Abstract
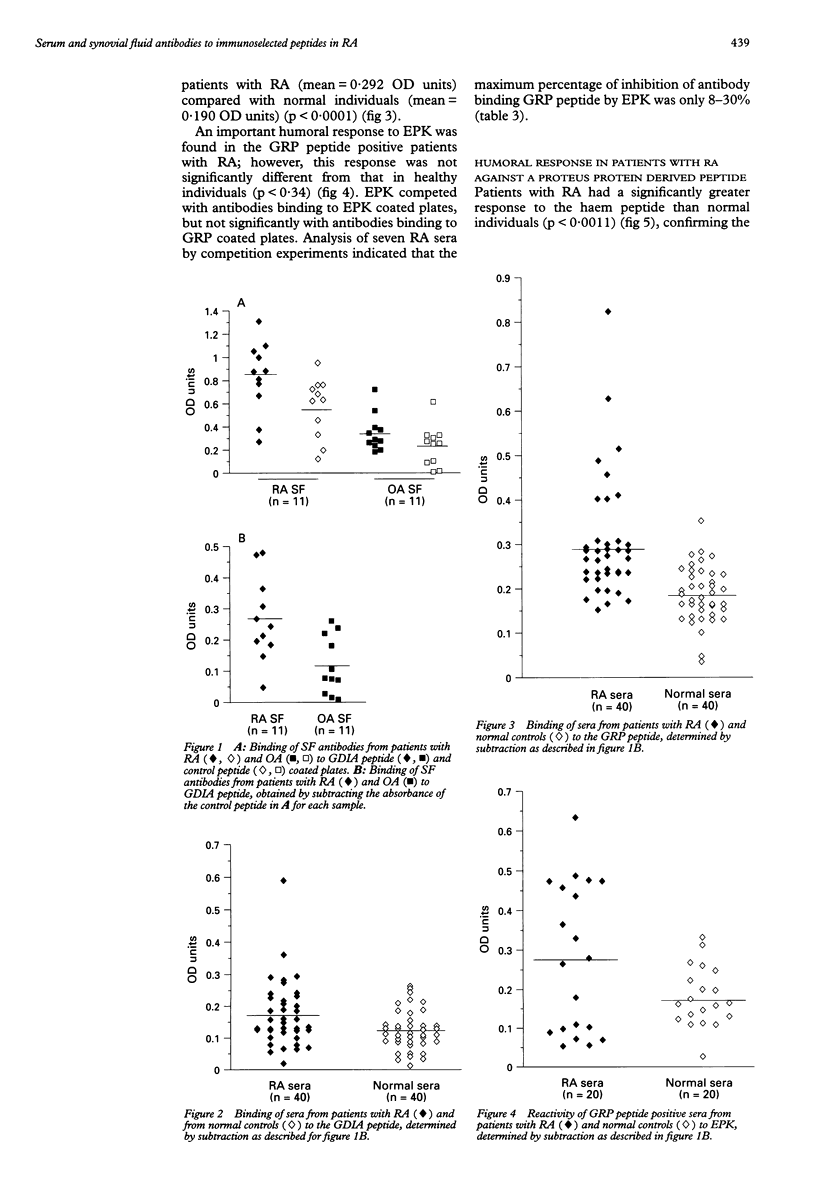
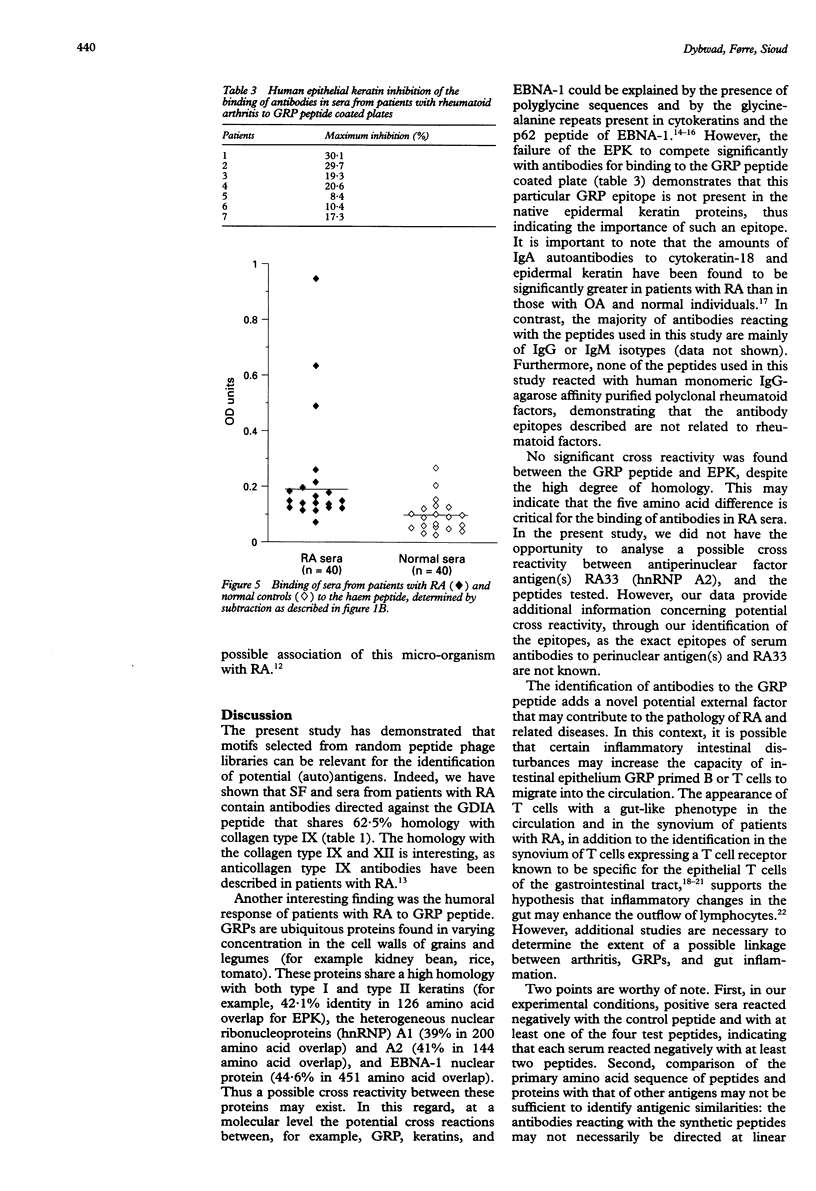
OBJECTIVES: To investigate the role of potential immunoselected phages displaying random peptides in addition to possible antigen leads in rheumatoid arthritis (RA) by assaying the levels of synovial fluid (SF) and serum antibodies to synthetic peptides. METHODS: Serum and SF antibodies from patients and controls were measured using an enzyme linked immunosorbent assay (ELISA). RESULTS: Sera and SF from RA patients reacted significantly more strongly to a 12 amino acid peptide, EFHELGDIAIAA, that shares a significant homology with collagen type IX, than did SF and sera from control groups (p < 0.0209 and p < 0.0115, respectively). In addition, the humoral responses to a 15 amino acid peptide, GGYGDGGAHGGGYGG, derived from the glycine-rich cell wall protein (GRP) 1.8, and to a 16 amino acid synthetic peptide, LGSISESRRALQDSQR, derived from the Proteus haemolysin protein were significantly stronger in RA patients compared with healthy individuals (p < 0.0001 and p < 0.0011, respectively). CONCLUSION: Our data indicate that peptide phage libraries can be used as tools for the identification of the (auto)antigen leads that may be responsible for the initiation, perpetuation, or both, of the immune response in patients with RA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho K., Palusuo T., Kurki P. Marker antibodies of rheumatoid arthritis: diagnostic and pathogenetic implications. Semin Arthritis Rheum. 1994 Jun;23(6):379–387. doi: 10.1016/0049-0172(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Baboonian C., Venables P. J., Williams D. G., Williams R. O., Maini R. N. Cross reaction of antibodies to a glycine/alanine repeat sequence of Epstein-Barr virus nuclear antigen-1 with collagen, cytokeratin, and actin. Ann Rheum Dis. 1991 Nov;50(11):772–775. doi: 10.1136/ard.50.11.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg A. A., Dawes P. T., Mattey D. L. Increased levels of IgA antibodies to cytokeratin-18 and epidermal keratin in rheumatoid arthritis. Arthritis Rheum. 1993 Feb;36(2):229–233. doi: 10.1002/art.1780360214. [DOI] [PubMed] [Google Scholar]
