display low cohesion. Consequently, CPCs may disintegrate and be washed out by blood, where leaked solid cement particles increase the risk of inflammation and pulmonary embolism [198]. In order to improve the adhesive strength to bone, orthophosphoric acid in a brushite CPC system was replaced with pyrophosphoric acid, since pyrophosphate ions have strong affinity for metallic ions including calcium and magnesium. A maximum adhesive strength of 1300 ± 800 kPa to cortical bone was reported, whereas the control cement containing orthophosphoric acid did not show any measurable adhesion to bone [197]. This modified CPC also displayed considerable adhesion to stainless steel and alumina, which may support its use to join medical prostheses and bone.
Various additives such as polymers and polypeptides have been added to CPCs to improve their physical properties, biocompatibility and osteogenic performance for bone repair [[198], [199], [200], [201]]. Inspired by nature, mixing calcium phosphate with the amino acid O-phospho-l-serine (OPLS), the organic component involved in wet adhesion mechanisms of sandcastle worm, has led to the development of a new class of modified cements. The bone-adhesive property of these phosphoserine-modified calcium phosphate (either tetracalcium or α-tricalcium phosphate) bone cements was confirmed in aqueous environments, rendering these cements attractive candidates for clinical use.
A specific type of these modified calcium phosphate cements, referred to as “Tetranite”, comprises phosphoserine and tetracalcium phosphate [202]. It is an injectable material that sets within 10–15 min, continues to harden after the initial setting, and reaches 90% hardening after 24 h. The reaction is proposed to proceed by two interdependent pathways involving hydroxyapatite and calcium phosphoserine formation [203]. Covalent bonds are not formed, thereby preventing exothermic reactions or leaching of cytotoxic substances during the setting reaction. Moreover, all precursor components and by-products involved in these two reactions are either consumed, metabolized or excreted in the human body [203]. The properties of phosphoserine/tetracalcium phosphate cement were further improved by i) formation of pores to promote fluid transport and bone cell infiltration and ii) addition of fillers such as poly (lactic-co-glycolic) (PLGA) fibers or chitosan lactate for mechanical reinforcement [204]. The adhesive strength of this cement to two cortical or cancellous porcine bone pieces was higher than 1000 kPa, which largely exceeds values reported for PMMA- or calcium phosphate-based cements. Phosphoserine/tetracalcium phosphate cement was significantly more effective than currently used non-invasive fracture fixation techniques in assisting the stabilization of ex vivo mandibular fractures in dogs [205].
The in vivo performance of phosphoserine/tetracalcium phosphate cement was evaluated by a number of preclinical studies. No evidence of cytotoxicity or abnormal local tissue effects were observed, while substantial osteointegration and osteoconduction were demonstrated upon implantation in critical size rabbit distal femur defects [97]. Degradation was mediated through bone remodeling, indicating that the material was recognized as a bone-like structure by the body. Phosphoserine/tetracalcium phosphate cement provided immediate stabilization, showed no inflammatory reaction, smoothly filled the void surrounding the implant, and demonstrated intimate contact with both the implant and bone via osseointegration-like bonds during dental implant stabilization of oversized osteotomies in a canine model [98]. After four months, the modified cement was replaced by bone, in contrast to the controls which demonstrated presence of either isolated graft particles or soft tissue. The strength of sheep cranial bone flaps fixated using phosphoserine/tetracalcium phosphate cement were significantly higher than flaps fixated with plates and screws [99]. Based on postoperative evaluation, both osteoconduction and almost complete osteointegration were observed.
A second chemically modified bone-adhesive calcium phosphate cement is “OsStic”, which comprises α-tricalcium phosphate and phosphoserine [100]. Detailed solid-state NMR spectroscopy evaluation indicated that the organic and inorganic components are strongly bound through electrostatic interactions and hydrogen bonding [206]. These interactions are suggested to trigger self-assembly of clusters to form an initial organic network which gradually transforms into a hierarchically organized composite material [100]. Specific orientation of bidentate calcium-binding motifs (phosphate and carboxylate) on the amino acid structure was found to mediate the high adhesive strength of these materials [207].
The adhesive strength of cortical bovine bone samples adhered together with phosphoserine/α-TCP cement and cured in liquid for 24 h were around 2500–4000 kPa [100]. This range is up to 40-fold and 100-fold greater than the adhesive strength of commercial cyanoacrylate and fibrin adhesives, respectively. According to a preclinical test model able to accurately and reproducibly assess the strength of bone adhesives upon reconstruction of fragments of metaphyseal bone, the bond strength of phosphoserine/α-TCP cement was more than 30-fold higher than commercial fibrin glue TISSEEL [101]. Phosphoserine/α-TCP cement was investigated ex vivo as a potential implant augmentation material using screws inserted into trabecular bone [208]. Screw fixation using this modified cement showed significantly higher pull-out load, stiffness and fracture energy than brushite cement-augmented or non-augmented screws used as controls. Initial in vivo safety assessment of phosphoserine/α-TCP cements was performed by subcutaneous implantation in a rat model. After implantation, no signs of inflammation, adverse reaction or significant difference in the gene expression were observed as compared to unmodified α-TCP control cements, indicating the absence of any excessive immune response [102].
2.3.2. Magnesium phosphate cements
Magnesium has been used in various bone-related applications including bone implants, scaffolds for bone regeneration as well as magnesium phosphate-based bone cements [209,210]. Magnesium phosphate cements (MPCs) have been reported to exhibit superior affinity to bone and a more rapid degradation than CPCs [103]. Mg2+ ions are known to stimulate osteoblast differentiation and inhibit osteoclast formation [211]. Furthermore, MPCs exhibit intrinsic antimicrobial activity against bacterial strains associated with bone and implant infections [212]. In an in vivo study investigating the biocompatibility of MPCs, no foreign body reaction, inflammation or necrosis was observed [213]. MPCs did not induce DNA damage or gene mutation during toxicologic evaluations [213]. However, similar to CPCs, MPCs also have a low cohesive strength due to their intrinsic brittleness. MPCs and CPCs have been combined together in order to obtain materials with improved characteristics [214]. Various calcium or magnesium phosphate-based bone cements are commercially available [153,154].
Novel MPCs developed by mixing farringtonite (Mg3(PO4)2) with phytic acid showed an initial adhesive strength of 1220 ± 410 kPa to bovine cortical bone. However, this value was reduced after 7 days of storage under aqueous conditions [103]. Preclinical studies with MPC bone void fillers sold under the brandname “Osteocrete” demonstrated successful results in various surgical applications such as tendon-to-bone healing, repair of osteotomies, healing of bone-screw interface, securing reduced cranial bone segments, and stabilization of dental implants [[104], [105], [106], [107], [108], [109]].
2.3.3. Glass ionomer cements
Glass ionomer cements (GIC), also known as glass polyalkenoate cements, were developed in the late 1960s for application in restorative dentistry [215]. This cement is made from a water-soluble polymeric acid such as poly (acrylic acid) crosslinked by metal ions released from ion-leachable glass particles [216]. Systems containing modified zinc oxide as metal ion responsible for cement crosslinking have been marketed as zinc polycarboxylate cements [215]. The polymeric acid can bind to calcium ions present in the mineral phase of teeth and bone, rendering GICs also promising for orthopedic applications (4). GICs rapidly set to yield a relatively brittle material which is prone to cohesive failure.
Reported shear strength values of for GICs range between 3000 and 6000 kPa [215]. To date, glass ionomer cements have been restricted to dental, ear, nose and throat applications due to concerns related to the release of aluminium ions from the glass phase of this type of cement [110]. Conventional GICs showed mild to severe in vivo toxicity in a rat model during a study performed to investigate their use in orthopedic surgery [217]. Hence, recently aluminium-free GICs have been developed and used for various bone repair applications, including fixation and stabilization of distal radius, sternum and percutaneous upper extremities in human cadaveric models [110,[111], [112], [113], [114]].
3. Fabrication of bone adhesives
Alongside with the product development, the manufacturing process should be designed and optimized to allow for industrial upscaling. The process of producing the bone adhesive should be feasible for upscaling in an industrial setting, since all formulations would not be easily scalable from lab scale (grams) to industrial scale (kilograms). The operations in terms of supply chain of raw materials should also be feasible for upscaling. The production of bone adhesives based on natural polymers would rely on supply of pure and uncontaminated raw materials derived from natural resources. Therefore, it is crucial to source out raw materials which have specifications suitable for producing medical products. Depending on the origin of the material, studies required for regulatory approval vary. This situation adds to upscaling challenges, especially for plant- or animal-based materials.
On the other hand, synthetic polymers often require multiple synthesis and purification steps, which could lower the overall yield. In addition, bone adhesives based on synthetic materials might include production steps which require high temperatures, low humidity etc. which would contribute to an increase in production costs. The production process for any kind of bone adhesive formulation, either of natural or synthetic origin, should provide control over the functional properties and lead to products with reproducible performance. To ensure stability during shelf-life, the stability of the developed materials should be assessed via either real time or accelerated stability studies in relevant biological conditions at an early stage. Moreover, identification and quantification of degradation products via in vitro and in vivo studies are also often required for regulatory approval.
The products should be highly purified and be void of any potential toxic components such as extractables and leachables to ensure patient safety. Since they are implanted in the human body for prolonged periods of time, tissue adhesives are generally classified as high-risk medical devices. Furthermore, effective sterilization methods which do no compromise the functional properties of the adhesives should be selected and tested. Hence, the identification and mitigation of device-related risks are crucial at every stage of the process.
The delivery system used for application of the bone adhesive should be carefully considered during product design. The product can be designed to be delivered in different forms such as tapes, patches, sprays, glues, gels etc. For example, recently developed phosphoserine-modified calcium phosphate based bone adhesive formulations “Tetranite” and “OsStic” are fabricated in the form of an injectable pliable paste and set within minutes after being applied [202,100]. Fiber-reinforced adhesive patches (FRAP) are proposed to be applied circumferentially around the bone fracture since applying adhesives directly onto bone fracture surfaces may interfere with the natural process of bone healing [179]. The primary and secondary packaging should also be designed in accordance with the delivery form of the product and in a way which will retain the functionality of the product during its shelf-life.
To obtain market approval, the manufacturing and testing facilities should comply with relevant regulations such as GMP, ISO standards (i.e. ISO 10993 for biological evaluation of medical devices and ISO 13485 for medical device quality management) and MDR. Currently, there is bone adhesive product commercially available. Most bone adhesive formulations specifically designed to adhere to bone are still in preclinical phase. Hence, these newly developed adhesives, due to their innovative features, are not equivalent to an already marketed device and have to go through stringent regulatory control for market approval which significantly increases the time for these products to reach the market.
