Abstract
Background
To compare the prevalence of refractive (RA), corneal (CA), and internal astigmatism (IA) in Hong Kong children and adults and evaluate the role of IA in compensating for total astigmatism and its relations to myopic traits.
Methods
The Hong Kong Children Eye Study is a population-based cross-sectional study. Totally 3704 school children (mean age 7.5 ± 1.0 years) and 5577 adults (mean age 41.1 ± 7.5 years), who were their parents, were recruited. Cycloplegic and non-cycloplegic refractive cylinders were obtained from children and adults, respectively. Spearman correlation was applied to detect associations between astigmatism, ocular biometrics, refraction, and lens power. Astigmatism compensation factor (CF) was derived from the power vector analysis J0 and J45.
Results
The prevalence of RA (≤−1.0 D), CA (≥+1.0 D) and IA (≥+1.0 D) was 21.9%, 63.9%, and 9.9% in children, and 30.9%, 39.5%, and 23.7% in adults respectively. The mean RA, CA and IA values in children and adults were −0.69 ± 0.66 D, +1.14 ± 0.61 D, +0.62 ± 0.32 D, and −0.80 ± 0.74 D, +0.97 ± 0.69 D, and +0.76 ± 0.43 D, respectively. In adults and children, IA was negatively correlated with axial length (p < 0.0001), but positively correlated with spherical values and equivalent (p < 0.0001), suggesting an association of astigmatism with myopic traits. A greater proportion of children exhibited compensation by IA than adults in J0 (86.6% vs. 66.0%, p < 0.0001) and J45 components (55.5% vs. 41.7%, p < 0.0001).
Conclusions
Chinese children in Hong Kong exhibit a higher prevalence of RA and CA than in other cities. Children displayed a greater compensation by IA than adults, suggesting an age-related attenuation of IA compensation. IA is associated with myopic traits.
Subject terms: Epidemiology, Education
Introduction
Astigmatism is a common type of refractive error in which incident light rays do not converge at a single focal point, leading to the formation of a blurred image. Its global pooled prevalence among subjects under 20 years of age and adults above 30 were 14.9% and 40.4% respectively [1].
Untreated astigmatism of >1 D poses an amblyogenic risk in children [2, 3]. Early detection is essential to guide appropriate intervention to preserve vision [4]. Unfortunately, high astigmatism (more than 3 D) may result in aberrations despite spectacle correction [5]. Contact lenses with toric design or orthokeratology lenses may help restore visual quality but are not without infection risks [6]. In addition, high astigmatism cannot be corrected entirely by keratorefractive procedures alone [7].
In a 10-year prevalence study of Hong Kong preschoolers, myopia was reported to have a threefold progression from 2.3% to 6.3% while astigmatism displayed a static trend between 5 and 6% [8]. Since then, no further studies explored the associations between myopia and astigmatism in Hong Kong. Different components of astigmatism have been studied in association with myopia development. Internal astigmatism, in particular, was found to be associated with greater axial lengths (AL) in school children [9]. Given the high prevalence of myopia of 26.2% in our study population [10], we herein study and evaluate the relationship between astigmatism and myopia in children and adults of Hong Kong.
In this study, we aim to (1) determine the prevalence of astigmatism in children and adults; and (2) evaluate the associations of different astigmatism components: refractive astigmatism (RA), corneal astigmatism (CA), internal astigmatism (IA) with spherical equivalent (SE), spherical value, axial length (AL), and lens power (LP) in children and adults.
Subjects and methods
The Hong Kong Children Eye Study is a territory-wide and population-based cohort that determines the prevalence of refractive errors and pediatric eye diseases among school children in Hong Kong. The study also includes ophthalmic investigations of the parents for refractive errors and ocular biometrics. The study design and protocols have been previously published [10–14]. The study population consisted of 9281 individuals, including 3704 school children (mean age 7.5 ± 1.0 years) and 5577 adults (mean age 41.1 ± 7.5 years) who were their parents. Children who failed the cycloplegic regimen were excluded. Adults who underwent prior keratorefractive surgery or had signs of corneal trauma or injury were also excluded. The project conformed to the tenets of the Declaration of Helsinki and was granted ethical approval from the Institutional Review Board of the Chinese University of Hong Kong. Informed consent was obtained from the parents of all children.
Ocular examination for children and adults
Cycloplegic autorefraction was performed for each child using an autorefractor (Nidek ARK-510A, Gamagori, Japan). Details of the cycloplegic protocol had been previously described [10].
Non-cycloplegic autorefraction was obtained from adults, along with corneal curvatures of the steep and flat meridian. The magnitude of CA was taken as the difference between the steepest and the flattest meridians. The axis of CA refers to the meridian of the flattest curvature. Both measurements of RA and CA were rounded off to the nearest 0.25 D for analyses. Axial length (AL) was measured using the Zeiss IOL Master (Carl Zeiss Meditec Inc., Dublin, CA), while corneal curvature (K) was measured using the auto-keratometer. Lens power (LP) was calculated using the modified Bennett–Rabbetts formula, by using measured values for spherical equivalent (SE), mean keratometry value, axial length, and anterior chamber depth.
All operators received appropriate training and were assessed before conducting examinations on study subjects. All examinations followed standard operating procedures and were supervised by the principal investigator (JCY).
Definitions
RA was expressed in negative cylinder notation and was defined as being ≤−1.0 D. CA was expressed in positive cylinder notation and was defined as being ≥+1.0 D. The presence of IA was defined as being ≥+1.0 D. Paired Wilcoxon signed-rank test was performed, demonstrating no statistically significant difference (p > 0.05) between both eyes data for subjects in this cohort. As such, only measurements from the subjects’ right eyes were used for analysis.
