Abstract
Background and Aims
Several studies showed muscularis macrophages (MMφ) are associated with GI motility disorders. The purpose of this study was to preliminary explore the association between MMφ and achalasia.
Methods
Tissue samples of the lower esophageal sphincter (LES) high‐pressure zone were obtained from 27 achalasia patients and 10 controls. Immunohistochemistry for MMφ, interstitial cells of Cajal (ICC), neuronal nitric oxide synthase (nNOS), and glial cells were conducted. Histological characteristics were compared between groups, and correlation analysis was performed.
Results
Fewer ICC was found in achalasia compared with controls (P = 0.018), and the level of M1 macrophages was higher than that in controls no matter in terms of the number or the proportion of M1(P = 0.026 for M1 and 0.037 for M1/MMφ). Statistical differences were found between two groups in terms of proportion of M2 and ratio of M1 to M2 (P = 0.048 for M2/ MMφ and < 0.001 for M1/M2). For the correlation analysis, significant correlations were detected between levels of nNOS, ICC, and glial cells in patients with achalasia (P = 0.026 for nNOS and ICC, 0.001 for nNOS and glial cells, 0.019 for ICC and glial cells). There were significant correlations between M2/MMφ and levels of ICC (P = 0.019), glial cells (P = 0.004), and nNOS (P = 0.135).
Conclusion
Patients with achalasia had a higher level of M1/M2 ratio in LES and significant correlations were found between M2/MMφ and numbers of ICC and glial cells, which suggested that MMφ were probably associated with occurrence and development of achalasia.
Keywords: Achalasia, Muscularis, Macrophage polarization, Interstitial Cells of Cajal (ICC)
Introduction
Achalasia is an esophageal motility disorder characterized by absence of peristalsis and impaired relaxation of the lower esophageal sphincter (LES) leading to symptoms including dysphagia, chest pain, reflux, and weight loss [1]. This motility disorder is associated with functional loss or reduction of myenteric plexus neurons, interstitial cells of Cajal (ICC), neuronal nitric oxide synthase (nNOS) mainly in the distal esophagus and LES [2–7]. Except for secondary achalasia due to Chagas disease, the etiology of idiopathic achalasia remains largely unknown.
Many studies indicated aberrant autoimmunity probably triggered by a viral infection may play an important part in neuronal degeneration of achalasia, particularly in genetically susceptible individuals [1]. Patients with achalasia are more likely to suffer from autoimmune diseases [8, 9] and specific autoantibodies associated with neuronal damage can be found in their sera [10, 11]. Moreover, infiltration in LES muscle by inflammatory cells including eosinophils, mast cells, and T cells was associated with loss of neuronal degeneration.
Macrophages are essential for innate immunity and play a critical role in inflammation and host defense [12]. Macrophages exhibit remarkable plasticity and can differentiate into classically activated macrophages (M1) or alternatively activated macrophages (M2), the type of activation depending on different microenvironments [13]. The M1 phenotype is pro‐inflammatory and expresses high levels of proinflammatory cytokines including interleukin‐6 (IL‐6), tumor necrosis factor‐alpha (TNF‐α), and inducible nitric oxide synthase (iNOS). In contrast, the M2 phenotype is anti-inflammatory, with high expression of galactose-type and the mannose receptor [14]. Macrophages in gastrointestinal (GI) can be divided into mucosal macrophages and muscularis macrophages (MMφ) according to their resident location [14]. Several studies showed MMφ are associated with GI motility disorders such as postoperative ileus, intestinal ischemia–reperfusion (I/R) damage and gastroparesis [15–18]. Activation status of MMφ can influence the function of smooth muscle, ICC (GI pacemaker cells), and neurons [15]. However, there is no study investigating the relationship between MMφ and esophageal motility diseases such as achalasia. Therefore, the purpose of this study was to preliminary explore the association between MMφ and achalasia and to investigate the correlation of M1 and M2 phenotype with ICC, nitrergic nerves, glial cells, as well as clinical characteristics.
Method
Patient Selection
The study included 27 patients diagnosed with achalasia undergoing peroral endoscopic myotomy (POEM) at our center from July 2020 to May 2021. Patients were excluded from this study based on the following criteria: (1) presence of esophagus disease that might interfere with the result of our study, such as Barrett’s esophageal lesions, esophageal stricture, esophageal varices, active esophagitis; (2) patients with serious underlying diseases such as liver cirrhosis, hematological disease, or coagulopathy; (3) pregnant and breastfeeding female patients; (4) patients with surgical contraindications for POEM.
