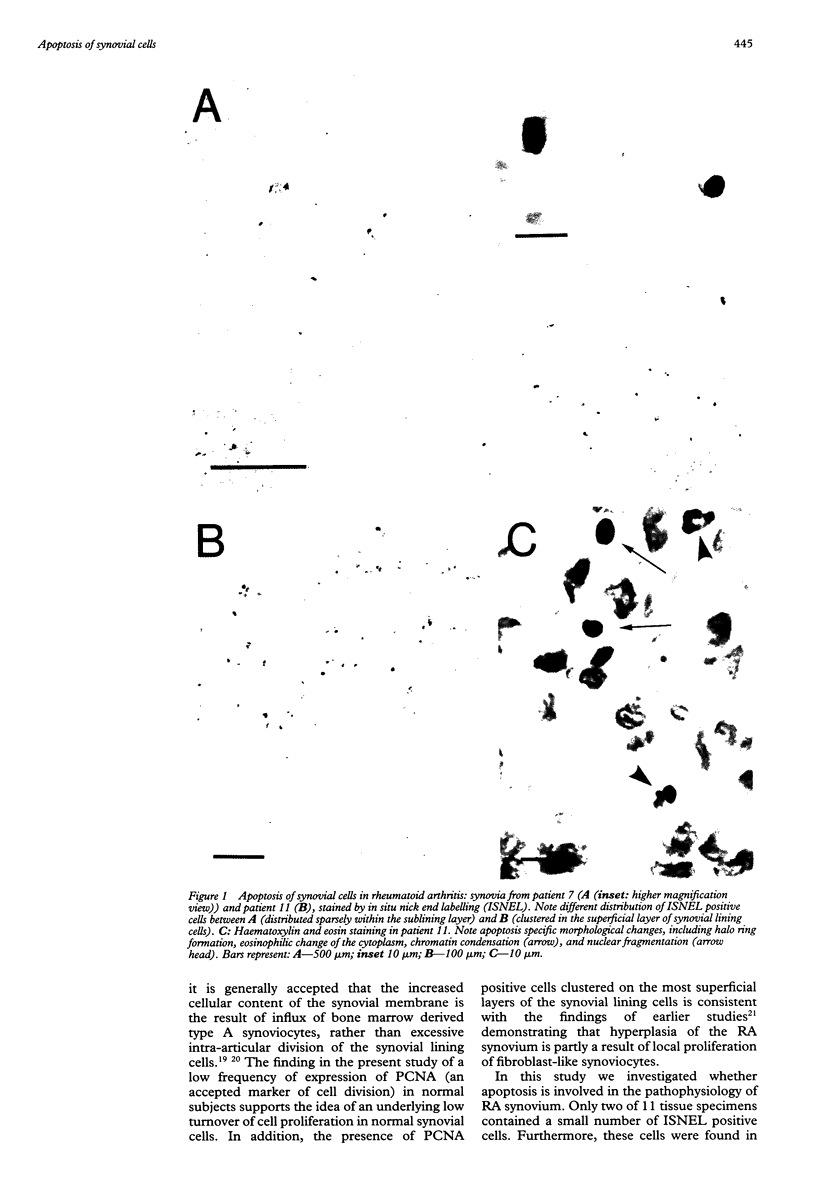
Abstract
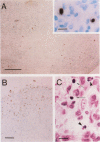
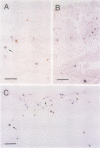
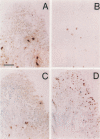
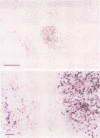
OBJECTIVES: To investigate whether apoptosis occurs in the synovium of rheumatoid arthritis (RA), and the intermediate molecules operating in this process. METHODS: DNA fragmentation was detected by in situ nick end labelling (ISNEL) in the synovium of patients with RA (n = 11) and control patients with femoral neck fracture (n = 5). The expression of proteins p53, p21WAFI/CIPI, c-myc, proliferating cell nuclear antigen (PCNA), and Bcl-2 was also examined by immunohistochemistry. RESULTS: ISNEL positive synovial cells with apoptosis specific morphology were detected in extremely limited areas in only two RA synovial tissue specimens. Proteins p53, p21WAFI/CIPI, and c-myc, known inducers of apoptosis or cell cycle arrest or both, were expressed in the sublining cells independent of ISNEL positive cells. PCNA, a marker for cell proliferation, was observed in the synovial lining cells. Bcl-2, an inhibitor of apoptosis, was expressed mainly in infiltrated lymphocytes and in parts of the sublining layer cells of RA; it also did not correspond with ISNEL staining. CONCLUSIONS: Our findings indicate that RA synovial cells undergo apoptosis in addition to cell proliferation, but the frequency of apoptosis was very low. We suspect that the apoptotic process in the RA synovium may be suppressed by over-expression of Bcl-2. Although expressed proteins p53, p21WAFI/CIPI, and c-myc were present in the RA synovium, these protooncogenes are probably not implicated in the apoptotic process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Berg E., Wainwright R., Barton B., Puchtler H., McDonald T. On the nature of rheumatoid rice bodies: an immunologic, histochemical, and electron microscope study. Arthritis Rheum. 1977 Sep-Oct;20(7):1343–1349. doi: 10.1002/art.1780200707. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Case J. P., Lafyatis R., Remmers E. F., Kumkumian G. K., Wilder R. L. Transin/stromelysin expression in rheumatoid synovium. A transformation-associated metalloproteinase secreted by phenotypically invasive synoviocytes. Am J Pathol. 1989 Dec;135(6):1055–1064. [PMC free article] [PubMed] [Google Scholar]
- Colby W. W., Chen E. Y., Smith D. H., Levinson A. D. Identification and nucleotide sequence of a human locus homologous to the v-myc oncogene of avian myelocytomatosis virus MC29. Nature. 1983 Feb 24;301(5902):722–725. doi: 10.1038/301722a0. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Cambridge J. Is rheumatoid arthritis a failure of B cell death in synovium? Ann Rheum Dis. 1995 Sep;54(9):696–700. doi: 10.1136/ard.54.9.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Willoughby D. A. Demonstration of bone marrow derived cells in synovial lining by means of giant intracellular granules as genetic markers. Ann Rheum Dis. 1982 Apr;41(2):177–182. doi: 10.1136/ard.41.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizenberg O., Faber-Elman A., Gottlieb E., Oren M., Rotter V., Schwartz M. Direct involvement of p53 in programmed cell death of oligodendrocytes. EMBO J. 1995 Mar 15;14(6):1136–1144. doi: 10.1002/j.1460-2075.1995.tb07097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fassbender H. G. Is pannus a residue of inflammation? Arthritis Rheum. 