Abstract
Background
No comprehensive multicenter study of sensitization patterns among patients with allergic rhinitis (AR) to various common pollen allergens was available nationwide, and risks factors of pollen-induced allergic rhinitis (PiAR) in mainland China was unclear. This study aimed to fill this gap.
Methods
A multicenter study was performed on 736 AR patients aged below 18 from four regions of mainland China. Patients completed a standardized questionnaire asking for the environmental risk factors and AR severity, and undertook skin prick tests (SPT) with 14 common pollen allergens.
Findings
Among the 736 patients, 341 patients (46.33%) suffered at least one positive pollen allergen sensitization. The positive rate of pollen allergens was significantly higher in the high-age group (Damato et al., 2007; Wang et al., 2018; Luo et al., 2016; Demoly et al., 2011; Sampson and Albergo, 1984; Li et al., 2009; Luo et al., 2021; Ziska and Beggs, 2011; Melén et al., 2020; Jensen-Jarolim, 2017; Rönmark et al., 2017; Ge et al., 2017) [6-17] than the low-age group ( ≤ 5), while no significant difference was found between the sexes. The sensitizations to pollen allergens varied widely among four geographical areas. The positive rate was higher in north China and west China than in east China, and south China had the lowest positive rate. The region of residence, ages, ethnic minorities, history of pollen exposure, the material of living room floor and material of pillow were statistically significant risks of PiAR.
Interpretation
This study provides new insights into the pollen allergens sensitization characteristics in AR and the factors affecting PiAR in mainland China.
Keywords: Allergic rhinitis, Pollen allergens sensitization, Risk factors
1. Introduction
Allergic rhinitis (AR) is one of the most common chronic disorders in the pediatric population and up to 40% of children are affected worldwide [1]. The occurrence of allergic diseases during childhood not only has persistent adverse effects on daily life, such as poor sleep quality and absence from school, but also increases the risk of sleep disordered breathing, and is associated with multiple co-morbidities like asthma, eczema, and otitis media [2]. Pollen is one of the common triggers of allergic rhinitis, and pollen-induced allergic rhinitis (PiAR) imposes a considerable burden on public health. High-risk atopic individual exposure to pollen leads the upper respiratory tract to release allergic mediators such as histamine and then triggers allergic reactions [3]. Allergic rhinitis caused by pollen exposure affects 60 million people per year and costs exceed 3 billion in medicine every year in the United States [4]. In Europe, 33 million people are sensitive to ragweed and the number is predicted to be more than double by 2041 to 2060(5). The types and concentrations of airborne pollens varied due to vegetation, geography, temperature, and climate [6].
China has a vast territory with significant differences in topography and climate, leading to differences in vegetation diversity and regional distribution. Though several studies have described the prevalence of pollen sensitization in China, most of them just focused on limited specific types of pollen allergens or in one geographic region of China [7,8]. One study conducted in the grasslands of north China found out that the most common pollen types among PiAR patients were artemisia, chenopodium, and humulus scandens [7]. Another study figured out that Cynodon dactylon was the most common grass pollen allergen among AR and/or asthma patients in south China [8]. Few studies focused on the pollen allergen distribution in east China and west China. The association of pollen allergenicity and the severity of AR was unclear. The correlation between skin index of pollen allergen sensitization and the severity of AR could offer a more precise diagnosis base for the clinicians. Exposure to pollen was considered to be a cause of PiAR, but few study figured out the influence of genetic characteristics and environmental exposure on the occurrence of PiAR. Finding out the factors affecting the development of PIAR and taking preventive measures could effectively reduce the prevalence of PIAR. Therefore, there is a strong need for a study to comprehensively investigate the pollen sensitization characteristics, to explore the association between pollen allergen sensitization and the severity of AR, and to explore the risks factors of PiAR across mainland China, not just limited to a few specific pollen allergens or in a specific geographic region of China. Here we conducted a multicenter study to address this need in the research on allergy.
Our study investigated the pollen allergen sensitization characteristics of allergy rhinitis among children from 11 hospitals located in four geographic regions of mainland China. In this study, the influence of sex, age and regions variants was investigated. Further, the effect of pollen allergens on allergic rhinitis severity was discussed. The association between PiAR and potential risk factors such as age, sex, ethnicity, parental allergy history, environmental exposure was explored. Compared with previous studies, this was a multicenter cross-sectional study concentrated in pollen allergens sensitization among AR in different regions of mainland China tested a variety of allergens including four grass pollens, six tree pollens and four weed pollens. Some of the pollen allergens included were common pollen allergens in mainland China and others were pollen allergens in northern and western parts of China, where plants were relatively widely distributed. The result of the study provided new insights into the pollen allergens sensitization characteristics in AR and the factors affecting disease manifestation and gave important clues for the prevention and immunotherapy of pollen allergens in mainland China.
