Abstract
Background and Aim
This study aimed to compare patients with and without sedation during emergency endoscopy for upper gastrointestinal bleeding (UGIB) and to clarify the safety and efficacy of sedation in emergency endoscopy.
Methods
We retrospectively collected 389 patients who underwent emergency endoscopy for UGIB at Ureshino Medical Center from 2016 to 2021. Patients were divided into two groups: sedation group during emergency endoscopy and nonsedation group. Clinical characteristics, patient status on admission, and UGIB etiology were evaluated. Treatment outcomes and adverse events were evaluated using propensity score matching (PSM), and risk factors for mortality from UGIB were investigated using Cox multivariate analysis.
Results
The sedation group was significantly younger, composed of a higher proportion of males, and had chronic liver disease. Blood pressure and hemoglobin level on admission were significantly higher in the sedation group. The main cause of bleeding was peptic ulcer, which was significantly higher in the nonsedation group. PSM created 133 matched pairs. The success rate of endoscopic hemostasis was similar in both groups, and procedure time was significantly shorter in the sedation group than in the nonsedation group (17.6 ± 10.0 versus 20.2 ± 10.2 min, P = 0.04). There were no significant differences in adverse events between groups. Cox multivariate analyses revealed that red blood cell transfusion [hazard ratio (HR) 4.45, P < 0.02] and rebleeding (HR 3.30, P = 0.03) were associated with increased risk of 30-day mortality from UGIB.
Conclusions
Sedation reduced the procedure time during emergency endoscopy for UGIB. Sedation during emergency endoscopy for UGIB is acceptable for safe endoscopic procedures.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-022-07740-0.
Keywords: Bleeding, Emergency endoscopy, Sedation, Mortality, Propensity score matching
Introduction
Upper gastrointestinal bleeding (UGIB) is the most common gastroenterological emergency, with an incidence of mortality of 5–10% [1]. UGIB is broadly classified into variceal and nonvariceal bleeding. Ruptured esophageal varices cause approximately 70% of all UGIB in cirrhosis [2, 3]. Among patients with nonvariceal UGIB, the major cause is peptic gastroduodenal ulcer [4–6]. Helicobacter pylori (H. pylori) infection and the use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, were the major causes of bleeding peptic ulcer disease in past years, but the epidemiology and pathophysiology of UGIB has changed with the widespread eradication of H. pylori and the increased use of antithrombotic drugs [7–12].
Emergency endoscopic hemostasis is useful in all cases of UGIB [13–15]. Regarding timeliness, urgent endoscopy within 24 h is reported to reduce the risk of mortality and surgical intervention in high-risk cases of UGIB, and it is recommended in guidelines that endoscopy be carried out within 24 h [16–19].
Sedation in gastroenterological endoscopy has become an important medical option in routine clinical care [20]. Sedation during emergency endoscopy is considered useful for safe and reliable emergency endoscopy when vital signs are stable, especially when the patient is agitated or anxious, although sedation may not be useful when the patient’s general condition deteriorates or the hemodynamics are unstable [21–24].
The guidelines for sedation in gastroenterological endoscopy by the Japan Gastroenterological Endoscopy Society and the Japanese Society of Anesthesiologists recommend the use of antagonists or sedatives with a short half-life as suitable sedation during emergency endoscopy with security monitoring and in an environment where emergency treatment can be conducted, but do not specify whether sedation during emergency endoscopy is safe and effective [20].
The aims of the present retrospective study were (1) to compare the clinical outcomes of patients with and without sedation during emergency endoscopy for UGIB and (2) to identify the risk factors for mortality from UGIB in these patients.
Methods
Study Design and Ethical Issues
This retrospective chart review included patients who underwent emergency endoscopy and endoscopic hemostasis for UGIB at the National Hospital Organization Ureshino Medical Center from January 2016 to December 2021. Patients older than 20 years who fulfilled the following criteria were candidates for the study: (1) suspected UGIB requiring emergency endoscopy; (2) underwent endoscopic hemostasis within 24 h of symptom onset; (3) had a clear level of consciousness as well as stable respiratory and circulatory dynamics. Patients who had been endotracheally intubated or whose respiratory and circulatory status was sufficiently unstable to preclude emergency endoscopy and those with missing data were excluded. Written informed consent was obtained from patients who met the inclusion criteria after endoscopic hemostasis.
This study was conducted in accordance with the Declaration of Helsinki and the guidelines of the Consolidated Standards of Reporting Trials (CONSORT). The study protocol and the consent procedure were approved by the Ethics Review Committee of the National Hospital Organization Ureshino Medical Center (approval number 20-86).
Study Endpoints
The primary endpoint was the success rate of endoscopic hemostasis according to use of sedation during emergency endoscopy for UGIB. Treatment success was defined as controlled by endoscopic hemostasis and no rebleeding within 5 days after endoscopic hemostasis.
The secondary endpoints were rebleeding, procedure time, adverse events, and 30-day mortality after emergency endoscopy for UGIB. Rebleeding was defined as follows: (1) follow-up endoscopy identified recurrent UGIB or the stigmata of recent hemorrhage within 30 days or (2) melena and progressive anemia with a decrease in hemoglobin level greater than 2 g/dL and/or with a decrease in systolic blood pressure < 80 mmHg. The procedure time was defined as the time interval between insertion and removal of the endoscope [25, 26].
Sedation and Monitoring During Emergency Endoscopy
For urgent endoscopic treatment, each of the 17 endoscopists performed emergency endoscopy with or without sedation, based on their judgment. Thereby patients were divided into two groups: those who underwent emergency endoscopy with sedation (sedation group) and those who underwent the same procedure without sedation (nonsedation group). Sedatives used during emergency endoscopy were midazolam, diazepam, or propofol, and analgesics were pentazocine or pethidine hydrochloride [20, 27]. The type of sedative and analgesic drugs was selected by each endoscopist considering patients’ age and physical condition. Details of sedative procedures are provided in Online Appendix S1.