- Bucht A., Söderström K., Hultman T., Uhlén M., Nilsson E., Kiessling R., Grönberg A. T cell receptor diversity and activation markers in the V delta 1 subset of rheumatoid synovial fluid and peripheral blood T lymphocytes. Eur J Immunol. 1992 Feb;22(2):567–574. doi: 10.1002/eji.1830220240. [DOI] [PubMed] [Google Scholar]
- Dybwad A., Førre O., Kjeldsen-Kragh J., Natvig J. B., Sioud M. Identification of new B cell epitopes in the sera of rheumatoid arthritis patients using a random nanopeptide phage library. Eur J Immunol. 1993 Dec;23(12):3189–3193. doi: 10.1002/eji.1830231222. [DOI] [PubMed] [Google Scholar]
- Dybwad A., Førre O., Natvig J. B., Sioud M. Structural characterization of peptides that bind synovial fluid antibodies from RA patients: a novel strategy for identification of disease-related epitopes using a random peptide library. Clin Immunol Immunopathol. 1995 Apr;75(1):45–50. doi: 10.1006/clin.1995.1051. [DOI] [PubMed] [Google Scholar]
- Ebringer A., Cunningham P., Ahmadi K., Wrigglesworth J., Hosseini R., Wilson C. Sequence similarity between HLA-DR1 and DR4 subtypes associated with rheumatoid arthritis and proteus/serratia membrane haemolysins. Ann Rheum Dis. 1992 Nov;51(11):1245–1246. doi: 10.1136/ard.51.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hassfeld W., Steiner G., Studnicka-Benke A., Skriner K., Graninger W., Fischer I., Smolen J. S. Autoimmune response to the spliceosome. An immunologic link between rheumatoid arthritis, mixed connective tissue disease, and systemic lupus erythematosus. Arthritis Rheum. 1995 Jun;38(6):777–785. doi: 10.1002/art.1780380610. [DOI] [PubMed] [Google Scholar]
- Keller B., Sauer N., Lamb C. J. Glycine-rich cell wall proteins in bean: gene structure and association of the protein with the vascular system. EMBO J. 1988 Dec 1;7(12):3625–3633. doi: 10.1002/j.1460-2075.1988.tb03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits A. I., Karsh J. Differential expression in rheumatoid synovium and synovial fluid of alpha 4 beta 7 integrin. A novel receptor for fibronectin and vascular cell adhesion molecule-1. J Immunol. 1993 Dec 1;151(11):6482–6489. [PubMed] [Google Scholar]
