Abstract
INTRODUCTION:
Synaptic degeneration is a key part of the pathophysiology of neurodegenerative diseases, and biomarkers reflecting the pathological alterations are greatly needed.
METHOD:
Seventeen synaptic proteins were quantified in a pathology-confirmed CSF cohort of patients with AD (n=63), frontotemporal lobar degeneration (FTLD) (n=53), and Lewy body spectrum of disorders (LBD) (n=21), as well as healthy controls (HC) (n=48).
RESULTS:
Comparisons revealed four distinct patterns: markers decreased across all neurodegenerative conditions compared to HC (the neuronal pentraxins), markers increased across all neurodegenerative conditions (14-3-3 zeta/delta), markers selectively increased in AD compared to other neurodegenerative conditions (neurogranin and beta-synuclein), and markers selectively decreased in LBD and FTLD compared to HC and AD (AP2B1 and syntaxin-1B).
DISCUSSION:
Several of the synaptic proteins may serve as biomarkers for synaptic dysfunction in AD, LBD, and FTLD. Additionally, differential patterns of synaptic protein alterations seem to be present across neurodegenerative diseases.
Keywords: Alzheimer’s disease, frontotemporal lobar degeneration, Lewy body spectrum of disorders, synaptic pathology, mass spectrometry, biomarkers
1. INTRODUCTION
Synaptic degeneration is a central pathophysiologic event of Alzheimer’s disease (AD) [1], with a stronger association with the degree of cognitive decline in AD than amyloid plaque pathology [2]. This correlation makes a convincing argument for the development and implementation of synaptic in vivo biomarkers -- not only in the routine clinical assessment of AD to facilitate diagnosis, disease staging, and progression -- but also to monitor the efficacy and endpoints of treatments in drug trials. Other neurodegenerative diseases, including frontotemporal lobar degeneration (FTLD) and Lewy body spectrum of disorders (LBD), are also marked by synaptic dysfunction and degeneration [3, 4]. It is thus of importance to study synaptic dysfunction across the range of neurodegenerative disorders.
Since the 1990s, when the first studies detecting synaptic proteins in the cerebrospinal fluid (CSF) emerged [5], various synaptic proteins have been studied as potential biomarkers, and several methods for their quantification have been developed [1]. To identify candidate synaptic biomarkers and to identify a multi-biomarker profile of synaptic dysfunction in AD, we successfully developed a mass spectrometry-based assay quantifying a panel of 17 potential synaptic proteins [6], selected based on a previous exploratory proteomics study [7]. The panel included syntaxins, vesicle-associated membrane protein 2 (VAMP-2), adaptor related protein complex 2 subunit beta 1 (AP2B1), complexin-2, synucleins, rab GDP dissociation inhibitor alpha (GDI1), neuronal pentraxins, phosphatidylethanolamine-binding protein 1 (PEBP-1), and members of the 14-3-3 protein family. The proteins all have a variety of synaptic functions and locations at the synapse, and several have been implicated in AD and other neurodegenerative diseases [1, 8-18].
When we studied the panel proteins in a small clinical sample, we found increased CSF levels of beta-synuclein, gamma-synuclein, neurogranin, PEBP-1, 14-3-3 proteins, and decreased CSF levels of the neuronal pentraxins in AD compared with healthy controls (HC), while the protein levels of complexin-2, the syntaxins, GDI1, and AP2B1 remained unchanged [6]. Thus, we concluded that several of the panel proteins could be potential synaptic pathology biomarkers for AD. However, it remained unclear if these protein alterations are specific to AD, or if any of these may also be markers of synaptic dysfunction in other neurodegenerative conditions. In this study, we aimed to validate these synaptic proteins in a larger independent sample of HC and AD, and we expand our study to other neurodegenerative diseases – LBD and FTLD.
2. METHOD
2.1. Patients and AD biomarker Analysis
Participants had autopsy-confirmed AD (n=63) and LBD (n=21), or had FTLD (n=53) pathologically confirmed by autopsy (n=42) or with a familial form (n=11, no autopsy data) determined by associated mutations (6 C9orf72, 2 GRN, 2 MAPT, 1 TARDBP) [19]. At autopsy, primary AD pathology was determined by board-certified neurologists (EBL, JQT) according to established criteria for high or intermediate AD neuropathologic change (ADNC) [20]; LBD pathology was determined by the accumulation of alpha-synuclein positive Lewy bodies [21], and FTLD pathology was determined by misfolded tau or transactive response DNA-binding protein of 43 kDa [22]. One FTLD patient and 9 LBD patients had concomitant AD (high/intermediate ADNC).
Patients had been assayed for CSF amyloid-β 1-42 (Aβ1-42), phosphorylated tau at Thr181 (p-tau181), and total tau (t-tau), as previously described [23]. In addition, we included 48 HC subjects who were cognitively unimpaired, with a mini-mental state exam (MMSE) score ≥ 28 [24], and who were biomarker-negative for AD with CSF Aβ1-42>192 pg/mL (no autopsy data). In all cases, we excluded other potential causes of cognitive decline such as hydrocephalus, closed head injury, a history of central nervous system surgery, stroke, infection, metabolic factors such as hypothyroidism, and primary psychiatric disorders. The demographics of the sample are shown group-wise in Table. Consent was obtained according to the Declaration of Helsinki and approved by the Penn Institutional Review Board.
Table.