2. Methods
2.1. Study population and definitions
This was a multicenter study conducted from October 2019 to March 2021 in 9 provinces with 11 participating centers from four geographic regions covering tropical, subtropical, warm-temperate, mid-temperate, cold-temperate, plateau sub-frigid, plateau temperate zones of mainland China. A total number of 736 children with clinical AR aged below 18 were included in this study. Clinical AR was defined as patients with classic symptoms of AR including sneezing, nasal congestion, rhinorrhea, and nasal pruritus, alongside ocular symptoms, such as redness and itching of eyes and lachrymation. Patients aged 0–5 were defined as the low-age group and subjects aged 6–17 were the high-age group. Patients attending outpatient clinics at 11 medical centers diagnosed with AR were eligible for enrolments in the study. After giving informed consent, the physicians or research nurses used a standardized questionnaire asking for demographic characteristics, birth mode, parental history of allergy, exposure to smokers, environmental factors, AR severity to conduct a clinical evaluation of AR patients. Parents were asked to help children fill in the questionnaire if children could not complete it independently. The severity of AR was assessed using the Allergic Rhinitis Control Test (ARCT), which was included in the questionnaire. ARCT evaluated the level of severity of the allergy rhinitis over the last 2 weeks by five items scored from 1 to 5, which were associated with impairments in patients' daily activities and uses of rescue and other AR medication [9]. Then, skin prick tests (Inmunotek, Inmunotek SL, Spain) were performed by the nurses for all the recruited patients (N = 736). The positive numbers of SPT among different regions were 52 (N = 71) in north China, 239 (N = 367) in west China, 43 (N = 193) in east China and 7 (N = 105)in south China. The wheal and flare results were recorded at 20 min and the orthogonal diameter greater than or equal to 3 mm were considered as SPT positive [10]. Skin index was calculated (SI = mean size of allergen weal/mean size of histamine wheal). To control the accuracy of the SPT results, the nurse carefully checked the patient's medical history to ensure that the patient did not take antihistamines or oral steroids in the last 7 days before SPT. Fourteen pollen allergens were tested in this study including four grass pollens (Cynodon dactylon, Phragmites communis, Phleum pratense, Triticum aestivum), six tree pollens (Betula verrucosa, Populus alba, Ulmus campestris, Salix fragilis, Cupressus sempervirens, Platanus acerifolia), and four weed pollens (Ambrosia artemisiifolia, Artemisia vulgaris, Chenopodium album, Brassica napus). Patients with one of the following items were excluded: 1. subjects with severe skin damage who could not conduct skin prick test; 2. subjects whose recent medications had affected the results of SPT; 3. subjects with immunodeficiency or autoimmune diseases; 4. subjects with a history of severe systemic allergic reactions. PiAR was confirmed by the following three items: 1. medical allergy history; 2. clinical symptoms; 3. positive SPT for at least one pollen allergen [7].
The study was approved by the ethics committees of the First Affiliated Hospital of Guangzhou Medical University (GYFYY-2018-93) and other 10 participating centers (2018-090, 2018–97, 2018-122, 20190577, KY201902, 2019-0328 F, 2019–03, 2019-03-2, 2019–04, KY-2021-008-04). This study was conducted in accordance with the Declaration of Helsinki. Subjects younger than 18 years of age, written informed consents were obtained from their parents or legal guardian.
2.2. Statistical analysis
Multiple imputation was conducted to deal with missing data in the questionnaire. The positive SPT for pollen allergens was calculated as percentages. Chi-square test and Fisher's exact test were used to estimate the differences between the rates of sensitization. The association between skin index of pollen allergen sensitizations and the severity of AR were explored by Spearman correlation analysis. Bivariate regression model and multiple regression model were used to investigate the association between PiAR and risk factors. All analyses were done using R studio 3.6.2.
3. Results
3.1. Pollen allergens sensitization characteristics
The pollen allergens in sensitization characteristics in the whole studied population and in subgroups in sex and age were shown in Table 1. Among 736 AR patients, 341 (46.33%) patients had at least one positive pollen sensitization among the 14 pollen allergens. The overall positive SPT responses to the 14 pollen allergens in mainland China from highest to lowest was 31.39% for Artemisia vulgaris, 21.60% for Cynodon dactyl, 21.06% for Ambrosia artemisiifolia, 20.92% for Chenopodium album, 20.79% for Ulmus campestris, 20.11% for Populus alba, 16.30% for Platanus acerifolia, 15.63% for Betula verrucosa, 15.22% for Salix fragillis, 14.95% for Brassica napus, 11.68% for Phleum pratense, 9.38% for Phragmites communis, 8.83% for Triticum aestivum, 7.20% for Cupressus sempervirens. There was a significant difference (P < 0.05) between the males and the females in the positive rate of Betula verrucosa allergen and Salix fragilis allergen. The males witnessed a higher but not significant positive rate of all pollen allergens than females except Artemisia vulgaris allergen and Chenopodium album allergen. Overall, the positive rate of pollen allergen was significantly higher in the high-age group than in the low-age group. There was a significant difference (P < 0.05) between the high-age group and the low-age group in the positive sensitization of Phragmites communis allergen, Phleum pratense allergen, Ulmus campestris allergen, Platanus acerifolia allergen, Ambrosia artemisiifolia allergen, Artemisia vulgaris allergen, Chenopodium album allergen and Brassica napus allergen.
Table 1.
