Abstract
Head and neck squamous cell carcinoma (HNSCC) is among the most severe and complex malignant diseases with a high level of heterogeneity and, as a result, a wide range of therapeutic responses, regardless of clinical stage. Tumor progression depends on ongoing co-evolution and cross-talk with the tumor microenvironment (TME). In particular, cancer-associated fibroblasts (CAFs), embedded in the extracellular matrix (ECM), induce tumor growth and survival by interacting with tumor cells. Origin of CAFs is quite varied, and the activation patterns of CAFs are also heterogeneous. Crucially, the heterogeneity of CAFs appears to play a key role in ongoing tumor expansion, including facilitating proliferation, enhancing angiogenesis and invasion, and promoting therapy resistance, through the production of cytokines, chemokines, and other tumor-promotive molecules in the TME. This review describes the various origin and heterogeneous activation mechanisms of CAFs, and biological heterogeneity of CAFs in HNSCC is also included. Moreover, we have highlighted versatility of CAFs heterogeneity in HNSCC progression, and have discussed different tumor-promotive functions of CAFs respectively. In the future, it is a promising strategy for the therapy of HNSCC that specifically targeting tumor-promoting CAF subsets or the tumor-promoting functional targets of CAFs.
Subject terms: Head and neck cancer, Cancer microenvironment
Fact
The origins of CAFs are quite varied, and the main sources of CAFs in TME are local NFs. Other sources of CAFs include MSCs, epithelial cells, adipocytes, pericytes and endothelial.
CAFs are divided into different subclusters (myCAFs, iCAFs and apCAFs) according to their characteristic marker genes, and these CAFs subclusters showed distinct phenotypes in HNSCC.
CAFs play a variety of roles in the progression of HNSCC (proliferation, invasion, migration, angiogenesis, lymphangiogenesis, EMT, and immunosuppression), and CAFs may lead to poor prognosis.
It is a promising strategy for the future therapy of HNSCC that specifically targeting tumor-promoting CAF subsets or the tumor-promoting functional targets of CAFs.
Open questions
What are the mechanisms and signaling pathways involved in the activation of CAFs in HNSCC?
What are the detailed molecular mechanisms by which the heterogeneous versatility of CAFs is involved in HNSCC progression?
CAFs may have complex functions as both tumor-promoting and tumor-suppressing agents, and how to specifically target the tumor-promoting function of CAFs?
Introduction
The most prevalent head and neck cancer type is squamous cell carcinoma (HNSCC), which develops from the mucosal epithelium of the oral cavity, hypopharynx, and larynx [1]. Despite recent advances in HNSCC therapy, the outcome of advanced patients remains poor [2]. Therefore, creating innovative and effective medicines powered by conceptually transformational basic science is urgent. Only a portion of the cancer development process can be explained by the conventional tumor cell-centric theory of the disease. Therefore, it is crucial to have a thorough understanding of the tumor microenvironment (TME).
Through paracrine, juxtacrine, and autocrine connections, the surrounding TME co-evolves into an active state during HNSCC development, resulting in a dynamic signaling circuitry that aids in the initiation, progression, and resistance to therapy [3]. Cancer-associated fibroblasts (CAFs) play numerous functions in the formation of tumors inside the TME, and related research has shown that CAFs can enhance tumor development in myriads of ways [4–6]. Our knowledge of CAF biology is incredibly dependent on their functional variations based on the heterogeneity of CAFs, various CAF subtypes, and their distinct impacts on tumor behavior. This review summarizes current findings about the significance of CAF heterogeneity in the development of HNSCC and discusses CAF targeting in anticancer therapies.
Origins of CAFs
The TME has been identified as one of the variables contributing to the growth and invasion of HNSCC [7]. The constantly active fibroblasts known as CAFs, which are found inside or close to the tumor mass, have been linked to having a solid HNSCC modifying impact and playing a significant role in areas such as drug resistance [7–9]. CAFs are spindle-shaped cells and a highly heterogeneous intra-tumoral population of fibroblasts expressing enhanced cellular migration, elevated proangiogenic cytokine signaling, and increased autocrine growth factor-induced signaling [3, 10].
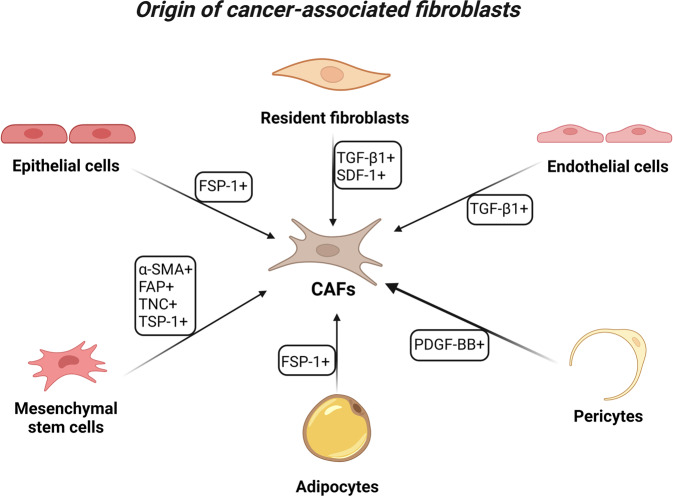
The origin of CAFs can be quite varied (Fig. 1), and the main sources of CAFs in TME are local normal fibroblasts (NFs). Tumor cells secrete growth factors, such as transforming growth factor-beta1 (TGF-β1) and stromal cell-derived factor-1 (SDF1), to facilitate the transformation of NFs into CAFs [11–14]. CAFs are drawn to the tumor site in a process similar to how they are pulled to the site of wound healing [15, 16]. The activated fibroblasts in the tumor matrix do not undergo apoptosis, a typical outcome of activated fibroblasts during typical wound healing. Instead, they constantly interact with tumors; hence, cancers are also known as “wounds that never heal” [17, 18].
Fig. 1. Different origins of cancer-associated fibroblasts (CAFs) in cancer.
The origin of CAFs can be quite heterogeneous, and the main sources of CAFs in TME are NFs. Growth factors like TGF-1 and stromal SDF-1 can be secreted by tumor cells to enable the conversion of NFs into CAFs, and CAFs in the tumor stroma do not undergo apoptosis. Other sources of CAFs include direct generation from MSCs, which can migrate to tumor sites in a way akin to fibroblast migration during wound healing. These migratory cells are drawn to cancer and differentiate into CAFs as a result of their attraction to the disease. In their cytoplasm, these CAFs exhibit particular markers such -SMA, FAP, TNC, and TSP-1. Moreover, epithelial cells can undergo EMT to develop into CAFs, and these CAFs maintain the genetic changes made to the parental genome. Due to the expression of mesenchymal lineage-committed marker genes, CAFs also originate from adipocytes. Endothelial and pericyte cells have the ability to transdifferentiate and add to the CAF population. Proliferating endothelial cells can undergo endothelial to mesenchymal conversions to develop CAFs under the effect of TGF-1 produced by cancer. By the influence of PDGF-BB, pericytes are also a source of CAFs. (Created with BioRender.com).
There are various other sources of CAFs, and direct production of CAFs from mesenchymal stem cells (MSCs) is possible [19], which also reach tumor sites using processes similar to fibroblast migration during wound healing. There is evidence that these migratory cells are attracted to cancer and undergo differentiation into CAFs. These CAFs express specific markers such as α-smooth muscle actin (α-SMA), fibroblast-activation protein (FAP), tenascin-C (TNC), and thrombospondin-1 (TSP-1) in their cytoplasm [20]. CAFs also arise from adipocytes due to the expression of mesenchymal lineage-committed marker genes [21]. Moreover, CAFs also can arise from epithelial cells via epithelial–mesenchymal transition (EMT), and EMT-derived CAFs retain the genetic alterations in their parental genome [4, 19]. Although EMT-derived CAFs seldom make up the majority of CAFs, several data point to the development of CAF mutations. However, Wang et al. [22] focused on the karyotype variations and biological characteristics between primary cultured CAFs and NFs. According to this model, HEp-2 laryngeal cancer cells could not produce CAFs via EMT during tumor growth. Pericytes and endothelial cells can transdifferentiate and contribute to the population of CAFs. Under the influence of TGF-β1 released by cancer, proliferating endothelial cells can undergo endothelial to mesenchymal transitions to become CAFs [19, 23]. Part of CAFs also derives from pericytes through the influence of platelet-derived growth factor-BB (PDGF-BB) [24]. All these CAF origins are not mutually exclusive and produce a vastly heterogeneous subpopulation of CAFs within individual cancer types. This could be the reason for the reported variations in the identification markers for CAFs.