Demographic characteristics for autopsy patients and healthy controls.
| Demographic Characteristics | HC | AD | LBD | FTLD | p-value |
|---|---|---|---|---|---|
| n | 48 | 63 | 21 | 53 | |
| Age at Onset (years) | -- | 68.0 [59.0, 73.2] | 64.0 [58.0, 66.0] | 63.0 [55.0, 66.5] | 0.004 |
| Age at CSF (years) | 66.5 [63.0, 72.0] | 72.0 [64.5, 78.5] | 71.0 [65.0, 78.0] | 66.0 [59.0, 71.0] | 0.001 |
| Age at Death (years) | 79.0 [70.0, 85.0] | 77.0 [71.0, 81.0] | 67.5 [61.0, 73.8] | <0.001 | |
| CSF to Death (years) | 6.0 [4.0, 8.0] | 5.0 [2.0, 8.0] | 3.0 [2.0, 5.0] | <0.001 | |
| CSF Aβ1-42 (pg/mL) | 299.8 [248.5, 374.2] | 132.0 [101.3, 156.3] | 196.5 [156.1, 215.0] | 258.5 [212.0, 304.5] | <0.001 |
| CSF p-tau181 (pg/mL) | 17.5 [13.4, 22.5] | 28.7 [17.5, 44.1] | 18.3 [11.8, 24.5] | 12.0 [9.8, 16.0] | <0.001 |
| CSF t-tau (pg/mL) | 45.0 [38.0, 61.0] | 100.0 [65.4, 152.6] | 37.0 [32.4, 59.5] | 56.0 [43.8, 78.2] | <0.001 |
| MMSE (max=30) | 29.0 [29.0, 30.0] | 23.0 [15.0, 26.0] | 27.0 [25.0, 28.0] | 23.0 [18.0, 27.2] | <0.001 |
| Education (years) | 16.0 [13.5, 18.0] | 16.0 [13.0, 18.0] | 16.0 [13.0, 18.0] | 16.0 [14.0, 18.0] | 0.860 |
| Sex = Male | 14 (29.2%) | 35 (55.6%) | 19 (90.5%) | 31 (58.5%) | <0.001 |
| ADNC | |||||
| Not | 0 (0.0%) | 3 (14.3%) | 18 (45.0%) | ||
| Low | 0 (0.0%) | 9 (42.9%) | 21 (52.5%) | ||
| Intermediate | 4 (6.5%) | 8 (38.1%) | 0 (0.0%) | ||
| High | 58 (93.5%) | 1 (4.8%) | 1 (2.5%) |
Notes: Median (interquartile range (IQR)) displayed for each demographic and pathologic variable. Kruskal-Wallis comparisons for continuous variables and chi-square goodness of fit test for the categorical variable performed across all groups: p-values are reported. In autopsy patients- Alzheimer’s disease neuropathologic change (ADNC) indicates severity of AD. Patients with intermediate or high levels of ADNC are considered positive for AD. Mini-mental state examination (MMSE) is a measure of global cognition- with higher scores indicating better cognitive function.
2.2. LC-MS/MS analysis
An internal standard consisting of a mixture of stable-isotope-labeled peptides was added (25 μL, 0.032 pmol/μL) to 100 μL of CSF. Reduction, alkylation, and tryptic digestion were performed, followed by solid-phase extraction for purification purposes (for detailed sample preparation, refer to [6]). A micro-high-performance liquid chromatography-mass-spectrometry system (6495 Triple Quadrupole LC/MS system, Agilent Technologies) equipped with a Hypersil Gold reversed-phase C18 column (dim.=100×2.1 mm, particle size=1.9 μm, Thermo Fisher Scientific) was utilized for quantitation, for detailed settings see Suppl. Table 1. Injections at regular intervals of two different quality control samples, consisting of pooled CSF samples, were used to monitor the performance of the assay over time.
2.3. Data processing and statistical analysis
For data analysis, including peak inspection and adjustment of the chromatographic spectra’s (Suppl. Fig. 1) Skyline 20.1 (MacCoss Lab Software) was used, and the relative peptide concentration was calculated using Suppl. Formula 1. R software (version 4.0.3) was used for statistical analysis and data visualization. Heatmap of the panel proteins was displayed by using the heatmap2 R package; synaptic proteins were grouped according to hierarchical clustering with Spearman’s correlation coefficient as distance. Demographic characteristics were evaluated by Kruskal-Wallis test and chi-square goodness of fit test for continuous and categorical variables, respectively (Table). Group-wise comparisons (HC, AD, FTLD, LBD) were assessed using rank-based ANCOVAs, including age and sex as covariates. Post-hoc analyses in autopsy-confirmed patients tested how two analytes of interest – 14-3-3 zeta/delta and beta-synuclein – differed by metrics AD pathological severity (ADNC, CERAD score, Braak stage); models included CSF to death interval and sex as covariates. All group comparisons were adjusted for multiple group comparisons with the false discovery rate approach. Associations between continuous variables were explored with Spearman rank correlation analysis. Receiver operating characteristic curve contrasted groups and provided the area under the curve (AUC) to evaluate the discriminatory ability of the biomarkers.
4. RESULTS
The analytical performance of the different proteins had a high precision within and between runs with a few exceptions (Suppl. Table. 2). For the proteins for which more than one peptide was analyzed, the peptide with the best analytical performance, in terms of repeatability and intermediate precision (lowest CV), was chosen for the statistical analysis. Analysis of 14-3-3 eta did not meet quality control standards and was excluded completely.