4. Clincial translation and commercialization of bone adhesives
The in-depth understanding of the composition, structure and function of bone tissue may serve as a guide to design novel bone-adhesive products with predictable performance and enhanced clinical outcome. Hence, rationally designed and specifically tailored adhesives capable of interacting with bone tissue would be optimal candidates for translation into clinical practice. Designing bone-adhesive materials with osteoconductive and osteoinductive features offers the opportunity to aid long-term bone healing after the initial short-term adhesion of bone fragments has been successfully achieved [218]. Osteoinduction is a process where bone formation, i.e. osteogenesis, is induced by stimulation of undifferentiated cells into pre-osteoblasts [219]. Osteoinduction can be achieved by incorporation of specific bioactive agents called osteopromoters, for example BMP-2, into the bone adhesive formulation. Osteoinduction is of utmost importance for effective bone healing after the occurrence of a bone fracture. Osteoconduction refers to the growth of bone along the surface of an implant [219]. This definition implies that the biocompatibility as well as the surface characteristics of the surface in which the bone cells are in contact with would affect bone growth. Incorporation of calcium phosphate-based or bioactive glass-based additives into the adhesive formulation are previously proven strategies to render polymers osteoconductive.
Another important requirement for successful development and clinical translation of bone adhesives relates to the fact that bone adhesives should not disrupt any of the subsequent phases of bone fracture healing. The type of bone healing depends on the anatomical condition of the bone after the fracture [220]. If the fractured bone pieces are in sufficiently close contact with each other (especially as a result of adequate fixation or reduction of these bone pieces), primary fracture healing occurs without callus formation. However, if a gap exists between both fractured ends, secondary fracture healing will inevitably take place. This process starts with the formation of a hematoma at the fracture site, followed by an inflammatory phase. Subsequently, stem cells are recruited and differentiated to form cartilaginous callus and primary bone. Meanwhile, angiogenesis is induced to supply blood to the healing site and facilitate bone healing. At later stages, active resorption of calcified cartilage and primary bone is initiated by osteoclasts. The process by which osteoblasts and osteoclasts orchestrate bone remodeling depending on biomechanical loading is the final long-term stage of the bone healing process. For effective bone healing the level of mechanical support provided by the bone adhesive to the fractured bone is a crucial factor determining the final clinical performance of these adhesives. Furthermore, since reduced blood supply to the healing site can result in delayed healing or even non-union, it is crucial that the application of a bone adhesive does not disrupt the vascular supply. Finally, bone-adhesive related infections should be avoided at any cost to avoid detrimental effects on fracture healing.
Researchers investigating novel bone adhesives should take clinical requirements into account as early as possible to achieve predictable outcome and minimize chances for inadequate performance of the material at later stages of research and development. Effective methods should be employed to monitor the long-term performance of the bone-adhesive materials after implantation. Moreover, the effect of swelling and degradation on the adhesive/cohesive performance of the material or immune response due to tissue-material interactions should be carefully assessed. For example, the degradation of the material may alter the pH and affect the adhesive performance, which may ultimately lead to product failure. Thus, a holistic approach which spans the entire life-time of the adhesive product should be adopted during the initial design and planning phase.
A major challenge in this research field involves the lack of well-established and standardized methods to evaluate the performance of a bone-adhesive product both in vitro and in vivo. In the literature, bone-adhesive materials are evaluated using different types of tests and test protocols. The wide variety of adhesion test parameters (in terms of test methods, types of bone, adhesion/storage conditions) and adhesion strength values underline the lack of standardized models, which severely complicates fair and scientifically sound comparison of the performance of novel materials with previously developed ones [221,222]. Such standardized bone adhesion tests should be designed according to the intended application of the bone-adhesive material, which is based on the type, location and characteristics of the specific bone fracture. Bones treated with bone adhesives are subject to different types of stress in the body, such as compression, shear, tensile and torsional stresses, depending on the anatomical site and other characteristics of the bone. Hence, suitable adhesion strength tests should be selected from the wide range of available methods such as lap shear, pull-off, three-point bending tests etc. in order to closely mimic the stresses experienced by the treated bone under physiological loading conditions [223]. Appropriate control groups should also be included in the study design to enable comparison and evaluation of the results.
The delivery system and the surgical procedure used for application of the bone adhesive are important factors determining clinical performance which should be carefully considered during product design. For instance, the final material should be easily prepared and applied to the surgical site, which stresses the need for adequate working and setting times, aiming to achieve bone adhesion within a clinically feasible time to minimize the duration of surgical application.
From a product point of view, the adhesives included in this study can be categorized as: 1) Formulations/products reaching late stage development which were not considered for further use due to safety concerns/complications, 2) Adhesives that already are clinically approved/commercially available for other indications with potential to provide bone-to-bone adhesion, or 3) Novel bone adhesives specifically designed for bone adhesion but yet not clinically approved/commercialized. Hence, no true bone adhesive product is currently commercially available. This can be understood from the extremely high number of requirements, which are hard to combine in a single product.
As can be expected, the formulations/products which were not considered for further clinical use due to safety concerns are mostly the synthetic bone adhesives. For example, gelatin-resorcinol-formaldehyde/glutaraldehyde (GRFG) systems referred to as “French adhesive” and ethyl 2-cyanoacrylate based adhesives “Cyacrin” and “Biobond” have raised concerns especially due to toxicity issues [118,129]. Polyurethane based bone adhesive “Ostamer” was investigated for bone fixation, however it was found that the adhesion was poor and the adhesive lead to complications such as infection and tissue necrosis [118]. Similarly, another polyurethane based bone adhesive “Kryptonite” initially showed promising results in clinical trials for sternal closure. However, later on unpredictable swelling and related complications were reported [141].
There is a number of commercially available products which are clinically approved for other purposes such as soft tissue applications or dental applications, but were later investigated for bone adhesion applications. For example, fibrin glue products are currently used in the clinic as a hemostat and/or sealant. However, due their low cohesive strength, they were shown to be useful only for fixation of bone fragments in non-load-bearing areas. N-butyl-2-cyanoacrylates “Histoacryl” and “Histoacryl Blue” as well as 2-octyl-2-cyanoacrylate “Dermabond” are cyanoacrylate-based adhesives approved for skin closure. Although these alkyl cyanoacrylates have been investigated for bone fixation applications, their use for bone fracture fixation still remains controversary, especially due to concerns regarding biocompatibility [148]. Besides soft tissue adhesives, dental materials were also considered as potential bone adhesives. Several commercial dental adhesives were shown to successfully adhere two pieces of bone samples to each other [79,80]. PMMA and calcium phosphate cements are clinically in use for dental and orthopedic applications [153,154]. However they interact with bone based on mechanical interlocking and cannot be regarded as true bone adhesives.
Several promising formulations have been recently designed for bone-adhesive purposes that are now reaching early stages of clinical testing. Inspired by wet adhesion mechanisms of sandcastle worm, a new class of modified calcium phosphate cements, i.e. phosphoserine-modified calcium phosphates were recently developed. One of these modified calcium phosphate cements, referred to as “Tetranite”, comprises phosphoserine and tetracalcium phosphate and a second one is “OsStic”, which comprises α-tricalcium phosphate and phosphoserine. Their bone adhesion capability under aqueous environments render these cements as promising candidates for clinical use. “OsStic” has already shown strong evidence to be effective in gluing bone pieces in preclinical studies [224]. “Tetranite” is soon to be tested in first-in-human clinical studies to assess the performance of the product for gluing dental implants.
5. Conclusion and future perspectives
This review provides a comprehensive overview of the large number of organic and inorganic formulations that have been proposed as bone adhesives for bone-reconstructive surgery. Most biomaterials which have been proposed as potential bone adhesives so far have not been developed for this specific purpose. Adhesives composed of natural materials were initially developed for other medical applications such as adhering/sealing soft tissues or controlling bleeding based on their hemostatic efficacy, and only considered for applications in bone-reconstructive surgery at later stages. Synthetic adhesives, on the other hand, were initially developed for non-medical applications, and adapted for use as bone adhesives much later. Consequently, these materials were not intentionally designed for bone reconstructive surgery, which explains the fact why one or more basic requirements are lacking for these bone adhesives, such as e.g. sufficient adhesion strength or biocompatibility.
Although natural materials are generally biocompatible, they typically exhibit comparably low cohesive strength, which hampers their clinical use as a bone adhesive. To improve cohesive properties of natural materials, various organic and/or inorganic additives such as fibres, which can provide structural support, have been incorporated into formulations. Furthermore, natural materials derived from animal sources pose the risk of disease transmission. In contrast, synthetic materials are capable of creating stronger bonds to bone, but they are associated with enhanced risk of toxicity upon long-term implantation.
Maintaining effective bone-adhesive strength in a wet environment while ensuring long-term biocompatibility of the biomaterial is still a major challenge. To this end, biomimetic strategies inspired by organisms capable of underwater adhesion may offer novel solutions to improve wet adhesion. However, only adhesive formulations which simultaneously combine high adhesive and cohesive strength would be suitable candidates for treatment of fractures in load-bearing bones.
Overcoming the abovementioned translational barriers related to product design and development, manufacturing process and market approval would eventually close the gap between the vast amount of research performed in this research field and the relatively low number of bone adhesive materials with translational promise. Hence, ongoing research in the field is expected to result in the development of commercial bone adhesives which fulfil a clear unmet clinical need and open new avenues on advanced treatment of complex bone fractures.
Funding
The Scientific and Technological Research Council of Turkey/BIDEB-2219 (HBB) and GATT Technologies are gratefully acknowledged for their financial support.
Credit author statement
Hatice B. Bingol: Project administration, Investigation, Methodology, Writing – original draft, Visualization, Johan C.M.E. Bender: Conceptualization, Writing – critical review & editing, Supervision, Funding acquisition. Joost A. Opsteen: Writing – critical review & diting, Supervision. Sander C.G. Leeuwenburgh: Conceptualization, Methodology, Writing – original draft, Writing – critical review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Hatice B. Bingol reports financial support was provided by Scientific and Technological Research Council of Turkey. Sander C.G. Leeuwenburgh reports a relationship with GATT Technologies that includes: board membership. Joost A. Opsteen reports a relationship with GATT Technologies that includes: employment. Hatice B. Bingol reports a relationship with GATT Technoogies that includes: employment. Johan C.M.E. Bender is the founder of GATT Technologies.
GATT Technologies has patent applications on bone adhesive materials. Since this manuscript is a review paper and not a research paper, the patent applications are not directly related to the submitted work.
Data availability
No data was used for the research described in the article.