All the 27 patients were received comprehensive preoperative clinical evaluation including Eckardt Score for symptoms of achalasia, high-resolution esophageal manometry (HREM), barium esophagogram and esophagogastroduodenoscopy (EGD). The Chicago Classification Criteria of esophageal motility disorders v3.0 was utilized for diagnosis and classification of achalasia [19].Clinical information was obtained by face-to-face inquiry, including age, gender, body mass index (BMI), history of tobacco and alcohol intake, history of prior treatments, disease duration.
The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2020-SR-380). Written informed consent was obtained from all the participants in our study.
Tissue Samples
As reported in previous studies [20, 21], tissue samples of muscle were obtained from the LES high‐pressure zone after myotomy during the POEM procedure. The 2–3 pieces of tissue were collected from each patient, and each of the tissue samples was generally 0.3 cm × 0.3 cm × 0.3 cm in size. Esophageal biopsy was performed at the same site from 10 patients who underwent surgery for esophageal or stomach neoplasms without invasion of the cardia at our center. The exclusive criteria were consistent with the above and none of the 10 patients had esophageal motility disorder including achalasia or autoimmune disease which might interfere with the result of our study. This method for obtaining control specimens was in accordance with previous studies [10, 20, 22, 23]. All the esophageal biopsies were conducted by the same endoscopist and surgeon to ensure the sampling sites were consistent and to avoid sampling error. The biopsy specimens were immediately immersed in 10% formalin and then embedded in paraffin.
Immunohistochemistry and Quantification
Consistent with previous studies of gastrointestinal motility [20, 24], antibodies to nNOS were used to examine nitrergic nerves and to S-100β to assess glial cells. The immunohistochemical staining for c‐kit was performed to assess the ICC networks [7]. The CD68 was used as a general marker for macrophages, and for the identification of macrophages phenotype, antibodies to inducible nitric oxide synthase (iNOS) were used to identify M1 macrophages and to CD206 to M2 macrophages [25].
The tissue samples were embedded in paraffin, sliced into 4‐μm sections, and mounted on slides. Following deparaffinization, rehydration, antigen retrieval and blocking of endogenous peroxidase, immunohistochemical staining was conducted using anti-CD68 antibody (ab955, Abcam, 1:3000 dilution), anti-iNOS antibody (abs131793, absin, Shanghai China, 1:200 dilution), anti-CD206 (ab64693, Abcam, 1:10,000 dilution), anti-c-kit antibody (ab32363, Abcam, 1:400 dilution), anti-nNOS antibody (ab5586, Abcam, 1:100 dilution), and anti S-100β antibody (ab52642, Abcam, 1:400 dilution). The immunohistochemical staining intensities of the interested proteins were quantified as integrated optical densities (IODs) utilizing Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA). The researcher who conducted the quantification did not know which study group the sample was in to avoid bias.
Follow-Up and Outcome Measures
Patients were regularly followed-up at 1, 3, 6, and 12 months by clinical interview. The Eckardt Score was performed for clinical assessment. HRM and barium esophagram were performed 3 months after POEM, and EGD 12 months after POEM. Eckardt Score > 3 after the operation was defined as clinical failure [26]. The last follow-up was in February 2022.
Statistical Analysis
Mean ± standard deviation (SD) or range was used to represent continuous variables, and the number of cases (ratio %) was utilized to express categorical variables. Differences between groups were compared using a two-sample independent t test for continuous variables and chi‐square test for categorical variables. Correlation between variables was evaluated using Spearman’s correlation or Pearson correlation analysis. SPSS 22.0 and GraphPad Prism 9 were used for data processing and statistical analysis. The P value < 0.05 was considered to be statistically significant.
Results
Demographic and Clinical Characteristics of all Subjects
The demographic characteristics of 27 patients with achalasia and 10 controls in this study are presented in Table 1. The mean age of patients with achalasia was younger compared to the control group (42.89 years vs 52.10 years, P = 0.003). There was no significant difference between two groups in terms of gender (P = 0.276), BMI (P = 0.074), history of alcohol (P = 1) or tobacco intake (P = 0.069). None of the participants had a definite history of herpes simplex virus (HSV), varicella zoster virus (VZV), measles and human papilloma virus (HPV) infection, and autoimmune disease.
Table 1.