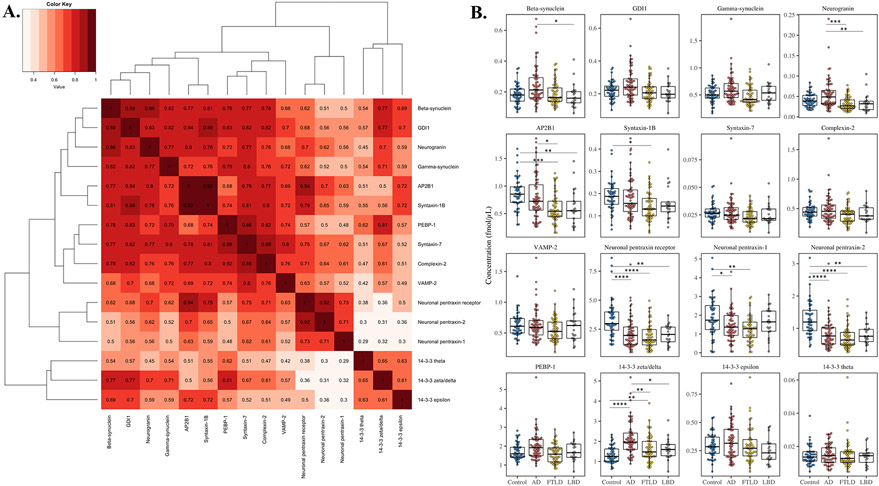
To investigate associations between the synaptic proteins, the cluster analysis (Fig. 1A) was performed, and it emerged that some of the measured proteins correlated strongly with each other (Spearman’s correlation coefficient (rho)>0.75, p≤0.0001). Additionally, we found that the levels of several of these proteins were altered in neurodegenerative patients compared with HC and each other, and four differential patterns emerged (Fig. 1B and Suppl. Table 3).
Figure 1.
(A) Hierarchical cluster analysis by using Spearman rank correlation coefficient as distance. From the cluster analysis it emerged that some of the measured proteins correlated strongly with each other. (B) MRM analysis of the synaptic panel proteins (one representative peptide for each protein) in the clinical sample consisting of healthy controls (HC, n=48), and pathology confirmed cases of Alzheimer’s disease (AD, n=63), Lewy body spectrum of disorders (LBD, n=21), and frontotemporal lobar degeneration (FTLD, n=53). Statistical comparison was performed with rank-based analyses of covariance, including age and sex as covariates, with p-value adjustment for multiple group comparisons. P-values: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 and **** p ≤ 0.0001.
First, increased neurogranin levels were found in AD compared with LBD (p=0.0074) and FTLD (p=0.00054) as well as increased beta-synuclein levels in AD compared with LBD (p=0.045). Taken together, these proteins seem to exhibit a pattern of AD-specific increase compared with other neurodegenerative diseases. In the cluster analysis, beta-synuclein and neurogranin were closely associated with each other as well as with gamma-synuclein and GDI1 (rho=0.77-0.89, p≤0.0001). However, despite a similar pattern, no changes were observed for GDI1 and gamma-synuclein.
Second, AP2B1 was found to have significantly decreased levels in FTLD compared with AD (p=0.039) and both LBD (p=0.0054) and FTLD (p=0.0017) compared with HC, but not AD. Similarly, syntaxin-1B was found to have decreased levels in FTLD compared with HC (p=0.014). Thus, AP2B1 and syntaxin-1B exhibited a pattern of non-AD-specific decrease compared with both HC and AD. AP2B1 and the syntaxin-1B correlated moderate-to-strongly with each other and with syntaxin-7, complexin-2, PEBP-1, and VAMP-2 (rho=0.68-0.92, p≤0.0001). However, syntaxin-7, complexin-2, PEBP-1, and VAMP-2 showed no group differences.
Third, neuronal pentraxin-2 and the receptor had decreased levels in AD (p≤0.0001), FTLD (p≤0.0001), and LBD (NPTX2; p=0.0012, NPTXR; p=0.0029) in comparison with HC. In the cluster analysis, neuronal pentraxin-2 and the receptor were found to be closely associated, and both also correlated well with neuronal pentraxin-1 (rho=0.71-0.92, p≤0.0001). Neuronal pentraxin-1, displayed decreased levels in AD (p=0.045) and FTLD (p=0.0051) compared to HC, but not in LBD. Together, the neuronal pentraxins exhibit a pattern of decreased levels of the same magnitude regardless of neurodegenerative disease.
Lastly, increased 14-3-3 zeta/delta levels were found in AD (p≤0.0001) and FTLD (p=0.0027) compared with HC, thus exhibiting a pattern of increased levels in neurodegenerative disease. Moreover, 14-3-3 zeta/delta was particularly elevated in AD, which also showed significantly increased levels in comparison to FTLD (p=0.0093) and LBD (p=0.013). 14-3-3 zeta/delta moderately correlated (rho=0.61-0.65, p≤0.0001) with 14-3-3 epsilon and theta, although neither showed significant differences between groups.
When splitting the LBD group based on the presence of concomitant AD, no difference was found for any of the synaptic proteins between the patients with or without concomitant AD (Suppl. Fig. 2). Of note, none of the peptide levels differed between LBD and FTLD; thus, they are combined into a “non-AD” group in subsequent analyses.
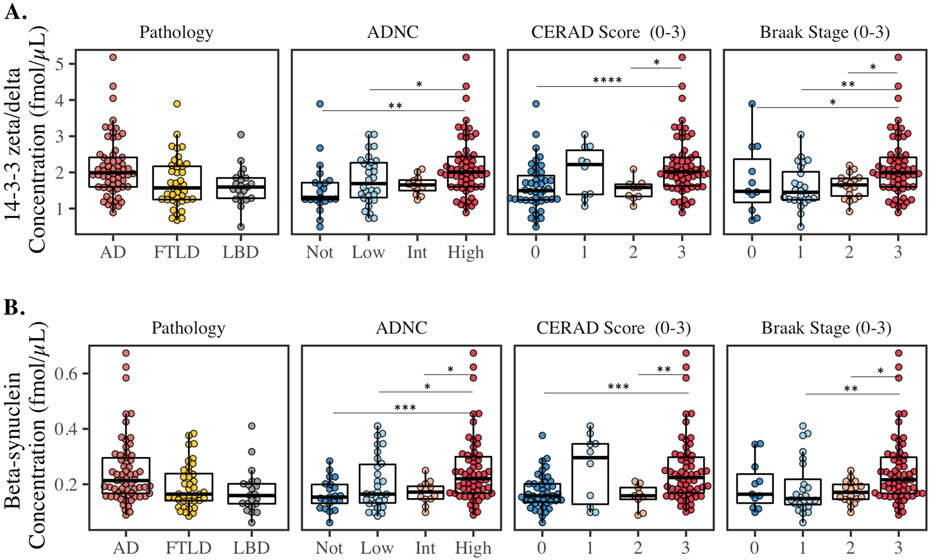
Post-hoc analyses examined the associations of the synaptic biomarkers with AD pathology (Fig. 2); 14-3-3 zeta/delta and beta-synuclein were chosen as representatives of the protein groupings, which showed elevated levels in AD (previously explored for neurogranin [25]). 14-3-3 zeta/delta and beta-synuclein were significantly increased for high ADNC compared to not and low (p<0.05), Braak stage 3 (widespread tau) compared to 1-2 (p<0.05), and CERAD score 3 (high plaque burden) compared to 0 and 2 (p<0.05).