References
- 1.Florencio-Silva R., Sasso G.R.D.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taljanovic M.S., Jones M.D., Ruth J.T., Benjamin J.B., Sheppard J.E., Hunter T.B. Fracture fixation. Radiographics. 2003;23:1569–1590. doi: 10.1148/rg.236035159. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-Fernández M.J., Hammoudeh H., Félix Lanao R.P., van Erk M., van Hest J.C.M., Leeuwenburgh S.C.G. Bone-adhesive materials: clinical requirements, mechanisms of action, and future perspective. Adv. Mater. Interfac. 2019;6 doi: 10.1002/admi.201802021. [DOI] [Google Scholar]
- 4.Tzagiollari A., McCarthy H.O., Levingstone T.J., Dunne N.J. Biodegradable and biocompatible adhesives for the effective stabilisation, repair and regeneration of bone. Bioengineering. 2022;9 doi: 10.3390/bioengineering9060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrar D.F. Bone adhesives for trauma surgery: a review of challenges and developments. Int. J. Adhesion Adhes. 2012;33:89–97. doi: 10.1016/j.ijadhadh.2011.11.009. [DOI] [Google Scholar]
- 6.Weber S.C., Chapman M.W. Adhesives in orthopedic surgery: a reivew of the literatures and in vitro bonding strengths of bone-bonding agents. Clin. Orthop. Relat. Res. 1984;191:249–261. [PubMed] [Google Scholar]
- 7.Böker K.O., Richter K., Jäckle K., Taheri S., Grunwald I., Borcherding K., von Byern J., Hartwig A., Wildemann B., Schilling A.F., Lehmann W. Current state of bone adhesives-Necessities and hurdles. Materials. 2019;12 doi: 10.3390/ma12233975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shokri M., Dalili F., Kharaziha M., Baghaban Eslaminejad M., Ahmadi Tafti H. Strong and bioactive bioinspired biomaterials, next generation of bone adhesives. Adv. Colloid Interface Sci. 2022;305 doi: 10.1016/j.cis.2022.102706. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Liu J., Zhu T., Le H., Wang X., Guo J., Liu G., Ding J. Functional macromolecular adhesives for bone fracture healing. ACS Appl. Mater. Interfaces. 2022;14 doi: 10.1021/acsami.1c17434. 1–19. [DOI] [PubMed] [Google Scholar]
- 10.Panagiotopoulou V.C., Santolini E., Jones E., Jha A., Giannoudis P.v. Adhesives for treatment of bone fractures: a review of the state-of-the art. Injury. 2022;53 doi: 10.1016/j.injury.2021.02.019. S20–S25. [DOI] [PubMed] [Google Scholar]
- 11.Shah N.v., Meislin R. Current state and use of biological adhesives in orthopedic surgery. Orthopedics. 2013;36:945–956. doi: 10.3928/01477447-20131120-09. [DOI] [PubMed] [Google Scholar]
- 12.Keller J., Andreassen T.T., Joyce F., Knudsen V.E., Jorgensen P.H., Lucht U. Fixation of osteochondral fractures- Fibrin sealant tested in dogs. Acta Orthop. Scand. 1985;56:323–326. doi: 10.3109/17453678508993025. [DOI] [PubMed] [Google Scholar]
- 13.Meyers M.H., Herron M. A fibrin adhesive seal for the repair of osteochondral fracture fragments. Clin. Orthop. Relat. Res. 1964;182:258–263. [PubMed] [Google Scholar]
- 14.Plaga B.R., Royster R.M., Donigian A.M., Wright G.B., Caskey P.M. Fixation of osteochondral fractures in rabbit knees a comparison of Kirschner wires, fibrin sealant, and polydioxanone pins. J. Bone Joint Surg. 1992;74:292–296. doi: 10.1302/0301-620X.74B2.1544972. [DOI] [PubMed] [Google Scholar]
- 15.Azarpira M.R., Vazani K., Ayatollahi M., Azarpira N., Kaviani M. Comparison of healing intra-articular fracture of distal femur using a Kirschner wire and autologous fibrin glue in an animal model. J. Pediatr. Orthop. B. 2017;26:454–457. doi: 10.1097/BPB.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 16.Shah M.A., Ebert A.M., Sanders W.E. Fibrin glue fixation of a digital osteochondral fracture: case report and review of the literature. J Hand Surg Am. 2002;27:464–469. doi: 10.1053/jhsu.2002.32957. [DOI] [PubMed] [Google Scholar]
- 17.Allende C.A., Allende B.T., Allende B.L., Bitar I., Gonzalez G. Intercondylar distal humerus fractures - surgical treatment and results. Chir. Main. 2004;23:85–95. doi: 10.1016/j.main.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Jeong H.S., Moon M.S., Lee H.K., Kim K.S. Use of fibrin glue for open comminuted nasal bone fractures. J. Craniofac. Surg. 2010;21:75–78. doi: 10.1097/SCS.0b013e3181c3ba30. [DOI] [PubMed] [Google Scholar]
- 19.Jo E.J., Yang H.J., Kim J.H. Fixation of fractured inferior orbital wall using fibrin glue in inferior blowout fracture surgery. J. Craniofac. Surg. 2015;26 doi: 10.1097/SCS.0000000000001293. e33–e36. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.T., Lee S.H. Reconstruction of inferior orbital wall fractures using bone fragments. J. Craniofac. Surg. 2015;26:2412–2414. doi: 10.1097/SCS.0000000000002090. [DOI] [PubMed] [Google Scholar]
- 21.Song S.H., Kyung H., Oh S.H., Kang N. Fixation of fractured anterior wall of maxillary sinus using fibrin glue in a zygomaticomaxillary complex fracture. J. Craniofac. Surg. 2014;25:919–921. doi: 10.1097/SCS.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 22.Kim J., Yang H.J., Kim J.H., Kim S.J. Reduction of the isolated anterior wall of the maxillary sinus fracture with double urinary balloon catheters and fibrin glue. Arch Craniofac Surg. 2017;18:238–242. doi: 10.7181/acfs.2017.18.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arce A.A., Marti Garin D., Molero Garcia V., Jansana J.P. Treatment of radial head fractures using a fibrin adhesive seal: a review of 15 cases. J. Bone Joint Surg. 1995;77:422–424. doi: 10.1302/0301-620X.77B3.7744928. [DOI] [PubMed] [Google Scholar]
- 24.Scapinelli R. Treatment of fractures of the humeral capitulum using fibrin sealant. Arch. Orthop. Trauma. Surg. 1990;109:235–237. doi: 10.1007/BF00453150. [DOI] [PubMed] [Google Scholar]
- 25.Kaplonyi G., Zimmerman I., Frenyo A.D., Farkas T., Nemes G. The use of fibrin adhesive in the repair of chondral and osteochondral injuries. Injury. 1988;19:267–272. doi: 10.1016/0020-1383(88)90043-5. [DOI] [PubMed] [Google Scholar]
- 26.Visuri T., Kuusela T. Fixation of large osteochondral fractures of the patella with fibrin adhesive system. Am. J. Sports Med. 1989;17:842–845. doi: 10.1177/036354658901700621. [DOI] [PubMed] [Google Scholar]
- 27.Angermann P., Riegels-Nielsen P. Fibrin fixation of osteochondral talar fracture. Acta Orthop. Scand. 1990;61:551–553. doi: 10.3109/17453679008993581. [DOI] [PubMed] [Google Scholar]
- 28.Stafford G.H., Bunn J.R., Villar R.N. Arthroscopic repair of delaminated acetabular articular cartilage using fibrin adhesive. Results at one to three years. Hip Int. 2011;21:744–750. doi: 10.5301/HIP.2011.8843. [DOI] [PubMed] [Google Scholar]
- 29.Chivers R.A., Wolowacz R.G. The strength of adhesive-bonded tissue joints. Int. J. Adhesion Adhes. 1997;17:127–132. doi: 10.1016/S0143-7496(96)00041-3. [DOI] [Google Scholar]
- 30.Cohen B., Panker M., Zuckerman E., Foox M., Zilberman M. Effect of calcium phosphate-based fillers on the structure and bonding strength of novel gelatin-alginate bioadhesives. J. Biomater. Appl. 2014;28:1366–1375. doi: 10.1177/0885328213509502. [DOI] [PubMed] [Google Scholar]
- 31.Mo J., Wang F., Xu Z., Feng C., Fang Y., Tang X., Shen X. Characterization and performance of soybean protein modified by tyrosinase. Int. J. Adhesion Adhes. 2019;92:111–118. doi: 10.1016/j.ijadhadh.2019.04.013. [DOI] [Google Scholar]
- 32.Cedano Serrano F.J., Pinzón L.M., Narváez D.M., Castro Paéz C.I., Moreno-Serrano C.L., Tabima D.M., Salcedo F., Briceno J.C., Casas-Rodriguez J.P. Evaluation of a water-resistant and biocompatible adhesive with potential use in bone fractures. J. Adhes. Sci. Technol. 2017;31:1480–1495. doi: 10.1080/01694243.2016.1263055. [DOI] [Google Scholar]
- 33.Vargas Villanueva J.G., Sarmiento Huertas P.A., Galan F.S., Esteban Rueda R.J., Briceño Triana J.C., Casas Rodriguez J.P. Bio-adhesion evaluation of a chitosan-based bone bio-adhesive. Int. J. Adhesion Adhes. 2019;92:80–88. doi: 10.1016/j.ijadhadh.2019.04.009. [DOI] [Google Scholar]
- 34.Liu Y., Zhu Z., Pei X., Zhang X., Cheng X., Hu S., Gao X., Wang J., Chen J., Wan Q. ZIF-8-modified multifunctional bone-adhesive hydrogels promoting angiogenesis and osteogenesis for bone regeneration. ACS Appl. Mater. Interfaces. 2020;12:36978–36995. doi: 10.1021/acsami.0c12090. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann B., Volkmer E., Kokott A., Augat P., Ohnmacht M., Sedlmayr N., Schieker M., Claes L., Mutschler W., Ziegler G. Characterisation of a new bioadhesive system based on polysaccharides with the potential to be used as bone glue. J. Mater. Sci. Mater. Med. 2009;20:2001–2009. doi: 10.1007/s10856-009-3782-5. [DOI] [PubMed] [Google Scholar]
- 36.Tang J., Xi K., Chen H., Wang L., Li D., Xu Y., Xin T., Wu L., Zhou Y., Bian J., Cai Z., Yang H., Deng L., Gu Y., Cui W., Chen L. Flexible osteogenic glue as an all-in-one solution to assist fracture fixation and healing. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202102465. [DOI] [Google Scholar]
- 37.Mandarino M.P., Salvatore J.E., Washington D.C. Polyurethane polymer; its use in fractured and diseased bones. Am. J. Surg. 1959;97:442–446. doi: 10.1016/0002-9610(59)90011-x. [DOI] [PubMed] [Google Scholar]
- 38.Erken M., Tevlek A., Hosseinian P., Topuz B., Aydin H.M. Effects of ceramic particle size on cell attachment and viability in polyurethane-based bone adhesive composites. J. Compos. Mater. 2020;54:2013–2022. doi: 10.1177/0021998319884729. [DOI] [Google Scholar]
- 39.Schreader K.J., Bayer I.S., Milner D.J., Loth E., Jasiuk I. A polyurethane-based nanocomposite biocompatible bone adhesive. J. Appl. Polym. Sci. 2013;127:4974–4982. doi: 10.1002/app.38100. [DOI] [Google Scholar]