Baseline characteristics of all subjects
| Variables | Achalasia | Controls | P value |
|---|---|---|---|
| Total number | 27 | 10 | – |
| Age, mean ± SD, years | 42.89 ± 13.63 | 58.10 ± 9.433 | 0.003* |
| Male (%) | 9 (33.3) | 6 (60) | 0.276 |
| BMI, mean ± SD | 22.04 ± 3.76 | 24.65 ± 4.04 | 0.074 |
| History of alcohol intake (%) | 3 (11.1) | 1 (10) | 1 |
| History of tobacco smoking (%) | 3 (11.1) | 4 (40) | 0.069 |
Values are n (%), unless otherwise noted
BMI Body Mass Index, SD standard deviation
*P < 0.05
As shown in Table 2, the median disease duration of patients with achalasia was 4 years (range 0.5‐20 years), and the median preoperative Eckardt score was 7 (range 6‐9). There were two patients with a history of prior treatments, including one patient with prior balloon dilation and esophageal stent, and the other with prior POEM. All patients with achalasia were received HREM, including 4 with type I and 20 with type II. The remaining 3 patients were unspecified subtypes due to the failure of catheter sensors to pass through EGJ (esophagogastric junction) because of strong tortuous angulation of the esophagus. The POEM was performed successfully in all 27 patients. The Eckardt score at 6 months after POEM was all less than 3 and was significantly decreased compared with pre-operation (P < 0.001).
Table 2.
Clinical characteristics of 27 patients with achalasia
| Characteristic | Value |
|---|---|
| History of prior treatments | 2 (7.4) |
| Disease duration, median (range), years | 4 (0.5–20) |
| Preoperative Eckardt score, median (range) | 7 (6–9) |
| Preoperative IRP, median (range), mmHg | 27.6 (15.9–56.6) |
| Chicago subtype (%) | |
| I | 4 (14.8) |
| II | 20 (74.1) |
| Unspecified | 3 (11.1) |
| Procedure time, mean ± SD, min | 52.78 ± 17.66 |
| Myotomy length, mean ± SD, cm | 10.07 ± 0.81 |
| Eckardt score at 6 months after POEM, median (range) | 1 (0–2) |
Values are n (%), unless otherwise noted. Unspecified, diagnosed by clinical symptoms, esophagogastroduodenoscopy and barium esophagram due to failure in high-resolution manometry classification
IRP integrated relaxation pressure, EGJ esophagogastric junction, SD standard deviation
Histological Evaluation of Tissue Samples
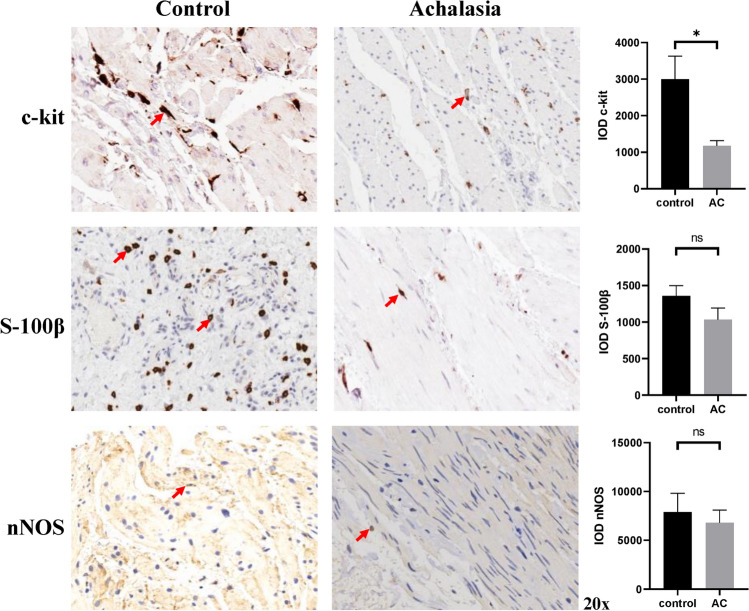
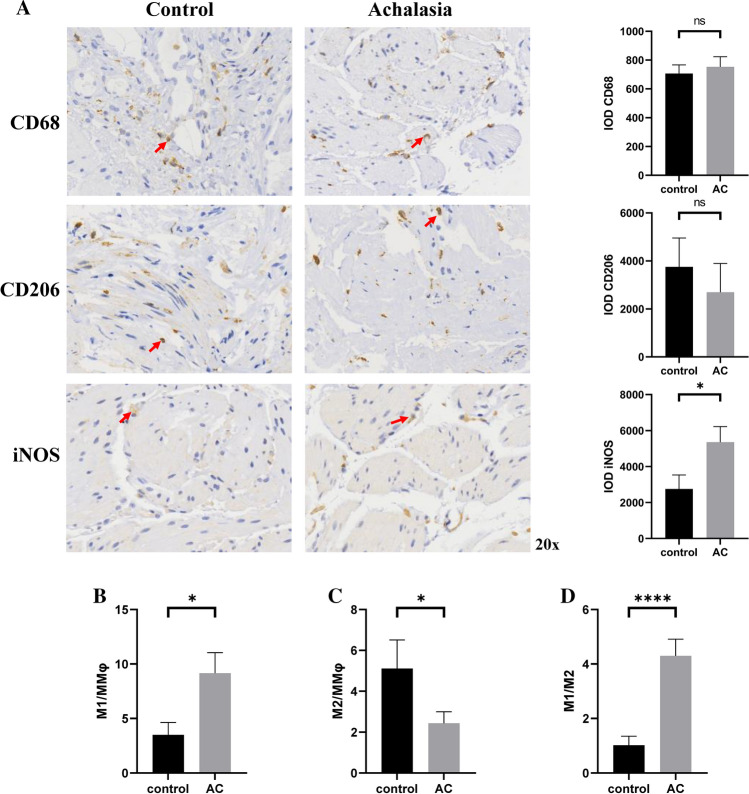
The results of immunoreactivity and quantification for ICC, glial cells, and nNOS was shown in Fig. 1. Interestingly, the staining for c‐kit showed significantly fewer ICC in patients with achalasia compared with controls (P = 0.018). However, no significant difference was found in glial cells and nNOS between two groups (P = 0.138 and 0.661, respectively). Figure 2A showed no considerable difference between two groups in neither total macrophages nor M2 macrophages (P = 0.621 and 0.539, respectively). In contrast, the level of M1 in patients with achalasia was found higher than that in controls no matter in terms of the absolute number or the proportion of M1 in the total macrophages (P = 0.026 for M1 and P = 0.037 for M1/MMφ; Fig. 2A, B). In addition, statistical differences were also found between two groups in terms of proportion of M2 in the total macrophages and ratio of M1 to M2 (P = 0.048 for M2/MMφ and P < 0.001 for M1/M2; Fig. 2C, D). The histological differences between different types of achalasia were also analyzed, but no statistical difference was found in terms of the levels of nNOS, ICC, glial cells, M1/MMφ, M2/MMφ, and M1/M2.