Figure 2.
CSF concentrations of 14-3-3 zeta/delta, and beta-synuclein in the pathology confirmed cases of Alzheimer’s disease (AD, n=63), Lewy body spectrum of disorders (LBD, n=21), and frontotemporal lobar degeneration (FTLD, n=53). From left to right; the groups are based on primary pathology group, ADNC (Not, Low, Intermediate [Int], High), CERAD score, and Braak stage. Statistical comparison was performed with rank-based ANCOVA adjusted for interval from CSF to death and sex. P-values: * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 and **** P ≤ 0.0001.
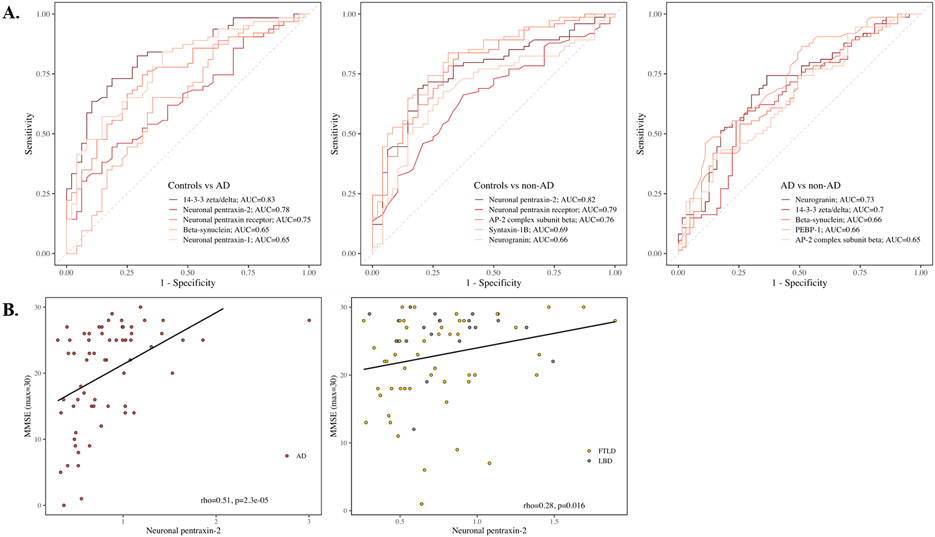
Next, ROC analyses tested how proteins discriminated AD and non-AD (Suppl. Table 4). 14-3-3 zeta/delta had the highest AUC (Fig. 3A, AUC=0.83, 95% confidence interval (CI95%)=0.75-0.90) for AD versus HC, closely followed by neuronal pentraxin-2 (AUC=0.78, CI95%=0.69-0.87). To discriminate non-AD from HC, neuronal pentraxin-2 had the highest AUC (AUC=0.82, CI95%=0.74-0.89), followed by neuronal pentraxin receptor (AUC = 0.79; 95% CI = 0.71 - 0.87). Performance by analytes was generally less robust when discriminating AD from non-AD, with neurogranin having the highest AUC (AUC=0.73, CI95%=0.65-0.82).
Figure 3.
(A) Receiver operating curves calculated for Alzheimer’s disease versus healthy controls, healthy controls vs non-Alzheimer’s disease, and Alzheimer’s disease versus non-Alzheimer’s disease for the five synaptic proteins with the highest area under the curve vales. (B) Association between neuronal pentraxin-2 and MMSE for Alzheimer’s disease and non-Alzheimer’s disease with Spearman rank correlation coefficient and p-value.
Because synaptic dysfunction has been linked to cognitive decline, we tested associations of synaptic proteins with MMSE. In AD, neuronal pentraxin-2 had the strongest correlation with MMSE (rho=0.51, p≤0.0001, Fig. 3B and Suppl. Table 5). Neuronal pentraxin-2 also had the strongest correlation with MMSE in the non-AD group (rho=0.28, p=0.016). Furthermore, we tested associations between synaptic proteins and core AD biomarkers Aβ1-42, t-tau, and p-tau181 (Suppl. Table 5). Within AD, all synaptic proteins correlated weak-to-strongly with t-tau (rho=0.34-0.81, p≤0.01) and weak-to-moderately with p-tau181 (rho=0.25-0.51, p≤0.05, except 14-3-3 theta), but not with Aβ1-42.
In the non-AD group, all synaptic proteins correlated weakly with p-tau181 (rho=0.24-0.48, p≤0.05, except 14-3-3 epsilon), as well as weak-to-moderately with t-tau (rho=0.30-0.71, p≤0.05, except 14-3-3 theta). Weak correlations with Aβ1-42 (rho=0.24-0.28, p≤0.05) were found for beta-synuclein, complexin-2, syntaxin-1B, AP2B1, and neuronal pentraxin receptor in the non-AD group.