Pollen allergens sensitization characteristics in sex and age.
| Allergen | Total | Grass pollen |
Tree pollen |
Weed pollen |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cynodon dactylon | Phragmites communis | Phleum pratense | Triticum aestivum | Betula verrucosa | Populus alba | Ulmus campestris | Salix fragilis | Cupressus sempervirens | Platanus acerifolia | Ambrosia artemisiifolia | Artemisia vulgaris | Chenopodium album | Brassica napus | |||
| Overall | n (%) | 341 (46.33) | 159 (21.60) | 69 (9.38) | 86 (11.68) | 65 (8.83) | 115 (15.63) | 148 (20.11) | 153 (20.79) | 112 (15.22) | 53 (7.20) | 120 (16.30) | 155 (21.06) | 231 (31.39) | 154 (20.92) | 110 (14.95) |
| Sex | ||||||||||||||||
| Female | n (%) | 110 (44.35) | 49 (19.76) | 22 (8.87) | 26 (10.48) | 16 (6.45) | 29 (11.69) | 48 (19.35) | 47 (18.95) | 28 (11.29) | 17 (6.85) | 40 (16.12) | 47 (18.95) | 78 (31.45) | 52 (20.97) | 34 (13.71) |
| Male | n (%) | 231 (47.34) | 110 (22.54) | 47 (9.63) | 60 (12.30) | 49 (10.04) | 86 (17.62) | 100 (20.49) | 106 (21.72) | 84 (17.21) | 36 (7.38) | 80 (16.39) | 108 (22.13) | 153 (31.35) | 102 (20.90) | 76 (15.57) |
| X2 | 0.47 | 0.60 | 0.04 | 0.36 | 2.20 | 3.95 | 0.07 | 0.61 | 4.02 | 0.01 | 0.00 | 0.82 | 0.00 | 0.00 | 0.32 | |
| P-value | 0.491 | 0.440 | 0.841 | 0.547 | 0.138 | 0.047 | 0.790 | 0.436 | 0.045 | 0.913 | 1.000 | 0.366 | 1.000 | 1.000 | 0.574 | |
| Age | ||||||||||||||||
| 0–5 | n (%) | 81 (36.49) | 39 (17.57) | 13 (5.86) | 13 (5.86) | 13 (5.86) | 26 (11.71) | 38 (17.12) | 31 (13.96) | 27 (12.16) | 14 (6.31) | 26 (11.71) | 24 (10.81) | 43 (19.37) | 33 (14.86) | 21 (9.46) |
| 6–17 | n (%) | 260 (50.58) | 120 (23.35) | 56 (10.89) | 73 (14.20) | 52 (10.12) | 89 (17.32) | 110 (21.40) | 122 (23.74) | 85 (16.54) | 39 (7.59) | 94 (18.29) | 131 (25.49) | 188 (36.58) | 121 (23.54) | 89 (17.32) |
| X2 | 11.83 | 2.73 | 4.06 | 9.67 | 2.99 | 3.28 | 2.73 | 8.41 | 1.97 | 0.21 | 4.44 | 19.21 | 20.52 | 6.54 | 6.92 | |
| P-value | <0.001 | 0.099 | 0.044 | 0.002 | 0.084 | 0.070 | 0.219 | 0.004 | 0.160 | 0.644 | 0.035 | <0.001 | <0.001 | 0.011 | 0.009 | |
Notes: significant difference: P < 0.05.
The detailed data of positive pollen allergens sensitization among different regions were shown in Table 2. The positive rate of SPT among different regions were 73.24% (52/71) in north China, 65.12% (239/367) in west China, 22.28% (43/193) in east China and 6.67% (7/105) in south China. For all pollen allergens, the positive rate was high in north China and west China with around 5% to 70%. The most common pollen allergen in these two regions was Artemisia vulgaris with 53.52% and 49.86%, respectively. In north China, Ambrosia artemisiifolia, Ulmus campestris, Chenopodium album, Cynodon dactylon and Populus alba showed a high positive rate which exceeded 30% with 46.48%, 40.85%, 36.63%, 35.21% and 30.99%, respectively. In west China, Chenopodium album, Cynodon dactylon, Populus alba and Ambrosia artemisiifolia had a high positive rate of 32.43%, 31.88%, 31.88% and 31.61%, respectively. In east China, the positive rate of all pollen allergens was low at below 10% and the positive rate in south China was the lowest at below 5%. Cynodon dactylon and Ulmus campestris were the most common pollen allergens in east China (7.25% and 7.25%, respectively) and south China (2.86% and 1.90%, respectively). In south China, the studied subjects only tested positive for five pollen allergens including Cynodon dactylon (2.86%), Ulmus campestris (1.90%), Phragmites communis (0.95%), Betula verrucosa (0.95%) and Populus alba (0.95%) among 14 pollen allergens. There was a significant difference (P < 0.05) among different regions in the positive rate of each pollen allergen.
Table 2.