The cylindrical axes for both RA and CA were classified as with-the-rule (WTR) when the flat meridian was at 180° ± 30°, against-the-rule (ATR) at 90° ± 30°, and oblique (OBL) when outside the ranges for both WTR and ATR. Both CA and RA were decomposed into their vector components J0 and J45, using the power vector approach described by Thibos and Wheeler [15] to allow comparison and visualization of astigmatism. Only subjects with both RA (i.e., ≤−1.0 D) or CA (i.e., ≥+1.0 D) were included in the analysis of different astigmatism types.
RA and CA were converted into power vector components using the equations:
C was the cylinder power and α was the measurement of the flat meridian. J0 was the vector component with reference to the cylinder power at 90° and 180°. Positive values of J0 indicate WTR astigmatism, and negative values indicate ATR astigmatism. J45 was the vector component at the 45° and 135° meridians and represented oblique astigmatism.
IA was given by the following formulae:
The relations between CA and IA was evaluated using the compensation factor (CF) [16], which was calculated using the equations: CF0 = − J0(IA)/J0(CA); CF45 = − J45(IA)/J45(CA). The compensation types were classified as: (1) <−0.1 same axis augmentation; (2) −0.1 to 0.1 no compensation; (3) 0.1 to 0.9 under compensation; (4) 0.9 to 1.1 full compensation; (5) 1.1 to 2 overcompensation; and (6) >2 opposite axis augmentation.
Statistical analysis
Prevalence with the corresponding 95% confidence intervals was determined for RA, CA, and IA across gender and age groups. A p value of less than 0.05 was considered statistically significant. All analyses were performed using Stata Statistical Software (version 14.0; StataCorp, College Station, Texas, USA). The Spearman correlation coefficient was used to determine the level of correlation between different types of astigmatism, as well as the SE, spherical value, AL, and LP. Independent samples t test was employed to compare different types of astigmatism between genders. Compensation for internal astigmatism will be regarded as the compensation factor subtypes including undercompensation, full compensation, and overcompensation.
Results
A total of 3704 children have completed cycloplegic refraction with astigmatism and ocular biometric data (response rate 74.1%), including 1926 boys and 1778 girls, and 5,577 adults, comprising 2383 men and 3194 women. The mean age was 7.5 ± 1.0 years and 41.1 ± 7.5 years in adults. There were more women (p < 0.0001) and boys (p = 0.004) in our cohort. This was compatible with the age-specific gender ratios according to the Census conducted in 2018 [17].
The prevalence and characteristics of astigmatisms in children and adults
CA was notably more prevalent in children than in adults (63.9% [95% CI: 62.4−65.5%] vs 39.5% [95% CI: 38.2–40.8%]) while RA and IA were more prevalent in adults than in children (RA: 30.9% [95% CI: 29.8–32.2%] vs 21.9% [95% CI: 20.6−23.3%]; IA [23.7% (95% CI: 22.6−24.8%) vs 9.9% (95% CI: 9.0−10.9%)] (Table 1 and Table 2). The mean RA, CA, and IA values in children and adults were −0.69 ± 0.66 D, +1.14 ± 0.61 D, +0.62 ± 0.32 D, and −0.80 ± 0.74 D, +0.97 ± 0.69 D, and +0.76 ± 0.43 D, respectively. WTR-RA constituted the most common astigmatism type, occurring in 793 of 813 (97.5%) astigmatic children and 1083 of 1,727 (62.7%) adults with RA. Similarly, WTR-CA was the most common type in 2361 of 2367 (99.7%) children and 2125 of 2202 (96.5%) adults with CA. The distribution of astigmatism types was summarized in supplementary table 1.
Table 1.