- Morgan K., Clague R. B., Collins I., Ayad S., Phinn S. D., Holt P. J. Incidence of antibodies to native and denatured cartilage collagens (types II, IX, and XI) and to type I collagen in rheumatoid arthritis. Ann Rheum Dis. 1987 Dec;46(12):902–907. doi: 10.1136/ard.46.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony S., Ferguson A. Small intestinal mucosal protection mechanisms and their importance in rheumatology. Ann Rheum Dis. 1991 May;50(5):331–336. doi: 10.1136/ard.50.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenstad B., Dybwad A., Lea T., Førre O., Vinje O., Sioud M. Evidence for monoclonal expansion of synovial T cells bearing V alpha 2.1/V beta 5.5 gene segments and recognizing a synthetic peptide that shares homology with a number of putative autoantigens. Immunology. 1995 Oct;86(2):168–175. [PMC free article] [PubMed] [Google Scholar]
- Rhodes G., Carson D. A., Valbracht J., Houghten R., Vaughan J. H. Human immune responses to synthetic peptides from the Epstein-Barr nuclear antigen. J Immunol. 1985 Jan;134(1):211–216. [PubMed] [Google Scholar]
- Simon M., Girbal E., Sebbag M., Gomès-Daudrix V., Vincent C., Salama G., Serre G. The cytokeratin filament-aggregating protein filaggrin is the target of the so-called "antikeratin antibodies," autoantibodies specific for rheumatoid arthritis. J Clin Invest. 1993 Sep;92(3):1387–1393. doi: 10.1172/JCI116713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Kjeldsen-Kragh J., Quayle A., Kalvenes C., Waalen K., Førre O., Natvig J. B. The V delta gene usage by freshly isolated T lymphocytes from synovial fluids in rheumatoid synovitis: a preliminary report. Scand J Immunol. 1990 Apr;31(4):415–421. doi: 10.1111/j.1365-3083.1990.tb02787.x. [DOI] [PubMed] [Google Scholar]
- Söderström K., Bucht A., Halapi E., Lundqvist C., Grönberg A., Nilsson E., Orsini D. L., van de Wal Y., Koning F., Hammarström M. L. High expression of V gamma 8 is a shared feature of human gamma delta T cells in the epithelium of the gut and in the inflamed synovial tissue. J Immunol. 1994 Jun 15;152(12):6017–6027. [PubMed] [Google Scholar]
- Venables P. J. Infection and rheumatoid arthritis. Curr Opin Rheumatol. 1989 Jun;1(1):15–20. doi: 10.1097/00002281-198901010-00005. [DOI] [PubMed] [Google Scholar]
- Wilson C., Ebringer A., Ahmadi K., Wrigglesworth J., Tiwana H., Fielder M., Binder A., Ettelaie C., Cunningham P., Joannou C. Shared amino acid sequences between major histocompatibility complex class II glycoproteins, type XI collagen and Proteus mirabilis in rheumatoid arthritis. Ann Rheum Dis. 1995 Mar;54(3):216–220. doi: 10.1136/ard.54.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]