- 40.Brandeis E., Katz D., Silbermann M., Zinman C. A new bioadhesive for in vivo bone adhesion. J. Mater. Sci. Mater. Med. 1993;4:543–546. doi: 10.1007/BF00125591. [DOI] [Google Scholar]
- 41.Lei K., Zhu Q., Wang X., Xiao H., Zheng Z. In vitro and in vivo characterization of a foam-like polyurethane bone adhesive for promoting bone tissue growth. ACS Biomater. Sci. Eng. 2019;5:5489–5497. doi: 10.1021/acsbiomaterials.9b00918. [DOI] [PubMed] [Google Scholar]
- 42.Fedak P.W.M., Kasatkin A. Enhancing sternal closure using kryptonite bone adhesive: technical report. Surg. Innovat. 2011;18 doi: 10.1177/1553350611412057. [DOI] [PubMed] [Google Scholar]
- 43.Fedak P.W.M., Kieser T.M., Maitland A.M., Holland M., Kasatkin A., Leblanc P., Kim J.K., King K.M. Adhesive-enhanced sternal closure to improve postoperative functional recovery: a pilot, randomized controlled trial. Ann. Thorac. Surg. 2011;92:1444–1450. doi: 10.1016/j.athoracsur.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Bayramoglu Z., Durak Y., Bayram M., Levent Ulusoy O., Caynak B., Sagbas E., Akpinar B. Bone cement-enhanced sternal closure technique in cardiac surgery: effects on sternal union, pain and life quality. J. Cardiothorac. Surg. 2013;8 doi: 10.1186/1749-8090-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spooner A.J., Mewhort H.E.M., DiFrancesco L.M., Fedak P.W.M. Case Rep Surg; 2017. Adhesive-enhanced Sternal Closure: Feasibility and Safety of Late Sternal Reentry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarnieri G., Tecame M., Izzo R., Vassallo P., Sardaro A., Iasiello F., Cavaliere C., Muto M. Vertebroplasty using calcium triglyceride bone cement (KryptoniteTM) for vertebral compression fractures: a single-centre preliminary study of outcomes at one-year follow-up. Intervent Neuroradiol. 2014;20:576–582. doi: 10.15274/INR-2014-10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uzun I., Keskin C., Guler B. The sealing ability of novel Kryptonite adhesive bone cement as a retrograde filling material. J. Dent. Res. Dent. Clin. Dent. Prospects. 2016;10:189–193. doi: 10.15171/joddd.2016.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper S.E., Durairaj V.D., Ramakrishnan V.R. Infectious complication following midface reconstruction with calcified triglyceride. Ophthalmic Plast. Reconstr. Surg. 2015;31 doi: 10.1097/IOP.0000000000000193. e157–e159. [DOI] [PubMed] [Google Scholar]
- 49.Kilpikari J., Lapinsuo M., Tormala P., Patiala H., Rokkanent P. Bonding strength of alkyl-2-cyanoacrylates to bone in vitro. J. Biomed. Mater. Res. 1986;20:1095–1102. doi: 10.1002/jbm.820200803. [DOI] [PubMed] [Google Scholar]
- 50.Kandalam U., Bouvier A.J., Casas S.B., Smith R.L., Gallego A.M., Rothrock J.K., Thompson J.Y., Huang C.Y.C., Stelnicki E.J. Novel bone adhesives: a comparison of bond strengths in vitro. Int. J. Oral Maxillofac. Surg. 2013;42:1054–1059. doi: 10.1016/j.ijom.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Cural U., Atalay B., Yildirim M.S. Comparison of mechanical stabilization of the mandibular angulus fracture fixation, with titanium plates and screws, resorbable plates and screws, and bone adhesives. J. Craniofac. Surg. 2018;29:1780–1787. doi: 10.1097/SCS.0000000000004866. [DOI] [PubMed] [Google Scholar]
- 52.Amarante M.T.J., Constantinescu M.A., O'Connor D. Cyanoacrylate fixation of the craniofacial skeleton. Plast. Reconstr. Surg. 1995;95:639–646. doi: 10.1097/00006534-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Sérgio M., Xavier V., Leite V.M. The effect of 2-butyl-cyanoacrylate adhesive in osteotomies and bone grafts in rabbits: macroscopic and radiographic characteristics. Rev Bras Ortop (Sao Paulo). 2012;47:639–645. doi: 10.1016/S2255-4971(15)30016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akif Akcal M., Poyanli O., Unay K., Esenkaya I., Gokcen B., Fıratligil A.S. Effect of N-butyl-cyanoacrylate on fracture healing in segmental rat tibia fracture model. J. Orthop. Surg. Res. 2014;9 doi: 10.1186/s13018-014-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dadas B., Alkan S., Cifci M., Basak T. Treatment of tripod fracture of zygomatic bone by N-2-butyl cyanoacrylate glue fixation, and its effects on the tissues. Eur. Arch. Oto-Rhino-Laryngol. 2007;264:539–544. doi: 10.1007/s00405-006-0227-3. [DOI] [PubMed] [Google Scholar]
- 56.Shermak M.A., Wong L., Inoue N., Crain B.J., Im M.J., Chao E.Y.S., Manson P.N. Fixation of the cranofacial skeleton with butyl-2-cyanoacrylate and its effects on histotoxicity and healing. Plast. Reconstr. Surg. 1998;102:309–318. doi: 10.1097/00006534-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Ahn D.K., Sims D., Randolph M.A., O'Connor D., Butler P.E.M., Amarante M.T.J., Yaremchuk M.J. Cranofacial skeletal fixation using biodegradable plates and cyanoacrylate glue. Plast. Reconstr. Surg. 1997;99:1508–1515. [PubMed] [Google Scholar]
- 58.Shermak M.A., Wong L., Inoue N., Chao E.Y.S., Manson P.N. Butyl-2-cyanoacrylate fixation of mandibular osteotomies. Plast. Reconstr. Surg. 1998;102:319–324. doi: 10.1097/00006534-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Bas B., Ozden B., Bekcioglu B., Sanal K.O., Gulbahar M.Y., Kabak Y.B. Screw fixation is superior to N-butyl-2-cyanoacrylate in onlay grafting procedure: a histomorphologic study. Int. J. Oral Maxillofac. Surg. 2012;41:537–543. doi: 10.1016/j.ijom.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 60.Gosain A.K., Song L., Corrao M.A., Pintar F.A. Biomechanical evaluation of titanium, biodegradable plate and screw, and cyanoacrylate glue fixation systems in craniofacial surgery. Plast. Reconstr. Surg. 1998;101:582–591. doi: 10.1097/00006534-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Mehta M.J., Shah K.H., Bhatt A.R.G. Osteosynthesis of mandibular fractures with N-Butyl cyanoacrylate: a pilot study. J. Oral Maxillofac. Surg. 1997;45:393–396. doi: 10.1016/0278-2391(87)90006-1. [DOI] [PubMed] [Google Scholar]
- 62.Yilmaz C., Kuyurtar F. Fixation of a talar osteochondral fracture with cyanoacrylate glue. Arthrosc. J. Arthrosc. Relat. Surg. 2005;21 doi: 10.1016/j.arthro.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 63.Sultan A., Mohamed A. Efficacy and safety of using N-butyl cyanoacrylate in cranial fixation following trauma and other pathologies. Turk Neurosurg. 2018;28:416–420. doi: 10.5137/1019-5149.JTN.20117-17.1. [DOI] [PubMed] [Google Scholar]
- 64.Kim Y.O. Use of cyanoacrylate in facial bone fractures. J. Craniofac. Surg. 1997;8:229–234. doi: 10.1097/00001665-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 65.Foresta E., Torroni A., Gasparini G., Saponaro G., Longo G., Boniello R., Cervelli D., Marianetti T.M., Pelo S., Moro A. Use of N-butyl-2-cyanoacrylate (Glubran2®) in fractures of orbital-maxillo-zygomatic complex. J Maxillofac Oral Surg. 2015;14:761–764. doi: 10.1007/s12663-015-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esteves J.C., Monteiro J.M., Aranega A.M., Betoni W., Sonoda C.K. Utilization of ethyl cyanoacrylate and 2-octyl cyanoacrylate adhesives for autogenous bone graft fixation: histomorphometric study in rats. J. Oral Implantol. 2014;40:411–417. doi: 10.1563/AAID-JOI-D-12-00063. [DOI] [PubMed] [Google Scholar]
- 67.Gomez V., Cairns M., Weinhold P., Jeffs A.D., Bortner B., v Paterno A., Dahners L., Draeger R.W. 2-Octyl cyanoacrylate (Dermabond®) inhibits bridging bone formation of articular fractures in a rat model. Cureus. 2021;13 doi: 10.7759/cureus.16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vieira J. de S., Santos F.R., de Freitas J.V., Baratto-Filho F., Gonzaga C.C., de Araujo M.R. Bond strength evaluation of cyanoacrylate-based adhesives and screws for bone fixation. Oral Maxillofac. Surg. 2016;20:157–160. doi: 10.1007/s10006-015-0541-2. [DOI] [PubMed] [Google Scholar]
- 69.Vainio’ J., Kilpikari J., Rokkanen’ P. Experimental fixation of bone cement and composite resins to bone. Arch. Orthop. Trauma Surg. 1979;94:191–195. doi: 10.1007/BF00618445. [DOI] [PubMed] [Google Scholar]
- 70.Heiss C., Hahn N., Wenisch S., Alt V., Pokinskyj P., Horas U., Kilian O., Schnettler R. The tissue response to an alkylene bis(dilactoyl)-methacrylate bone adhesive. Biomaterials. 2005;26:1389–1396. doi: 10.1016/j.biomaterials.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 71.Heiss C., Schettler N., Wenisch S., Cords S., Schilke F., Lips K.S., Alt V., Schnettler R. Bond strength of an alkylene bis(dilactoyl)-methacrylate bone adhesive: a biomechanical evaluation in sheep. J. Biomater. Sci. Polym. Ed. 2010;21:1345–1358. doi: 10.1163/092050609X12517190417759. [DOI] [PubMed] [Google Scholar]
- 72.Grossterlinden L., Janssen A., Schmitz N., Priemel M., Pogoda P., Amling M., Rueger J.M., Linhart W. Deleterious tissue reaction to an alkylene bis(dilactoyl)-methacrylate bone adhesive in long-term follow up after screw augmentation in an ovine model. Biomaterials. 2006;27:3379–3386. doi: 10.1016/j.biomaterials.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 73.Ishihara K., Arai H., Nakabayashi N., Morita S., Furuya K. Adhesive bone cement containing hydroxyapatite particle as bone compatible filler. J. Biomed. Mater. Res. 1992;26:937–945. doi: 10.1002/jbm.820260708. [DOI] [PubMed] [Google Scholar]
- 74.Bauer N.B., Brinke N., Heiss C., Skorupa A.B., Peters F., Kraus R., Schnettler R., Moritz A. Biodegradable β-tri-calciumphosphate/hydroxyethyl methacrylate enhanced three component bone adhesive demonstrates biocompatibility without evidence of systemic toxicity in a rabbit model. J. Biomed. Mater. Res. B Appl. Biomater. 2009;90 B:767–777. doi: 10.1002/jbm.b.31346. [DOI] [PubMed] [Google Scholar]