Vital signs including blood pressure, heart rate, and blood oxygen saturation were recorded before the introduction of sedatives. During the endoscopy, vital signs were monitored every 5 min. When oxygen saturation was below 92%, nasal oxygen supplementation (2 L/min) was initiated. When the vital signs fluctuated by 20% or more compared with baseline values, the endoscopic procedure was temporarily stopped until the recovery of those values. Flumazenil, an antagonist of benzodiazepines, was administered after endoscopy as necessary.
Endoscopic Hemostasis
Endoscopic hemostasis was performed mainly by high-frequency soft coagulation, hemoclipping, or endoscopic variceal ligation (EVL) [26, 28–30]. The choice of procedure was at the endoscopists’ discretion. Details of endoscopic hemostasis are presented in Online Appendix S2. Each procedure was repeated until hemostasis was endoscopically confirmed. Interventional radiology and/or surgery were implemented when endoscopic hemostasis was considered ineffective.
After endoscopic hemostasis was achieved, all patients were hospitalized and managed conventionally (fasting with peripheral parenteral nutrition and intravenous proton pump inhibitors). The indications for red blood cell (RBC) transfusion were a hemoglobin level < 6 g/dL on admission or a rapid drop in hemoglobin level > 2 g/dL in patients with hemoglobin levels < 10 g/dL at baseline. Follow-up endoscopy was performed on patients for whom it was judged necessary by the endoscopist 48–72 h after the initial endoscopy, and repeat endoscopic hemostasis was applied in cases where recurrent UGIB was detected. Interventional radiology and/or surgery were implemented when endoscopic hemostasis was considered ineffective.
Endoscopic hemostasis was performed by all 17 endoscopists, comprising 7 specialists and 10 trainees. Specialists were defined as endoscopists who had performed endoscopy for more than 5 years with experience in more than 40 endoscopic submucosal dissection procedures after mastering the required fundamental skills and knowledge [31, 32].
Clinical Outcomes and Statistical Analysis
Clinical data collected in the retrospective cohort were age, gender, alcohol consumption, smoking habit, H. pylori infection, American Society of Anesthesiologists physical status, use of antithrombotic agents, nonsteroidal anti-inflammatory drugs (NSAIDs), or gastric acid secretion inhibitor, and comorbidity (including Charlson comorbidity score). H. pylori infection was diagnosed by the serum levels of anti-H. pylori antibodies, the urea breath test, or the rapid urease test. Patient status on admission, including initial symptoms, initial vital signs, initial laboratory data, and transfusion volume, was collected. Three scoring systems (Rockall score, Glasgow-Blatchford score, and AIMS65 score) have been reported to be useful in predicting mortality, rebleeding, need for transfusion, and hemostasis [33–36]. These scoring systems were applied, on the basis of admission history, clinical and laboratory data, endoscopic findings, treatment, and clinical follow-up. Accumulated information concerning the cause of UGIB, endoscopic findings, and types of anesthesia drugs was also collected.
Treatment outcomes and adverse events were compared between the two groups using propensity score matching analysis. This method was applied to adjust significant differences in the baseline characteristics of the patients and reduce the influence of possible confounding factors [37]. The two groups were matched at a 1:1 ratio (133 patients in each group) with adjustment for 13 covariates (age, gender, H. pylori infection, use of antithrombotic agents, use of NSAIDs, chronic liver damage, Rockall score, Glasgow-Blatchford score, AIMS65 score, systolic blood pressure, diastolic blood pressure, hemoglobin level, and peptic ulcer) to minimize inherent bias. These 13 covariates were selected on the basis of the opinions of seven expert endoscopists. This model yielded a C statistic of 0.705, indicating a preferable ability for comparison between two groups. The caliper width of propensity score matching was 0.20.
Categorical data were expressed as a number (percentage), and chi-squared test was used to identify differences between the two groups. Numerical data for normal distribution were expressed as the mean ± standard deviation, and Student’s t-test was used to determine differences between the two groups. Numerical data for a skewed distribution were expressed as median interquartile range, and the Mann–Whitney U-test was applied. Levels of significance for all comparisons made were reported, whether significant or not, with P values or confidence intervals. A P value of < 0.05 was statistically significant for each test. Survival was analyzed using Kaplan–Meier plots with log-rank tests. The Cox proportional hazard model was then used to adjust confounding factors in the analysis of 30-day mortality from UGIB. All statistical analyses were performed with JMP version 13.0.0 (SAS Institute, Tokyo, Japan).
Results
Clinical Characteristics and Endoscopic Findings of Patients with UGIB
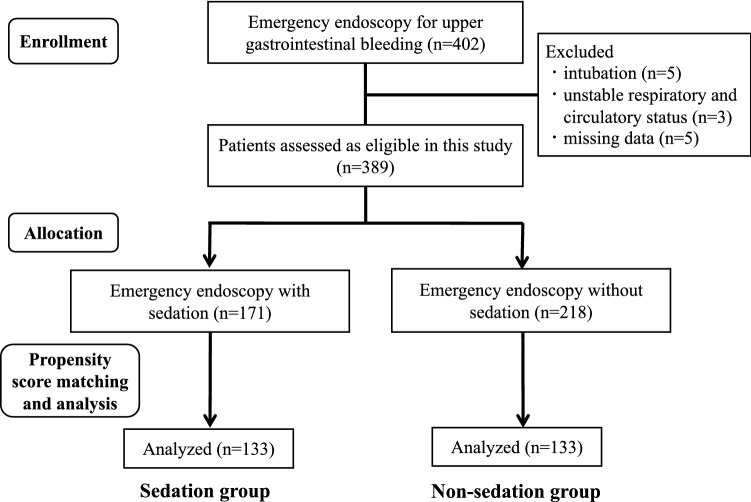
During the period between January 2016 and December 2021, we underwent emergency endoscopy for UGIB in 402 patients. Among them, endotracheal intubation was applied in five patients, respiratory and circulatory status was unstable in three patients, and missing data were found in five patients. Thirteen patients were thus excluded, and 389 patients were assessed as eligible for the present study.