Pollen allergens sensitization characteristics in regions.
| Region | East China |
West China |
North China |
South China |
X2 |
P |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | N | n (%) | |||
| At least one pollen allergen sensitization | 43 | 22.28 | 239 | 65.12 | 52 | 73.24 | 7 | 6.67 | 184.00 | <0.001 |
| Sensitization to pollen allergens by SPT | ||||||||||
| Grass pollen | ||||||||||
| Cynodon dactylon | 14 | 7.25 | 117 | 31.88 | 25 | 35.21 | 3 | 2.86 | 75.90 | <0.001 |
| Phragmites communis | 4 | 2.07 | 55 | 14.99 | 9 | 12.68 | 1 | 0.95 | 35.39 | <0.001 |
| Phleum pratense | 6 | 3.11 | 71 | 19.35 | 9 | 12.68 | 0 | 0.00 | 48.59 | <0.001 |
| Triticum aestivum | 6 | 3.11 | 52 | 14.17 | 7 | 9.86 | 0 | 0.00 | 31.10 | <0.001 |
| Tree pollen | ||||||||||
| Betula verrucosa | 10 | 5.18 | 91 | 24.80 | 13 | 18.31 | 1 | 0.95 | 56.91 | <0.001 |
| Populus alba | 8 | 4.15 | 117 | 31.88 | 22 | 30.99 | 1 | 0.95 | 91.48 | <0.001 |
| Ulmus campestris | 14 | 7.25 | 108 | 29.43 | 29 | 40.85 | 2 | 1.90 | 78.19 | <0.001 |
| Salix fragilis | 5 | 2.59 | 91 | 24.80 | 16 | 22.54 | 0 | 0.00 | 71.74 | <0.001 |
| Cupressus sempervirens | 4 | 2.07 | 44 | 11.99 | 5 | 7.04 | 0 | 0.00 | 28.34 | <0.001 |
| Platanus acerifolia | 5 | 2.59 | 98 | 26.70 | 17 | 23.94 | 0 | 0.00 | 79.17 | <0.001 |
| Weed pollen | ||||||||||
| Ambrosia artemisiifolia | 6 | 3.11 | 116 | 31.61 | 33 | 46.48 | 0 | 0.00 | 117.58 | <0.001 |
| Artemisia vulgaris | 10 | 5.18 | 183 | 49.86 | 38 | 53.52 | 0 | 0.00 | 183.91 | <0.001 |
| Chenopodium album | 9 | 4.66 | 119 | 32.43 | 26 | 36.62 | 0 | 0.00 | 98.54 | <0.001 |
| Brassica napus | 7 | 3.63 | 87 | 23.71 | 16 | 22.54 | 0 | 0.00 | 63.27 | <0.001 |
Notes: significant difference: P < 0.05; N: the number of positive cases; n (%): the percentage of positive cases.
3.2. Pollen allergen sensitization and the severity of AR
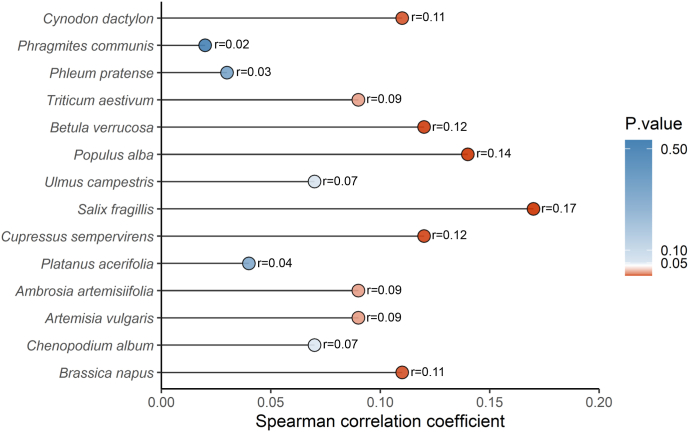
The association between pollen allergen sensitization and the severity of AR were explored using Spearmen correlation analysis, and the result was shown in Fig. 1. The severity of AR was correlated with the skin index of reactivity to all pollen allergens, and all Spearmen correlation coefficients were above 0. Skin index of sensitization to Cynodon dactylon, Triticum aestivum, Betula verrucosa, Populus alba, Salix fragilis, Cupressus sempervirens, Ambrosia artemisiifolia, Artemisia vulgaris and Brassica napus were significantly positively correlated with the severity of AR (P < 0.05) with Spearman correlation coefficient of 0.11, 0.09, 0.12, 0.14, 0.17, 0.12, 0.09, 0.09 and 0.11, respectively. The symptom severity of AR patients was significantly different in different seasons (P < 0.001). The AR severity score of patients in summer and autumn was significantly higher than that in spring and winter (Table 3).
Fig. 1.
Correlations between skin index of pollen allergen sensitizations and the severity of AR with Spearman's analysis. Legends: r: Spearman correlation coefficient; significant difference: P < 0.05.
Table 3.
Relationship between different seasons and AR severity score.
| Month (N) | AR severity score (mean ± sd) | P-value |
|---|---|---|
| 1-3 (183) | 15.77 ± 4.79 | <0.001 |
| 4-6 (113) | 18.74 ± 3.73* | |
| 7-9 (161) | 17.89 ± 4.11# | |
| 10-12 (279) | 16.19 ± 4.90 |
Notes: significant difference: P < 0.05.