Fig. 1.
Immunohistochemistry and quantification of ICC, glial cells, and nNOS in LES tissue samples obtained from patients with achalasia and controls. The c‐kit staining was used to examine ICC, S-100β staining was used to assess glial cells and nNOS to nitrergic nerves. LES lower esophageal sphincter, ICC interstitial cells of Cajal, nNOS neuronal nitric oxide synthase. *P < 0.05. ns not significant
Fig. 2.
Immunohistochemistry and quantification of macrophages in LES tissue samples obtained from patients with achalasia and controls. A The CD68 was used as general marker for macrophages, and antibodies to iNOS were used to identify M1 macrophages and to CD206 to M2 macrophages. Quantification analysis showed that no considerable difference between two groups in total macrophages and M2 macrophages, but level of M1 macrophages in achalasia was higher than that in controls no matter in terms of the absolute number or the proportion of M1 in the total macrophages (B). Statistical differences were also found between two groups in terms of proportion of M2 in the total macrophages (C) and ratio of M1 to M2 (D). iNOS inducible nitric oxide synthase, MMφ muscularis propria macrophages. *P < 0.05; ***P < 0.001. ns not significant
Correlation Analysis of Histological and Clinical Characteristics in Achalasia
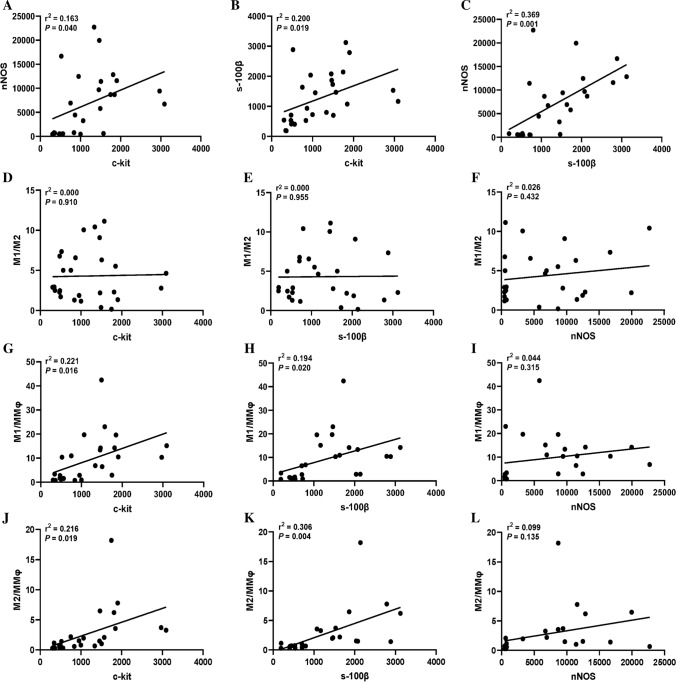
For the correlation analysis of histological characteristics, significant correlations were detected between levels of nNOS, ICC, and glial cells (P = 0.026 for nNOS and ICC, P = 0.001 for nNOS and glial cells, P = 0.019 for ICC and glial cells, Fig. 3A–C). Moreover, as shown in Fig. 3G–I, the results indicated that there were significant correlations between M1/MMφ and levels of ICC (P = 0.016) and glial cells (P = 0.020), but no clear relationship was found between M1/MMφ and nNOS (P = 0.315) in patients with achalasia. Similar results were also found between M2/MMφ and levels of ICC (P = 0.019), glial cells (P = 0.004), and nNOS (P = 0.135) (Fig. 3J–L). However, no significant correlations were found between M1/M2 and other histological characteristics (Fig. 3D–F). To further explore the role of macrophages in achalasia, correlation analysis between clinical characteristics and M1/M2, M1/MMφ, and M2/MMφ was conducted. However, none of them had a significant association with clinical characteristics including gender, age, BMI, disease duration, Chicago subtype, preoperative Eckardt score, IRP and decrease of Eckardt score after POEM.
Fig. 3.
Correlation analysis of histological characteristics in patients with achalasia. Significant correlations were detected between levels of nNOS, ICC, and glial cells (A–C). There was no significant correlations between M1/M2 and levels of nNOS, ICC, and glial cells (D–F). Significant correlations was found between M1/MMφ and levels of ICC and glial cells (G, H), but no clear relationship was found between M1/MMφ and nNOS (I). Similar results were also found inM2/MMφ (J–L)
Discussion