- 75.Shahbazi S., Moztarzadeh F., Sadeghi G.M.M., Jafari Y. In vitro study of a new biodegradable nanocomposite based on polypropylene fumarate as bone glue. Mater. Sci. Eng. C. 2016;69:1201–1209. doi: 10.1016/j.msec.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 76.Ishihara K., Nakabayashi N. Adhesive bone cement both to bone and metals: 4-META in MMA initiated with tri-n-butyl borane. J. Biomed. Mater. Res. 1989;23:1475–1482. doi: 10.1002/jbm.820231209. [DOI] [PubMed] [Google Scholar]
- 77.Lee R.R., Ogiso M., Watanabe A., Ishihara K. Examination of hydroxyapatite filled 4-META/MMA-TBB adhesive bone cement in in vitro and in vivo environment. Inc. J Biomed Mater Res (Appl Biomater). 1997;38:11–16. doi: 10.1002/(sici)1097-4636(199721)38:1<11::aid-jbm2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 78.Sakai T., Morita S., Shinomiya K.-I., Watanabe A., Nakabayashi N., Ishihara K. In vivo evaluation of the bond strength of adhesive 4-META/MMA-TBB bone cement under weight-bearing conditions. J. Biomed. Mater. Res. 2000;52:128–134. doi: 10.1002/1097-4636(200010)52:1<128::aid-jbm16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 79.Maurer P., Bekes K., Gernhardt C.R., Schaller H.G., Schubert J. Comparison of the bond strength of selected adhesive dental systems to cortical bone under in vitro conditions. Int. J. Oral Maxillofac. Surg. 2004;33:377–381. doi: 10.1016/j.ijom.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 80.Ortiz Ruiz A.J., Vicente A., Camacho Alonso F., López Jornet P. A new use for self-etching resin adhesives: cementing bone fragments. J. Dent. 2010;38:750–756. doi: 10.1016/j.jdent.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Sánchez-Fernández M.J., Rutjes J., Félix Lanao R.P., Bender J.C.M.E., van Hest J.C.M., Leeuwenburgh S.C.G. Bone-adhesive hydrogels based on dual crosslinked poly(2-oxazoline)s. Macromol. Biosci. 2021;21 doi: 10.1002/mabi.202100257. [DOI] [PubMed] [Google Scholar]
- 82.Wistlich L., Rücker A., Schamel M., Kübler A.C., Gbureck U., Groll J. A bone glue with sustained adhesion under wet conditions. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201600902. [DOI] [PubMed] [Google Scholar]
- 83.Wang J., Liu C., Lu X., Yin M. Co-polypeptides of 3,4-dihydroxyphenylalanine and l-lysine to mimic marine adhesive protein. Biomaterials. 2007;28:3456–3468. doi: 10.1016/j.biomaterials.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Lu D., Wang H., Li T., Li Y., Dou F., Sun S., Guo H., Liao S., Yang Z., Wei Q., Lei Z. Mussel-inspired thermoresponsive polypeptide-pluronic copolymers for versatile surgical adhesives and hemostasis. ACS Appl. Mater. Interfaces. 2017;9:16756–16766. doi: 10.1021/acsami.6b16575. [DOI] [PubMed] [Google Scholar]
- 85.Lu D., Wang H., Li T., Li Y., Wang X., Niu P., Guo H., Sun S., Wang X., Guan X., Ma H., Lei Z. Versatile surgical adhesive and hemostatic Mmterials: synthesis, properties, and application of thermoresponsive polypeptides. Chem. Mater. 2017;29:5493–5503. doi: 10.1021/acs.chemmater.7b00255. [DOI] [Google Scholar]
- 86.Lu D., Li Y., Wang X., Li T., Zhang Y., Guo H., Sun S., Wang X., Zhang Y., Lei Z. All-in-one hyperbranched polypeptides for surgical adhesives and interventional embolization of tumors. J. Mater. Chem. B. 2018;6:7511–7520. doi: 10.1039/C8TB01015C. [DOI] [PubMed] [Google Scholar]
- 87.Lu D., Wang H., Wang X., Li Y., Guo H., Sun S., Zhao X., Yang Z., Lei Z. Biomimetic chitosan-graft-polypeptides for improved adhesion in tissue and metal. Carbohydr. Polym. 2019;215:20–28. doi: 10.1016/j.carbpol.2019.03.065. [DOI] [PubMed] [Google Scholar]
- 88.Xie D., Guo J., Mehdizadeh M.R., Tran R.T., Chen R., Sun D., Qian G., Jin D., Bai X., Yang J. Development of injectable citrate-based bioadhesive bone implants. J. Mater. Chem. B. 2015;3:387–398. doi: 10.1039/c4tb01498g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puertas-Bartolomé M., Fernández-Gutiérrez M., García-Fernández L., Vázquez-Lasa B., San Román J. Biocompatible and bioadhesive low molecular weight polymers containing long-arm catechol-functionalized methacrylate. Eur. Polym. J. 2018;98:47–55. doi: 10.1016/j.eurpolymj.2017.11.011. [DOI] [Google Scholar]
- 90.Yan S., Wang W., Li X., Ren J., Yun W., Zhang K., Li G., Yin J. Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties. J. Mater. Chem. B. 2018;6:6377–6390. doi: 10.1039/C8TB01928B. [DOI] [PubMed] [Google Scholar]
- 91.Cui C., Fan C., Wu Y., Xiao M., Wu T., Zhang D., Chen X., Liu B., Xu Z., Qu B., Liu W. Water-triggered hyperbranched polymer universal adhesives: from strong underwater adhesion to rapid sealing hemostasis. Adv. Mater. 2019;31 doi: 10.1002/adma.201905761. [DOI] [PubMed] [Google Scholar]
- 92.Shao H., Bachus K.N., Stewart R.J. A water-borne adhesive modeled after the sandcastle glue of P. californica. Macromol. Biosci. 2009;9:464–471. doi: 10.1002/mabi.200800252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao H., Stewart R.J. Biomimetic underwater adhesives with environmentally triggered setting mechanisms. Adv. Mater. 2010;22:729–733. doi: 10.1002/adma.200902380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winslow B.D., Shao H., Stewart R.J., Tresco P.A. Biocompatibility of adhesive complex coacervates modeled after the sandcastle glue of Phragmatopoma californica for craniofacial reconstruction. Biomaterials. 2010;31:9373–9381. doi: 10.1016/j.biomaterials.2010.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhagat V., O'Brien E., Zhou J., Becker M.L. Caddisfly inspired phosphorylated poly(ester urea)-based degradable bone Adhesives. Biomacromolecules. 2016;17:3016–3024. doi: 10.1021/acs.biomac.6b00875. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto H., Nagai A. Polypeptide models of the arthropodin protein of the barnacle Balanus balanoides. Mar. Chem. 1992;37:131–143. doi: 10.1016/0304-4203(92)90061-E. [DOI] [Google Scholar]
- 97.Kirillova A., Kelly C., von Windheim N., Gall K. Bioinspired mineral-organic bioresorbable bone adhesive. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800467. [DOI] [PubMed] [Google Scholar]
- 98.Cochran D., Jones A., Sugita R., Brown M., Guda T., Prasad H., Ong J., Pollack A., Kay G. Immediate dental implant stabilization in a canine model using a novel mineral-organic adhesive: 4-month results. Int. J. Oral Maxillofac. Implants. 2020;35:39–51. doi: 10.11607/jomi.7891. [DOI] [PubMed] [Google Scholar]
- 99.Foley K.T., Woodard E.J., Slotkin J.R., Mayotte C.K., Baldwin A.C., Brown M.C., Hess B.J. Cranial flap fixation in sheep using a resorbable bone adhesive. J. Neurosurg. 2021;134:621–629. doi: 10.3171/2019.11.JNS192806. [DOI] [PubMed] [Google Scholar]
- 100.Pujari-Palmer M., Guo H., Wenner D., Autefage H., Spicer C.D., Stevens M.M., Omar O., Thomsen P., Edén M., Insley G., Procter P., Engqvist H. A novel class of injectable bioceramics that glue tissues and biomaterials. Materials. 2018;11 doi: 10.3390/ma11122492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Procter P., Pujari-Palmer M., Hulsart-Billström G., Wenner D., Insley G., Larsson S., Engqvist H. A biomechanical test model for evaluating osseous and osteochondral tissue adhesives. BMC Biomed Eng. 2019;1 doi: 10.1186/s42490-019-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hulsart-Billström G., Stelzl C., Procter P., Pujari-Palmer M., Insley G., Engqvist H., Larsson S. In vivo safety assessment of a bio-inspired bone adhesive. J. Mater. Sci. Mater. Med. 2020;31 doi: 10.1007/s10856-020-6362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brückner T., Meininger M., Groll J., Kübler A., Gbureck U. Magnesium phosphate cement as mineral bone adhesive. Materials. 2019;12:3819. doi: 10.3390/ma12233819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gulotta L.v., Kovacevic D., Ying L., Ehteshami J.R., Montgomery S., Rodeo S.A. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am. J. Sports Med. 2008;36:1290–1297. doi: 10.1177/0363546508314396. [DOI] [PubMed] [Google Scholar]
- 105.Waselau M., Samii V.F., Weisbrode S.E., Litsky A.S., Bertone A.L. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am. J. Vet. Res. 2007;68:370–378. doi: 10.2460/ajvr.68.4.370. [DOI] [PubMed] [Google Scholar]
- 106.Hirvinen L.J.M., Litsky A.S., Samii V.F., Weisbrode S.E., Bertone A.L. Influence of bone cements on bone-screw interfaces in the third metacarpal and third metatarsal bones of horses. Am. J. Vet. Res. 2009;70:964–972. doi: 10.2460/ajvr.70.8.964. [DOI] [PubMed] [Google Scholar]
- 107.Sehlke B.M., Wilson T.G., Jones A.A., Yamashita M., Cochran D.L. The use of a magnesium-based bone cement to secure immediate dental implants. Int. J. Oral Maxillofac. Implants. 2013;28 doi: 10.11607/jomi.te16. e357–e367. [DOI] [PubMed] [Google Scholar]
- 108.Schendel S.A., Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J. Craniofac. Surg. 2009;20:461–464. doi: 10.1097/SCS.0b013e31819b9819. [DOI] [PubMed] [Google Scholar]