Activation of CAFs in cancer
A sea of signaling pathways regulates the activation and reprogramming of CAFs. Shimoda et al. [25] confirmed that complete knockout of tissue inhibitor of metalloproteinases (TIMP) is sufficient to obtain the hallmark CAFs function. Similarly, loss of expression of the tumor suppressor p85α results in fibroblasts in the stroma that can acquire characteristics of CAFs [26]. Failure of the Notch effector protein CSL [27] or upregulation of the proto-oncogene YAP1 [28] in fibroblasts is sufficient to activate CAFs and promote tumorigenesis. However, it is still unclear which epithelial-stromal cell or stromal-stromal cell cross-talk and other mechanisms activate fibroblasts in fibroblasts harboring mutations or alterations in the expression of these genes. Numerous, and possibly separate, external cues that activate fibroblasts in the TME are present in various tumor types. The anti-invasive or pro-invasive effects of CAFs may be activated by a particular combination of growth factors and cytokines that are unique to each tumor type. In this regard, the contractile and invasive characteristics of CAFs have been linked to the leukemia inhibitory factor (LIF) [29, 30]. Increasing WNT7A synthesis by invasive cancer cells in breast cancer may improve TGF-receptor signaling related to the invasive characteristics of CAFs [31]. Vitamin D receptor activation may decrease the CAFs’ tumor-promoting secretome in pancreatic ductal adenocarcinoma (PDAC) [32]. Therefore, most current studies are more inclined to believe that various regulators released by tumor cells activate quiescent fibroblasts or other stromal target cells and reprogram their activation into irreversible CAFs [33, 34].
In a host of studies on HNSCC, NFs are fibroblasts extracted from normal tissue distant from cancer. They can be activated in vitro or in vivo, and activated NFs are found to possess abilities that facilitate cancer [7, 35–37]. Kim et al. found that this process can be induced via co-culturing NFs with oral squamous carcinoma cells (OSCCs) in vitro [38, 39]. Other studies have also reflected a familiar phenomenon in the treatment with TNF-α [40] and TGF-β1 [41–43]. TGF-β1 is regarded as a traditional activator of the phenotype known as myofibroblastic CAFs, which is distinguished by the upregulation of α-SMA. HNSCC cells reportedly interact with skin dermal fibroblasts [44, 45], according to investigations that administered either TGF-β1 or LIF, two distinct activation activators, which led to the same epigenetically controlled CAFs phenotype [45]. Notably, TGF-β1 and fibroblast growth factor (FGF) cause opposing regulation of CAF effector genes, demonstrating that various activation approaches may result in various CAF phenotypes, with consequent diverse effects on the growth of cancer [45]. However, in contrast to an experimental context, the interactions that result in the in vivo activity of fibroblasts are more complex. In addition, extracellular vesicles (EVs) are substances with lipid bilayers that most cells release into their environment [46, 47], and EVs have been thought to be a way of expelling toxic or unnecessary internal substances from cells, and they are also significant facilitators of cell-to-cell communication [48]. EVs can transport a variety of cargoes that can send messages to destination cells to cause metabolic responses, they can also translocate into the cytoplasm of target cells, become active, and then regulate how secretory cells interact with the extracellular matrix there [49]. The stromal target cells of tumor cell-derived EVs are mainly fibroblasts, which dynamically regulate each other in the tumor microenvironment, diverse HNSCC-derived EVs cargoes, such as nucleic acids, signaling proteins, and metabolites, contribute to the activation of CAFs (Table 1).
Table 1.
Fibroblast activation and reprogramming mediated by HNSCC-derived EVs.
| Cancer type | EVs-secreted content | Stromal target cells | Mechanism | References |
|---|---|---|---|---|
| Head and neck squamous cell carcinoma | miR-192/215 | Human foetal lung fibroblasts (MRC-5cells) | Targeting regulating Caveolin-1 | [134] |
| Oral squamous cell carcinoma | LncRNA CAFs | Oral fibroblasts | Regulating IL-33 | [135] |
| Head and neck squamous cell carcinoma | TGFβ1 | Head and neck fibroblasts | Regulating Fibronectin | [43] |
| Nasopharyngeal carcinoma | LMP1 | Nasopharyngeal fibroblasts | NF-κB p65 signaling pathway | [103] |
Biological heterogeneity of CAFs in HNSCC
To isolate or identify CAFs, a variety of intracellular, extracellular, and cell surface proteins have been utilized. However, there is no common marker for CAF research. For instance, upregulation of α-SMA has previously been thought to be a distinguishing factor between activated CAFs and NFs [50]. However, a recent study by Michael Bartoschek et al. demonstrated that the existence of reduced α-SMA CAFs highlights the necessity of taking into account a number of characteristics when analyzing CAFs activation [51–53]. Furthermore, pericytes, lymphatic endothelial cells, and fibroblastic reticular cells, in that order, show significant levels of some proteins that are extensively expressed among CAFs, including α-SMA, podoplanin (PDPN), and FAP [4]. A selection of the reported markers of HNSCC CAFs is summarized in Table 2.
Table 2.
Markers and signature genes of HNSCC CAFs.
| CAFs markers/ signature genes | Description of markers/ genes | Expression level in HNSCC CAFs | Biological functions/ notes | References |
|---|---|---|---|---|
| Vimentin | Type III intermediate filament protein | Upregulated | Promoting tumor EMT/ poor outcome | [70] |
| α-SMA | Actin isoform | Upregulated | Related with CAFs activation/ poor outcome | [136] |
| FSP1 | Calcium-binding protein containing 2 EF-hand calcium-binding motifs | Upregulated | Identifying CAFs in vitro | [70] |
| FAP | Membrane-bound gelatinase | Upregulated | Poor outcome | [60, 136] |
| PDGFRα | Protein tyrosine kinase receptor | Upregulated | Mediating mesenchymal stromal cell chemotaxis to tumor | [137] |
| PDGFRβ | Protein tyrosine kinase receptor | Upregulated | Signing stromal activation in tumor | [60, 138] |
| Caveolin-1 | Scaffolding protein within caveolar membranes | Upregulated | Inhibited the TGF-β/SMAD signaling and promoting CAFs activation | [134, 139] |
| Periostin | Secreted extracellular matrix protein, a ligand for α-V/β-3 and α-V/β-5 integrins | Upregulated | Promoting tumor stemness/ poor outcome | [60, 85] |
| Podoplanin | Mucin-type protein, heavily O-glycosylated glycoprotein | There is no relationship directly | Promoting lymph nodes metastasis/ poor outcome | [140] |
| CD44 | Non-kinase transmembrane glycoprotein | Upregulated | Mediating immunosuppression/ poor outcome | [141] |
| AKT3 | A serine/ threonine protein kinas that modulates various cellular responses via the PI3K-AKT pathway | Upregulated | Mediating immunosuppression and promoting myofibroblastic phenotype/ poor outcome | [60] |
| COX-2 | An inducible enzyme responsible for the production of prostaglandins at sites of inflammation and wound‐healing. | Upregulated | Promoting tumor migration, angiogenesis and invasiveness/ poor outcome | [95, 125] |
The roles of CAFs are as varied as their identification markers, and they have dynamically heterogeneous impacts on cancer at various stages [54–56].According recent research, CAFs were divided into “myofibroblastic CAFs” (myCAFs), “inflammatory CAFs” (iCAFs)and “antigen-presenting CAFs” (apCAFs) according to their characteristic marker genes, and these CAFs subclusters showed distinct phenotypes enriched in myofibroblast function, ECM remodeling and antigen-presenting function respectively [57–59]. Among them, myCAFs are characterized by myofibroblasts with high α-SMA expression and enrichment in smooth muscle contraction [57], and ECM-receptor interaction, vascular smooth muscle contraction, and focal adhesion are enriched in myCAFs [59]. Related study found that CAFs in HNSCC mainly comprise myCAFs that play key roles in HNSCC progression, including matrix remodeling, the production of growth factors and cytokines, and metabolic effects [60]. CXCL12 is a vital chemokine, which can promote cancer cells proliferation, angiogenesis, and metastasis [61], and Wang et al. found that CXCL12 is mainly secreted by iCAFs in OSCC [59]. That means iCAFs may facilitate HNSCC progression via some mechanisms. Recent studies have revealed a novel apCAFs in pancreatic ductal adenocarcinoma [62], it was observed that apCAFs were capable of expressing genes associated with the major histocompatibility complex (MHC) class II family. Further evidence that apCAFs might deliver antigens to T cells came from in vivo testing, the expression of costimulatory genes, which are necessary for T cell proliferation, was low in the apCAFs [30]. This suggested that apCAFs could be involved in TME immunosuppression. Zhang et al. found that Cluster 4 expressed high levels of MHC class II family, which was characterized as antigen-presenting CAFs, and that revealed that perhaps apCAFs also exist in HNSCC tissue [57].