3.3. Factors associated with pollen-induced allergic rhinitis
Associations between PiAR and potential risk factors were shown in Table 4 and Table S1. In multiple regression analysis, compared with living in east China, living in north China and west China was associated with a higher risk of PiAR (aOR = 5.95, 95% CI: 2.95–12.32; aOR = 4.44, 95% CI: 2.55–7.83), while living in south China had a lower risk of PiAR (aOR = 0.30, 95% CI: 0.11–0.70). The high-age group was displayed a positive correlation with the risk of PiAR (aOR = 1.95, 95% CI: 1.31–2.90). Ethnic minorities were at a greater risk of PiAR (aOR = 2.23, 95% CI: 1.14–4.62) compared with Han nationality. History of pollen exposure was displayed to increase the risk of PiAR (aOR = 2.10, 95% CI = 1.41–3.16). Living room with carpet, and pillow made of the plant showed higher risks of PiAR (aOR = 2.21, 95% CI: 1.12–4.42; aOR = 1.74, 95% CI: 1.06–2.85) compared with the most common materials.
Table 4.
Factors associated with pollen allergy among allergy rhinitis of children in mainland China.
| Predictors | Frequency |
Bivariate |
Multivariable |
||
|---|---|---|---|---|---|
| N (%) | OR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Region | |||||
| East | 193 (26.22) | 1 | |||
| West | 367 (49.86) | 6.51 (4.39,9.82) | <0.001 | 4.44 (2.55,7.83) | <0.001 |
| North | 71 (9.65) | 9.55 (5.19,18.21) | <0.001 | 5.95 (2.95,12.32) | <0.001 |
| South | 105 (14.27) | 0.25 (0.10,0.54) | 0.001 | 0.30 (0.11,0.70) | 0.009 |
| Age | |||||
| 0–5 | 222 (30.16) | 1 | |||
| 6–17 | 514 (69.84) | 1.78 (1.29,2.47) | <0.001 | 1.95 (1.31,2.90) | 0.001 |
| Ethnic | |||||
| Han | 677 (91.98) | 1 | |||
| other | 59 (8.01) | 4.14 (2.29,7.95) | <0.001 | 2.23 (1.14,4.62) | 0.023 |
| Smoker | |||||
| Nonsmoker at home | 374 (50.82) | ||||
| Smoker at home | 362 (49.18) | 1.34 (1.00,1.79) | 0.050 | 1.13 (0.79,1.61) | 0.501 |
| History of pollen exposure | |||||
| No | 529 (71.88) | 1 | |||
| Yes | 207 (28.13) | 2.54 (1.83,3.55) | <0.001 | 2.10 (1.41,3.16) | <0.001 |
| House age | |||||
| ∼2010 | 408 (55.43) | ||||
| 2011–2015 | 217 (29.48) | 1.14 (0.82,1.59) | 0.424 | 1.14 (0.76,1.73) | 0.521 |
| 2016–2021 | 111 (15.08) | 1.39 (0.91,2.11) | 0.128 | 1.16 (0.70,1.95) | 0.563 |
| Living area | |||||
| Rural area | 198 (26.90) | 1 | |||
| Urban area | 538 (73.10) | 1.51 (1.09,2.12) | 0.014 | 1.31 (0.86, 2.01) | 0.212 |
| cockroach | |||||
| No | 527 (71.60) | 1 | |||
| Yes | 209 (28.40) | 0.28 (0.20,0.40) | <0.001 | 0.67 (0.42,1.06) | 0.088 |
| Air conditioner | |||||
| No | 272 (36.96) | 1 | |||
| Yes | 464 (63.04) | 0.27 (0.20,0.37) | <0.001 | 0.88 (0.56,1.39) | 0.575 |
| Material of living room floor | |||||
| Tile or cement | 524 (71.20) | 1 | |||
| Wood material | 113 (15.35) | 1.05 (0.70,1.58) | 0.803 | 1.14 (0.69,1.88) | 0.603 |
| Composite material | 44 (5.98) | 0.83 (0.44,1.53) | 0.548 | 0.79 (0.37,1.67) | 0.533 |
| Carpet | 55 (7.47) | 1.54 (0.88,2.72) | 0.131 | 2.21 (1.12,4.42) | 0.023 |
| Material of pillow | |||||
| Synthetic materials | 275 (37.36) | 1 | |||
| Cotton | 124 (16.85) | 1.13 (0.73, 1.73) | 0.588 | 1.58 (0.92,2.73) | 0.101 |
| Sponge | 101 (13.72) | 0.52 (0.31, 0.84) | 0.010 | 0.63 (0.34,1.16) | 0.141 |
| Plant | 187 (25.41) | 3.39 (2.29, 5.06) | <0.001 | 1.74 (1.06,2.85) | 0.028 |
| Feather | 49 (6.66) | 0.51 (0.25, 0.98) | 0.052 | 0.60 (0.26,1.36) | 0.229 |
| Material of quilt | |||||
| Synthetic materials | 161 (21.88) | 1 | |||
| Cotton | 420 (57.07) | 1.09 (0.76, 1.58) | 0.625 | 1.43 (0.88,2.33) | 0.150 |
| Blanket | 24 (3.26) | 0.53 (0.21, 1.28) | 0.171 | 1.67 (0.52,5.17) | 0.380 |
| Other material | 131 (17.80) | 0.50 (0.31, 0.81) | 0.005 | 1.31 (0.70,2.47) | 0.403 |
Notes: significant difference of Bivariate regression analysis: P < 0.15.