Prevalence of astigmatism magnitudes across age in children and adults.
| Children | n | Boys (n = 1926) | Girls (n = 1778) | |||||
|---|---|---|---|---|---|---|---|---|
| Diopter magnitude (absolute value) | ||||||||
| Age group (years) | ≥0.5 D | ≥1.0 D | ≥2.0 D | n | ≥0.5 D | ≥1.0 D | ≥2.0 D | |
| Corneal astigmatism | ||||||||
| 5.5≥ × <6.0 | 45 | 42 (93.3%) | 27 (60.0%) | 8 (17.8%) | 40 | 38 (95.0%) | 32 (80.0%) | 6 (15.0%) |
| 6.0≤ × <7.0 | 579 | 553 (95.6%) | 353 (61.0%) | 65 (11.2%) | 550 | 535 (97.3%) | 369 (67.1%) | 53 (9.6%) |
| 7.0≤ × <8.0 | 730 | 694 (95.1%) | 462 (63.3%) | 69 (9.5%) | 684 | 656 (95.9%) | 454 (66.4%) | 76 (11.1%) |
| 8.0≤ × <9.0 | 572 | 544 (95.1%) | 345 (60.3%) | 55 (9.6%) | 504 | 485 (96.2%) | 325 (64.5%) | 42 (8.3%) |
| Overall | (95.2%) | (61.7%) | (10.2%) | (96.4%) | (66.4%) | (10.0%) | ||
| Refractive astigmatism | ||||||||
| 5.5≥ × <6.0 | 45 | 29 (64.4%) | 13 (28.9%) | 5 (11.1%) | 40 | 30 (75.0%) | 9 (22.5%) | 3 (7.5%) |
| 6.0≤ × <7.0 | 579 | 371 (64.1%) | 147 (25.4%) | 38 (6.6%) | 550 | 326 (59.3%) | 102 (18.5%) | 26 (4.7%) |
| 7.0≤ × <8.0 | 730 | 465 (63.7%) | 160 (21.9%) | 39 (5.3%) | 684 | 448 (65.5%) | 150 (21.9%) | 52 (7.6%) |
| 8.0≤ × <9.0 | 572 | 356 (62.2%) | 130 (22.7%) | 37 (6.5%) | 504 | 330 (65.5%) | 102 (20.2%) | 27 (5.4%) |
| Overall | (63.3%) | (23.4%) | (6.2%) | (63.8%) | (20.4%) | (6.1%) | ||
| Internal astigmatism | ||||||||
| 5.5≥ × <6.0 | 45 | 28 (62.2%) | 6 (13.3%) | 1 (0.2%) | 40 | 26 (65.0%) | 7 (17.5%) | 0 (0.0%) |
| 6.0≤ × <7.0 | 579 | 340 (58.7%) | 59 (10.2%) | 4 (0.7%) | 550 | 351 (63.8%) | 69 (12.5%) | 2 (0.4%) |
| 7.0≤ × <8.0 | 730 | 442 (60.5%) | 66 (9.0%) | 1 (0.1%) | 684 | 446 (65.2%) | 74 (10.8%) | 2 (0.3%) |
| 8.0≤ × < | 572 | 326 (57.0%) | 42 (7.3%) | 3 (0.5%) | 504 | 311 (61.7%) | 45 (8.9%) | 2 (0.4%) |
| Overall | (59.0%) | (9.0%) | (0.5%) | (78.3%) | (20.5%) | (0.7%) | ||
| Adults | Men (n = 2383) | Women (n = 3194) | ||||||
| Corneal astigmatism | ||||||||
| 24≥ × <30 | 13 | 12 (92.3%) | 7 (53.9%) | 0 (0.0%) | 104 | 78 (75.0%) | 38 (36.5%) | 11 (10.6%) |
| 30≥ × <40 | 677 | 531 (78.3%) | 288 (42.5%) | 63 (9.3%) | 1617 | 1278 (79.0%) | 655 (40.5%) | 118 (7.3%) |
| 40≥ × <50 | 1367 | 1011 (74.0%) | 526 (38.5%) | 113 (8.3%) | 1437 | 1090 (75.9%) | 577 (40.2%) | 86 (6.0%) |
| 50≥ × ≤72 | 326 | 208 (63.8%) | 100 (30.7%) | 13 (4.0%) | 36 | 23 (63.9%) | 11 (30.6%) | 4 (11.1%) |
| Overall | (73.9%) | (38.6%) | (7.9%) | (77.3%) | (40.1%) | (6.9%) | ||
| Refractive astigmatism | ||||||||
| 24≥ × <30 | 13 | 9 (69.2%) | 5 (38.5%) | 0 (0.0%) | 104 | 63 (60.6%) | 26 (25.0%) | 8 (7.7%) |
| 30≥ × <40 | 677 | 510 (75.3%) | 253 (37.4%) | 61 (9.0%) | 1617 | 1067 (66.0%) | 411 (25.4%) | 105 (6.5%) |
| 40≥ × <50 | 1367 | 1005 (73.5%) | 506 (37.2%) | 137 (10.2%) | 1437 | 981 (68.5%) | 380 (26.4%) | 89 (6.2%) |
| 50≥ × ≤72 | 326 | 259 (79.4%) | 132 (40.5%) | 30 (9.2%) | 36 | 29 (80.6%) | 14 (38.9%) | 4 (11.1%) |
| Overall | (74.8%) | (37.6%) | (9.6%) | (65.2%) | (26.0%) | (6.5%) | ||
| Internal astigmatism | ||||||||
| 24≥ × <30 | 13 | 11 (84.6%) | 5 (38.5%) | 0 (0.0%) | 104 | 70 (67.3%) | 13 (12.5%) | 1 (1.0%) |
| 30≥ × <40 | 677 | 488 (72.1%) | 126 (18.6%) | 6 (8.9%) | 1617 | 1227 (75.9%) | 372 (23.0%) | 10 (0.6%) |
| 40≥ × <50 | 1367 | 947 (69.3%) | 298 (21.8%) | 16 (1.2%) | 1437 | 1103 (76.8%) | 399 (27.8%) | 11 (0.8%) |
| 50≥ × ≤72 | 326 | 246 (75.5%) | 95 (29.1%) | 3 (0.9%) | 36 | 28 (77.8%) | 12 (33.3%) | 1 (2.8%) |
| Overall | (71.0%) | (22.0%) | (1.1%) | (76.0%) | (25.0%) | (0.7%) | ||
Table 2.
Spearman correlations between age, astigmatism, and myopic ocular traits in children and adults.