Although it has been 340 years since achalasia was first described in 1674 by Sir Thomas Willis [1], the etiology of achalasia remains unclear. Many studies indicated that autoimmune-mediated inflammation may be the main cause of achalasia [1]. A matched case–control study which enrolled 6769 cases and 27,076 controls, found that the presence of autoimmune conditions and viral infections was also associated with an increased risk of achalasia [27]. The prevalence of autoimmune diseases in patients with achalasia is 7.42% (502/6,769) versus 4.02% (1,088/27,076) in controls, and the prevalence of viral infections is 1.58% (107/6,769) versus 0.82% (221/27,076). This result was in accordance with other previous studies [8, 10, 28] and suggested that aberrant autoimmune and viral infections may contribute to the occurrence of achalasia. In our study, we collected clinical information from 27 patients with achalasia and 10 controls, but none of them had a definite history of HSV, VZV, HPV, and measles infection, or autoimmune diseases. This result may be attributed to the small number of cases in our study, and some of the patients with viral infections or autoimmune diseases may be excluded due to the surgical contraindications and other exclusive criteria as mentioned above.
ICC are recognized as pacemaker cells and generate spontaneous electrical slow waves regulating gastrointestinal motility, and they are also associated with the transfer of neurotransmitters [3]. The nNOS is a kind of inhibitory neurotransmitter, which help produce NO in nervous tissue to regulate muscle relaxation [29]. Previous studies showed that the main pathological feature of achalasia is characterized by the decrease of esophageal myenteric plexus neurons, ICC and nNOS in the LES [2–7]. This pathological change leads to the aperistalsis and impaired relaxation of the LES. In this study, although a significant difference was found only in ICC, LES of patients with achalasia displayed fewer glial cells and nNOS than controls. For the correlation analysis, significant correlations were detected between levels of nNOS, ICC, and glial cells. A study conducted by Ward et al. indicated that ICC may be the effectors that transduce NO signals into hyperpolarizing responses, and loss of ICC may impair relaxations and normal motility of LES [30]. Another study also suggested that reduction of nNOS release might underlie the profound ICC impairment, which could impair the relaxation of LES in patients with achalasia. However, no clear correlation between the reduction degree of ICC and that of nNOS was found in other studies [3, 31]. Moreover, an animal study also found that the reduction of ICC and nNOS can cause dysfunction of the LES and esophageal peristalsis, but they might be independent relevant causes [32]. Enteric glial cells are thought to function as intermediaries in enteric neurotransmission, thus reduction of them might weaken the neurenteric balance [33]. Although published studies found that reduction of ICC and glial cells are present in gastrointestinal motor abnormalities such as slow transit constipation [34], colonic diverticular disease [35] and achalasia [20], to our knowledge, no study has reported a clear correlation between levels of ICC and glial cells in these diseases. We speculated that the causes of reduction of nNOS, ICC, and glial cells were consistent, and the levels of them reflected the degree of the damage, so there were correlations among them. Overall, it is not very clear the role of and relationships between ICC, nNOS, and glial cells in achalasia, further studies are still needed to be conducted.