Significant difference of multiple regression analysis: P < 0.05.
OR: odds ratio.
aOR: adjusted odds ratio.
4. Discussion
Countrywide epidemiological data on pollen allergens were scarce and risks factors of PiAR in mainland China were unclear. This multicenter study detected four grass pollens, six tree pollens and four weed pollens, which covered the majority of pollen allergen types and could provide a more comprehensive map of pollen allergens sensitization distribution in mainland China.
46.33% of AR patients showed positive SPT reactions to pollen in mainland China. In our study, Artemisia vulgaris, one of the weed pollens, was the most common pollen allergen in mainland China with a positive rate up to 31.39% (Table 1). Our study found that symptoms were most severe in patients with AR in autumn. Previous studies in China also found Artemisia, Chenopodium, and Humulus scandens were the top three pollens found in July, August and September [7]. These results indicated that Artemisia, Chenopodium, and Humulus scandens had aggravating effects on AR symptoms in autumn [7]. Compared with a previous study, there was a significant increase in the positive rate of grass pollens, tree pollens and weed pollens by year. A study conducted from 2006 to 2007 showed that the overall prevalence of Artemisia vulgaris positive rate among asthma or rhinitis was between 1.6% and 19.7% in children which was dramatically lower than our findings [11]. For Ambrosia artemisiifolia allergen, it also witnessed a growing positive rate from below 10% to 21.6% from 2006 to 2021(11). Similar tendency was showed for the grass pollens and tree pollens [11,12]. The continued rise of pollen sensitization was also found in Europe and it might result from climate change [5]. Warmer temperatures and higher levels of Carbon dioxide led to vigorously plant growth and pollen production [13].
The positive rate of pollen allergens was also higher in males than females except Artemisia vulgaris and Chenopodium album, but most of them did not have statistically significant differences. The positive rate of Artemisia vulgaris and Chenopodium album were quite similar between males and females. A population-based cohort study found that the significant difference of pollen sensitization among sexes gradually appeared by age [14]. It might be related to the difference in sexual hormones and lifestyle such as hormonal medications and sports habits [15]. Further study needed to be carried out.
Allergen sensitization varied in different life-course. The high-age group had a higher positive rate than the low-age group in all pollens among AR patients. Our result was consistent with a longitudinal study conducted in Northern Sweden which found that the positive rates of Phleum pratense, Artemisia vulgaris allergen and Betula verrucosa allergen increased from the aged 7–8 to 19(16). High incidence and persistence of the increasing prevalence of pollen allergen sensitization by age might be the potential explanation [16].
The pollen allergen sensitization characteristics were widely different in regions. Overall, the positive rate was higher in north China and west China than in east China, and south China had the lowest positive sensitization. Fourteen pollen allergens were detected positive in AR patients in north, west and east China, where only five pollen allergens positive in south China. The differences might result from geographic and climatic differences. On one hand, there were vast grasslands and forests in the northern and western regions of China which were the natural environment suitable for various plants growth. On the other hand, most of the northern and western parts of China belong to the temperate monsoon climate, the temperate continental climate, the plateau mountain climate, which had the characteristics of greater wind and relatively aridity and was more conducive to the spread of pollen [17,18]. In the northern and western parts of China, the positive rate of Artemisia vulgaris pollen was extremely high at around 50% (Table 2). Artemisia vulgaris, which were used for prevention of desertification, was significantly excessive due to its vegetation abundance [17]. The eastern and southern parts of China were plain areas with densely populated populations and low vegetation coverage. The southern part had a subtropical monsoon climate, with long rainy seasons and heavy rainfall, which reduced the spread of pollen. Cynodon dactylon was the most common pollen allergen in east China and south China. because it was widely grown for the improvement of urban infrastructure such as parks, lawns, golf courses [8].
Skin index of reactivity to Ambrosia artemisiifolia and Artemisia vulgaris pollens was significantly positively correlated with the severity of AR, which was consistent with a previous study [11]. It was found for the first time that the skin index of reactivity to Cynodon dactylon, Triticum aestivum, Betula verrucosa, Populus alba, Salix fragilis, Cupressus sempervirens and Brassica napus pollens was all significantly positively associated with the severity of AR. In addition, a previous study found that skin index of reactivity to mixed grass pollen and mixed tree pollen was not significantly associated with the severity of AR(11). In our study, grass pollens including Phragmites communis, Phleum pratense, tree pollens including Ulmus campestris, Platanus acerifolia, and weed pollen including Chenopodium album pollen were not significantly related to the severity of AR. Different types of pollen had different effects on patients with AR, and the reason might be related to the type of protein contained in each pollen [19]. Based on the geographic distribution of pollen allergy and association between pollen allergen sensitization and the severity of AR, it raised an emergent need to conduct prevention and treatment for the Artemisia vulgaris sensitization in north China and west China.