| CA | RA | IA | Age | |||||
|---|---|---|---|---|---|---|---|---|
|
Adults (n = 5577) |
Children (n = 3704) |
Adults (n = 5577) |
Children (n = 3704) |
Adults (n = 5577) |
Children (n = 3704) |
Adults (n = 5577) |
Children (n = 3704) |
|
| SEQ |
r = −0.2485* p < 0.0001 |
r = 0.0419* p = 0.0108 |
r = 0.3228 p < 0.0001 |
r = 0.0301 p = 0.0673 |
r = 0.1881 p < 0.0001 |
r = 0.0746 p < 0.0001 |
r = 0.0282* p = 0.038 |
r = −0.2361* p < 0.0001 |
| Spherical value |
r = −0.2022* p < 0.0001 |
r = 0.1927* p < 0.0001 |
r = 0.2064* p < 0.0001 |
r = −0.1751* p < 0.0001 |
r = 0.1981 p < 0.0001 |
r = 0.0308 p = 0.0611 |
r = 0.0333* p = 0.015 |
r = −0.2401* p < 0.0001 |
| Axial length |
r = 0.1390* p < 0.0001 |
r = −0.1690* p < 0.0001 |
r = −0.2439* p < 0.0001 |
r = 0.0727* p < 0.0001 |
r = −0.2431 p < 0.0001 |
r = −0.1698 p < 0.0001 |
r = 0.1112 p < 0.0001 |
r = 0.2752 p < 0.0001 |
| Lens power |
r = 0.1418 p < 0.0001 |
r = 0.0544 p = 0.0009 |
r = 0.0084* p = 0.5326 |
r = −0.0455* p = 0.0056 |
r = 0.1155 p < 0.0001 |
r = 0.0569 p = 0.0005 |
r = −0.3441 p < 0.0001 |
r = −0.3251 p < 0.0001 |
| CA |
r = −0.3997 p < 0.0001 |
r = −0.6843 p < 0.0001 |
r = 0.0387 p = 0.0038 |
r = 0.3036 p < 0.0001 |
r = −0.0762 p < 0.0001 |
r = −0.0341 p = 0.036 |
||
| RA |
r = −0.3997 p < 0.0001 |
r = −0.6843 p < 0.0001 |
r = 0.0051 p = 0.7020 |
r = 0.2124 p < 0.0001 |
r = −0.0601 p < 0.0001 |
r = −0.0062 p = 0.729 |
||
| IA |
r = 0.0387 p = 0.0038 |
r = 0.3036 p < 0.0001 |
r = 0.0051 p = 0.7020 |
r = 0.2124 p < 0.0001 |
r = 0.0301* p = 0.025 |
r = −0.0611* p < 0.0001 |
Asterisk (*) indicates an opposite direction of association in children and adults while bolded p values indicate clinical significance.
The differential compensation of IA in children and adults
Figure 1 summarized the distribution for CF among children and adults. A greater proportion of children than adults compensated their total J0 and J45 in varying degrees (CF > 0.1 and CF < 2) with internal J0 and J45 (J0 [86.6% vs 66.0%, p < 0.0001]; J45 [55.5% vs 41.7%, p < 0.0001]). The proportion of under compensation, full compensation, and overcompensation of CF0 were 71.3%, 9.1%, and 6.2% in children, and 39.2%, 9.77%, and 17.1% in adults respectively, while that for CF45 were 32.5%, 11.4% and 11.5 in children, and 24.8%, 8.3%, and 8.6% in adults, respectively.
Fig. 1. Compensation by internal astigmatism in children and adults.
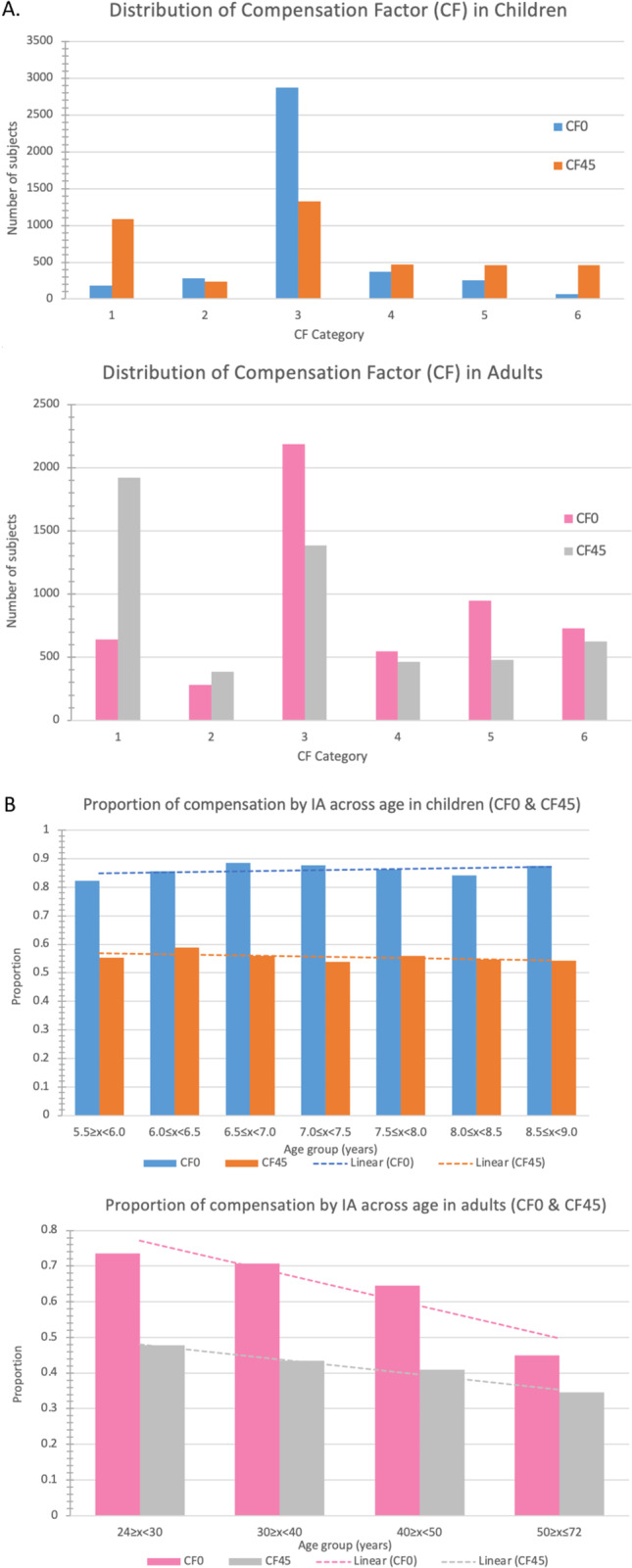
A The distribution of compensation factors (CF) in children and adults. B The proportion of compensation by internal astigmatism (IA) in CF0 and CF45 across age in children and adults.