- 109.Thomopoulos S., Zampiakis E., Das R., Kim H.M., Silva M.J., Havlioglu N., Gelberman R.H. Use of a magnesium-based bone adhesive for flexor tendon-to-bone healing. J. Hand Surg. 2009;34:1066–1073. doi: 10.1016/j.jhsa.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khader B., Peel S., Towler M. An injectable glass polyalkenoate cement engineered for fracture fixation and stabilization. J. Funct. Biomater. 2017;8 doi: 10.3390/jfb8030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehrvar C., Kuzyk P., Cohen G., Safir O., Zalzal P., Alhalawani A., Towler M.R., Papini M. Novel adhesives for sternal fixation and stabilization: a biomechanical analysis. Clin. BioMech. 2019;62:66–71. doi: 10.1016/j.clinbiomech.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 112.Mehrvar C., Kuzyk P., Shamlou J., Safir O., Zalzal P., Alhalawani A., Towler M.R., Papini M. Novel adhesives for distal radius fixation: a biomechanical analysis. J. Mech. Behav. Biomed. Mater. 2019;89:99–106. doi: 10.1016/j.jmbbm.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 113.Zalzal P., Safir O., Alhalawani A., Papini M., Towler M. Percutaneous upper extremity fracture fixation using a novel glass-based adhesive. J. Orthop. 2018;15:67–69. doi: 10.1016/j.jor.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khader B.A., Rodriguez O., Towler M.R. The effect of Mg2+ incorporation into the glass phase of zinc-based glass polyalkenoate cements. J. Non-Cryst. Solids. 2018;483:106–117. doi: 10.1016/j.jnoncrysol.2018.01.007. [DOI] [Google Scholar]
- 115.Cronkite E.P., Lozner E.L., Deaver J.M. Use of thrombin and fibrinogen in skin grafting: preliminary report. J. Am. Med. Assoc. 1944;124:976–978. doi: 10.1001/jama.1944.02850140022006. [DOI] [Google Scholar]
- 116.Thoms R.J., Marwin S.E. The role of fibrin sealants in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2009;17:727–736. doi: 10.5435/00124635-200912000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Donkerwolcke M., Burny F., Muster D. Tissues and bone adhesives-Historical aspects. Biomaterials. 1998;19:1461–1466. doi: 10.1016/S0142-9612(98)00059-3. [DOI] [PubMed] [Google Scholar]
- 118.Heiss C., Kraus R., Schluckebier D., Stiller A.C., Wenisch S., Schnettler R. Bone adhesives in trauma and orthopedic surgery. Eur. J. Trauma. 2006;32:141–148. doi: 10.1007/s00068-006-6040-2. [DOI] [Google Scholar]
- 119.Spotnitz W.D. Fibrin sealant: the only approved hemostat, sealant, and adhesive—a laboratory and clinical perspective. ISRN Surg. 2014:1–28. doi: 10.1155/2014/203943. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhagat V., Becker M.L. Degradable adhesives for surgery and tissue engineering. Biomacromolecules. 2017;18:3009–3039. doi: 10.1021/acs.biomac.7b00969. [DOI] [PubMed] [Google Scholar]
- 121.Schlag G., Redl H. Fibrin sealant in orthopedic surgery. Clin. Orthop. Relat. Res. 1968;227:269–285. [PubMed] [Google Scholar]
- 122.Sierra D.H., Feldman D.S., Saltzt R., Huang S. A method to determine shear adhesive strength of fibrin sealants. J. Appl. Biomater. 1992;3:147–151. doi: 10.1002/jab.770030210. [DOI] [PubMed] [Google Scholar]
- 123.Arbes H., Bösch P., Lintner F., Salzer M. First clinical experience with heterologous aancellous bone grafting combined with the fibrin adhesive system (FAS) Arch. Orthop. Trauma. Surg. 1981;98:183–188. doi: 10.1007/BF00632975. [DOI] [PubMed] [Google Scholar]
- 124.Giebel G., Rimpler M. Klebungen am skelettsystem: klebstoffe, 50 jahre hilfsstoffe für den chirurgen. Teil 1. Biomed. Tech. 1981;26:35–40. doi: 10.1515/bmte.1981.26.3.35. [DOI] [PubMed] [Google Scholar]
- 125.Iranmanesh P., Gowdini M., Khademi A., Dehghani M., Latifi M., Alsaadi N., Hemati M., Mohammadi R., Saber-Samandari S., Toghraie D., Khan A. Bioprinting of three-dimensional scaffold based on alginate-gelatin as soft and hard tissue regeneration. J. Mater. Res. Technol. 2021;14:2853–2864. doi: 10.1016/j.jmrt.2021.08.069. [DOI] [Google Scholar]
- 126.Cheng Y., Morovvati M.R., Huang M., Shahali M., Saber-Samandari S., Niazi Angili S., Ghadiri Nejad M., Shakibaie M., Toghraie D. A multilayer biomimetic chitosan-gelatin-fluorohydroxyapatite cartilage scaffold using for regenerative medicine application. J. Mater. Res. Technol. 2021;14:1761–1777. doi: 10.1016/j.jmrt.2021.07.052. [DOI] [Google Scholar]
- 127.Zare-Harofteh A., Saber-Samandari S., Saber-Samandari S. The effective role of akermanite on the apatite-forming ability of gelatin scaffold as a bone graft substitute. Ceram. Int. 2016;42:17781–17791. doi: 10.1016/j.ceramint.2016.08.106. [DOI] [Google Scholar]
- 128.Walker J.D., Kratz J.M., Basler C.G., Meck L.P., Stratton J.R., Kribbs S.B., Crawford F.A., Spinale F.G. Fate of gelatin-resorcinol-formaldehyde/glutaraldehyde adhesive on femoral vessel morphology. J. Surg. Res. 1997;71:73–78. doi: 10.1006/jsre.1997.5128. [DOI] [PubMed] [Google Scholar]
- 129.Albes J.M., Krettek C., Hausen B., Rohde R., Haverich A., Borst H.G. Biophysical properties of the gelatin-resorcinformaldehyde/glutaraldehyde adhesive. Ann. Thorac. Surg. 1993;56:910–915. doi: 10.1016/0003-4975(93)90354-K. [DOI] [PubMed] [Google Scholar]
- 130.Graham L.D., Glattauer V., Huson M.G., Maxwell J.M., Knott R.B., White J.W., Vaughan P.R., Peng Y., Tyler M.J., Werkmeister J.A., Ramshaw J.A. Characterization of a protein-based adhesive elastomer secreted by the Australian frog Notaden bennetti. Biomacromolecules. 2005;6:3300–3312. doi: 10.1021/bm050335e. [DOI] [PubMed] [Google Scholar]
- 131.Graham L.D., Danon S.J., Johnson G., Braybrook C., Hart N.K., Varley R.J., Evans M.D.M., McFarland G.A., Tyler M.J., Werkmeister J.A., Ramshaw J.A.M. Biocompatibility and modification of the protein-based adhesive secreted by the Australian frog Notaden bennetti. J. Biomed. Mater. Res. 2010;93:429–441. doi: 10.1002/jbm.a.32559. [DOI] [PubMed] [Google Scholar]
- 132.Szomor Z.L., Murrell G.A.C., Appleyard R.C., Tyler M.J. Meniscal repair with a new biological glue: an ex vivo study. Tech. Knee Surg. 2008;7:261–265. doi: 10.1097/BTK.0b013e31818f8e7f. [DOI] [Google Scholar]
- 133.Millar N.L., Bradley T.A., Walsh N.A., Appleyard R.C., Tyler M.J., Murrell G.A.C. Frog glue enhances rotator cuff repair in a laboratory cadaveric model. J. Shoulder Elbow Surg. 2009;18:639–645. doi: 10.1016/j.jse.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 134.Sidle D.M., Maas C.S. Determination of shear strength of periosteum attached to bone with BioGlue surgical adhesive. Arch. Facial Plast. Surg. 2008;10:316–320. doi: 10.1001/archfaci.10.5.316. [DOI] [PubMed] [Google Scholar]
- 135.Bouten P.J.M., Zonjee M., Bender J., Yauw S.T.K., van Goor H., van Hest J.C.M., Hoogenboom R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014;39:1375–1405. doi: 10.1016/j.progpolymsci.2014.02.001. [DOI] [Google Scholar]
- 136.Zaokari Y., Persaud A., Ibrahim A. Biomaterials for adhesion in orthopedic applications: a review. Engineered Regeneration. 2020;1:51–63. doi: 10.1016/j.engreg.2020.07.002. [DOI] [Google Scholar]
- 137.Katz D., Brandeis E., Klein J. Biomedical adhesive compositions. 1993;US5266608 [Google Scholar]
- 138.Ferreira P., Pereira R., Coelho J.F.J., Silva A.F.M., Gil M.H. Modification of the biopolymer castor oil with free isocyanate groups to be applied as bioadhesive. Int. J. Biol. Macromol. 2007;40:144–152. doi: 10.1016/j.ijbiomac.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 139.Fedak P.W.M., Kolb E., Borsato G., Frohlich D.E.C., Kasatkin A., Narine K., Akkarapaka N., King K.M. Kryptonite bone cement prevents pathologic sternal displacement. Ann. Thorac. Surg. 2010;90:979–985. doi: 10.1016/j.athoracsur.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 140.Doumit G.D., Meisler E., Sidaoui J., Zins J.E., Papay F.A. The expansile properties of Kryptonite relating to cranioplasty. J. Craniofac. Surg. 2014;25:880–883. doi: 10.1097/SCS.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 141.Ball C.G., Grondin S.C., Pasieka J.L., Kirkpatrick A.W., Maclean A.R., Cantle P., Dixon E., Schneider P., Hamilton M. Examples of dramatic failures and their effectiveness in modern surgical disciplines: can we learn from our mistakes? J Comp Eff Res. 2018;7:709–720. doi: 10.2217/cer-2017-0090. [DOI] [PubMed] [Google Scholar]
- 142.García Cerdá D., Ballester A.M., Aliena-Valero A., Carabén-Redaño A., Lloris J.M. Use of cyanoacrylate adhesives in general surgery. Surg. Today. 2015;45:939–956. doi: 10.1007/s00595-014-1056-4. [DOI] [PubMed] [Google Scholar]
- 143.Mehdizadeh M., Yang J. Design strategies and applications of tissue bioadhesives. Macromol. Biosci. 2013;13:271–288. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sohn J.J., Gruber T.M., Zahorsky-Reeves J.L.L.G.W. Comparison of 2-ethyl-cyanoacrylate and 2-butyl-cyano acrylate for use on the calvaria of CD1 mice. J Am Assoc Lab Anim Sci. 2016;55:199–203. [PMC free article] [PubMed] [Google Scholar]
- 145.Caldeira J., Borrasca A.G., Aranega A.M., Rangel I., Junior G., Filho O.M. Histomorphometric analysis of the repair process of autogenous bone grafts fixed at rat calvaria with cyanoacrylate. J. Appl. Oral Sci. 2011;19:529–534. doi: 10.1590/s1678-77572011000500016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hunter K.M.S. Cyanoacrylate tissue adhesive in osseous repair. Br. J. Oral Surg. 1976;14:80–86. doi: 10.1016/0007-117x(76)90098-6. [DOI] [PubMed] [Google Scholar]
- 147.Singer A.J., Thode H.C. A review of the literature on octylcyanoacrylate tissue adhesive. Am. J. Surg. 2004;187:238–248. doi: 10.1016/j.amjsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 148.Papatheofanis F.J. Cytotoxicity of alkyl-2-cyanoacrylate adhesives. J. Biomed. Mater. Res. 1989;23:661–668. doi: 10.1002/jbm.820230609. [DOI] [PubMed] [Google Scholar]
- 149.Benthien J.P., Russlies M., Behrens P. Investigating the effects of bone cement, cyanoacrylate glue and marine mussel adhesive protein from Mytilus edulis on human osteoblasts and fibroblasts in vitro. Ann. Anat. 2004;186:561–566. doi: 10.1016/S0940-9602(04)80108-0. [DOI] [PubMed] [Google Scholar]