MMφ were first described as a macrophage subtype residing in the myenteric plexus associated with both ICC and enteric neurons in the early 90 s [36]. Although insight into the function of MMφ in gastrointestinal is still limited, several studies indicated a key role in regulation of gastrointestinal motility in both pathological and physiological conditions. Previous reports showed that M1-like macrophages could impair smooth muscle function and impair gut motility by producing pro-inflammatory cytokines such as IL-6 both in postoperative ileus and intestinal I/R injury [15]. This motility disorder was also associated with functional changes of ICC networks which was most likely caused by the inflammatory process [37, 38]. Moreover, inhibition of TNF-α derived from M1 macrophage or suppressing M1 macrophage activation by blockade of IL-17A could alleviate the injury of the ICC [39, 40]. Emerging evidence about diabetic gastroparesis has revealed that heme oxygenase 1 (HO1) which is expressed by M2 macrophages, could protect ICC and nNOS expression in enteric neurons from oxidative damage associated with diabetes and prevent development of delayed gastric emptying [14]. In contrast, activation of M1 macrophages which lack HO1 could cause significant damage to ICC and enteric neurons and influence gastric motility [14, 15]. Furthermore, reduction of CD206-positive cells was found in full thickness biopsies of the gastric body from patients with diabetic gastroparesis, which was associated with loss of ICC [41]. In addition to regulation of inflammation to influence the gastrointestinal motility, MMφ could also regulate peristaltic activity of the colon in the steady state through the secretion of bone morphogenetic protein 2 (BMP2), which activates BMP receptor (BMPR) expressed by enteric neurons [42]. In a previous work, Luo et al. demonstrated that MMφ could directly affect the function of intestinal smooth muscle cells by expressing the transient receptor potential vanilloid 4 (TRPV4) channel, without the enteric nervous system involvement [43]. Nevertheless, no evidence suggested these mechanisms are associated with the etiology of achalasia.
In our study, we found patients with achalasia had a higher level of M1/MMφ and a lower level of M2/MMφ in their LES than controls. Moreover, significant positive correlations were detected between M2/MMφ and numbers of ICC and glial cells. These results indicated that M1 macrophages might underlie the reduction of ICCs and glial cells due to their pro-inflammatory functions, and M2 macrophages might play a protective role against injury. Since the median disease duration of patients with achalasia in this study was 4 years and no significant correlation was found between disease duration and levels of M1/M2, M1/MMφ, and M2/MMφ, we thought that the inflammatory injury to ICC and glial cells might be persistent. However, positive correlations were found between M1/MMφ and numbers of ICC and glial cells, which were contrary to our expectations. These results were difficult to explain, we hypothesized that M1 macrophages can impair ICC and glial cells through secreting pro-inflammatory mediators, and measuring the number of M1 macrophages without detecting pro-inflammatory cytokines and chemokines they produced could not fully reflect the intensity of inflammation in LES of achalasia patients. In addition, other inflammatory cells such as mast cells and eosinophils also affected the degree of injury to cells related to GI motility, which were not included in the study.
As we know, this is the first pilot study investigating the relationship between macrophages and achalasia, but it had several limitations. Firstly, the number of patients with achalasia was limited due to the low incidence. This may reduce the efficiency of detecting positive results during statistical analysis and hinder subgroup analysis among different types of achalasia. Secondly, the controls are patients who underwent surgery for esophageal or stomach neoplasms instead of age-gender matched healthy people, and tissue samples are obtained in different ways between the two groups. Although this method has been widely used in previous studies [20, 21], it also had inherent biases. In addition, tissue samples obtained from the procedure of POEM were small compared to controls, and a multi-site biopsy of the esophagus is needed, which could reflect the pathological features of the whole esophagus. Finally, Western blot and fluorescence-activated cell sorting (FACS) for quantification were not performed. In our future study, we will expand the sample size, take related inflammatory cytokines and chemokines into analysis, and explore mechanisms underlying the role of MMφ in achalasia.