IA and its relations to lens power
The mean lens power was 24.3 ± 1.9 D (range: 6.9–35.9 D) in children and 21.9 ± 2.1 D (range: −6.1–37.9 D) in adults. Lens power decreased with age in both populations (r = −0.3249, p < 0.0001 in children; r = −0.3436, p < 0.0001 in adults) as shown in Fig. 2 and was lower in male gender (children: 23.7 ± 1.7 D vs 24.9 ± 1.8 D, p < 0.0001; adults 21.0 ± 1.9 D vs 22.6 ± 2.0 D, p < 0.0001). A greater lens power was associated with a higher mean IA value in both children and adult (children: r = 0.0569, p = 0.0005; adults: r = 0.1155, p < 0.0001).
Fig. 2. The distribution of mean Internal Astigmatism (IA) and mean Lens Power (LP) in children and adults.
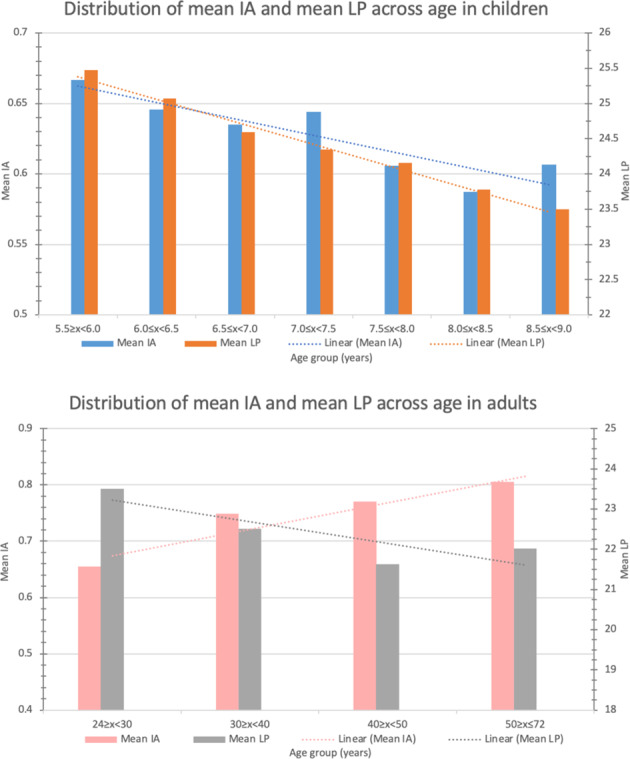
Both the mean IA and mean LP decreased with age in children. This is in contrast to the mean LP which decreased with age while the mean IA increased with age in adults.
Associations of RA, CA, and IA with myopic parameters
Increasing ametropia was associated with greater magnitudes of mean RA and CA observed in both children and adults (Fig. 3). IA was positively associated with SE and spherical value in both children and adults [(SE: children: r = 0.0746, p < 0.0001, adults: r = 0.1881, p < 0.0001); spherical value: children: r = 0.0308, p = 0.0611, adults: r = 0.1981, p < 0.0001]]. In contrast, IA was negatively associated with AL in both populations (SE: children: r = −0.1698, p < 0.0001, adults: r = −0.2431, p < 0.0001). In children, CA was positively associated with SE and spherical value, but negatively associated with AL. In contrast, CA was positively correlated with AL, but negatively correlated with SE and spherical values in adults (Table 2).
Fig. 3. The distribution of mean CA, RA, IA and spherical equivalent in children and adults.
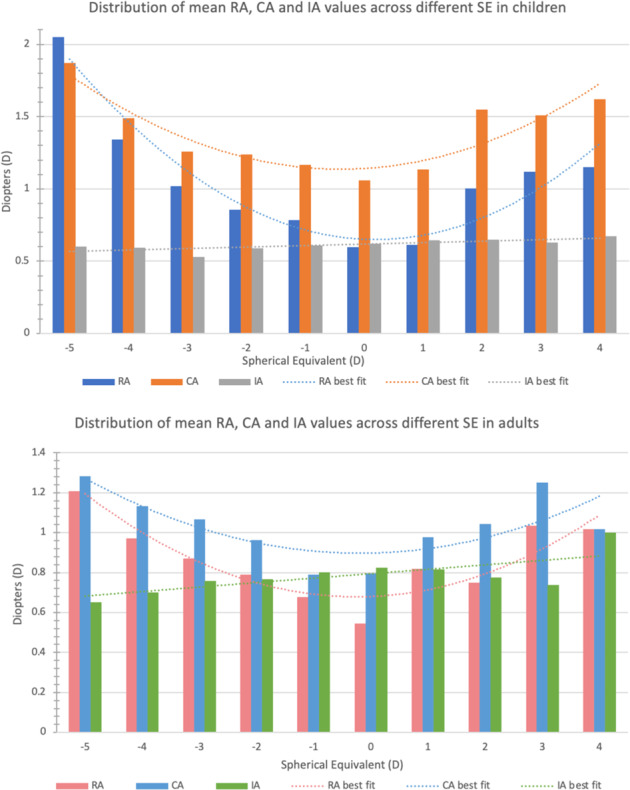
Increasing ametropia was associated with greater magnitudes of mean RA and CA observed in both children and adults. IA was positively associated with SE and spherical value in both children and adults.
The change of astigmatism with age and sex
In children, all CA, RA and IA marginally decreased with age. In adults, IA increased whereas both RA and CA decreased with age (Table 2). In children, CA and IA were significantly correlated with sex (CA: r = 0.0397, p = 0.0157; IA: r = 0.0789, p < 0.0001, respectively), but not with RA (r = 0.0089, p = 0.5878). In adults, mean RA and IA were correlated with sex (p < 0.0001 in RA and IA) but not CA (p = 0.0310). Females had a more negative value of RA and more positive CA value than males (Supplementary Table 2). Mean IA values were significantly higher in females (p = 0.0003 in adults, and p < 0.0001 in children).