In addition, despite the overwhelming theories that CAFs are positive regulators of cancer, however, recent research has shown that some CAFs subtypes can inhibit tumor development through certain mechanisms as well [4, 19]. CAFs have been defined as C1 and C2 types according to low and high α-SMA expression in OSCCs, respectively, and C1-type CAFs suppress the self-renewal of oral stem-like cancer cells (SLCCs) [63]. And that may provide new strategy for anti-cancer therapy of HNSCC in the future.
Versatility of CAFs heterogeneity in HNSCC progression
In human samples, spindle-shaped stromal cells that express the myofibroblastic marker α-SMA are often used to identify CAFs in HNSCC [64, 65]; other markers are less frequently co-expressed [66, 67]. There may be a subgroup of non-myofibroblastic CAFs, whose functional importance remains unclear, given that positivity for α-SMA is very varied [64, 65, 67]. Chemotherapy could also result in upregulation of α-SMA [68]. Based on the myofibroblastic phenotype, the clinical and prognostic characteristics of CAFs have been discovered. Vered et al. found that worse clinical parameters [69, 70] and terrible outcome [67, 71–73] are likely linked with high numbers of CAFs. CAF-derived products grant tumors different promoting abilities [63, 74, 75], and the possible subtypes of CAFs must be considered when assessing those differences [63, 74, 75]. For example, NFs were induced by HPV-negative oropharyngeal cells to release hepatocyte growth factor (HGF) and IL-6, which might encourage tumor cell formation by activating c-met and STAT3. While Oropharyngeal cells that were HPV-positive did not work [76]. A selection of the reported markers and genes of CAFs is summarized in Table 2.
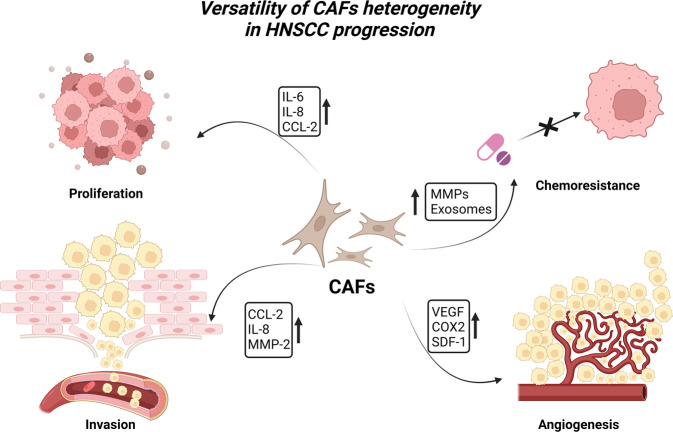
The procedure for promoting tumor growth involves several channels and signals. Numerous studies have demonstrated that CAFs play a variety of roles in carcinogenesis, including proliferation, invasion, and metastasis, through the production of cytokines, chemokines, and the extracellular matrix (ECM) in the TME [4, 19, 70, 77–79] (Fig. 2).
Fig. 2. Versatility of cancer-associated fibroblasts (CAFs) heterogeneity in HNSCC progression.
By the synthesis of cytokines, chemokines, and the extracellular matrix (ECM) in the TME, CAFs perform a range of roles in carcinogenesis, including proliferation, chemoresistance, invasion, and angiogenesis. As epithelial cells undergo the transformation to become CAFs during the premalignant stage, it is believed that the primary function of CAFs in the development of cancer is to stimulate the proliferation of cancer cells. In the coculture of CAFs/NFs-OCCs, CAFs but not NFs facilitated OCC cell proliferation and invasion, and MMP-2 derived from senescent CAFs induced keratinocyte dis-cohesion and epithelial invasion into collagen gels in a TGF-β-dependent manner. In addition, CAFs can cause angiogenesis in HNSCC by producing PGE2 through COX-2, and VEGF and SDF-1 are required for CAFs to stimulate neoangiogenesis in the tissue of NPC. Moreover, by secreting exosomes to the tumor, CAFs can confer HNSCC therapy resistance, such as chemoresistance. (Created with BioRender.com).
CAFs contribute to proliferation and stemness in cancer
The primary role of CAFs in carcinogenesis is assumed to be related to the process of epithelial cells transforming into CAFs during the premalignant stage. This property of CAFs is thought to be the stimulation of cancer expansion [80]. Simona et al. demonstrated that OSCC CAFs-derived microfibrillar-associated protein five could increase phosphorylation of PDK1 and Akt and decrease cRAF and PTEN to induce tumor proliferation [81]. Qin et al. found that HNSCC CAFs-derived IL-6 can promote tumor proliferation by regulating osteopontin expression in a STAT3 dependent-way [82]. In a related study [83], CAFs–OSCC coculture creates a favorable cytokine-rich microenvironment. CAFs produce high chemokine ligand 2 (CCL-2) and promote oral cancer cells (OCCs) proliferation, migration, invasion, and tumor growth.
CAFs can also maintain cancer cell stemness or even encourage the reacquisition of stem-like features via producing insulin-like growth factor (IGF)-II [84]. CAFs play a crucial function in controlling the TME and promoting cancer development because they have a variety of effects on cancer stemness and proliferation. According to a related study [85], Binbin Yu et al. demonstrated a notable relationship among PTK7, Wnt/β-catenin pathway, and aggressive clinicopathologic features in HNSCC. Further, a co-immunoprecipitation (co-IP) assay confirmed that periostin secreted by CAFs might act as a receptor because it is a potential upstream ligand of PTK7. Further analysis revealed that periostin boosted the tumor stem cell (CSC)-like phenotype through PTK7-Wnt/β-catenin signaling, inducing HNSCC cell migration in vitro. The study also assessed tumor initiation and progression in vivo. The results suggest that the combination of periostin and PTK7 might be a potential prognostic and diagnostic indicator and a promising therapeutic target.
CAFs promote invasion, migration, and EMT in cancer
Accumulating evidence suggests that CAFs can promote tumor development in other ways, such as invasion and migration, via certain mechanisms [86]. For instance, Li et al. demonstrated that in a coculture of CAFs/NFs-OCCs, CAFs but not NFs promoted OCC cell proliferation and migration [87]. Matrix metalloproteinase (MMP)-2 derived from senescent CAFs-CM induced keratinocyte dis-cohesion and epithelial invasion into collagen gels in a TGF-β-dependent manner. Hassona et al. [88] demonstrated that senescent CAFs from genetically unstable OSCC promote a more aggressive oral cancer phenotype through the production of active MMP-2, disruption of epithelial adhesion, and induction of keratinocyte invasion. According to a study by Qin et al. [89], periostin is overexpressed in HNSCC and linked with the proliferation and spread of tumor cells. Periostin was found to be substantially expressed in CAFs and hardly expressed in NFs according to western blotting, real-time PCR, and semi-quantitative RT-PCR examinations. Therefore, they thought that the primary source of periostin in HNSCC tissues was the cancer stroma, particularly CAFs.
Among the most important ways that CAFs promote carcinogenesis is via EMT. This mechanism gives tumor cells the ability to move about and invade nearby tissues, which leads to the dispersal of tumor cells and the progress of metastatic disease [90]. Upregulation of EMT markers is common in primary and metastatic cancer, as shown by immunohistochemical analyses of SCC, indicating that there is a strong connection between CAFs and cancer-promoting functions, such as EMT induction and metastasis [91]. Furthermore, TNFa facilitates the myofibroblast differentiation of NFs to CAFs. CAF-derived SDF1 can separately serve as an independent factor to enhance metastasis, EMT, and angiogenesis of tongue cancer [40].