In summary, the main finding of this study showed patients with achalasia had a higher level of M1/M2 ratio in LES and significant correlations were found between M2/MMφ and numbers of ICC and glial cells, which suggested that MMφ were probably associated with occurrence and development of achalasia. Further research should be undertaken to explore the mechanisms underlying the role of MMφ in achalasia and to illuminate the relationship between MMφ and nNOS, ICC and glial cells.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81900485).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haisheng Qian and Yanjuan Wang have contributed equally to this work.
References
- 1.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383:83–93. doi: 10.1016/S0140-6736(13)60651-0. [DOI] [PubMed] [Google Scholar]
- 2.Muir A, Falk GW. Eosinophilic esophagitis: a review. JAMA. 2021;326:1310–1318. doi: 10.1001/jama.2021.14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Zhang M, Liang M, et al. The number of interstitial cells of cajal differs among different subtypes of achalasia and is related to patients' prognosis. Clin. Transl. Gastroenterol. 2021;12:e388. doi: 10.14309/ctg.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur. J. Clin. Investig. 1993;23:724–728. doi: 10.1111/j.1365-2362.1993.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 5.De Giorgio R, Di Simone MP, Stanghellini V, et al. Esophageal and gastric nitric oxide synthesizing innervation in primary achalasia. Am. J. Gastroenterol. 1999;94:2357–2362. doi: 10.1111/j.1572-0241.1999.01357.x. [DOI] [PubMed] [Google Scholar]
- 6.Gockel I, Bohl JR, Eckardt VF, Junginger T. Reduction of interstitial cells of cajal (icc) associated with neuronal nitric oxide synthase (n-nos) in patients with achalasia. Am. J. Gastroenterol. 2008;103:856–864. doi: 10.1111/j.1572-0241.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 7.Zarate N, Wang XY, Tougas G, et al. Intramuscular interstitial cells of cajal associated with mast cells survive nitrergic nerves in achalasia. Neurogastroenterol. Motil. 2006;18:556–568. doi: 10.1111/j.1365-2982.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 8.Booy JD, Takata J, Tomlinson G, Urbach DR. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis. Esophagus. 2012;25:209–213. doi: 10.1111/j.1442-2050.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 9.Romero-Hernandez F, Furuzawa-Carballeda J, Hernandez-Molina G, et al. Autoimmune comorbidity in achalasia patients. J. Gastroenterol. Hepatol. 2018;33:203–208. doi: 10.1111/jgh.13839. [DOI] [PubMed] [Google Scholar]
- 10.Furuzawa-Carballeda J, Aguilar-Leon D, Gamboa-Dominguez A, et al. Achalasia–an autoimmune inflammatory disease: a cross-sectional study. J. Immunol. Res. 2015;2015:729217. doi: 10.1155/2015/729217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pressman A, Behar J. Etiology and pathogenesis of idiopathic achalasia. J. Clin. Gastroenterol. 2017;51:195–202. doi: 10.1097/MCG.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 12.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen HB. Interstitial cells of cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J. Cell. Mol. Med. 2010;14:818–832. doi: 10.1111/j.1582-4934.2010.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis–the missing link? Neurogastroenterol. Motil. 2015;27:7–18. doi: 10.1111/nmo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Schepper S, Stakenborg N, Matteoli G, Verheijden S, Boeckxstaens GE. Muscularis macrophages: key players in intestinal homeostasis and disease. Cell. Immunol. 2018;330:142–150. doi: 10.1016/j.cellimm.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan S. Macrophages: the missing link in diabetic gastroparesis? Cell. Mol. Gastroenterol. Hepatol. 2016;2:5–6. doi: 10.1016/j.jcmgh.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farro G, Stakenborg M, Gomez-Pinilla PJ, et al. Ccr2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut. 2017;66:2098–2109. doi: 10.1136/gutjnl-2016-313144. [DOI] [PubMed] [Google Scholar]
- 18.Liu WF, Wen SH, Zhan JH, et al. Treatment with recombinant trichinella spiralis cathepsin b-like protein ameliorates intestinal ischemia/reperfusion injury in mice by promoting a switch from m1 to m2 macrophages. J. Immunol. 2015;195:317–328. doi: 10.4049/jimmunol.1401864. [DOI] [PubMed] [Google Scholar]