Discussion
In this population-based study of 3704 children and 5577 adults, we have revealed several notable findings. First, there is a high prevalence of RA and CA in school children in Hong Kong. Second, a greater proportion of children compensated their total astigmatism with IA than adults. Third, a linear relationship existed between IA and SE among both children and adults. Myopic subjects had lower mean IA values than hyperopic subjects, and the mean IA value was higher among females of both age groups. Fourth, adults displayed an association between lens power and IA that was twice as strong as that for children, suggesting an age-related change in the contribution of the crystalline lens to IA.
A high prevalence of RA in parallel with a high prevalence of myopia in Children of Hong Kong
In this study, the prevalence of RA in Hong Kong school children was higher than in other counterparts worldwide, such as Singapore (19.2%) [18], Taiwan (13.0%) [19], Iran (6.4%) [20], and Denmark (4.3%) [21] using the same 1.0 D threshold (Supplementary Tables 3–6). Compared to the meta-analysis on global estimates of the prevalence of refractive errors, which included studies measuring either non-cycloplegic or cycloplegic refraction and utilizing lower thresholds to diagnose astigmatism [1], the adjusted prevalence of RA in children and adults in our study became 37.4% and 46.7% (with ≤−0.75 D) and 63.5% and 70.3% (with ≤−0.5 D), respectively.
Our prevalence of RA (≤−0.5 D) is the highest among all 16 included studies (0.76–59.7%) [1]. Our prevalence of astigmatism >0.5 D in adults (70.3%) is greater than that in southwestern China (56.8% in 1626 adults) [22]. Our prevalence of astigmatism >1.0 D in adults is lower (30.9%) than the NHANES study from the United States (36.2% of 12,010 adults) [23].
Astigmatism is known for its association with myopia progression [24, 25]. The high prevalence of astigmatism in our current study may be explained by the concurrent high prevalence of myopia in our population [10, 14]. The prevalence of CA in children in our study was more than double that of a multi-ethnic cohort of Australian children (25.6–27.7%) [26–28]. However, if only the data for East Asian children were extracted, we found a similar prevalence of CA between our study (63.7%) and theirs (prevalence of CA: 50.0% at 12 years [27] and 67.2% at 6 years [26]. Thus CA was thought to be significantly associated with ethnicity in childhood. A multi-center collaborative study identified that Asian children had the greatest corneal power in the vertical meridian among different ethnic groups [29]. Our findings among children of Han Chinese origin supported an ethnic variation in astigmatism.
Age-related attenuation of the degree of astigmatism compensation from childhood to adulthood
A significantly greater proportion of children compensated their total astigmatism with IA (86.6% in J0 and 55.5% in J45) compared to adults (66.0% in J0 and 41.7% in J45). This suggested an age-related attenuation of IA in compensating for the overall amount of astigmatism.
The Nanjing Eye study found even higher degrees of compensation (91.5% in J0 and 77.2% in J45) in younger children (48–60 months) [16], whereas Liu et al. [30] reported similar findings (80.6% in J0 and 58.3% in J45) among children aged 6–11 in Beijing. Nonetheless, the Nanjing Eye study used non-cycloplegic refraction which may not be comparable to our cohort as cycloplegia is known to influence the J0 of RA [16, 31, 32]. Despite having employed cycloplegic refraction in Liu’s study, the analysis of astigmatism compensation was limited to a subset of 206 children [30].
In adults, a South Korean study on 178 subjects with a younger mean age of 27.6 years showed that 80% of J0 and 59% of J45 exhibited some degree of IA compensation [33]. Our adult data, which included older subjects (mean 41.1 years), revealed a smaller proportion of IA compensation in both J0 (66.0%) and J45 (41.7%). These results, altogether, were in line with our hypothesized age-related attenuation in IA compensation.
IA as a potential biomarker for myopia incidence and progression in childhood
Our study shows a strong association between myopic parameters across all types of astigmatisms among both children and adults. The mean IA power was also smaller in myopic children. Given the relations between CA, IA, and CF, if the CA value in children is held constant, a smaller degree of IA compensation (a lower CF) would attribute to a smaller mean IA power. As IA was negatively correlated with AL among children, it is reasonable to extrapolate that a smaller amount of IA compensation (a lower CF) is associated with myopia and/or myopic progression. The rate of age-related attenuation of IA compensation may therefore be a biomarker for predicting myopia incidence and/or progression.
The hypothesized role of the crystalline lens and vitreous humor in IA
IA is thought to be contributed by both the lens and vitreous humor. Active fine-tuning of crystalline lines' shape and position were also postulated mechanisms for compensating corneal astigmatism [34]. Our study showed that the mean LP decreased with age in both children and adult populations. However, the mean IA power decreased with age in children but increased with age in adults (Fig. 2). Hence, we hypothesize that the increase in mean IA power among adults could be a result of a progressive, age-related change in the vitreous humor, outweighing the decreasing lens power of an aging crystalline lens.
Strengths and limitations
The strengths of our paper include a large sample of children in a territory-wide and population-based setting which minimizes selection bias. The inclusion of children of a narrow age range and adults who are parents of these children and of the same population allows comparison of astigmatism patterns between the two distinct age populations. We employed stringent cycloplegia for children for more accurate refractive data. The limitations of our study were that only the parents of children were selected to study the prevalence of astigmatism. The use of non-cycloplegic refraction for adults, which can overestimate myopic parameters (SE, spherical values), may affect the consistency in the correlation analyses with astigmatism. Only the right eyes of the subjects were adopted for analyses, and we did not correct for the effect of ocular dominance in ametropia and astigmatism. This study also adopted stringent criteria in defining astigmatism of 1.0 D, instead of 0.5 D or 0.75 D, because of its clinical relevance and its potential to cause meridional amblyopia. For better comparisons with other studies, the prevalence of astigmatism in our cohort as defined by 0.5 D and 0.75 D was also provided. Furthermore, the autokeratometry only analyzed the anterior cornea without accounting for posterior corneal astigmatism. Due to the lack of an air–cornea interface at the posterior corneal surface, the effect of posterior corneal astigmatism in the overall refraction was much smaller. Future studies utilizing more sophisticated tomography systems to look at both anterior and posterior cornea separately may allow us to study their roles in overall astigmatism.