CAFs promote angiogenesis and lymphangiogenesis
Prior research concentrated on the TME’s vascularization and immunological response because of their potential as therapeutic targets [92, 93]. Many models have supported the contribution of CAFs to tumor vascularization. For instance, PDGFR-expressing CAFs promote cancer cell growth and angiogenesis in a model of HPV cervical carcinogenesis. Cancer cell-derived PDGF promotes FGF2 production, which produces a proangiogenic impact. This effect is eliminated by pharmaceutical therapy that blocks stromal PDGFR signaling [94]. CAFs are reported to induce angiogenesis in HNSCC via COX-2-mediated production of prostaglandin E2 (PGE2) [95, 96]. In addition to increasing invasion and vein formation, oral CAFs have been demonstrated to induce tumor vascularization [40]. In nasopharyngeal carcinoma (NPC), Wang et al. found that CAFs are closely linked to angiogenesis. They showed that fibroblasts from NPC tissues have elevated levels of α-SMA expression, and that the stroma of NPC tissues also included elevated endothelial progenitor cells, which promote neoangiogenesis in a VEGF- and SDF-1 dependent way [97]. Further, they demonstrated that fibroblasts from NPC tissues have much elevated levels of α-SMA expression, and that the stroma of NPC tissues also included elevated endothelial progenitor cells, which promote angiogenesis in a VEGF- and SDF-1-dependent way. In the TME, lymphatic vessel remodeling facilitates cancer progression and metastasis [98]. To efficiently study the role of CAFs in lymphatic vessel conditioning in the context of HNSCC, Karina et al. built lymphatic organotypic coculture models using HNSCC-derived CAFs [9]. They found that HNSCC-derived CAFs can induce lymphatic vessel sprouting to alter lymphatic vessel permeability and cause gene expression changes in lymphatic vessels.
CAFs regulate the immune systems
Cytokines released by CAFs also control the polarization and recruitment of immune cells. Hideyuki et al. [99] found that compared with control cells, CAF-educated cells suppressed T cell proliferation more strongly, and the neutralization of TGF-β, IL-10, or arginase I significantly restored T cell proliferation. According to their findings, CAFs regulate the tumor immunosuppressive microenvironment in OSCCs by fostering the protumoral phenotype of tumor-associated macrophages (TAMs). Furthermore, this potential role of CAFs in regulating immune cells and vascular demonstrates the complications of CAFs and their potential application in anticancer therapies. In the follow-up study, the team demonstrated that AKT3 expression in CAFs promoted immunosuppression in HNSCC and related to poor prognosis [60]. T cells cocultured with AKT3 knockdown CAFs proliferated more than control cells. In addition, AKT3 knockdown CAFs showed reduced proliferation and migration compared with controls, and overall survival (OS) was significantly shorter in patients with AKT3-positive CAFs.
Furthermore, in order to compared the effects between CAFs and NFs on T cell proliferation in HNSCC, Hideyuki et al. co-cultured CAFs and NFs with fluorescent cell staining dye -labeled T cells, and found that the suppressor activity of CAFs was greater than that of NFs [100]. In addition, the suppression of T cell proliferation by the culture supernatant from CAFs was higher than that from NFs. And they demonstrated that the supernatant from CAFs induced T cells apoptosis and regulatory T cells. Those results elucidated that CAFs collaborated with tumor cells in the TME to establish an immunosuppressive network that facilitated tumor evasion from immunological destruction. Moreover, Huang et al. [101] found that there is a negative correlation between WNT2+ CAFs and active CD8+ T cells was detected in OSCC, and their subsequent mechanistic analyses demonstrated that CAFs-derived WNT2 inhibit the dendritic cell (DC) -mediated anticancer T-cell response via the SOCS3/p- JAK2/p- STAT3 signalling cascades.
CAFs induce cancer progression via metabolic changes
Metabolic reprogramming most frequently results from the TME. Tumor cells are well recognized for altering their metabolism to sustain high proliferation rates and survive in adverse conditions with limited oxygen and nutritional deficiencies [102]. A growing body of research indicates that CAFs play a crucial role in regulating tumor metabolism, particularly through dysregulating various metabolic pathways, such as glucose, amino acids, and lipid metabolism. These actions aid in the development, spread, and resistance of tumor cells to treatments [103–106].
Cancer cells perform glycolysis rapidly, even under aerobic conditions, thus increasing glucose uptake and lactate secretion, an effect called the “Warburg effect” or “aerobic glycolysis” [107]. Tumor cells hijack CAFs and reprogram their metabolism; under the impact of cancer cells, CAFs display comparable aerobic glycolysis, which is known as the reverse “Warburg effect” [106]. In contrast to NFs, CAFs significantly switch from aerobic glycolysis to oxidative phosphorylation, and can secrete products such as pyruvate and lactate to meet the metabolic needs of cancer cells. Zhang and colleagues observed that OSCC-derived CAFs exhibited significantly higher integrin beta 2 (ITGB2), and increased expression of ITGB2 in CAFs was associated with a poor prognosis. ITGB2 controls the PI3K/AKT/mTOR pathway, which increases CAFs’ glycolytic activity. Lactate generated by CAFs with ITGB2 overexpression is subsequently absorbed by cancer cells, promoting the growth of OSCCs [108]. NPC has been linked to the lactate shuttle phenomenon [103]. The investigators noted that inhibiting monocarboxylate transporter 4 (MCT4) upregulation in activated CAFs significantly reduced nasopharyngeal carcinoma cell proliferation, invasion, and colony formation. Moreover, Dhruv Kumar et al. [109] found that CAFs-secreted HGF facilitates HNSCC progression: HNSCC cancer cells and CAFs have a metabolic relationship in which CAFs secrete HGF to induce a glycolytic switch in HNSCC cells, and HNSCC cells secrete basic FGF to promote lactate consumption by CAFs.
CAFs confer cancer therapy resistance
The TME can impose a substantial impact on how well a therapy works since it continuously supplies pro-tumorigenic substances both during and after therapy, reducing the lethal effect on tumor cells [110, 111]. Standard therapies for HNSCC have not changed much over the years, and treatment resistance is still an issue [112]. According to investigations, CAFs promote the survival of HPV-negative HNSCC after cisplatin treatment [7, 113], as well as HPV-positive and negative cells that receive cetuximab [114, 115] and mTOR inhibitor therapy [114]. MMP-1 was shown to be more highly expressed in both the cancer cells and the CAFs when HNSCC were cocultured with them, according to Johansson et al. [115]. Furthermore, the addition of an MMP inhibitor partially eliminated CAF-induced tolerance. The fact that CAFs treated with siRNA directed against MMP-1 continued to shield cancer cells from cetuximab therapy suggests that either several MMPs may work together to promote resistance, or that a different MMP family member underlies the protective effect. Johansson et al. discovered that cetuximab-induced growth suppression was lessened when cancer cells were cocultured with CAFs in a transwell system, indicating that the resistance to treatment was caused by soluble substances produced from CAFs [115]. When HNSCC cell lines and CAFs were cocultured, MMP-1 expression was increased in both the cancer cells and the CAFs. Furthermore, the addition of an MMP inhibitor partially eliminated CAF-induced resistance. These findings indicate that the unique modification of cetuximab sensitivity is CAF-dependent and imply that blocking MMPs could enhance the benefits of EGFR-targeted treatment.
Furthermore, CAFs can target specific drugs or treatment modalities through EV-mediated tumor therapy resistance, making the results more accurate. Qin et al. [7] showed that HNSSC CAFs are intrinsically resistant to cisplatin, and CAFs can secrete miR-196a to tumor cells through exosomes. Consequently, miR-196a released from exosomes can confer cisplatin resistance to cancer cells by novel binding targets, CDKN1B and ING5, demonstrating that miR-196a could be a potential predictor in HNSCC and may become a potential therapeutic target for cisplatin resistance. Moreover, Peltanova et al. [116] found that CAFs from different patients had different sensitivity for cisplatin, and demonstrated that CAFs could enhance and/or inhibit colony-forming capability and chemoresistance in HNSCC cells via paracrine effects and subsequent changes in gene expression of cancer-associated genes in cancer cells.
There are various immune escape mechanisms in cancer, such as suppression of lymphocyte infiltration into the tumor mass. Ford et al. [117] found that CAF induce immunotherapies resistance via specifically excluding CD8 + T-cells from the tumor mass, and they found that by the inhibition of NOX4, CAF can be precisely targeted to decrease and revert CAF differentiation, inducing CD8+ T-cell infiltration, and restore the sensitivity of CAF-rich tumor to immunotherapy. In the therapy of anticancer, there exist association between CAFs subtypes and immunotherapy resistance: different CAF subtypes express distinct immunosuppressive factors [118]. CAF subtypes were linked to various clinical prognosis, and researchers discovered important molecular pathways that might either trigger or repress cancer growth, or were involved in resistance to antiPD1 or anti-PD-L1 immunotherapy [118]. In addition, Ksenis et al. [119] showed that higher TGF-β pathway activity in CAFs is correlated with HNSCC resistance to cetuximab. These TGF-activated CAFs produce substances that limit cetuximab’s ability to fight tumors, both in vitro and in vivo. Cetuximab’s effectiveness was increased, and HNSCC development was stopped by just blocking TGF-β signaling.