5. DISCUSSION
There is a need in clinical practice and trials to implement biomarkers that can reflect synaptic pathology, not only as early indicators of AD, but also to predict cognitive decline, monitor synaptic health, and facilitate differential diagnosis. In our previous study of the synaptic panel, we found that levels of beta-synuclein, gamma-synuclein, neurogranin, PEBP-1, and 14-3-3 proteins were increased while levels of the neuronal pentraxins were decreased in AD compared with HC [6] and identified these proteins as potential synaptic pathology biomarkers for AD. In the current study, we found four distinct biomarker patterns while validating this panel of synaptic proteins in an independent autopsy-confirmed sample of AD and expanding on our previous work to include two other neurodegenerative diseases: LBD and FTLD.
The first pattern was specifically increased CSF levels in AD compared to other neurodegenerative diseases, represented by beta-synuclein and neurogranin. Beta-synuclein, together with gamma-synuclein, is part of the presynaptic synuclein family also containing alpha-synuclein. Together, the synucleins have all been widely associated with neurodegenerative diseases [26]. Especially alpha-synuclein, which is well-researched due to it being a major component of Lewy bodies as well as amyloid plaques [27, 28]. The synucleins have been found to have increased CSF concentrations in AD compared with controls [29], and more recently, this pattern has also been observed for serum beta-synuclein [30]. In corroboration of our findings of AD-specific changes of beta-synuclein, previous work also finds no difference in CSF in PD and in amyotrophic lateral sclerosis compared with non-neurodegenerative controls [31]. In general, the measured panel of proteins seems to have a better ability to discriminate HC from non-AD or AD patients than to discriminate non-AD from AD. One of the exceptions seems to be neurogranin, which is a postsynaptic protein involved in the regulation of calmodulin and, consequently calcium-mediated signaling pathways [15]. Both ELISAs and mass spectrometric methods have repeatedly found increased levels of neurogranin in AD in comparison with controls [15, 32], and also several meta-analyses have confirmed this [32, 33]. We detected no significant difference when comparing AD with HC; however, we did observe significantly increased neurogranin CSF levels in AD compared to other neurodegenerative diseases (non-AD). In fact, supporting our results, neurogranin has previously been reported to be specifically increased in AD not only in comparison with HC but also to other neurodegenerative diseases [25]. To summarize, we have found novel increased levels of beta-synuclein and neurogranin in AD in comparison with non-AD. However, we were not able to validate our earlier findings of increased levels in AD compared with HC for neurogranin and the synucleins [6], possibly due to rigorous correction for multiple comparisons across multiple disease groups. We additionally observed novel findings of unchanged levels in non-AD neurodegenerative diseases for these proteins in comparison with HC. Neurogranin and beta-synuclein should therefore be studied further to discern their potential as possible diagnostic biomarkers of AD and how they are specifically associated with AD, in order to cast light on possible AD specific mechanisms.
The second pattern is represented by AP2B1 and syntaxin-1B, which have significantly decreased levels in non-AD compared with HC, but no change in AD. Syntaxin-1B is involved in vesicle exocytosis at the synapse and, consequently, neurotransmitter release as a SNARE protein [34]. AP2B1 belongs to a family of adaptor proteins involved in mediating endocytosis by linking clathrin to the plasma membrane [35]. This novel pattern is particularly interesting since these proteins do not show a significant difference between AD and HC, as in past findings [6], but show significantly decreased levels in FTLD and LBD (only AP2B1) compared with HC. For AP2B1, this is also corroborated by Sjödin et.al., finding no difference in AD but decreased levels in PD [36]. Additionally, decreased levels in LBD compared with controls have also been previously reported [37]. Endocytic impairment has been implicated to be a feature of many neurodegenerative diseases, not the least in AD [38]. However, we show that endocytic impairment, reflected by AP2B1 CSF levels, seems to be a more prominent feature of FTLD and LBD pathology than of AD. The similar findings for the CSF levels of syntaxin-1B might hence also be indicative of specific impairment of synaptic transport processes in FTLD pathology. The reason for the specific change in non-AD but not in AD compared with HC is certainly interesting but remains elusive. Further studies of AP2B1 and syntaxin-1B should investigate this in-depth and also include a wider range of neurodegenerative diseases to discern the specificity of the changes.
The third pattern is decreased CSF levels in all explored diseases (AD, FTLD, and LBD), represented by the neuronal pentraxins. The neuronal pentraxins are proposed to be involved in the modulation of synaptic plasticity by AMPA-type glutamate receptors recruitment during exocytosis [39, 40]. The interest in the pentraxin family in the context of neurodegenerative diseases has increased recently since several studies have reported reduced CSF levels of the neuronal pentraxins in AD [8-12, 41], DLB [42], and FTLD [43] compared with controls. Furthermore, the levels have been found to constantly decrease from cognitively normal controls to MCI and lastly to AD [8]. Interestingly, in the present study, neuronal pentraxin-2 is the best correlate with MMSE in AD, corroborated by several studies [6, 8], and provides one of the best separations (AUC=0.78) between AD and HC of all panel proteins. The pentraxin levels also correlate with MMSE in non-AD patients (LBD and FTLD) and are all decreased in FTLD and LBD pathologies compared with HC. In fact, neuronal pentraxin-2 of all panel proteins shows the best separation (AUC=0.82) between non-AD and HC. Thus, the neuronal pentraxins may be potential monitoring biomarkers of general cognitive decline across neurodegenerative diseases, which should be further explored.