In this study, the region of residence was associated with pollen-induced AR among AR patients. Living in north China and west China was positively associated with suffering from pollen-induced AR. It might be because of the mass pollen types and high pollen content in these areas. The high-age group was displayed as a risk factor. Ethnic minorities were at a greater risk of PiAR compared with Han nationality (Table 4). In our study, ethnic minorities were mainly concentrated in northern and western China, accounting for 14.08% and 12.43% of the northern and western study populations, respectively. While only 1.90% and 0.52% of the population were ethnic minorities in southern and eastern China. The greater risk of PiAR in ethnic minorities might be caused by different genetic makeup and lifestyle habits. A previous study found out that genetic variants greatly affected the development of autoimmune and inflammatory diseases [20]. Genetic predisposition might explain the differences. Ethnic minorities like the Tibetans and Mongolians were nomads and lived in the grasslands which increased the probability of exposure to pollens. History of pollen exposure was a significant risk of PiAR and it could be explained that pollens were a major cause of PiAR.
There are four kinds of materials for the floor of the living room and tile/cement was the most common one. Carpet displayed a higher risk of PiAR. The potential interpretation was that there was an interaction between pollens and carpet, which might need further research. Among the five common material types of pillows, synthetic materials were the highest used and pillow containing plant was a risk factor of PiAR (Table 4). In addition, previous studies found that people living in urban areas were more likely to be affected by PiAR than people living in rural areas because of the urbanization and high level of vehicle emissions [21,22]. However, no statistically significant difference in PiAR sensitivity was found between AR patients living in urban areas and AR patients living in rural areas in this study (Table 4). It might be because of a higher smoking rate and frequency use of burning coal in the countryside in mainland China, which increased the pollution particulate matter content in the air [23,24].
There were several limitations in this study. The temporal and spatial distributions of allergic pollen types were both important for the prevention and diagnosis of allergic diseases. In this study, only the spatial distribution was explored. Further study could focus on the temporal distribution of pollen allergens to give a deeper insight into the prevalence patterns in mainland China and to help clinicians identify the triggering factors of allergic diseases more accurately and carry out the targeted treatment. In addition, the sample size was not large enough, but our finding was consistent with previous studies, which showed that the study subjects were representative [7,8].
In conclusion, around 50% of patients aged below 18 with AR had identifiable sensitizations to pollen detected by SPT. The positive rate of pollen allergens was significantly higher in the high-age group [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]] than the low-age group ( ≤ 5), while no significant difference was found between the sexes. Overall, the positive rate among different regions from highest to lowest was 73.24% in north China, 65.12% in west China, 22.28% in east China and 6.67% in south China. The positive rate of skin reactivity to Ambrosia artemisiifolia, Artemisia vulgaris, Cynodon dactylon, Triticum aestivum, Betula verrucosa, Populus alba, Salix fragilis, Cupressus sempervirens and Brassica napus pollens was significantly associated with the severity of AR. The region of residence, ages, ethnic minorities, history of pollen exposure, material of living room floor and the material of pillow were significant risks of PiAR. This study revealed the pollen allergens sensitization characteristics in AR in mainland China and explored the factors affecting PiAR, providing important clues for the prevention and immunotherapy of pollen allergens in mainland China.
Data availability
The datasets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval
The study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (GYFYY-2018-93).
Production notes
Author contribution
Conceived and designed the experiments: Wenting Luo, Yusi Li, Jingping Zheng, Chuangli Hao and Baoqing Sun.
Analyzed and interpreted the data: Yusi Li, Wenting Luo and Teng Zhang.
Performed the experiments and contributed reagents, materials, analysis tools: Lina Xu, Yongmei Yu, Jinhai Ma, Yu Wang, Yi Wang, Huajie Wu, Meng Xv and Liting Wu.
Wrote the paper: Wenting Luo and Yusi Li.
Funding statement
This study was supported by the National Natural Science Foundation of China (81871736), the Guangzhou Science and Technology Foundation (202102010327), the Foundation of SKLRD (SKLRD-Z-2022-09).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no competing interests.
Acknowledgement
Thanks Dr Dongming Huang, Dr Xiaoluan Li, Dr Chunhua Wei, Dr Rongfang Zhang, Dr Guoping Li, Dr Peiru Xv, Dr Xiaowen Huang, Dr Yang Liu, Dr Shuang Ren, Dr Shuping Zhang, Dr Hui Yang, Dr Junyi Wang, Dr Xiangping Ma, Dr Tianhao Chen for their contribution to the case and data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14914.
Contributor Information
Jingping Zheng, Email: jpzhenggy@163.com.
Chuangli Hao, Email: hcl_md@163.com.