- 150.Ekelund A., Nilsson O.S. Tissue adhesives inhibit experimental new bone formation. Int. Orthop. 1991;15:331–334. doi: 10.1007/BF00186872. [DOI] [PubMed] [Google Scholar]
- 151.Montanaro L., Arciola C.R., Cenni E., Ciapetti G., Savioli F., Filippini F., Barsanti L.A. Cytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical use. Biomaterials. 2001;22:59–66. doi: 10.1016/s0142-9612(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 152.Kusleika R., Stupp S.I. Mechanical strength of poly(methyl methacrylate) cement-human bone interfaces. J. Biomed. Mater. Res. 1983;17:441–458. doi: 10.1002/jbm.820170305. [DOI] [PubMed] [Google Scholar]
- 153.Yousefi A.M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019;17 doi: 10.1177/2280800019872594. [DOI] [PubMed] [Google Scholar]
- 154.Cervantes-Uc J.M., Cauich-Rodríguez J.v., Hernández-Sánchez F., Chan-Chan L.H. Encyclopedia of Biomedical Polymers and Polymeric Biomaterials. Taylor & Francis; 2015. Bone cements: formulation, modification, and characterization; pp. 1053–1066. [DOI] [Google Scholar]
- 155.O'Dowd-Booth C.J., White J., Smitham P., Khan W., Marsh D.R. Bone cement: perioperative issues, orthopaedic applications and future developments. J. Perioperat. Pract. 2011;21:304–308. doi: 10.1177/175045891102100902. [DOI] [PubMed] [Google Scholar]
- 156.Sallent I., Capella-Monsonís H., Procter P., Bozo I.Y., Deev R.v., Zubov D., Vasyliev R., Perale G., Pertici G., Baker J., Gingras P., Bayon Y., Zeugolis D.I. The few who made it: commercially and clinically successful innovative bone grafts. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Erli H.J., Marx R., Paar O., Niethard F.U., Weber M., Wirtz D.C. Surface pretreatments for medical application of adhesion. Biomed. Eng. Online. 2003;2 doi: 10.1186/1475-925X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ho S.M., Young A.M. Synthesis, polymerisation and degradation of poly(lactide-co-propylene glycol) dimethacrylate adhesives. Eur. Polym. J. 2006;42:1775–1785. doi: 10.1016/j.eurpolymj.2006.03.018. [DOI] [Google Scholar]
- 159.Young A.M., Man Ho S., Abou Neel E.A., Ahmed I., Barralet J.E., Knowles J.C., Nazhat S.N. Chemical characterization of a degradable polymeric bone adhesive containing hydrolysable fillers and interpretation of anomalous mechanical properties. Acta Biomater. 2009;5:2072–2083. doi: 10.1016/j.actbio.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 160.Gellynck K., Abou Neel E.A., Li H.Y., Mardas N., Donos N., Buxton P., Young A.M. Cell attachment and response to photocured, degradable bone adhesives containing tricalcium phosphate and purmorphamine. Acta Biomater. 2011;7:2672–2677. doi: 10.1016/j.actbio.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 161.Abou Neel E.A., Palmer G., Knowles J.C., Salih V., Young A.M. Chemical, modulus and cell attachment studies of reactive calcium phosphate filler-containing fast photo-curing, surface-degrading, polymeric bone adhesives. Acta Biomater. 2010;6:2695–2703. doi: 10.1016/j.actbio.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 162.Neel E.A.A., Salih V., Revell P.A., Young A.M. Viscoelastic and biological performance of low-modulus, reactive calcium phosphate-filled, degradable, polymeric bone adhesives. Acta Biomater. 2012;8:313–320. doi: 10.1016/j.actbio.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ignatius A.A., Augat P., Ohnmacht M., Pokinskyj P., Kock H.-J.R., Claes L.E. A new bioresorbable polymer for screw augmentation in the osteosynthesis of osteoporotic cancellous bone: a biomechanical evaluation. J. Biomed. Mater. Res. 2001;58:254–260. doi: 10.1002/1097-4636(2001)58:3<254::aid-jbm1014>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 164.Morita S., Furuya K., Ishihara K., Nakabayashi N. Performance of adhesive bone cement containing hydroxyapatite particles. Biomaterials. 1998;19:1601–1606. doi: 10.1016/s0142-9612(97)00120-8. [DOI] [PubMed] [Google Scholar]
- 165.Moszner N., Salz U., Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent. Mater. 2005;21:895–910. doi: 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 166.Park K.H., Seong K.Y., Yang S.Y., Seo S. Advances in medical adhesives inspired by aquatic organisms' adhesion. Biomater. Res. 2017;21 doi: 10.1186/s40824-017-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rathi S., Saka R., Domb A.J., Khan W. Protein-based bioadhesives and bioglues. Polym. Adv. Technol. 2019;30:217–234. doi: 10.1002/pat.4465. [DOI] [Google Scholar]
- 168.Bré L.P., Zheng Y., Pêgo A.P., Wang W. Taking tissue adhesives to the future: from traditional synthetic to new biomimetic approaches. Biomater. Sci. 2013;1:239–253. doi: 10.1039/c2bm00121g. [DOI] [PubMed] [Google Scholar]
- 169.Hofman A.H., van Hees I.A., Yang J., Kamperman M. Bioinspired underwater adhesives by using the supramolecular toolbox. Adv. Mater. 2018;30 doi: 10.1002/adma.201704640. [DOI] [PubMed] [Google Scholar]
- 170.Sun J., Su J., Ma C., Göstl R., Herrmann A., Liu K., Zhang H. Fabrication and mechanical properties of engineered protein-based adhesives and fibers. Adv. Mater. 2020;32 doi: 10.1002/adma.201906360. [DOI] [PubMed] [Google Scholar]
- 171.Yang Y.J., Jung D., Yang B., Hwang B.H., Cha H.J. Aquatic proteins with repetitive motifs provide insights to bioengineering of novel biomaterials. Biotechnol. J. 2014;9:1493–1502. doi: 10.1002/biot.201400070. [DOI] [PubMed] [Google Scholar]
- 172.Pandey N., Soto-Garcia L.F., Liao J., Zimmern P., Nguyen K.T., Hong Y. Mussel-inspired bioadhesives in halthcare: design parameters, current trends, and future perspectives. Biomater. Sci. 2020;8:1240–1255. doi: 10.1039/c9bm01848d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee B.P., Messersmith P.B., Israelachvili J.N., Waite J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Silverman H.G., Roberto F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007;9:661–681. doi: 10.1007/s10126-007-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guo Q., Chen J., Wang J., Zeng H., Yu J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale. 2020;12:1307–1324. doi: 10.1039/c9nr09780e. [DOI] [PubMed] [Google Scholar]
- 176.Kord Forooshani P., Lee B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Polym. Chem. 2017;55:9–33. doi: 10.1002/pola.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krtička M., Michlovská L., Nekuda V., Poláček P., Valová K., Žídek J., Kaiser J., Zikmund T., Vojtová L. Ex-vivo biomechanical testing of pig femur diaphysis B type fracture fixed by novel biodegradable bone glue. J. Mech. Behav. Biomed. Mater. 2021;115 doi: 10.1016/j.jmbbm.2020.104249. [DOI] [PubMed] [Google Scholar]
- 178.Zhang H., Bré L., Zhao T., Newland B., da Costa M., Wang W. A biomimetic hyperbranched poly(amino ester)-based nanocomposite as a tunable bone adhesive for sternal closure. J. Mater. Chem. B. 2014;2:4067–4071. doi: 10.1039/c4tb00155a. [DOI] [PubMed] [Google Scholar]
- 179.Axel Nordberg K.P. Fiber reinforced adhesive patch (FRAP): a new technology for minimal invasive treatments of bone fractures. J. Trauma Treat. 2014;s2 doi: 10.4172/2167-1222.S2-007. [DOI] [Google Scholar]
- 180.Granskog V., García-Gallego S., von Kieseritzky J., Rosendahl J., Stenlund P., Zhang Y., Petronis S., Lyvén B., Arner M., Håkansson J., Malkoch M. High-performance thiol–ene composites unveil a new era of adhesives suited for bone repair. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201800372. [DOI] [Google Scholar]
- 181.Nordberg A., Antoni P., Montañez M.I., Hult A., von Holst H., Malkoch M. Highly adhesive phenolic compounds as interfacial primers for bone fracture fixations. ACS Appl. Mater. Interfaces. 2010;2:654–657. doi: 10.1021/am100002s. [DOI] [PubMed] [Google Scholar]
- 182.Olofsson K., Granskog V., Cai Y., Hult A., Malkoch M. Activated dopamine derivatives as primers for adhesive-patch fixation of bone fractures. RSC Adv. 2016;6:26398–26405. doi: 10.1039/c5ra23142f. [DOI] [Google Scholar]
- 183.Hed Y., Öberg K., Berg S., Nordberg A., von Holst H., Malkoch M. Multipurpose heterofunctional dendritic scaffolds as crosslinkers towards functional soft hydrogels and implant adhesives in bone fracture applications. J. Mater. Chem. B. 2013;1:6015–6019. doi: 10.1039/c3tb21061h. [DOI] [PubMed] [Google Scholar]
- 184.Jensen R.A., Morse D.E. The bioadhesive of Phragmatopoma californica tubes: a silk-like cement containing l-DOPA. J. Comp. Physiol. B. 1988;158:317–324. doi: 10.1007/BF00695330. [DOI] [Google Scholar]
- 185.Stewart R.J., Wang C.S., Song I.T., Jones J.P. The role of coacervation and phase transitions in the sandcastle worm adhesive aystem. Adv. Colloid Interface Sci. 2017;239:88–96. doi: 10.1016/j.cis.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stewart R.J., Wang C.S., Shao H. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci. 2011;167:85–93. doi: 10.1016/j.cis.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Basiri Z., Rezayan A.H., Akbari B., Aghdam R.M., Tafti H.A. Developing new synthetic biomimetic nanocomposite adhesives: synthesis and evaluation of bond strength and solubilization. React. Funct. Polym. 2018;127:85–93. doi: 10.1016/j.reactfunctpolym.2018.04.004. [DOI] [Google Scholar]
- 188.Addison J.B., Ashton N.N., Weber W.S., Stewart R.J., Holland G.P., Yarger J.L. β-sheet nanocrystalline domains formed from phosphorylated serine-rich motifs in caddisfly larval silk: a solid state NMR and XRD study. Biomacromolecules. 2013;14:1140–1148. doi: 10.1021/bm400019d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liang C., Strickland J., Ye Z., Wu W., Hu B., Rittschof D. Biochemistry of barnacle adhesion: an updated review. Front. Mar. Sci. 2019;6 doi: 10.3389/fmars.2019.0056. [DOI] [Google Scholar]