Of the 389 patients, 171 patients (44.0%) received sedation during emergency endoscopy, and the remaining 218 patients (56.0%) did not receive sedation during the procedure (Fig. 1). Baseline patient characteristics are presented in Table 1. The sedation group was significantly younger with more male patients, and had H. pylori infection and chronic liver disease. The use of antithrombotic agents and NSAIDs was higher in the sedation group. Three scoring systems (Rockall score, Glasgow-Blatchford score, and AIMS65 score) were significantly higher for the sedation group.
Fig. 1.
Flow diagram showing selection of patients with UGIB who underwent emergency endoscopy
Table 1.
Characteristics of patients
| Sedation | Nonsedation | P value | |
|---|---|---|---|
| Number of patients (N) | 171 | 218 | |
| Age (years) | 70.1 ± 13.9 | 74.8 ± 12.3 | < 0.01 |
| Gender, males | 123 (71.9%) | 133 (61.0%) | 0.03 |
| BMI (%) | 21.5 ± 3.9 | 21.2 ± 3.8 | 0.48 |
| ASA-PS classification I–II | 136 (79.5%) | 169 (77.5%) | 0.71 |
| Alcohol drinking | 76 (44.4%) | 80 (36.7%) | 0.14 |
| Smoking | 81 (47.4%) | 91 (41.7%) | 0.30 |
| Helicobacter pylori infection | 50 (29.2%) | 41 (18.8%) | 0.02 |
| Using antithrombotic agents | 45 (26.3%) | 86 (39.5%) | 0.01 |
| Using NSAIDs | 31 (18.1%) | 61 (28.0%) | 0.03 |
| Using gastric acid secretion inhibitor | 57 (33.3%) | 81 (37.2%) | 0.46 |
| Comorbidity | |||
| Cardiovascular diseases | 22 (12.9%) | 43 (19.7%) | 0.08 |
| Cerebrovascular diseases | 16 (9.4%) | 27 (12.4%) | 0.42 |
| Chronic kidney diseases | 28 (16.4%) | 46 (21.1%) | 0.25 |
| Chronic liver diseases | 46 (26.9%) | 38 (17.4%) | 0.03 |
| Diabetes mellitus | 49 (28.7%) | 56 (25.7%) | 0.57 |
| Hypertension | 90 (52.6%) | 123 (56.4%) | 0.47 |
| Malignant diseases | 51 (29.8%) | 56 (25.7%) | 0.42 |
| Charlson comorbidity score | 2.2 ± 1.8 | 2.0 ± 1.8 | 0.40 |
| Scoring system | |||
| Rockall score | 4.4 ± 1.4 | 4.7 ± 1.5 | 0.01 |
| Glasgow-Blatchford score | 9.8 ± 4.2 | 10.9 ± 3.8 | 0.01 |
| AIMS65 score | 1.4 ± 0.9 | 1.6 ± 1.0 | < 0.01 |
BMI body mass index, ASA-PS American Society of Anesthesiologists physical status, NSAIDs nonsteroidal anti-inflammatory drugs
Results are presented as mean ± SD or number of patients
UGIB patient status on admission is presented in Table 2. Systolic and diastolic blood pressure and hemoglobin levels were significantly higher in the sedation group. Initial laboratory data other than hemoglobin level did not differ between the sedation and nonsedation groups. There were no significant differences between the two groups in the number of patients who received RBC transfusions and the total volume of RBC transfused.
Table 2.