The last distinct pattern with higher CSF protein levels across all the investigated patient groups compared with HC was found for 14-3-3 protein zeta/delta, which belongs to a synapse enriched seven protein family [13]. The protein family has been associated with wide modulation abilities and a high number of binding partners and is consequently implicated in a number of neuronal functions. At the synapse, they regulate transmission and plasticity; however, their functions are still largely unknown in detail. The protein family are established biomarkers of Creutzfeldt-Jakob disease but are also associated with other neurodegenerative diseases [13]. In relation to AD, 14-3-3 proteins have been discovered to both colocalize in neurofibrillary tangles and interact with the key AD pathology protein tau [44, 45]. They have similarly been found to be present in Lewy bodies [46]. To our knowledge, this is the first targeted CSF method to be used in the study of zeta/delta in neurological disease, even though several exploratory proteomics studies have suggested not only zeta/delta but also the other proteins in the family as potential biomarkers [47, 48]. In the current study, 14-3-3 protein zeta/delta concentration was higher in the CSF of AD patients compared with HC, with the best separation of all the biomarkers (AUC=0.83), while the rest of the 14-3-3 protein family members did not show any notable differences between any of the groups. 14-3-3 zeta/delta having the highest diagnostic potential of all synaptic proteins is corroborated by earlier exploratory data [7], and it has been implicated to have the strongest connection with tau and its phosphorylation of all the 14-3-3s [49]. Increased 14-3-3 zeta/delta levels were also observed in FTLD patients compared with HC. However, 14-3-3 zeta/delta seems to have a stronger association with AD pathology, with higher levels in AD than in FTLD and LBD. Thus, 14-3-3 zeta/delta separated AD from non-AD (AUC=0.70) almost as well as neurogranin (AUC=0.73). We hence validate our previous findings that 14-3-3 zeta/delta is particularly increased in AD [6] and observe novel findings that the protein is also increased in non-AD albeit not to the same degree. This indicates that 14-3-3 zeta/delta may be a general indicator of neurodegeneration and or cognitive decline across neuropathology, particularly affected by AD pathology.
Lastly, VAMP-2, PEBP-1, GDI1, syntaxin-7, and complexin-2 showed no significant differential patterns, which replicates our earlier findings [6], with the exception of PEBP-1. These proteins seem accordingly to not be potential biomarkers for AD, FTLD, or LBD but should be confirmed in additional studies and possibly explored in other neurodegenerative diseases.
Two major strengths of this study were the use of gold-standard autopsy-confirmed pathology and the use of multiplexed mass spectrometry. The method allows for quantification in a small sample volume with high specificity of a range of biomarkers with diverse functions and localizations and has the ability to possibly discover, distinguish and differentiate among general and specific pathological patterns. When the aim is to differentiate a range of neurodegenerative diseases and track disease progression, this is especially important due to the high complexity and pathological heterogeneity. Thus, unbiased mass spectrometry studies are an important step to biomarker discovery. However, these kinds of multiplex assays also carry the limitation of an increased analytical challenge due to the nonspecific sample preparation, which leads to a relatively broad concentration range and substantial general protein background. Several steps are needed to translate our findings here from bench to bedside [50, 51]. Foremost, a challenge of future work is the “cross-technology translation gap” [50] and the need to bridge mass spectrometry findings to immunoassay to be implemented in a clinical context. Further analytical validation in clinical cohorts will be necessary, and histopathological studies may be needed to understand the mechanism underlying the differences in synaptic protein patterns we observed between AD and FTLD/LBD. Another caveat to consider when interpreting our findings is that mixed pathology in all neurodegenerative diseases is common. Indeed, 1 FTLD patient and 9 LBD patients had co-occurring AD pathology. While we did not see significant differences in analyte levels across LBD with and without AD, small sample sizes may preclude sufficient power to detect differences. Future work should further explore how co-pathologies might interact with synaptic CSF levels that we observed here. Additionally, while a strength of this study is the assessment of biomarkers across multiple neurodegenerative diseases, in this clinically and pathologically heterogeneous sample, we lacked a global measure of disease stage to be applied in the primary analyses. However, analyses within autopsy-confirmed patients included adjustment for CSF-to-death interval to help account for differences in disease severity at CSF collection and the lag to pathological assessment. Finally, while we studied CSF analytes in rare patients with known pathology, our groups were small, and we performed rigorous correction for multiple comparisons. Comparative studies are incredibly important for differential diagnosis, and we were able to identify a pattern of selective change in some analytes; however, additional work is needed to confirm our observations in larger sample sets as well as in longitudinal studies to explore the biomarker changes over time.
6. CONCLUSION
The present study validated our previously published results that several of the synaptic proteins of our in-house mass spectrometric panel have the potential to be synaptic degeneration biomarkers in AD. We also find evidence that several of the proteins were also altered in the other neurodegenerative diseases: FTLD and LBD. Together, our results indicate differential patterns of synaptic protein alterations across neurodegenerative diseases. Thus, the synaptic panel proteins not only show promise as possible complements to other CSF and imaging markers to guide diagnostics, as prognostics, stage biomarkers or track cognitive decline, but may also give invaluable mechanistic input into the complex overlapping neuropathologies in neurodegenerative diseases and their differential impacts on synaptic function.
Supplementary Material
Highlights.
Panel of synaptic proteins quantified in the CSF using mass spectrometry
Compared Alzheimer’s disease, frontotemporal degeneration, and Lewy body spectrum of disorders
Pathology was confirmed by autopsy or familial mutations
Synaptic biomarkers for synaptic degeneration and cognitive decline
Differential patterns of synaptic proteins across neurodegenerative diseases
RESEARCH IN CONTEXT.
Systematic review: In the available scientific literature, numerous synaptic proteins have been identified as candidate biomarkers of synaptic degeneration. However, the validation of synaptic proteins across neurodegenerative diseases is needed to improve our understanding of both synaptic pathology and their potential as biomarkers of cognitive decline. We perform comparisons of a panel of synaptic proteins in rare pathology-confirmed cases of AD, LBD, and FTLD.
Interpretation: Our findings identify several synaptic dysfunction biomarkers for AD, LBD, and FTLD that could be utilized as possible prognostic and diagnostic biomarkers. We show differential patterns of synaptic protein alterations, both general to neurodegenerative disease and specific to AD. These findings have implications of overlapping and distinct pathological features of the synapse in AD, LBD, and FTLD.