Conclusion
Refractive and corneal astigmatisms are more prevalent in Hong Kong school children and adults than in other cities. Children displayed a greater compensation by IA than adults, suggesting an age-related attenuation of IA compensation. A correlation existed between IA and myopia traits for both children and adults, which may serve as a potential biomarker for myopic development or progression. The differential role of IA between children and adults may explain the disparity in RA prevalence between the two age groups. Vitreous humor may play a role in determining IA.
Supplemental material is available on Eye’s website.
Summary
What was known before
Few epidemiological studies looked into astigmatism in Hong Kong children and adults, as well as the astigmatism components. No prior studies explored the associations between myopia and astigmatism in Hong Kong children and adults.
What this study adds
Chinese children in Hong Kong exhibit a higher prevalence of refractive astigmatism and corneal astigmatism than in other cities. Children displayed a greater compensation for internal astigmatism than adults, suggesting an age-related attenuation of internal astigmatism compensation. Internal astigmatism is associated with myopic traits.
Supplementary information
Acknowledgements
The authors would like to acknowledge Ms. Mandy Pui Har Ng for her tremendous contributions to the Hong Kong Children Eye Study.
Author contributions
JCY, KKW, and ASH conceived the idea. KKW, ASH, and RCY performed data interpretation, and data analysis and wrote the manuscript. YZ performed data collection, extraction, and streamlined data analysis. XZ, YMW, SLL, LJC, ALY, CCT, and CPP critically revised the manuscript. JCY supervised the work and critically revised the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported in part by the General Research Fund (GRF), Research Grants Council, Hong Kong (14111515 and 14103419 [JCY]); Collaborative Research Fund (C7149-20G [JCY]); Health and Medical Research Fund (HMRF), Hong Kong (5160836, [LJC] and 07180826 [XJZ]), and the Direct Grants of the Chinese University of Hong Kong, (4054193 [LJC] and 4054121 & 4054199 [JCY] and 4054634 [XJZ]); the Innovation and Technology Fund (7010590 [JCY]), the UBS Optimus Foundation Grant 8984 (JCY); the Centaline Myopia Fund [JCY]; the Lim Por-yen Eye Genetics Research Centre; the CUHK Jockey Club Children’s Eye Care Programme, and the CUHK Jockey Club Myopia Prevention Programme.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ka Wai Kam, Arnold Shau Hei Chee.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02072-9.
References
- 1.Hashemi H, Fotouhi A, Yekta A, Pakzad R, Ostadimoghaddam H, Khabazkhoob M. Global and regional estimates of prevalence of refractive errors: systematic review and meta-analysis. J Curr Ophthalmol. 2018;30:3–22. doi: 10.1016/j.joco.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascual M, Huang J, Maguire MG, Kulp MT, Quinn GE, Ciner E, et al. Risk factors for amblyopia in the vision in preschoolers study. Ophthalmology. 2014;121:622–629 e621. doi: 10.1016/j.ophtha.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margines JB, Huang C, Young A, Mehravaran S, Yu F, Mondino BJ, et al. Refractive errors and amblyopia among children screened by the UCLA Preschool Vision Program in Los Angeles County. Am J Ophthalmol. 2020;210:78–85. doi: 10.1016/j.ajo.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Harvey EM. Development and treatment of astigmatism-related amblyopia. Optom Vis Sci. 2009;86:634–639. doi: 10.1097/OPX.0b013e3181a6165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cakir B, Aksoy NO, Ozmen S, Bursali O, Celik E, Horozoglu F. Corneal topography, anterior segment and high-order aberration assessments in children with ≥2 diopter astigmatism. Int Ophthalmol 2020;40:1461–1467. [DOI] [PubMed]
- 6.Kam KW, Yung W, Li GKH, Chen LJ, Young AL. Infectious keratitis and orthokeratology lens use: a systematic review. Infection. 2017;45:727–735. doi: 10.1007/s15010-017-1023-2. [DOI] [PubMed] [Google Scholar]
- 7.Chow SSW, Chow LLW, Lee CZ, Chan TCY. Astigmatism correction using SMILE. Asia Pac J Ophthalmol (Philos) 2019;8:391–396. doi: 10.1097/01.APO.0000580140.74826.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan DS, Lai C, Lau HH, Cheung EY, Lam DS. Change in vision disorders among Hong Kong preschoolers in 10 years. Clin Exp Ophthalmol. 2011;39:398–403. doi: 10.1111/j.1442-9071.2010.02470.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Weng C, Xia F, Wang X, Zhou X. Internal astigmatism and its role in the growth of axial length in school-age children. J Ophthalmol. 2018;2018:1686045. doi: 10.1155/2018/1686045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yam JC, Tang SM, Kam KW, Chen LJ, Yu M, Law AK, et al. High prevalence of myopia in children and their parents in hong kong chinese population: the Hong Kong Children Eye Study. Acta Ophthalmol 2020. [DOI] [PubMed]
- 11.Cheung CY, Li J, Yuan N, Lau GYL, Chan AYF, Lam A, et al. Quantitative retinal microvasculature in children using swept-source optical coherence tomography: the Hong Kong Children Eye Study. Br J Ophthalmol 2018. [DOI] [PubMed]
- 12.Wong ES, Zhang XJ, Yuan N, Li J, Pang CP, Chen L, et al. Association of optical coherence tomography angiography metrics with detection of impaired macular microvasculature and decreased vision in amblyopic eyes: the Hong Kong Children Eye Study. JAMA Ophthalmol 2020;138:858–865. [DOI] [PMC free article] [PubMed]
- 13.Yuan N, Li J, Tang S, Li FF, Lee CO, Ng MPH, et al. Association of secondhand smoking exposure with choroidal thinning in children aged 6 to 8 years: the Hong Kong Children Eye Study. JAMA Ophthalmol 2019;137:1406–1414. [DOI] [PMC free article] [PubMed]
- 14.Tang SM, Kam KW, French AN, Yu M, Chen LJ, Young AL, et al. Independent influence of parental myopia on childhood myopia in a dose-related manner in 2055 trios: the Hong Kong Children Eye Study. Am J Ophthalmol 2020;218:199–207. [DOI] [PubMed]
- 15.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Huang D, Chen X, Zhu H, Sun Q, Wang Y, et al. Preschool children exhibit evident compensatory role of internal astigmatism in distribution of astigmatism: the Nanjing Eye Study. Invest Ophthalmol Vis Sci. 2019;60:73–81. doi: 10.1167/iovs.18-24799. [DOI] [PubMed] [Google Scholar]
- 17.Table 1A: Population by Age and Sex Group. Census and Statistics Department. Available at: https://www.censtatd.gov.hk/en/web_table.html?id=1A#. Accessed 29/3/2022, 2022.