Patient status on admission
| Sedation | Nonsedation | P value | |
|---|---|---|---|
| Initial symptoms | |||
| Vomiting blood | 83 (48.5%) | 96 (44.0%) | 0.41 |
| Black stools | 86 (50.3%) | 108 (49.5%) | 0.92 |
| Fainting attack | 7 (4.1%) | 10 (4.6%) | 1.00 |
| Initial vital signs | |||
| Systolic blood pressure (mmHg) | 121.0 ± 24.4 | 112.7 ± 26.7 | < 0.01 |
| Diastolic blood pressure (mmHg) | 66.0 ± 14.4 | 61.3 ± 15.3 | < 0.01 |
| Pulse rate (beats/min) | 89.6 ± 19.2 | 87.4 ± 22.1 | 0.29 |
| Respiratory rate (breaths/min) | 18.3 ± 4.1 | 18.9 ± 3.8 | 0.12 |
| Arterial oxygen saturation (%) | 97.8 ± 2.1 | 97.3 ± 2.7 | 0.06 |
| Initial laboratory data | |||
| White blood count (k/μL) | 9.03 ± 5.16 | 8.34 ± 6.89 | 0.29 |
| Hemoglobin (g/dL) | 8.9 ± 3.0 | 8.1 ± 2.6 | < 0.01 |
| Platelets (k/μl) | 25.4 ± 39.1 | 33.0 ± 48.4 | 0.09 |
| Albumin (mg/dL) | 3.1 ± 0.7 | 3.0 ± 0.8 | 0.38 |
| Serum creatinine (mg/dL) | 1.52 ± 1.78 | 1.59 ± 1.64 | 0.68 |
| Serum BUN (g/dL) | 39.2 ± 29.8 | 44.1 ± 32.3 | 0.12 |
| Serum AST (U/L) | 45.2 ± 90.8 | 55.1 ± 179.5 | 0.48 |
| Serum ALT (U/L) | 26.5 ± 36.3 | 37.6 ± 117.9 | 0.19 |
| Serum total bilirubin (mg/dL) | 1.3 ± 2.7 | 0.8 ± 1.8 | 0.07 |
| Prothrombin time (s) | 16.6 ± 11.4 | 16.3 ± 8.5 | 0.79 |
| APTT (s) | 35.3 ± 8.2 | 35.4 ± 9.6 | 0.95 |
| INR | 1.26 ± 0.4 | 1.32 ± 0.6 | 0.26 |
| RBC transfusion | 107 (62.6%) | 156 (71.6%) | 0.06 |
| Amount of blood transfused (U) | 3.3 ± 5.1 | 3.5 ± 3.2 | 0.76 |
BUN blood urea nitrogen, AST aspartate aminotransferase, ALT alanine aminotransferase, APTT activated partial thromboplastin time, PT-INR international normalized ratio of prothrombin time, RBC red blood cell
Results are presented as mean ± SD or number of patients
Table 3 demonstrates the endoscopic findings of UGIB. The main cause of bleeding was peptic ulcers (49.1% in the sedation group and 59.6% in the nonsedation group, P = 0.04). The number and size of peptic ulcers did not differ significantly between the two groups.
Table 3.
Endoscopic findings of UGIB
| Sedation | Nonsedation | P value | |
|---|---|---|---|
| Causes of UGIB | |||
| Peptic ulcer | 84 (49.1%) | 130 (59.6%) | 0.04 |
| Esophageal varices | 34 (20.0%) | 20 (9.2%) | |
| GERD | 10 (5.9%) | 16 (7.3%) | |
| Post ESD ulcer | 15 (8.8%) | 10 (4.6%) | |
| Angioectasia | 11 (6.4%) | 13 (6.0%) | |
| Mallory–Weiss syndrome | 5 (2.9%) | 11 (5.1%) | |
| Malignant tumor | 6 (3.5%) | 10 (4.6%) | |
| Gastritis | 3 (1.8%) | 4 (1.8%) | |
| Polyps | 3 (1.8%) | 4 (1.8%) | |
| Location of ulcer | |||
| Gastric ulcer | 60 (71.4%) | 98 (75.4%) | 0.61 |
| Upper third | 13 (15.5%) | 21 (16.2%) | |
| Middle third | 32 (25.7%) | 43 (33.1%) | |
| Lower third | 15 (17.9%) | 34 (26.1%) | |
| Duodenal ulcer | 24 (28.6%) | 32 (24.6%) | |
| Number of ulcers | |||
| Single | 64 (76.2%) | 103 (79.8%) | 0.61 |
| Multiple | 20 (23.8%) | 26 (20.2%) | |
| Size of ulcer (mm) | |||
| 0–10 | 35 (41.7%) | 60 (46.2%) | 0.57 |
| > 11 | 49 (58.3%) | 70 (53.8%) | |
| Forrest classification | |||
| Ia | 13 (15.5%) | 14 (10.8%) | 0.22 |
| Ib | 29 (34.5%) | 34 (26.1%) | |
| IIa | 21 (25.0%) | 43 (33.1%) | |
| IIb | 21 (25.0%) | 39 (30.0%) | |
| Atrophic gastritis | |||
| Closed type | 88 (51.5%) | 105 (48.2%) | 0.54 |
| Open type | 83 (48.5%) | 113 (51.8%) | |
UGIB upper gastrointestinal bleeding, GERD gastroesophageal reflux disease, ESD endoscopic submucosal dissection
Administered dosages of sedatives are presented in Table S1. One hundred and thirteen patients (66.1%) were sedated using midazolam, for whom the mean dose of midazolam was 4.2 ± 1.8 mg. In addition, 54 (31.6%) and 4 (2.3%) patients underwent sedation using diazepam and propofol.
Comparison of Clinical Outcomes Between the Two Groups by Propensity Score Matching
Table 4 compares the clinical characteristics between the two groups before and after propensity score matching. Before propensity score matching, mean age, gender, H. pylori infection, use of antithrombotic agents, use of NSAIDs, chronic liver damage, Rockall score, Glasgow-Blatchford score, AIMS65 score, systolic and diastolic blood pressure, hemoglobin level, and peptic ulcer were significantly different between the two groups. Propensity score matching subsequently created 133 matched pairs in the present study and averaged the differences in 13 covariates.
Table 4.