Anticancer therapy based on targeting CAFs
Cancer cells, immune cells, and other stromal cells, such as CAFs, constantly interact with one another. Over the past 10 years, anticancer treatments that target CAFs have attracted a lot of attention. FAP is a kind of serine protease that can regulate the physiological function of myofibroblasts and is frequently utilized in studying the activation of CAFs [120]. Targeting FAP in preclinical animals has demonstrated antitumor effectiveness. In mice, the reduction of cells that produce FAP results in a therapeutic vaccine against cancers with TNF and IFN [121]. A related study reported that CAFs, but not HNSCC cell lines, secrete HGF [122], both HGF and c-Met levels are increased in HNSCC compared with normal mucosa, and that HGF acts in a paracrine manner to facilitate HNSCC cell proliferation and invasion [123]. Dhruv Kumar et al. [124] have demonstrated for the first time that the HGF-targeted antibody ficlatuzumab inhibits CAFs-facilitated HNSCC cell migration, invasion, and proliferation. Li et al. [87] demonstrated the dysregulation of miR-124 in oral CAFs and OSCCs compared with matched NFs, and showed that restoring miR-124 expression by lentiviral infection or formulated miR-124 injection inhibited cancer growth in vivo, indicating miR-124 rescue may become a potential therapeutic application in OSCC in the future. Furthermore, Zhu et al. discovered that COX-2 elevates tumor necrosis factor-α expression in CAFs to promote NPC cell migration and invasiveness [125]. Importantly, they discovered that inhibiting COX-2 and prostaglandin E2, a downstream product of COX-2, as well as knocking down COX-2 expression in vivo, induced invasion and metastasis in vitro, indicating that the identification of this COX-2-mediated axis may present odds for targeting CAFs in advanced NPC. In addition, Hideyuki et al. [60] elucidated the function of AKT3 of CAFs in mediating immunosuppression and promoting myCAFs, and OS was significantly shorter in patients with AKT3-positive CAFs. T cells that were cocultured with conditioned medium from AKT3 knockdown CAFs proliferated more than control cells; AKT3 knockdown CAFs showed a reduced proliferation and migration compared with controls. These findings suggest the viability of a new therapeutic modality that targets CAFs in HNSCC.
It is a kind of intriguing strategy: focusing on cytokines and other elements that are connected to CAF biology [126]. The JAK2/STAT3 pathways are intriguing targets for CAF activation, while the constitutive activation of STAT3 by numerous signaling pathways and other variables has made the creation of STAT3 inhibitors challenging over time [127]. Notably, Kasembeli et.al developed TTI-101, a competitive inhibitor of STAT3 that has demonstrated target engagement, little toxicity, and evidence of clinical benefit in a Phase I study in patients with solid tumors (NCT03195699). And they found TTI-101 didn’t have an impact on mitochondrial activity, STAT3 aggregation, chemical modification, or neuropathic pain in subsequent research [128]. Furthermore, both in vivo and in vitro JAK2 inhibitors have demonstrated to reduce HNSCC proliferation in previous study [129]; Ruxolitinib, a JAK1/2 inhibitor approved for myelofibrosis, has being evaluated in operable HNSCC (NCT03153982). In addition, FAP is expressed scarcely in common condition, and its high and restricted expression on reactive stroma in TME makes FAP a promising target for anticancer therapy. FAP-IL2v is an immunocytokine comprising an antibody against FAP and an IL-2 variant, and FAP-IL2v demonstrated ability of targeting tumor according to imaging studies results [130]. Moreover, in vitro and vivo, FAP-IL2v significantly promoted the activity of therapeutic antibodies that mediate antibody-dependent or T cell-dependent cellular cytotoxicity (TDCC) and of programmed death-ligand 1 (PD-L1) checkpoint inhibition. In the clinical trial about combination therapy of FAP- IL2v with trastuzumab and cetuximab (NCT02627274), rapid expansion of CD8 cells and NK cells, but not Tregs, was observed in treated patients. The result indicated that FAP-IL2v is a promising immunocytokine that potentiates the efficacy of anticancer immunotherapies.
Conclusion and perspective
Treatment options for HNSCC are increasingly abundant, but the prognosis of patients is still unsatisfactory. CAFs play an essential role in HNSCC progression, and their functional differences are based on the heterogeneity of CAFs. Some studies have suggested that CAFs or fibroblasts have detrimental impacts on tumors that include a number of factors, which is contrary to the predominant idea that CAFs always facilitate tumor [131–133]. Oral CAF subtypes with a lower score for α-SMA (C1-type CAFs) were demonstrated to be more likely to induce cell proliferation but suppress the self-renewal growth of oral SLCCs, and Ankit et al. found the decisive role of bone morphogenetic protein 4 in C1-type CAFs-mediated suppression of self-renewal of oral SLCCs [63].
However, the heterogeneity of CAFs has not been studied extensively in HNSCC, and the mechanism of their impact on tumor progression remains unclear. The actual biological functions of different groups of CAFs in tumorigenesis still need to be validated using various tumor models and clinical data. Given that CAFs may have complex functions as both tumor-promoting and tumor-suppressing agents, potential therapeutic strategies targeting CAFs should specifically target tumor-promoting CAF subsets or the tumor-promoting functional targets of CAFs.
Acknowledgements
The authors have no relevant financial or non-financial interests to disclose.
Author contributions
CH, YZ: Conceptualization, Writing—original draft, Writing—review & editing. CW, QH: Conceptualization, Supervision, Writing—review & editing. All authors read and approved the final manuscript.
Funding
The present study was supported by the grants from Shanghai Sailing Program (23YF1404700) and the Special Fund for Clinical Research in Health Industry of Shanghai Municipal Health Commission (No.202240320).
Data availability
All data included in this review are available upon request by contact with the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chen Hu, Yifan Zhang
Contributor Information
Chunping Wu, Email: wcpeent@163.com.
Qiang Huang, Email: huangq16@fudan.edu.cn.