- 18.Tong L, Saw SM, Carkeet A, Chan WY, Wu HM, Tan D. Prevalence rates and epidemiological risk factors for astigmatism in Singapore school children. Optom Vis Sci. 2002;79:606–613. doi: 10.1097/00006324-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Shih YF, Hsiao CK, Tung YL, Lin LL, Chen CJ, Hung PT. The prevalence of astigmatism in Taiwan schoolchildren. Optom Vis Sci. 2004;81:94–98. doi: 10.1097/00006324-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hashemi H, Asharlous A, Khabazkhoob M, Yekta A, Emamian MH, Fotouhi A. The profile of astigmatism in 6-12-year-old children in Iran. J Optom. 2020;14:58–68. doi: 10.1016/j.optom.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandfeld L, Weihrauch H, Tubaek G, Mortzos P. Ophthalmological data on 4.5- to 7-year-old Danish children. Acta Ophthalmol. 2018;96:379–383. doi: 10.1111/aos.13650. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Cui J, Shan G, Peng X, Pan L, Yan Z, et al. Prevalence and risk factors of refractive error: a cross-sectional study in Han and Yi adults in Yunnan, China. BMC Ophthalmol. 2019;19:33. doi: 10.1186/s12886-019-1042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitale S, Ellwein L, Cotch MF, Ferris FL, 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111–119. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twelker JD, Miller JM, Sherrill DL, Harvey EM. Astigmatism and myopia in Tohono O’odham Native American children. Optom Vis Sci. 2013;90:1267–1273. doi: 10.1097/OPX.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan DS, Rao SK, Cheung EY, Islam M, Chew S, Lam DS. Astigmatism in Chinese preschool children: prevalence, change, and effect on refractive development. Br J Ophthalmol. 2004;88:938–941. doi: 10.1136/bjo.2003.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huynh SC, Kifley A, Rose KA, Morgan I, Heller GZ, Mitchell P. Astigmatism and its components in 6-year-old children. Invest Ophthalmol Vis Sci. 2006;47:55–64. doi: 10.1167/iovs.05-0182. [DOI] [PubMed] [Google Scholar]
- 27.Huynh SC, Kifley A, Rose KA, Morgan IG, Mitchell P. Astigmatism in 12-year-old Australian children: comparisons with a 6-year-old population. Invest Ophthalmol Vis Sci. 2007;48:73–82. doi: 10.1167/iovs.06-0263. [DOI] [PubMed] [Google Scholar]
- 28.Sanfilippo PG, Yazar S, Kearns L, Sherwin JC, Hewitt AW, Mackey DA. Distribution of astigmatism as a function of age in an Australian population. Acta Ophthalmol. 2015;93:e377–385. doi: 10.1111/aos.12644. [DOI] [PubMed] [Google Scholar]
- 29.Twelker JD, Mitchell GL, Messer DH, Bhakta R, Jones LA, Mutti DO, et al. Children’s ocular components and age, gender, and ethnicity. Optom Vis Sci. 2009;86:918–935. doi: 10.1097/OPX.0b013e3181b2f903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Cheng Y, Zhang Y, Zhang L, Zhao M, Wang K. Evaluating internal and ocular residual astigmatism in Chinese myopic children. Jpn J Ophthalmol. 2017;61:494–504. doi: 10.1007/s10384-017-0532-y. [DOI] [PubMed] [Google Scholar]
- 31.Asharlous A, Hashemi H, Jafarzadehpur E, Mirzajani A, Yekta A, Nabovati P, et al. Does astigmatism alter with cycloplegia? J Curr Ophthalmol. 2016;28:131–136. doi: 10.1016/j.joco.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Tong H, Hao Q, Chen X, Zhu H, Huang D, et al. Risk factors for astigmatic components and internal compensation: the Nanjing Eye Study. Eye (Lond) 2021;35:499–507. doi: 10.1038/s41433-020-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CY, Oh JH, Chuck RS. Predicting ocular residual astigmatism using corneal and refractive parameters: a myopic eye study. Curr Eye Res. 2013;38:851–861. doi: 10.3109/02713683.2013.790976. [DOI] [PubMed] [Google Scholar]
- 34.Artal P, Benito A, Tabernero J. The human eye is an example of robust optical design. J Vis. 2006;6:1–7. doi: 10.1167/6.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