Characteristics of patients before and after propensity score matching
| Sedation | Nonsedation | P value | Standardized difference | |
|---|---|---|---|---|
| Before propensity score matching | ||||
| Number of patients (N) | 171 | 218 | ||
| Age (years) | 70.1 ± 13.9 | 74.8 ± 12.3 | < 0.01 | 0.36 |
| Gender, males | 123 (71.9%) | 133 (61.0%) | 0.03 | 0.23 |
| Helicobacter pylori infection | 50 (29.2%) | 41 (18.8%) | 0.02 | 0.25 |
| Using antithrombotic agents | 45 (26.3%) | 86 (39.5%) | 0.01 | 0.28 |
| Using NSAIDs | 31 (18.1%) | 61 (28.0%) | 0.03 | 0.24 |
| Chronic liver damage | 46 (26.9%) | 38 (17.4%) | 0.03 | 0.23 |
| Rockall score | 4.4 ± 1.4 | 4.7 ± 1.5 | 0.01 | 0.21 |
| Glasgow-Blatchford score | 9.8 ± 4.2 | 10.9 ± 3.8 | 0.01 | 0.27 |
| AIMS65 score | 1.4 ± 0.9 | 1.6 ± 1.0 | < 0.01 | 0.21 |
| Systolic blood pressure (mmHg) | 121.0 ± 24.4 | 112.7 ± 26.7 | < 0.01 | 0.32 |
| Diastolic blood pressure (mmHg) | 66.0 ± 14.4 | 61.3 ± 15.3 | < 0.01 | 0.32 |
| Hemoglobin (g/dL) | 8.9 ± 3.0 | 8.1 ± 2.6 | < 0.01 | 0.28 |
| Peptic ulcer | 84 (49.1%) | 130 (59.6%) | 0.04 | 0.22 |
| After propensity score matching | ||||
| Number of patients (N) | 133 | 133 | ||
| Age (years) | 71.1 ± 13.8 | 72.7 ± 12.6 | 0.31 | 0.12 |
| Gender, males | 91 (68.4%) | 92 (69.2%) | 1.00 | 0.02 |
| Helicobacter pylori infection | 34 (25.6%) | 35 (26.3%) | 1.00 | 0.02 |
| Using antithrombotic agents | 40 (30.1%) | 40 (30.1%) | 1.00 | 0.00 |
| Using NSAIDs | 27 (20.3%) | 27 (20.3%) | 1.00 | 0.00 |
| Chronic liver damage | 34 (25.6%) | 32 (24.1%) | 0.89 | 0.03 |
| Rockall score | 4.5 ± 1.4 | 4.5 ± 1.4 | 0.96 | 0.00 |
| Glasgow-Blatchford score | 10.5 ± 3.7 | 10.1 ± 4.1 | 0.34 | 0.10 |
| AIMS65 score | 1.5 ± 1.0 | 1.5 ± 1.0 | 0.95 | 0.00 |
| Systolic blood pressure (mmHg) | 118.9 ± 23.4 | 119.4 ± 28.0 | 0.88 | 0.02 |
| Diastolic blood pressure (mmHg) | 64.4 ± 13.7 | 62.9 ± 15.9 | 0.40 | 0.10 |
| Hemoglobin (g/dL) | 8.4 ± 2.7 | 8.6 ± 2.7 | 0.43 | 0.07 |
| Peptic ulcer | 69 (51.9%) | 72 (54.1%) | 0.81 | 0.04 |
NSAIDs nonsteroidal anti-inflammatory drugs
Results are presented as mean ± SD or number of patients
Table 5 compares the treatment outcomes after propensity score matching between the two groups. The success rate of endoscopic hemostasis was similar in both groups, with a significantly shorter procedure time in the sedation group than in the nonsedation group (17.6 ± 10.0 versus 20.2 ± 10.2 min, P = 0.04). The main modality of hemostasis was soft coagulation between the two groups. Adverse events such as aspiration pneumonia, rebleeding, and 30-day mortality did not differ significantly between the two groups.
Table 5.
Treatment outcomes and adverse events after propensity score matching
| Sedation | Nonsedation | P value | |
|---|---|---|---|
| Number of patients (N) | 133 | 133 | |
| Successful endoscopic hemostasis | 123 (92.5%) | 123 (92.5%) | 1.00 |
| Mean procedure time (min) | 17.6 ± 10.0 | 20.2 ± 10.2 | 0.04 |
| Time to emergency endoscopy ≤ 3 h | 88 (66.2%) | 86 (64.7%) | 0.90 |
| Main modality of hemostasis | |||
| Soft coagulation | 63 (47.4%) | 63 (47.4%) | 1.00 |
| EVL | 23 (17.3%) | 14 (10.6%) | 0.16 |
| Thrombin spraying | 17 (12.8%) | 14 (10.6%) | |
| Hemoclips | 12 (9.0%) | 16 (12.0%) | |
| APC | 3 (2.3%) | 4 (3.0%) | |
| HSE | 1 (0.8%) | 3 (2.2%) | |
| EMR | 2 (1.5%) | 0 (0%) | |
| EIS | 2 (1.5%) | 0 (0%) | |
| Operator of hemostasis | |||
| Trainees | 77 (57.9%) | 89 (66.9%) | 0.16 |
| Specialists | 56 (42.1%) | 44 (33.1%) | |
| Surgery performed | 0 (0%) | 0 (0%) | 1.00 |
| IVR performed | 3 (2.3%) | 0 (0%) | 0.25 |
| Adverse events | |||
| Hypotension | 4 (3.0%) | 7 (5.3%) | 0.54 |
| Bradycardia | 3 (2.3%) | 3 (2.3%) | 1.00 |
| Hypoxia | 2 (1.5%) | 4 (3.0%) | 0.68 |
| Aspiration pneumonia | 10 (7.5%) | 7 (5.3%) | 0.62 |
| Rebleeding | 10 (7.5%) | 10 (7.5%) | 1.00 |
| 30-Day mortality | 12 (9.0%) | 9 (6.8%) | 0.65 |
EVL endoscopic variceal ligation, APC argon plasma coagulation, HSE hypertonic saline-epinephrine, EMR endoscopic mucosal resection, EIS endoscopic injection sclerotherapy, IVR interventional radiology
Results are presented as mean ± SD or number of patients
Survival Analysis and the Risk Factors for Mortality from UGIB
In the survival analysis, the Kaplan–Meier plot demonstrated no impact of sedation on mortality within 30 days (Fig. S1). Table 6 lists the risk factors for 30-day mortality from UGIB. The Cox proportional hazard model demonstrated that RBC transfusion and rebleeding were risk factors for 30-day mortality from UGIB: hazard ratio (HR) [95% confidence interval] 4.45 [1.50–19.11], P < 0.01; and HR 3.30 [1.14–8.36], P = 0.03. Nevertheless, sedation endoscopy was not associated with 30-day mortality after adjusting for potential confounders: HR 1.39 [0.64–3.02], P = 0.40.