Baoqing Sun, Email: sunbaoqing@vip.163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bousquet J., Khaltaev N., Cruz A.A., Denburg J., Fokkens W.J., Togias A., et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Mir E., Panjabi C., Shah A. Impact of allergic rhinitis in school going children. Asia Pac. Aller. 2012;2(2):93–100. doi: 10.5415/apallergy.2012.2.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein D.I., Schwartz G., Bernstein J.A. Allergic rhinitis: mechanisms and treatment. Immunol. Allergy Clin. 2016;36(2):261–278. doi: 10.1016/j.iac.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Allergens and Pollen Centers for Diseases Control and Prevention. 2020. https://www.cdc.gov/climateandhealth/effects/allergen.htm Available from: [Google Scholar]
- 5.Lake I.R., Jones N.R., Agnew M., Goodess C.M., Giorgi F., Hamaoui-Laguel L., et al. Climate change and future pollen allergy in Europe. Environ. Health Perspect. 2017;125(3):385–391. doi: 10.1289/EHP173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damato G., Cecchi L., Bonini S., Nunes C., Annesi-Maesano I., Behrendt H., et al. Allergenic pollen and pollen allergy in Europe. Allergy (Cph.) 2007;62(9):976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.Y., Ma T.T., Wang X.Y., Zhuang Y., Wang X.D., Ning H.Y., et al. Prevalence of pollen‐induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy (Cph.) 2018;73(6):1232–1243. doi: 10.1111/all.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo W., Huang H., Zheng P., Wei N., Luo J., Sun B., et al. Major grass pollen allergens and components detected in a southern Chinese cohort of patients with allergic rhinitis and/or asthma. Mol. Immunol. 2016;78:105–112. doi: 10.1016/j.molimm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Demoly P., Jankowski R., Chassany O., Bessah Y., Allaert F.A. Validation of a self-questionnaire for assessing the control of allergic rhinitis. Clin. Exp. Allergy. 2011;41(6):860–868. doi: 10.1111/j.1365-2222.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 10.Sampson H.A., Albergo R. Comparison of results of skin tests, RAST, and double-blind, placebo-controlled food challenges in children with atopic dermatitis. J. Allergy Clin. Immunol. 1984;74(1):26–33. doi: 10.1016/0091-6749(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Sun B., Huang Y., Lin X., Zhao D., Tan G., et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy (Cph.) 2009;64(7):1083–1092. doi: 10.1111/j.1398-9995.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 12.Luo W., Wang D., Zhang T., Zheng P., Leng D., Li L., et al. Prevalence patterns of allergen sensitization by region, gender, age, and season among patients with allergic symptoms in mainland China: a four‐year multicenter study. Allergy (Cph.) 2021;76(2):589–593. doi: 10.1111/all.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziska L.H.P., Beggs P.J.P. Anthropogenic climate change and allergen exposure: the role of plant biology. J. Allergy Clin. Immunol. 2011;129(1):27–32. doi: 10.1016/j.jaci.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Melén E., Bergström A., Kull I., Almqvist C., Andersson N., Asarnoj A., et al. Male sex is strongly associated with IgE‐sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin. Transl. Allergy. 2020;10(1):1–n/a. doi: 10.1186/s13601-020-00319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen-Jarolim E. Gender effects in allergology - secondary publications and update. World Aller. Organiz. J. 2017;10(1):47. doi: 10.1186/s40413-017-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rönmark E., Warm K., Bjerg A., Backman H., Hedman L., Lundbäck B. High incidence and persistence of airborne allergen sensitization up to age 19 years. Allergy (Cph.) 2017;72(5):723–730. doi: 10.1111/all.13053. [DOI] [PubMed] [Google Scholar]
- 17.Ge Y., Li Y., Bunting M.J., Li B., Li Z., Wang J. Relation between modern pollen rain, vegetation and climate in northern China: implications for quantitative vegetation reconstruction in a steppe environment. Sci. Total Environ. 2017;586:25–41. doi: 10.1016/j.scitotenv.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Rojo J., Rapp A., Lara B., Fernández-González F., Pérez-Badia R. Effect of land uses and wind direction on the contribution of local sources to airborne pollen. Sci. Total Environ. 2015;538:672–682. doi: 10.1016/j.scitotenv.2015.08.074. [DOI] [PubMed] [Google Scholar]
- 19.Biedermann T., Winther L., Till S.J., Panzner P., Knulst A., Valovirta E. Birch pollen allergy in Europe. Allergy (Cph.) 2019;74(7):1237–1248. doi: 10.1111/all.13758. [DOI] [PubMed] [Google Scholar]
- 20.Demenais F., Margaritte-Jeannin P., Barnes K.C., Cookson W.O.C., Altmüller J., Ang W., et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat. Genet. 2018;50(1):42–53. doi: 10.1038/s41588-017-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira M., Slezakova K., Delerue-Matos C., Pereira M.C., Morais S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: a review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019;124:180–204. doi: 10.1016/j.envint.2018.12.052. [DOI] [PubMed] [Google Scholar]
- 22.D'Amato Urban air pollution and plant-derived respiratory allergy. Clin. Exp. Allergy. 2000;30(5):628–636. doi: 10.1046/j.1365-2222.2000.00798.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang M., Luo X., Xu S., Liu W., Ding F., Zhang X., et al. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Respir. Med. 2019;7(1):35–45. doi: 10.1016/S2213-2600(18)30432-6. [DOI] [PubMed] [Google Scholar]
- 24.Pang Y., Zhang B., Xing D., Shang J., Chen F., Kang H., et al. Increased risk of carotid atherosclerosis for long-term exposure to indoor coal-burning pollution in rural area, Hebei Province, China. Environ. Pollut. 2019;255(Pt 2) doi: 10.1016/j.envpol.2019.113320. 1987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
Data will be made available on request.