References
- 1.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Den Bossche V, Zaryouh H, Vara-Messler M, Vignau J, Machiels JP, Wouters A, et al. Microenvironment-driven intratumoral heterogeneity in head and neck cancers: clinical challenges and opportunities for precision medicine. Drug Resist Updat. 2022;60:100806. doi: 10.1016/j.drup.2022.100806. [DOI] [PubMed] [Google Scholar]
- 3.Kanzaki R, Pietras K. Heterogeneity of cancer-associated fibroblasts: opportunities for precision medicine. Cancer Sci. 2020;111:2708–17.. doi: 10.1111/cas.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021;101:147–76.. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–88.e16. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 7.Qin X, Guo H, Wang X, Zhu X, Yan M, Wang X, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20:12. doi: 10.1186/s13059-018-1604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matos LL, Menderico Junior GM, Theodoro TR, Pasini FS, Ishikawa MM, Ribeiro AAB, et al. Cancer-associated fibroblast regulation by microRNAs promotes invasion of oral squamous cell carcinoma. Oral Oncol. 2020;110:104909. doi: 10.1016/j.oraloncology.2020.104909. [DOI] [PubMed] [Google Scholar]
- 9.Lugo-Cintrón KM, Ayuso JM, Humayun M, Gong MM, Kerr SC, Ponik SM, et al. Primary head and neck tumour-derived fibroblasts promote lymphangiogenesis in a lymphatic organotypic co-culture model. EBioMedicine. 2021;73:103634. doi: 10.1016/j.ebiom.2021.103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–905. doi: 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher PG, Bao Y, Prorock A, Zigrino P, Nischt R, Politi V, et al. Gene expression profiling reveals cross-talk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res. 2005;65:4134–46. doi: 10.1158/0008-5472.CAN-04-0415. [DOI] [PubMed] [Google Scholar]
- 12.Buess M, Nuyten DS, Hastie T, Nielsen T, Pesich R, Brown PO. Characterization of heterotypic interaction effects in vitro to deconvolute global gene expression profiles in cancer. Genome Biol. 2007;8:R191. doi: 10.1186/gb-2007-8-9-r191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107:20009–14. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller L, Goumas FA, Affeldt M, Sandtner S, Gehling UM, Brilloff S, et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol. 2007;171:1608–18. doi: 10.2353/ajpath.2007.060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stellos K, Kopf S, Paul A, Marquardt JU, Gawaz M, Huard J, et al. Platelets in regeneration. Semin Thrombosis Hemost. 2010;36:175–84. doi: 10.1055/s-0030-1251502. [DOI] [PubMed] [Google Scholar]
- 16.Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122:2550–4. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- 17.Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 18.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 19.Bu L, Baba H, Yoshida N, Miyake K, Yasuda T, Uchihara T, et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 2019;38:4887–901.. doi: 10.1038/s41388-019-0765-y. [DOI] [PubMed] [Google Scholar]
- 20.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PloS ONE. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–68. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Wu CP, Pan JY, Zheng WW, Cao XJ, Fan GK. Cancer-associated fibroblasts in a human HEp-2 established laryngeal xenografted tumor are not derived from cancer cells through epithelial-mesenchymal transition, phenotypically activated but karyotypically normal. PloS ONE. 2015;10:e0117405. doi: 10.1371/journal.pone.0117405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 24.Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci USA. 2016;113:E5618–27. doi: 10.1073/pnas.1608384113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimoda M, Principe S, Jackson HW, Luga V, Fang H, Molyneux SD, et al. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat Cell Biol. 2014;16:889–901. doi: 10.1038/ncb3021. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Zeng C, Zhan Y, Wang H, Jiang X, Li W. Aberrant low expression of p85α in stromal fibroblasts promotes breast cancer cell metastasis through exosome-mediated paracrine Wnt10b. Oncogene. 2017;36:4692–705.. doi: 10.1038/onc.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Procopio MG, Laszlo C, Al Labban D, Kim DE, Bordignon P, Jo SH, et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol. 2015;17:1193–204.. doi: 10.1038/ncb3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen T, Li Y, Zhu S, Yu J, Zhang B, Chen X, et al. YAP1 plays a key role of the conversion of normal fibroblasts into cancer-associated fibroblasts that contribute to prostate cancer progression. J Exp Clin Cancer Res. 2020;39:36. doi: 10.1186/s13046-020-1542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albrengues J, Bourget I, Pons C, Butet V, Hofman P, Tartare-Deckert S, et al. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014;7:1664–78.. doi: 10.1016/j.celrep.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 31.Avgustinova A, Iravani M, Robertson D, Fearns A, Gao Q, Klingbeil P, et al. Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun. 2016;7:10305. doi: 10.1038/ncomms10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorsam B, Bosl T, Reiners KS, Barnert S, Schubert R, Shatnyeva O, et al. Hodgkin Lymphoma-Derived Extracellular Vesicles Change the Secretome of Fibroblasts Toward a CAF Phenotype. Front Immunol. 2018;9:1358. doi: 10.3389/fimmu.2018.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naito Y, Yamamoto Y, Sakamoto N, Shimomura I, Kogure A, Kumazaki M, et al. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene. 2019;38:5566–79.. doi: 10.1038/s41388-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Qin X, Yan M, Shi J, Xu Q, Li Z, et al. Loss of exosomal miR-3188 in cancer-associated fibroblasts contributes to HNC progression. J Exp Clin Cancer Res. 2019;38:151. doi: 10.1186/s13046-019-1144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YY, Tao YW, Gao S, Li P, Zheng JM, Zhang SE, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209–20.. doi: 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang E, Xu Z, Wang M, Yan T, Huang C, Zhou X, et al. Tumoral microvesicle-activated glycometabolic reprogramming in fibroblasts promotes the progression of oral squamous cell carcinoma. FASEB J. 2019;33:5690–703.. doi: 10.1096/fj.201802226R. [DOI] [PubMed] [Google Scholar]
- 38.Kim DK, Kim EK, Jung DW, Kim J. Cytoskeletal alteration modulates cancer cell invasion through RhoA-YAP signaling in stromal fibroblasts. PloS ONE. 2019;14:e0214553. doi: 10.1371/journal.pone.0214553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YY, Zhou CX, Gao Y. Interaction between oral squamous cell carcinoma cells and fibroblasts through TGF-β1 mediated by podoplanin. Exp Cell Res. 2018;369:43–53. doi: 10.1016/j.yexcr.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 40.Zhou B, Zhuang XM, Wang YY, Lin ZY, Zhang DM, Fan S, et al. Tumor necrosis factor α induces myofibroblast differentiation in human tongue cancer and promotes invasiveness and angiogenesis via secretion of stromal cell-derived factor-1. Oral Oncol. 2015;51:1095–102. doi: 10.1016/j.oraloncology.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Wei LY, Lee JJ, Yeh CY, Yang CJ, Kok SH, Ko JY, et al. Reciprocal activation of cancer-associated fibroblasts and oral squamous carcinoma cells through CXCL1. Oral Oncol. 2019;88:115–23. doi: 10.1016/j.oraloncology.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Melling GE, Flannery SE, Abidin SA, Clemmens H, Prajapati P, Hinsley EE, et al. A miRNA-145/TGF-β1 negative feedback loop regulates the cancer-associated fibroblast phenotype. Carcinogenesis. 2018;39:798–807. doi: 10.1093/carcin/bgy032. [DOI] [PubMed] [Google Scholar]
- 43.Huang Q, Hsueh CY, Shen YJ, Guo Y, Huang JM, Zhang YF, et al. Small extracellular vesicle-packaged TGFβ1 promotes the reprogramming of normal fibroblasts into cancer-associated fibroblasts by regulating fibronectin in head and neck squamous cell carcinoma. Cancer Lett. 2021;517:1–13. doi: 10.1016/j.canlet.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Albrengues J, Bertero T, Grasset E, Bonan S, Maiel M, Bourget I, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat Commun. 2015;6:10204. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bordignon P, Bottoni G, Xu X, Popescu AS, Truan Z, Guenova E, et al. Dualism of FGF and TGF-β signaling in heterogeneous cancer-associated fibroblast activation with ETV1 as a critical determinant. Cell Rep. 2019;28:2358–72.e6. doi: 10.1016/j.celrep.2019.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 47.Witwer KW, Goberdhan DC, O’driscoll L, THéRY C, Welsh JA, Blenkiron C, et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182. doi: 10.1002/jev2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tkach M, THéRY C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–32.. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 49.Nogues L, Benito-Martin A, Hergueta-Redondo M, Peinado H. The influence of tumour-derived extracellular vesicles on local and distal metastatic dissemination. Mol Asp Med. 2018;60:15–26. doi: 10.1016/j.mam.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA. 1993;90:999–1003. doi: 10.1073/pnas.90.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard V, Semaan A, Huang J, San Lucas FA, Mulu FC, Stephens BM, et al. Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin Cancer Res: Off J Am Assoc Cancer Res. 2019;25:2194–205. doi: 10.1158/1078-0432.CCR-18-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9:282–301. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchsbaum RJ, Oh SY. Breast cancer-associated fibroblasts: where we are and where we need to go. Cancers. 2016;8:19. doi: 10.3390/cancers8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun DY, Wu JQ, He ZH, He MF, Sun HB. Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-β signaling pathway. Life Sci. 2019;235:116791. doi: 10.1016/j.lfs.2019.116791. [DOI] [PubMed] [Google Scholar]