Table 6.
Univariate and multivariate analysis of risk factors for 30-day mortality of UGIB
| Factors | Kaplan–Meier | Cox multivariate analysis | |||
|---|---|---|---|---|---|
| P value | Hazard ratio | 95% CI | P value | ||
| Age, > 70 years | 0.66 | 1.03 | 0.44 | 2.51 | 0.94 |
| Sex, male | 0.43 | 1.38 | 0.61 | 3.43 | 0.45 |
| Using antithrombotic agents | 0.74 | 1.01 | 0.42 | 2.29 | 0.97 |
| Chronic liver damage | 0.41 | 1.56 | 0.61 | 3.71 | 0.34 |
| Malignant diseases | 0.20 | 0.54 | 0.18 | 1.34 | 0.19 |
| AIMS65 score ≥ 2 | 0.74 | 0.97 | 0.43 | 2.15 | 0.97 |
| Systolic blood pressure, < 110 mmHg | 0.24 | 1.48 | 0.67 | 3.29 | 0.33 |
| RBC transfusion | 0.01 | 4.45 | 1.50 | 19.11 | < 0.01 |
| Peptic ulcer | 0.66 | 0.68 | 0.32 | 1.47 | 0.33 |
| Sedation endoscopy | 0.41 | 1.39 | 0.64 | 3.02 | 0.40 |
| Successful endoscopic hemostasis | 0.65 | 1.02 | 0.22 | 3.14 | 0.98 |
| Mean procedure time, 20 > min | 0.82 | 1.08 | 0.49 | 2.33 | 0.84 |
| Aspiration pneumonia | 0.54 | 0.99 | 0.23 | 2.91 | 0.99 |
| Rebleeding | 0.01 | 3.30 | 1.14 | 8.36 | 0.03 |
UGIB upper gastrointestinal bleeding, RBC red blood cell, 95% CI 95% confidence interval
Discussion
Endoscopic hemostasis is the gold standard for the treatment of UGIB, and more stable techniques are required in emergency situations [13–19]. The benefits of sedation in endoscopy have already been recognized, and the frequency of sedation endoscopy is increasing [20, 21]. Sedation is effective for conducting safe and secure emergency treatment if vital signs are stable, particularly when the patient is in an agitated or anxious state [38], but sedation may not be effective depending on the patient’s performance status or extent of symptoms [22]. Some endoscopists hesitate to use sedation during emergency endoscopy for gastrointestinal bleeding because of concerns about potential adverse events related to sedatives, such as respiratory depression and cardiovascular instability [24]. The present study focused on whether sedatives are safe and effective when administered during emergency endoscopy in patients with UGIB.
Some cohort studies reported the safety of sedation during emergency gastrointestinal endoscopy for variceal bleeding or peptic ulcers, respectively [21, 26]. In practice, however, the source of bleeding, whether variceal or nonvariceal, is not known at the time when sedation is initiated. Therefore, this study investigated the usefulness of sedation during emergency endoscopy in patients with UGIB, including both nonvariceal and variceal bleeding. A previous report has shown that shock is more common with variceal than with nonvariceal bleeding [39]. Thus, given that there are some differences in patient background between nonvariceal and variceal bleeding, in this study we analyzed the data using propensity score matching to equalize the patient background.
In the present study, emergency endoscopy for UGIB could be performed in a shorter time in the sedation group than in the nonsedation group. It is speculated that sedation may have calmed the patient’s agitation, stabilized body movements, and allowed the endoscopist to perform emergency endoscopy more easily. Furthermore, success rates of endoscopic hemostasis were similar between the sedation group and nonsedation group. These data may indicate that the use of sedation in emergency endoscopy is useful.
Sedation endoscopy did not increase the incidence of adverse events such as aspiration pneumonia, rebleeding, and mortality compared with nonsedation endoscopy. This was also true when comparing sedated (n = 38) and nonsedated (n = 85) patients excluded from this propensity score matching analysis. The incidence of adverse events (16.5% versus 15.8%; P = 1.00) and 30-day mortality (7.9% versus 5.9%; P = 0.70) were comparable between the two groups outside the propensity score matching analysis. In addition, the incidence of these adverse events was similar to that in some previous reports [26, 40, 41]. Therefore, we believe that sedatives can be safely used during emergency endoscopy for UGIB.
In the Cox multivariate analysis, risk factors for 30-day mortality after endoscopic hemostasis with UGIB were RBC transfusion and rebleeding, which have previously been reported as risk factors for mortality from UGIB [26, 33], and the present study was no different in this regard. Sedation endoscopy did not increase mortality in patients with UGIB, demonstrating that sedation is not directly related to the cause of death in patients with UGIB. Sedation is considered safe in emergency endoscopy because it does not adversely affect the course of treatment of UGIB in the short term.
The present study has several limitations. It was a single-center study, and the number of patients was small. Being a retrospective study there was a patient selection bias, and the use of sedatives was at the discretion of the endoscopist. Although propensity score matching analysis was applied to minimize the difference in clinical characteristics between the two groups, the results of the present study should be interpreted with caution. Further prospective studies with a larger number of subjects should be carried out to validate our results.