- 190.Yamamoto H., Nagai A., Okada T., Nishida A. Synthesis and adhesive studies of barnacle model proteins. Mar. Chem. 1989;26:331–338. doi: 10.1016/0304-4203(89)90038-8. [DOI] [Google Scholar]
- 191.Ambard A.J., Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J. Prosthodont. 2006;15:321–328. doi: 10.1111/j.1532-849X.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 192.Schmit J.P., Hollinger J.0, Milan S.B. Reconstruction of bone using calcium phosphate bone cements: a critical review. J. Oral Maxillofac. Surg. 1999;57:122–126. doi: 10.1016/s0278-2391(99)90338-5. [DOI] [PubMed] [Google Scholar]
- 193.Basirun W.J., Nasiri-Tabrizi B., Baradaran S. Overview of hydroxyapatite–graphene nanoplatelets composite as bone graft substitute: mechanical behavior and in-vitro biofunctionality. Crit. Rev. Solid State Mater. Sci. 2018;43:177–212. doi: 10.1080/10408436.2017.1333951. [DOI] [Google Scholar]
- 194.Rad M.M., Saber-Samandari S., Bokov D.O., Suksatan W., Esfahani M.M., Yusof M.Y.P.M., El-Shafay A.S. Fabrication of elastin additive on polymethyl methacrylate and hydroxyapatite-based bioactive bone cement. Mater. Chem. Phys. 2022;280 doi: 10.1016/j.matchemphys.2022.125783. [DOI] [Google Scholar]
- 195.Li X., Saeed S.S., Beni M.H., Morovvati M.R., Angili S.N., Toghraie D., Khandan A., Khan A. Experimental measurement and simulation of mechanical strength and biological behavior of porous bony scaffold coated with alginate-hydroxyapatite for femoral applications. Compos. Sci. Technol. 2021;214 doi: 10.1016/j.compscitech.2021.108973. [DOI] [Google Scholar]
- 196.de Lacerda Schickert S., Pinto J.C., Jansen J., Leeuwenburgh S.C.G., van den Beucken J.J.J.P. Tough and injectable fiber reinforced calcium phosphate cement as an alternative to polymethylmethacrylate cement for vertebral augmentation: a biomechanical study. Biomater. Sci. 2020;8:4239–4250. doi: 10.1039/d0bm00413h. [DOI] [PubMed] [Google Scholar]
- 197.Grover L.M., Gbureck U., Farrar D., Barralet J.E. Adhesion of a novel calcium phosphate cement to cortical bone and several common biomaterials. Key Eng. Mater. 2006;309–311:849–852. doi: 10.4028/www.scientific.net/kem.309-311.849. [DOI] [Google Scholar]
- 198.Zhong W., Sun L., Yu T., Zhou C. Preparation and characterization of calcium phosphate cement with enhanced tissue adhesion for bone defect repair. Ceram. Int. 2021;47:1712–1720. doi: 10.1016/j.ceramint.2020.08.288. [DOI] [Google Scholar]
- 199.Jang J.H., Shin S., Kim H.J., Jeong J., Jin H.E., Desai M.S., Lee S.W., Kim S.Y. Improvement of physical properties of calcium phosphate cement by elastin-like polypeptide supplementation. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-23577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu F., Ngothai Y., Wei J., Liu C., O'Neill B., Wu Y. Premixed, injectable PLA-modified calcium deficient apatite biocement (cd-AB) with washout resistance. Colloids Surf. B Biointerfaces. 2012;92:113–120. doi: 10.1016/j.colsurfb.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 201.Chew K.K., Low K.L., Sharif Zein S.H., McPhail D.S., Gerhardt L.C., Roether J.A., Boccaccini A.R. Reinforcement of calcium phosphate cement with multi-walled carbon nanotubes and bovine serum albumin for injectable bone substitute applications. J. Mech. Behav. Biomed. Mater. 2011;4:331–339. doi: 10.1016/j.jmbbm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 202.Norton M.R., Kay G.W., Brown M.C., Cochran D.L. Bone glue - the final frontier for fracture repair and implantable device stabilization. Int. J. Adhesion Adhes. 2020;102 doi: 10.1016/j.ijadhadh.2020.102647. [DOI] [Google Scholar]
- 203.Kesseli F.P., Lauer C.S., Baker I., Mirica K.A., van Citters D.W. Identification of a calcium phosphoserine coordination network in an adhesive organo-apatitic bone cement system. Acta Biomater. 2020;105:280–289. doi: 10.1016/j.actbio.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kirillova A., Nillissen O., Liu S., Kelly C., Gall K. Reinforcement and fatigue of a bioinspired mineral–organic bioresorbable bone adhesive. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202001058. [DOI] [PubMed] [Google Scholar]
- 205.Geddes A.T., Thatcher G.P., Hetzel S., McCabe R.P., Vandereby R., Snyder C.J. Biomechanical testing of a calcium phosphate-phosphoserine–based mineral-organic adhesive for non-invasive fracture repair of mandibular fractures in dogs. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mathew R., Pujari-Palmer M., Guo H., Yu Y., Stevensson B., Engqvist H., Edén M. Solid-state NMR rationalizes the bone-adhesive properties of serine- and phosphoserine-bearing calcium phosphate cements by unveiling their organic/inorganic interface. J. Phys. Chem. C. 2020;124:21512–21531. doi: 10.1021/acs.jpcc.0c06224. [DOI] [Google Scholar]
- 207.Spicer C.D., Pujari-Palmer M., Autefage H., Insley G., Procter P., Engqvist H., Stevens M.M. Synthesis of phospho-amino acid analogues as tissue adhesive cement additives. ACS Cent. Sci. 2020;6:226–231. doi: 10.1021/acscentsci.9b01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu D., Pujari-Palmer M., Bojan A., Palmquist A., Procter P., Öhman-Mägi C., Ferguson S.J., Isaksson P., Persson C. The effect of two types of resorbable augmentation materials – a cement and an adhesive – on the screw pullout resistance in human trabecular bone. J. Mech. Behav. Biomed. Mater. 2020;110 doi: 10.1016/j.jmbbm.2020.103897. [DOI] [PubMed] [Google Scholar]
- 209.Qin W., Kolooshani A., Kolahdooz A., Saber-Samandari S., Khazaei S., Khandan A., Ren F., Toghraie D. Coating the magnesium implants with reinforced nanocomposite nanoparticles for use in orthopedic applications. Colloids Surf. A Physicochem. Eng. Asp. 2021;621 doi: 10.1016/j.colsurfa.2021.126581. [DOI] [Google Scholar]
- 210.Angili S.N., Morovvati M.R., Kardan-Halvaei M., Saber-Samandari S., Razmjooee K., Abed A.M., Toghraie D., Khandan A. Fabrication and finite element simulation of antibacterial 3D printed Poly L-lactic acid scaffolds coated with alginate/magnesium oxide for bone tissue regeneration. Int. J. Biol. Macromol. 2023;224:1152–1165. doi: 10.1016/j.ijbiomac.2022.10.200. [DOI] [PubMed] [Google Scholar]
- 211.Nabiyouni M., Brückner T., Zhou H., Gbureck U., Bhaduri S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018;66:23–43. doi: 10.1016/j.actbio.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 212.Mestres G., Abdolhosseini M., Bowles W., Huang S.H., Aparicio C., Gorr S.U., Ginebra M.P. Antimicrobial properties and dentin bonding strength of magnesium phosphate cements. Acta Biomater. 2013;9:8384–8393. doi: 10.1016/j.actbio.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 213.Yu Y., Wang J., Liu C., Zhang B., Chen H., Guo H., Zhong G., Qu W., Jiang S., Huang H. Evaluation of inherent toxicology and biocompatibility of magnesium phosphate bone cement. Colloids Surf. B Biointerfaces. 2010;76:496–504. doi: 10.1016/j.colsurfb.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 214.Wu F., Wei J., Guo H., Chen F., Hong H., Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4:1873–1884. doi: 10.1016/j.actbio.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 215.Nicholson J.W. Adhesive dental materials - a review. Int. J. Adhesion Adhes. 1998;18:229–236. doi: 10.1016/S0143-7496(98)00027-X. [DOI] [Google Scholar]
- 216.Nicholson J.W. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19:485–494. doi: 10.1016/s0142-9612(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 217.Lucksanasombool P., Higgs W.A.J., Higgs R.J.E.D., v Swain M., Howlett C.R. Effects of glass ionomer cements on bone tissue. J. Mater. Sci. Mater. Med. 2002;13:203–210. doi: 10.1023/a:1013890331714. [DOI] [PubMed] [Google Scholar]
- 218.Zhang M., Liu J., Zhu T., Le H., Wang X., Guo J., Liu G., Ding J. Functional macromolecular adhesives for bone fracture healing. ACS Appl. Mater. Interfaces. 2022;14 doi: 10.1021/acsami.1c17434. 1–19. [DOI] [PubMed] [Google Scholar]
- 219.Albrektsson T., Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001;10:S96. doi: 10.1007/s005860100282. –S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Morgan E.F., de Giacomo A., Gerstenfeld L.C. Overview of skeletal repair (fracture healing and its assessment) Methods Mol. Biol. 2014;1130:13–31. doi: 10.1007/978-1-62703-989-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bou-Francis A., Ghanem A. Standardized methodology for in vitro assessment of bone-to-bone adhesion strength. Int. J. Adhesion Adhes. 2017;77:96–101. doi: 10.1016/j.ijadhadh.2017.03.014. [DOI] [Google Scholar]
- 222.van Erk M., van Luijk J., Yang F., Leeuwenburgh S.C.G., Sánchez-Fernández M.J., Hermans E., Félix Lanao R.P., van Goor H. A systematic review and meta-analyses on animal models used in bone adhesive research. J. Orthop. Res. 2021 doi: 10.1002/jor.25057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ge L., Chen S. Recent advances in tissue adhesives for clinical medicine. Polymers. 2020;12 doi: 10.3390/polym12040939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bojan A.J., Stadelmann V.A., Wu D., Pujari-Palmer M., Insley G., Sundh D., Persson C., Engqvist H., Procter P. A new bone adhesive candidate- does it work in human bone? An ex-vivo preclinical evaluation in fresh human osteoporotic femoral head bone. Injury. 2022;53:1858–1866. doi: 10.1016/j.injury.2022.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.