- 56.Mcandrews KM, Chen Y, Darpolor JK, Zheng X, Yang S, Carstens JL, et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer. Cancer Discov. 2022;12:1580–97.. doi: 10.1158/2159-8290.CD-20-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q, Wang Y, Xia C, Ding L, Pu Y, Hu X, et al. Integrated analysis of single-cell RNA-seq and bulk RNA-seq reveals distinct cancer-associated fibroblasts in head and neck squamous cell carcinoma. Ann Transl Med. 2021;9:1017. doi: 10.21037/atm-21-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song H, Lou C, Ma J, Gong Q, Tian Z, You Y, et al. Single-cell transcriptome analysis reveals changes of tumor immune microenvironment in oral squamous cell carcinoma after chemotherapy. Front Cell Dev Biol. 2022;10:914120. doi: 10.3389/fcell.2022.914120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Zhang H, Zhai Y, Li F, Shi X, Ying M. Single-cell profiling reveals heterogeneity of primary and lymph node metastatic tumors and immune cell populations and discovers important prognostic significance of CCDC43 in oral squamous cell carcinoma. Front Immunol. 2022;13:843322. doi: 10.3389/fimmu.2022.843322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi H, Rokudai S, Kawabata-Iwakawa R, Sakakura K, Oyama T, Nishiyama M, et al. AKT3 is a novel regulator of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancers. 2021;13:1233. doi: 10.3390/cancers13061233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Loberg R, Taichman RS. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006;25:573–87. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- 62.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–23.. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel AK, Vipparthi K, Thatikonda V, Arun I, Bhattacharjee S, Sharan R, et al. A subtype of cancer-associated fibroblasts with lower expression of alpha-smooth muscle actin suppresses stemness through BMP4 in oral carcinoma. Oncogenesis. 2018;7:78. doi: 10.1038/s41389-018-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valach J, Fík Z, Strnad H, Chovanec M, Plzák J, Cada Z, et al. Smooth muscle actin-expressing stromal fibroblasts in head and neck squamous cell carcinoma: increased expression of galectin-1 and induction of poor prognosis factors. Int J Cancer. 2012;131:2499–508. doi: 10.1002/ijc.27550. [DOI] [PubMed] [Google Scholar]
- 65.Lin NN, Wang P, Zhao D, Zhang FJ, Yang K, Chen R. Significance of oral cancer-associated fibroblasts in angiogenesis, lymphangiogenesis, and tumor invasion in oral squamous cell carcinoma. J Oral Pathol Med. 2017;46:21–30. doi: 10.1111/jop.12452. [DOI] [PubMed] [Google Scholar]
- 66.Zeltz C, Alam J, Liu H, Erusappan PM, Hoschuetzky H, Molven A, et al. α11β1 integrin is induced in a subset of cancer-associated fibroblasts in desmoplastic tumor stroma and mediates in vitro cell migration. Cancers. 2019;11:765. doi: 10.3390/cancers11060765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Zhang J, Chen SW, Liu LL, Li L, Gao F, et al. Cancer-associated fibroblasts provide a suitable microenvironment for tumor development and progression in oral tongue squamous cancer. J Transl Med. 2015;13:198. doi: 10.1186/s12967-015-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang L, Luo H, He Q, You Y, Fan Y, Liang J. Investigation of cancer-associated fibroblasts and p62 expression in oral cancer before and after chemotherapy. J Cranio-Maxillo-Facial Surg. 2018;46:605–10.. doi: 10.1016/j.jcms.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 69.Vered M, Shnaiderman-Shapiro A, Zlotogorski-Hurvitz A, Salo T, Yahalom R. Cancer-associated fibroblasts in the tumor microenvironment of tongue carcinoma is a heterogeneous cell population. Acta Histochemica. 2019;121:151446. doi: 10.1016/j.acthis.2019.151446. [DOI] [PubMed] [Google Scholar]
- 70.Custódio M, Biddle A, Tavassoli M. Portrait of a CAF: The story of cancer-associated fibroblasts in head and neck cancer. Oral Oncol. 2020;110:104972. doi: 10.1016/j.oraloncology.2020.104972. [DOI] [PubMed] [Google Scholar]
- 71.Bello IO, Vered M, Dayan D, Dobriyan A, Yahalom R, Alanen K, et al. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33–8. doi: 10.1016/j.oraloncology.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 72.Parajuli H, Teh MT, Abrahamsen S, Christoffersen I, Neppelberg E, Lybak S, et al. Integrin α11 is overexpressed by tumour stroma of head and neck squamous cell carcinoma and correlates positively with alpha smooth muscle actin expression. J Oral Pathol Med. 2017;46:267–75.. doi: 10.1111/jop.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470–81. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]
- 74.Costea DE, Hills A, Osman AH, Thurlow J, Kalna G, Huang X, et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res. 2013;73:3888–901. doi: 10.1158/0008-5472.CAN-12-4150. [DOI] [PubMed] [Google Scholar]
- 75.Hassona Y, Cirillo N, Lim KP, Herman A, Mellone M, Thomas GJ, et al. Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-β. Carcinogenesis. 2013;34:1286–95. doi: 10.1093/carcin/bgt035. [DOI] [PubMed] [Google Scholar]
- 76.Bolt R, Foran B, Murdoch C, Lambert DW, Thomas S, Hunter KD. HPV-negative, but not HPV-positive, oropharyngeal carcinomas induce fibroblasts to support tumour invasion through micro-environmental release of HGF and IL-6. Carcinogenesis. 2018;39:170–9. doi: 10.1093/carcin/bgx130. [DOI] [PubMed] [Google Scholar]
- 77.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 78.Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, Rawal S, et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 2015;75:4681–7. doi: 10.1158/0008-5472.CAN-15-1633. [DOI] [PubMed] [Google Scholar]
- 79.Cazet AS, Hui MN, Elsworth BL, Wu SZ, Roden D, Chan CL, et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun. 2018;9:2897. doi: 10.1038/s41467-018-05220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 81.Principe S, Mejia-Guerrero S, Ignatchenko V, Sinha A, Ignatchenko A, Shi W, et al. Proteomic analysis of cancer-associated fibroblasts reveals a paracrine role for MFAP5 in human oral tongue squamous cell carcinoma. J Proteome Res. 2018;17:2045–59.. doi: 10.1021/acs.jproteome.7b00925. [DOI] [PubMed] [Google Scholar]
- 82.Qin X, Yan M, Wang X, Xu Q, Wang X, Zhu X, et al. Cancer-associated fibroblast-derived IL-6 promotes head and neck cancer progression via the osteopontin-NF-kappa B signaling pathway. Theranostics. 2018;8:921–40.. doi: 10.7150/thno.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X, Xu Q, Wu Y, Li J, Tang D, Han L, et al. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis. 2014;35:1362–70. doi: 10.1093/carcin/bgu046. [DOI] [PubMed] [Google Scholar]
- 84.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014;5:3472. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 85.Yu B, Wu K, Wang X, Zhang J, Wang L, Jiang Y, et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018;9:1082. doi: 10.1038/s41419-018-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Fan Q, Li J, Song J, Gu Y. MiR-124 down-regulation is critical for cancer associated fibroblasts-enhanced tumor growth of oral carcinoma. Exp Cell Res. 2017;351:100–8. doi: 10.1016/j.yexcr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Hassona Y, Cirillo N, Heesom K, Parkinson EK, Prime SS. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br J Cancer. 2014;111:1230–7. doi: 10.1038/bjc.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv Z, et al. TGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep. 2016;6:20587. doi: 10.1038/srep20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–32. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vered M, Dayan D, Yahalom R, Dobriyan A, Barshack I, Bello IO, et al. Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int J Cancer. 2010;127:1356–62. doi: 10.1002/ijc.25358. [DOI] [PubMed] [Google Scholar]