Future directions: Further studies in larger cohorts will be needed to validate the specificity of the biomarkers and the differential patterns presented herein.
ACKNOWLEDGEMENTS
This work was supported by the Eivind and Elsa K: Son Sylvan Foundation, the Märta and Gustaf Ågren Foundation, the Herbert and Karin Jacobsson Foundation, the Gun and Bertil Stohne Foundation, the Foundation for Gamla Tjänarinnor, the Felix Neubergh Foundation, Demensfonden, Rune and Ulla Almlöv Foundation, an anonymous donor and NIH (AG066597, AG054529). KAQC is supported by the Alzheimer's Association (AARF-D-619473, AARF-D-619473-RAPID), P30 AG072979 and the FTLD PPG clinical core AG066597. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden(#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), European UADRCnion Joint Program for Neurodegenerative Disorders (JPND2021-00694), and the UK Dementia Research Institute at UCL. KB is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986), European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), and the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495). JG is supported by Alzheimerfonden (AF-930934) and the Foundation of Gamla Tjänarinnor. AB is supported by European Union Joint Program for Neurodegenerative Disorders (“PreSSAD” JPND2021-650-272). LMS receives support from the NIA: P30 AG010124, U19 AG024904. MG is supported by NIH (AG066597, AG054519, AG062418), The Samuel Newhouse Foundation, The Robinson Family Foundation, and the Peisach Family Foundation.
ABBREVIATIONS
- AP2B1
adaptor related protein complex 2 subunit beta 1
- AD
Alzheimer’s disease
- AUC
area under the curve
- Aβ1-42
Aβ peptide 1-42
- CI95%
95% confidence interval
- CJD
Creutzfeldt-Jakob disease
- CSF
cerebrospinal fluid
- CV
coefficient of variation
- FTLD
Frontotemporal lobar degeneration
- FTD
Frontotemporal dementia
- HC
Healthy controls
- LBD
Lewy body spectrum of disorders
- LC-MS
liquid chromatography-mass spectrometry
- MCI
mild cognitive impairment
- MMSE
mini-mental state exam
- MRM
multiple reaction monitoring
- PEBP-1
phosphatidylethanolamine-binding protein 1
- P-tau181
tau phosphorylated at Thr181
- QC
quality control
- GDI1
rab GDP dissociation inhibitor alpha
- ROC
receiver operating characteristic curve
- rho
Spearman’s correlation coefficient
- SNAP-25
synaptosomal-associated protein 25
- T-tau
total tau
- VAMP-2
vesicle-associated membrane protein 2
- ADNC
AD neuropathologic change
- GAP-43
Growth Associated Protein 43
- PD
Parkinson’s disease
- PET
Positron emission tomography
Footnotes
DISCLOSURES
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. LMS has served on scientific advisory boards and/or as a consultant for Biogen, Roche Diagnostics, Fujirebio, Siemens and Diadem and has given lectures for Biogen, Roche, and Fujirebio. The rest of the authors have no disclosures to report.
REFERENCES
- 1.Camporesi E, et al. , Fluid Biomarkers for Synaptic Dysfunction and Loss. Biomarker Insights, 2020. 15: p. 1177271920950319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terry RD, et al. , Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 1991. 30(4): p. 572–580. [DOI] [PubMed] [Google Scholar]
- 3.Overk CR and Masliah E, Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochemical pharmacology, 2014. 88(4): p. 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber N, et al. , Deficient neurotransmitter systems and synaptic function in frontotemporal lobar degeneration—Insights into disease mechanisms and current therapeutic approaches. Molecular psychiatry, 2021: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidsson P, Puchades M, and Blennow K, Identification of synaptic vesicle, pre- and postsynaptic proteins in human cerebrospinal fluid using liquid-phase isoelectric focusing. Electrophoresis, 1999. 20(3): p. 431–437. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson J, et al. , Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer's disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring, 2021. 13(1): p. e12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tijms BM, Gobom J, Reus L, Jansen I, Hong S, Dobricic V, … & Visser PJ (2020). Pathophysiological subtypes of Alzheimer’s disease based on cerebrospinal fluid proteomics. Brain, 143(12), 3776–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galasko D, et al. , Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer's disease. Alzheimers Dement (N Y), 2019. 5: p. 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellman DS, et al. , Development and evaluation of a multiplexed mass spectrometry based assay for measuring candidate peptide biomarkers in Alzheimer's Disease Neuroimaging Initiative (ADNI) CSF. Proteomics Clin Appl, 2015. 9(7-8): p. 715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson A, Willette A, and A.s.D.N. Initiative, Neuronal pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer’s disease spectrum. Brain, behavior, and immunity, 2016. 58: p. 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begcevic I, et al. , Neuronal pentraxin receptor-1 is a new cerebrospinal fluid biomarker of Alzheimer's disease progression. F1000Res, 2018. 7: p. 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim B, et al. , Cerebrospinal fluid neuronal pentraxin receptor as a biomarker of long-term progression of Alzheimer’s disease: a 24-month follow-up study. Neurobiology of Aging, 2020. [DOI] [PubMed] [Google Scholar]
- 13.Foote M and Zhou Y, 14-3-3 proteins in neurological disorders. International journal of biochemistry and molecular biology, 2012. 3(2): p. 152. [PMC free article] [PubMed] [Google Scholar]
- 14.Guadagno NA and Progida C, Rab GTPases: switching to human diseases. Cells, 2019. 8(8): p. 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvartsberg H, et al. , Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer's disease, in Alzheimer's & Dementia. 2015. p. 1180–1190. [DOI] [PubMed] [Google Scholar]
- 16.Honer WG, et al. , The synaptic pathology of cognitive life. Dialogues in clinical neuroscience, 2019. 21(3): p. 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoentgen F and Jonic S, PEBP1/RKIP behavior: A mirror of actin-membrane organization. Cellular and Molecular Life Sciences, 2020. 77(5): p. 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson PT, Fardo DW, and Katsumata Y, The MUC6/AP2A2 Locus and Its Relevance to Alzheimer’s Disease: A Review. Journal of Neuropathology & Experimental Neurology, 2020. 79(6): p. 568–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woollacott IO and Rohrer JD, The clinical spectrum of sporadic and familial forms of frontotemporal dementia. Journal of Neurochemistry, 2016. 138: p. 6–31. [DOI] [PubMed] [Google Scholar]