- 19.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu ZQ, Chen WF, Wang Y, et al. Mast cell infiltration associated with loss of interstitial cells of cajal and neuronal degeneration in achalasia. Neurogastroenterol. Motil. 2019;31:e13565. doi: 10.1111/nmo.13565. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima N, Sato H, Takahashi K, et al. Muscle layer histopathology and manometry pattern of primary esophageal motility disorders including achalasia. Neurogastroenterol. Motil. 2017;29:e12968. doi: 10.1111/nmo.12968. [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Wang B, Zhang LL, Zhao W. Activated eosinophils are present in esophageal muscle in patients with achalasia of the esophagus. Med. Sci. Monit. 2018;24:2377–2383. doi: 10.12659/MSM.909727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Zhao W, Zheng Z, et al. Reduction of hydrogen sulfide synthesis enzymes in the esophagus of patients with achalasia: effect of hydrogen sulfide in achalasia. Neurogastroenterol. Motil. 2015;27:1274–1281. doi: 10.1111/nmo.12621. [DOI] [PubMed] [Google Scholar]
- 24.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garaicoa-Pazmino C, Fretwurst T, Squarize CH, et al. Characterization of macrophage polarization in periodontal disease. J. Clin. Periodontol. 2019;46:830–839. doi: 10.1111/jcpe.13156. [DOI] [PubMed] [Google Scholar]
- 26.Liu XY, Cheng J, Chen WF, et al. A risk-scoring system to predict clinical failure for patients with achalasia after peroral endoscopic myotomy. Gastrointest. Endosc. 2020;91:33–40. doi: 10.1016/j.gie.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 27.Gaber CE, Cotton CC, Eluri S, et al. Autoimmune and viral risk factors are associated with achalasia: a case-control study. Neurogastroenterol. Motil. 2021;34:e14312. doi: 10.1111/nmo.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus dna in the oesophageal myenteric plexus in achalasia. Gut. 1993;34:299–302. doi: 10.1136/gut.34.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/S0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 31.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nnos(-/-) and hypotensive in w/w(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 32.Müller M, Colcuc S, Drescher DG, et al. Murine genetic deficiency of neuronal nitric oxide synthase (nnos-/-) and interstitial cells of cajal (w/wv): implications for achalasia? J. Gastroen. Hepatol. 2014;29:1800–1807. doi: 10.1111/jgh.12600. [DOI] [PubMed] [Google Scholar]
- 33.Ruhl A, Nasser Y, Sharkey KA. Enteric glia. Neurogastroenterol. Motil. 2004;16:44–49. doi: 10.1111/j.1743-3150.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- 34.Bassotti G, Villanacci V, Maurer CA, et al. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41–46. doi: 10.1136/gut.2005.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of cajal, enteric nerves, and glial cells in colonic diverticular disease. J. Clin. Pathol. 2005;58:973–977. doi: 10.1136/jcp.2005.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol. Histopathol. 1995;10:719–736. [PubMed] [Google Scholar]
- 37.Deng J, Yang S, Yuan Q, et al. Acupuncture ameliorates postoperative ileus via il-6-mir-19a-kit axis to protect interstitial cells of cajal. Am. J. Chin. Med. 2017;45:737–755. doi: 10.1142/S0192415X17500392. [DOI] [PubMed] [Google Scholar]
- 38.Shimojima N, Nakaki T, Morikawa Y, et al. Interstitial cells of cajal in dysmotility in intestinal ischemia and reperfusion injury in rats. J. Surg. Res. 2006;135:255–261. doi: 10.1016/j.jss.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Eisenman ST, Gibbons SJ, Verhulst PJ, et al. Tumor necrosis factor alpha derived from classically activated "m1" macrophages reduces interstitial cell of cajal numbers. Neurogastroenterol. Motil. 2017;29:e12984. doi: 10.1111/nmo.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Kong P, Chen C, et al. Targeting il-17a improves the dysmotility of the small intestine and alleviates the injury of the interstitial cells of cajal during sepsis. Oxid. Med. Cell Longev. 2019;2019:1475729. doi: 10.1155/2019/1475729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard CE, Gibbons SJ, Mann IS, et al. Association of low numbers of cd206-positive cells with loss of icc in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol. Motil. 2014;26:1275–1284. doi: 10.1111/nmo.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J, Qian A, Oetjen LK, et al. Trpv4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity. 2018;49:107–119. doi: 10.1016/j.immuni.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]