- 92.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–25. doi: 10.1016/S0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 93.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–91. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alcolea S, Antón R, Camacho M, Soler M, Alfranca A, Avilés-Jurado FX, et al. Interaction between head and neck squamous cell carcinoma cells and fibroblasts in the biosynthesis of PGE2. J Lipid Res. 2012;53:630–42. doi: 10.1194/jlr.M019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dudás J, Fullár A, Bitsche M, Schartinger V, Kovalszky I, Sprinzl GM, et al. Tumor-produced, active interleukin-1β regulates gene expression in carcinoma-associated fibroblasts. Exp Cell Res. 2011;317:2222–9. doi: 10.1016/j.yexcr.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang S, Ma N, Kawanishi S, Hiraku Y, Oikawa S, Xie Y, et al. Relationships of alpha-SMA-positive fibroblasts and SDF-1-positive tumor cells with neoangiogenesis in nasopharyngeal carcinoma. Biomed Res Int. 2014;2014:507353. doi: 10.1155/2014/507353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–72. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 99.Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–47.. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi H, Sakakura K, Kawabata-Iwakawa R, Rokudai S, Toyoda M, Nishiyama M, et al. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol, immunotherapy. 2015;64:1407–17. doi: 10.1007/s00262-015-1742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang TX, Tan XY, Huang HS, Li YT, Liu BL, Liu KS, et al. Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut. 2022;71:333–44.. doi: 10.1136/gutjnl-2020-322924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Z, Sun C, Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021;11:8322–36.. doi: 10.7150/thno.62378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu X, Zhou Z, Xu S, Liao C, Chen X, Li B, et al. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020;478:93–106. doi: 10.1016/j.canlet.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 104.Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, et al. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 2016;24:685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 2019;9:617–27.. doi: 10.1158/2159-8290.CD-18-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S, et al. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle. 2011;10:2504–20. doi: 10.4161/cc.10.15.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 2016;41:211–8. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, Dong Y, Zhao M, Ding L, Yang X, Jing Y, et al. ITGB2-mediated metabolic switch in CAFs promotes OSCC proliferation by oxidation of NADH in mitochondrial oxidative phosphorylation system. Theranostics. 2020;10:12044–59.. doi: 10.7150/thno.47901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar D, New J, Vishwakarma V, Joshi R, Enders J, Lin F, et al. Cancer-associated fibroblasts drive glycolysis in a targetable signaling loop implicated in head and neck squamous cell carcinoma progression. Cancer Res. 2018;78:3769–82.. doi: 10.1158/0008-5472.CAN-17-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–8. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 111.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 112.Alsahafi E, Begg K, Amelio I, Raulf N, Lucarelli P, Sauter T, et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liao JK, Zhou B, Zhuang XM, Zhuang PL, Zhang DM, Chen WL. Cancer-associated fibroblasts confer cisplatin resistance of tongue cancer via autophagy activation. Biomed Pharmacother. 2018;97:1341–8. doi: 10.1016/j.biopha.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 114.Ayuso JM, Vitek R, Swick AD, Skala MC, Wisinski KB, Kimple RJ, et al. Effects of culture method on response to EGFR therapy in head and neck squamous cell carcinoma cells. Sci Rep. 2019;9:12480. doi: 10.1038/s41598-019-48764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johansson AC, Ansell A, Jerhammar F, Lindh MB, Grénman R, Munck-Wikland E, et al. Cancer-associated fibroblasts induce matrix metalloproteinase-mediated cetuximab resistance in head and neck squamous cell carcinoma cells. Mol Cancer Res. 2012;10:1158–68. doi: 10.1158/1541-7786.MCR-12-0030. [DOI] [PubMed] [Google Scholar]
- 116.Peltanova B, Liskova M, Gumulec J, Raudenska M, Polanska HH, Vaculovic T, et al. Sensitivity to cisplatin in head and neck cancer cells is significantly affected by patient-derived cancer-associated fibroblasts. Int J Mol Sci. 2021;22:1912. doi: 10.3390/ijms22041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ford K, Hanley CJ, Mellone M, Szyndralewiez C, Heitz F, Wiesel P, et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 2020;80:1846–60.. doi: 10.1158/0008-5472.CAN-19-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galbo PM, Jr., Zang X, Zheng D. Molecular features of cancer-associated fibroblast subtypes and their implication on cancer pathogenesis, prognosis, and immunotherapy resistance. Clin Cancer Res. 2021;27:2636–47. doi: 10.1158/1078-0432.CCR-20-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yegodayev KM, Novoplansky O, Golden A, Prasad M, Levin L, Jagadeeshan S, et al. TGF-beta-activated cancer-associated fibroblasts limit cetuximab efficacy in preclinical models of head and neck cancer. Cancers. 2020;12:339. doi: 10.3390/cancers12020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU, et al. Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Invest Dermatol. 2003;120:182–8. doi: 10.1046/j.1523-1747.2003.12035.x. [DOI] [PubMed] [Google Scholar]
- 121.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 122.Kan M, Zhang GH, Zarnegar R, Michalopoulos G, Myoken Y, Mckeehan WL, et al. Hepatocyte growth factor/hepatopoietin A stimulates the growth of rat kidney proximal tubule epithelial cells (RPTE), rat nonparenchymal liver cells, human melanoma cells, mouse keratinocytes and stimulates anchorage-independent growth of SV-40 transformed RPTE. Biochem Biophys Res Commun. 1991;174:331–7. doi: 10.1016/0006-291X(91)90524-B. [DOI] [PubMed] [Google Scholar]
- 123.Knowles LM, Stabile LP, Egloff AM, Rothstein ME, Thomas SM, Gubish CT, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–50. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kumar D, Kandl C, Hamilton CD, Shnayder Y, Tsue TT, Kakarala K, et al. Mitigation of tumor-associated fibroblast-facilitated head and neck cancer progression with anti-hepatocyte growth factor antibody Ficlatuzumab. JAMA Otolaryngol—Head Neck Surg. 2015;141:1133–9. doi: 10.1001/jamaoto.2015.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu Y, Shi C, Zeng L, Liu G, Jiang W, Zhang X, et al. High COX-2 expression in cancer-associated fibiroblasts contributes to poor survival and promotes migration and invasiveness in nasopharyngeal carcinoma. Mol Carcinog. 2020;59:265–80.. doi: 10.1002/mc.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Economopoulou P, Kotsantis I, Psyrri A. Tumor microenvironment and immunotherapy response in head and neck cancer. Cancers. 2020;12:3377. doi: 10.3390/cancers12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wong ALA, Hirpara JL, Pervaiz S, Eu JQ, Sethi G, Goh BC. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin Investig. Drugs. 2017;26:883–7. doi: 10.1080/13543784.2017.1351941. [DOI] [PubMed] [Google Scholar]
- 128.Kasembeli MM, Singhmar P, Ma J, Edralin J, Tang Y, Adams C, 3rd, et al. TTI-101: A competitive inhibitor of STAT3 that spares oxidative phosphorylation and reverses mechanical allodynia in mouse models of neuropathic pain. Biochem. Pharmacol. 2021;192:114688. doi: 10.1016/j.bcp.2021.114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sen M, Pollock NI, Black J, Degrave KA, Wheeler S, Freilino ML, et al. JAK kinase inhibition abrogates STAT3 activation and head and neck squamous cell carcinoma tumor growth. Neoplasia. 2015;17:256–64. doi: 10.1016/j.neo.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Waldhauer I, Gonzalez-Nicolini V, Freimoser-Grundschober A, Nayak TK, Fahrni L, Hosse RJ, et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. mAbs. 2021;13:1913791. doi: 10.1080/19420862.2021.1913791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brechbuhl HM, Finlay-Schultz J, Yamamoto TM, Gillen AE, Cittelly DM, Tan AC, et al. Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clin Cancer Res. 2017;23:1710–21. doi: 10.1158/1078-0432.CCR-15-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 133.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu G, Cao B, Liang X, Li L, Hao Y, Meng W, et al. Small extracellular vesicles containing miR-192/215 mediate hypoxia-induced cancer-associated fibroblast development in head and neck squamous cell carcinoma. Cancer Lett. 2021;506:11–22. doi: 10.1016/j.canlet.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Ding L, Ren J, Zhang D, Li Y, Huang X, Hu Q, et al. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis. 2018;39:397–406. doi: 10.1093/carcin/bgy006. [DOI] [PubMed] [Google Scholar]
- 136.Xie J, Qi X, Wang Y, Yin X, Xu W, Han S, et al. Cancer-associated fibroblasts secrete hypoxia-induced serglycin to promote head and neck squamous cell carcinoma tumor cell growth in vitro and in vivo by activating the Wnt/β-catenin pathway. Cell Oncol (Dordr) 2021;44:661–71.. doi: 10.1007/s13402-021-00592-2. [DOI] [PubMed] [Google Scholar]
- 137.Watts TL, Cui R, Szaniszlo P, Resto VA, Powell DW, Pinchuk IV. PDGF-AA mediates mesenchymal stromal cell chemotaxis to the head and neck squamous cell carcinoma tumor microenvironment. J Transl Med. 2016;14:337. doi: 10.1186/s12967-016-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kartha VK, Stawski L, Han R, Haines P, Gallagher G, Noonan V, et al. PDGFRβ is a novel marker of stromal activation in oral squamous cell carcinomas. PloS ONE. 2016;11:e0154645. doi: 10.1371/journal.pone.0154645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaya S, Wiesmann N, Goldschmitt J, Krüger M, Al-Nawas B, Heider J. Differences in the expression of caveolin-1 isoforms in cancer-associated and normal fibroblasts of patients with oral squamous cell carcinoma. Clin Oral Investig. 2021;25:5823–31.. doi: 10.1007/s00784-021-03887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–72. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 141.Lee JC, Wu ATH, Chen JH, Huang WY, Lawal B, Mokgautsi N, et al. HNC0014, a multi-targeted small-molecule, inhibits head and neck squamous cell carcinoma by suppressing c-Met/STAT3/CD44/PD-L1 oncoimmune signature and eliciting antitumor immune responses. Cancers. 2020;12:3759. doi: 10.3390/cancers12123759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this review are available upon request by contact with the corresponding author.