- 20.Montine TJ, et al. , National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta neuropathologica, 2012. 123(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeith IG, et al. , Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology, 2005. 65(12): p. 1863–1872. [DOI] [PubMed] [Google Scholar]
- 22.Mackenzie IR, et al. , Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta neuropathologica, 2010. 119(1): p. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LM, et al. , Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta neuropathologica, 2011. 121(5): p. 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roalf DR, et al. , Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimer's & Dementia, 2013. 9(5): p. 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portelius E, et al. , Cerebrospinal fluid neurogranin concentration in neurodegeneration: relation to clinical phenotypes and neuropathology. Acta Neuropathol, 2018. 136(3): p. 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brás J, Gibbons E, and Guerreiro R, Genetics of synucleins in neurodegenerative diseases. Acta Neuropathologica, 2021. 141(4): p. 471–490. [DOI] [PubMed] [Google Scholar]
- 27.Burré J, The synaptic function of α-synuclein. Journal of Parkinson's disease, 2015. 5(4): p. 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uéda K, et al. , Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proceedings of the National Academy of Sciences, 1993. 90(23): p. 11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oeckl P, et al. , Alpha-, Beta-, and Gamma-synuclein Quantification in Cerebrospinal Fluid by Multiple Reaction Monitoring Reveals Increased Concentrations in Alzheimer′ s and Creutzfeldt-Jakob Disease but No Alteration in Synucleinopathies. Molecular & Cellular Proteomics, 2016. 15(10): p. 3126–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oeckl P, et al. , Targeted mass spectrometry suggests beta-synuclein as synaptic blood marker in Alzheimer’s disease. Journal of proteome research, 2020. 19(3): p. 1310–1318. [DOI] [PubMed] [Google Scholar]
- 31.Halbgebauer S, et al. , Beta-synuclein in cerebrospinal fluid as an early diagnostic marker of Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 2021. 92(4): p. 349–356. [DOI] [PubMed] [Google Scholar]
- 32.Mavroudis IA, et al. , A meta-analysis on CSF neurogranin levels for the diagnosis of Alzheimer's disease and mild cognitive impairment. Aging Clin Exp Res, 2019. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, et al. , Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer’s disease and mild cognitive impairment. Translational psychiatry, 2020. 10(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng FYH, Wang Y, and Tang BL, The syntaxins. Genome biology, 2001. 2(11): p. reviews3012. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royle SJ, The cellular functions of clathrin. Cellular and Molecular Life Sciences CMLS, 2006. 63(16): p. 1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjödin S, et al. , Endo-lysosomal proteins and ubiquitin CSF concentrations in Alzheimer’s and Parkinson’s disease. Alzheimer's research & therapy, 2019. 11(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerche S, et al. , CSF protein level of neurotransmitter secretion, synaptic plasticity, and autophagy in PD and DLB. Movement Disorders, 2021. 36(11): p. 2595–2604. [DOI] [PubMed] [Google Scholar]
- 38.Root J, et al. , Lysosome dysfunction as a cause of neurodegenerative diseases: Lessons from frontotemporal dementia and amyotrophic lateral sclerosis. Neurobiology of disease, 2021. 154: p. 105360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu D, et al. , Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron, 2003. 39(3): p. 513–528. [DOI] [PubMed] [Google Scholar]
- 40.Lee S-J, et al. , Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. Journal of Neuroscience, 2017. 37(5): p. 1062–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libiger O, et al. , Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer's disease. Alzheimer's & Dementia, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boiten WA, et al. , Pathologically decreased CSF levels of synaptic marker NPTX2 in DLB are correlated with levels of alpha-synuclein and VGF. Cells, 2021. 10(1): p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergström S, et al. , A panel of CSF proteins separates genetic frontotemporal dementia from presymptomatic mutation carriers: a GENFI study. Molecular neurodegeneration, 2021. 16(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umahara T, et al. , 14-3-3 proteins and zeta isoform containing neurofibrillary tangles in patients with Alzheimer’s disease. Acta neuropathologica, 2004. 108(4): p. 279–286. [DOI] [PubMed] [Google Scholar]
- 45.Drummond E, et al. , Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain, 2020. 143(9): p. 2803–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giusto E, et al. , Pathways to Parkinson’s disease: a spotlight on 14-3-3 proteins. npj Parkinson's Disease, 2021. 7(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke MTM, et al. , CSF synaptic protein concentrations are raised in those with atypical Alzheimer's disease but not frontotemporal dementia. Alzheimers Res Ther, 2019. 11(1): p. 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sathe G, et al. , Quantitative Proteomic Profiling of Cerebrospinal Fluid to Identify Candidate Biomarkers for Alzheimer's Disease. Proteomics Clin Appl, 2019. 13(4): p. e1800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pair FS and Yacoubian TA, 14-3-3 Proteins: Novel Pharmacological Targets in Neurodegenerative Diseases. Trends in Pharmacological Sciences, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teunissen CE, et al. , White paper by the Society for CSF Analysis and Clinical Neurochemistry: Overcoming barriers in biomarker development and clinical translation. Alzheimer's research & therapy, 2018. 10(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agrawal I, Tripathi P, and Biswas S, Mass spectrometry based protein biomarkers and drug target discovery and clinical diagnosis in Age-Related progressing neurodegenerative diseases. Drug Metabolism Reviews, 2022. 54(1): p. 22–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.