In conclusion, sedation reduces endoscopic procedure time for UGIB, and sedatives can be used safely during emergency endoscopy.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file3 (PDF 67 KB) Overall survival curves for 30 days after emergency endoscopy with sedation and non-sedation of UGIB patients
Acknowledgments
We thank Hugh McGonigle, from Edanz (https://www.jp.edanz.com/ac), for editing a draft of this manuscript.
Abbreviations
- UGIB
Upper gastrointestinal bleeding
- PSM
Propensity score matching
- EVL
Endoscopic variceal ligation
- RBC
Red blood cell
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- HR
Hazard ratio
Author’s contribution
D.Y. designed the research; D.Y., G.N., Y.M., T.N., A.J, K.G., R.A., S.I., S.K., S.F., A.S., A.J., Y.T., K.I., Y.T., W.Y., N.H., T.M., K.A., and S.T. performed sedative endoscopy, acquired the patient data, and made substantial contributions to the conception; D.Y. designed the work; D.Y. analyzed the data; D.Y., Y.S., and M.E. wrote the paper; M.E. was a major contributor in writing the manuscript. All authors read and approved the submitted version, and approved the final manuscript. All authors have agreed both to be personally accountable for the author’s contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated and resolved, and the resolution documented in the literature.
Funding
No specific grants from any funding agencies in the public, commercial, or not-for-profit sectors were received for this research.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and the guidelines of the Consolidated Standards of Reporting Trials (CONSORT). The study protocol and the consent procedure were approved by the Ethics Review Committee of the National Hospital Organization Ureshino Medical Center (approval number 20-86).
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107:1190–1195. doi: 10.1038/ajg.2012.168. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G, De Franchis R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi D, Stanley AJ, Hayes PC, et al. Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680–1704. doi: 10.1136/gutjnl-2015-309262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ootani H, Iwakiri R, Shimoda R, et al. Role of Helicobacter pylori infection and nonsteroidal anti-inflammatory drug use in bleeding peptic ulcers in Japan. J Gastroenterol. 2006;41:41–46. doi: 10.1007/s00535-005-1720-y. [DOI] [PubMed] [Google Scholar]
- 5.Fujishiro M, Iguchi M, Kakushima N, et al. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc. 2016;28:363–378. doi: 10.1111/den.12639. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi D, Sakata Y, Yoshida H, et al. Effectiveness of endoscopic hemostasis with soft coagulation for non-variceal upper gastrointestinal bleeding over a 12-year period. Digestion. 2017;95:319–326. doi: 10.1159/000477439. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa K, Sugiyama T, Kato M, et al. Non-Helicobacter pylori and non-NSAID peptic ulcer disease in the Japanese population. Eur J Gastroenterol Hepatol. 2000;12:635–640. doi: 10.1097/00042737-200012060-00010. [DOI] [PubMed] [Google Scholar]
- 8.Kamada T, Haruma K, Ito M, et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter. 2015;20:192–198. doi: 10.1111/hel.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi D, Sakata Y, Tsuruoka N, et al. Upper gastrointestinal bleeding in Japanese patients prescribed antithrombotic drugs: differences in trends over time. Hepatogastroenterology. 2014;61:1055–1062. [PubMed] [Google Scholar]
- 10.Johnsen SP, Sørensen HT, Mellemkjoer L, et al. Hospitalisation for upper gastrointestinal bleeding associated with use of oral anticoagulants. Thromb Haemost. 2001;86:563–568. doi: 10.1055/s-0037-1616087. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi D, Sakata Y, Tsuruoka N, et al. Characteristics of patients with non-variceal upper gastrointestinal bleeding taking antithrombotic agents. Dig Endosc. 2015;27:30–36. doi: 10.1111/den.12316. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama M, Iwakiri R, Hara M, et al. Low-dose aspirin is a prominent cause of bleeding ulcers in patients who underwent emergency endoscopy. J Gastroenterol. 2009;44:912–918. doi: 10.1007/s00535-009-0074-2. [DOI] [PubMed] [Google Scholar]
- 13.ASGE Technology Committee. Wong Kee Song LM, Banerjee S, Barth BA, et al. Emerging technologies for endoscopic hemostasis. Gastrointest Endosc. 2012;75:933–937. doi: 10.1016/j.gie.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Mullady DK, Wang AY, Waschke KA. AGA clinical practice update on endoscopic therapies for non-variceal upper gastrointestinal bleeding: expert review. Gastroenterology. 2020;159:1120–1128. doi: 10.1053/j.gastro.2020.05.095. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi D, Tominaga N, Miyahara K, et al. Safety and efficacy of the noncessation method of antithrombotic agents after emergency endoscopic hemostasis in patients with nonvariceal upper gastrointestinal bleeding: a multicenter pilot study. Can J Gastroenterol Hepatol. 2021;2021:6672440. doi: 10.1155/2021/6672440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jairath V, Kahan BC, Logan RF, et al. Outcomes following acute nonvariceal upper gastrointestinal bleeding in relation to time to endoscopy: results from a nationwide study. Endoscopy. 2012;44:723–730. doi: 10.1055/s-0032-1309736. [DOI] [PubMed] [Google Scholar]
- 17.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut. 2010;59:1022–1029. doi: 10.1136/gut.2008.174599. [DOI] [PubMed] [Google Scholar]