1984 Aug;27(8):956–957. doi: 10.1002/art.1780270819. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Yeo M., Zvaifler N. J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995 Sep;96(3):1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E., Chayen J. Cell division in the synovial lining in experimental allergic arthritis: proliferation of cells during the development of chronic arthritis. Ann Rheum Dis. 1982 Jun;41(3):275–281. doi: 10.1136/ard.41.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeking H., Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994 Sep 30;265(5181):2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinouchi T., Saiki S., Naoe T., Uenaka A., Kotake T., Shiku H., Nakayama E. Correlation of c-myc expression with nuclear pleomorphism in human renal cell carcinoma. Cancer Res. 1989 Jul 1;49(13):3627–3630. [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Krey P. R., Cohen A. S., Smith C. B., Finland M. The human fetal synovium. Histology, fine structure and changes in organ culture. Arthritis Rheum. 1971 May-Jun;14(3):319–341. doi: 10.1002/art.1780140303. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Maltzman W., Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984 Sep;4(9):1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. J., Cheung H. S. Origin and significance of rice bodies in synovial fluid. Lancet. 1982 Sep 25;2(8300):715–716. doi: 10.1016/s0140-6736(82)90735-8. [DOI] [PubMed] [Google Scholar]
- Mohr W., Beneke G., Mohing W. Proliferation of synovial lining cells and fibroblasts. Ann Rheum Dis. 1975 Jun;34(3):219–224. doi: 10.1136/ard.34.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Aono H., Hasunuma T., Yamamoto K., Shirai T., Hirohata K., Nishioka K. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995 Apr;38(4):485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Faure M. P., DiBattista J. A., Wilhelm S., Visco D., Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993 Jan;142(1):95–105. [PMC free article] [PubMed] [Google Scholar]
- Qu Z., Garcia C. H., O'Rourke L. M., Planck S. R., Kohli M., Rosenbaum J. T. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum. 1994 Feb;37(2):212–220. doi: 10.1002/art.1780370210. [DOI] [PubMed] [Google Scholar]
- Schmitz G. G., Walter T., Seibl R., Kessler C. Nonradioactive labeling of oligonucleotides in vitro with the hapten digoxigenin by tailing with terminal transferase. Anal Biochem. 1991 Jan;192(1):222–231. doi: 10.1016/0003-2697(91)90212-c. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Trabandt A., Aicher W. K., Gay R. E., Sukhatme V. P., Nilson-Hamilton M., Hamilton R. T., McGhee J. R., Fassbender H. G., Gay S. Expression of the collagenolytic and Ras-induced cysteine proteinase cathepsin L and proliferation-associated oncogenes in synovial cells of MRL/I mice and patients with rheumatoid arthritis. Matrix. 1990 Dec;10(6):349–361. doi: 10.1016/s0934-8832(11)80142-3. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Grunwald D., Wilder S., Kimchi A., May E., Lawrence J. J., May P., Oren M. p53-mediated cell death: relationship to cell cycle control. Mol Cell Biol. 1993 Mar;13(3):1415–1423. doi: 10.1128/mcb.13.3.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995 Jul 1;55(13):2910–2919. [PubMed] [Google Scholar]