- 18.Sarin N, Monga N, Adams PC. Time to endoscopy and outcomes in upper gastrointestinal bleeding. Can J Gastroenterol. 2009;23:489–493. doi: 10.1155/2009/604639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung JJ, Chiu PW, Chan FKL, et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67:1757–1768. doi: 10.1136/gutjnl-2018-316276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoda T, Akamatsu T, Abe S, et al. Guidelines for sedation in gastroenterological endoscopy (second edition) Dig Endosc. 2021;33:21–53. doi: 10.1111/den.13882. [DOI] [PubMed] [Google Scholar]
- 21.Lohse N, Lundstrøm LH, Vestergaard TR, et al. Anaesthesia care with and without tracheal intubation during emergency endoscopy for peptic ulcer bleeding: a population-based cohort study. Br J Anaesth. 2015;114:901–908. doi: 10.1093/bja/aev100. [DOI] [PubMed] [Google Scholar]
- 22.Duch P, Haahr C, Møller MH, et al. Anaesthesia care for emergency endoscopy for peptic ulcer bleeding. A nationwide population-based cohort study. Scand J Gastroenterol. 2016;51:1000–1006. doi: 10.3109/00365521.2016.1164237. [DOI] [PubMed] [Google Scholar]
- 23.Laursen SB, Leontiadis GI, Stanley AJ, Møller MH, Hansen JM, Schaffalitzky de Muckadell OB. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: a nationwide cohort study. Gastrointest Endosc. 2017;85:936–944. doi: 10.1016/j.gie.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 24.Tohda G, Higashi S, Sakumoto H, Sumiyoshi K, Kane T. Efficacy and safety of nurse-administered propofol sedation during emergency upper endoscopy for gastrointestinal bleeding: a prospective study. Endoscopy. 2006;38:684–689. doi: 10.1055/s-2006-925374. [DOI] [PubMed] [Google Scholar]
- 25.Albillos A, Zamora J, Martínez J, et al. Stratifying risk in the prevention of recurrent variceal hemorrhage: results of an individual patient meta-analysis. Hepatology. 2017;66:1219–1231. doi: 10.1002/hep.29267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park CH, Park SW, Jung JH, et al. Clinical outcomes of sedation during emergency endoscopic band ligation for variceal bleeding: multicenter cohort study. Dig Endosc. 2020;32:894–903. doi: 10.1111/den.13610. [DOI] [PubMed] [Google Scholar]
- 27.Kiriyama S, Gotoda T, Sano H, et al. Safe and effective sedation in endoscopic submucosal dissection for early gastric cancer: a randomized comparison between propofol continuous infusion and intermittent midazolam injection. J Gastroenterol. 2010;45:831–837. doi: 10.1007/s00535-010-0222-8. [DOI] [PubMed] [Google Scholar]
- 28.Arima S, Sakata Y, Ogata S, et al. Evaluation of hemostasis with soft coagulation using endoscopic hemostatic forceps in comparison with metallic hemoclips for bleeding gastric ulcers: a prospective, randomized trial. J Gastroenterol. 2010;45:501–505. doi: 10.1007/s00535-009-0186-8. [DOI] [PubMed] [Google Scholar]
- 29.Shimoda R, Iwakiri R, Sakata H, et al. Evaluation of endoscopic hemostasis with metallic hemoclips for bleeding gastric ulcer: comparison with endoscopic injection of absolute ethanol in a prospective, randomized study. Am J Gastroenterol. 2003;98:2198–2202. doi: 10.1111/j.1572-0241.2003.07692.x. [DOI] [PubMed] [Google Scholar]
- 30.Shimoda R, Iwakiri R, Sakata H, et al. Endoscopic hemostasis with metallic hemoclips for iatrogenic Mallory–Weiss tear caused by endoscopic examination. Dig Endosc. 2009;21:20–23. doi: 10.1111/j.1443-1661.2008.00825.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji Y, Ohata K, Sekiguchi M, et al. An effective training system for endoscopic submucosal dissection of gastric neoplasm. Endoscopy. 2011;43:1033–1038. doi: 10.1055/s-0031-1291383. [DOI] [PubMed] [Google Scholar]
- 32.Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition) Dig Endosc. 2021;33:4–20. doi: 10.1111/den.13883. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura S, Matsumoto T, Sugimori H, Esaki M, Kitazono T, Hashizume M. Emergency endoscopy for acute gastrointestinal bleeding: prognostic value of endoscopic hemostasis and the AIMS65 score in Japanese patients. Dig Endosc. 2014;26:369–376. doi: 10.1111/den.12187. [DOI] [PubMed] [Google Scholar]
- 34.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318–1321. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 36.Saltzman JR, Tabak YP, Hyett BH, et al. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74:1215–1224. doi: 10.1016/j.gie.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 37.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 38.Waye JD. Intubation and sedation in patients who have emergency upper GI endoscopy for GI bleeding. Gastrointest Endosc. 2000;51:768–771. doi: 10.1016/S0016-5107(00)70104-0. [DOI] [PubMed] [Google Scholar]
- 39.Park CH, Han DS, Jeong JY, et al. Outcomes of propofol sedation during emergency endoscopy performed for upper gastrointestinal bleeding. Dig Dis Sci. 2016;61:825–834. doi: 10.1007/s10620-015-3942-z. [DOI] [PubMed] [Google Scholar]
- 40.Hayat U, Lee PJ, Ullah H, Sarvepalli S, Lopez R, Vargo JJ. Association of prophylactic endotracheal intubation in critically ill patients with upper GI bleeding and cardiopulmonary unplanned events. Gastrointest Endosc. 2017;86:500–509.e1. doi: 10.1016/j.gie.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhuri D, Bishay K, Tandon P, et al. Prophylactic endotracheal intubation in critically ill patients with upper gastrointestinal bleed: a systematic review and meta-analysis. JGH Open. 2019;4:22–28. doi: 10.1002/jgh3.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file3 (PDF 67 KB) Overall survival curves for 30 days after emergency endoscopy with sedation and non-sedation of UGIB patients
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.