Abstract
OBJECTIVE:
Endoscopic sleeve gastroplasty (ESG) has gained global adoption but our understanding of its mechanism(s) of action and durability of efficacy is limited. We sought to determine changes in gastric emptying (GE), gastric motility (GM), hormones, and eating behaviors after ESG.
DESIGN:
A priori-designed single-center substudy of a large U.S. randomized clinical trial, adults with obesity were randomized to ESG or lifestyle interventions (LS) alone. We measured GE, hormones, and weight loss and assessed eating behaviors. In a subset of ESG patients, we assessed GM. The primary outcome was the change in T1/2 (minutes) at 3 months, and secondary outcomes were changes in weight, GE, GM, hormones, and eating behaviors. We used t-test analyses and regression to determine the association between GE and weight loss.
RESULTS:
36 (ESG=18; LS=18) participated in this substudy. Baseline characteristics were similar between the two groups. At 3 months, T1/2 was delayed in the ESG group (n=17) compared to the LS group (n=17) (152.3 ± 47.3 vs. 89.1 ±27.9; p<0.001). At 12 months, T1/2 remained delayed in the ESG group (n=16) vs. control group (n=14) (137 ± 37.4 vs. 90.1 ± 23.4; p<0.001). Greater delays in GE at 3 months were associated with greater weight loss. Gastric motility was preserved and fasting ghrelin, GLP-1, and PYY significantly increased 18 months after ESG.
CONCLUSION:
ESG promotes weight loss through several key mechanistic pathways involving gastric emptying and hormones while preserving gastric motility. These findings further support clinical adoption of this technique for the management of obesity.
Keywords: ESG, Obesity, Gastric Emptying, Hormones
INTRODUCTION
Endoscopic sleeve gastroplasty (ESG) has gained global popularity over the past decade due to increasing evidence surrounding the procedure’s safety and efficacy for the treatment of obesity. Data from over 1600+ procedures have been reported worldwide in both academic and community settings, all with varying degrees of success 1, 2. The procedure has been compared to intensive lifestyle interventions 3, other endoscopic bariatric therapies 4–6 and surgical sleeve gastrectomy7–10 showing this non-surgical weight loss procedure is closing the therapeutic gap in the management of obesity11. With a clinically significant 16% total body weight loss (%TBWL) over 18 months and a serious adverse event rate of around 1%, the procedure has also been used in children and adolescents with success12 and has shown improvement in obesity-related complications13. The procedure is thought to produce weight loss by reducing the stomach’s capacity by approximately 80%, and by altering gastrointestinal physiology through delayed gastric emptying at 3 months 14. The mechanism(s) of delayed gastric emptying (GE) after ESG are unknown. Similarly, intragastric balloons (IGBs) have been shown to produce weight loss through changes in GE, and selecting patients based on their baseline physiology shows promise 15, 16. The effect of IGBs on GE is however dependent on device indwelling time and whether the ESG produces long-term changes in GE, alterations in gastric motility (GM) and hormones remains to be investigated.
Thus, our major aim was to determine the changes in GE with the ESG versus lifestyle changes at 3 and 12 months. We also investigated the changes in eating behaviors at 6 months, changes in obesity-related hormones, ghrelin, polypeptide YY (PYY), and Glucagon Like-Peptide 1 (GLP-1) at 12 months, changes in gastric accommodation, and changes in gastric motility at 3 and 12 months. Finally, we investigated whether the change in gastric emptying was predictive of weight loss. We hypothesized that the ESG would delay gastric emptying throughout time, the delay would correlate with weight loss, and that the delay would lead to minimal to no changes in ghrelin, PYY, and GLP-1 despite weight loss.
MATERIALS AND METHODS
Ethical Approval and Patient Involvement
The study was approved by Mayo Clinic Institutional Review Board on 1/19/2018 (IRB: 17-008902). Written informed consent was received from participants before inclusion in the study. Patients were not involved in the design, conduct, reporting, or dissemination plans of this research study. This was a sub-study of a larger registered randomized prospective clinical trial (ClinicalTrials.gov: NCT03406975). The authors had access to the study data and reviewed and approved the final manuscript.
Study Design
In a priori-designed single-center nested sub-study of ag multicenter U.S. randomized, controlled clinical trial (MERIT trial), we evaluated the effects of the ESG versus moderate-intensity lifestyle interventions on gastric emptying of solids, weight loss, gastrointestinal hormones, and eating habits.17 The main study participants were allocated in a 1:1.5 fashion using computer-generated variable block randomization stratified by hypertension and type 2 diabetes. Allocation was not concealed. Personnel analyzing the primary outcome of gastric emptying and hormone assays were blinded as to their group assignment. Gastric emptying was measured using an FDA-approved 4-hour solid meal breath test (GEBT) at baseline, 3 and 12 months into the study. 18 Weight loss was reported as percent total body weight lost (%TBWL) at 3, 6, and 12 months. Fasting levels of ghrelin, GLP-1, and PYY were analyzed from plasma samples at baseline, and at 12 months. Eating habits were assessed using the three-factor eating questionnaire at baseline and 6 months. (Figure 1) In a subset of participants, gastric motility and accommodation was assessed with magnetic resonance imaging (MRI) at baseline, 3, and 12 months after ESG.
Figure 1:
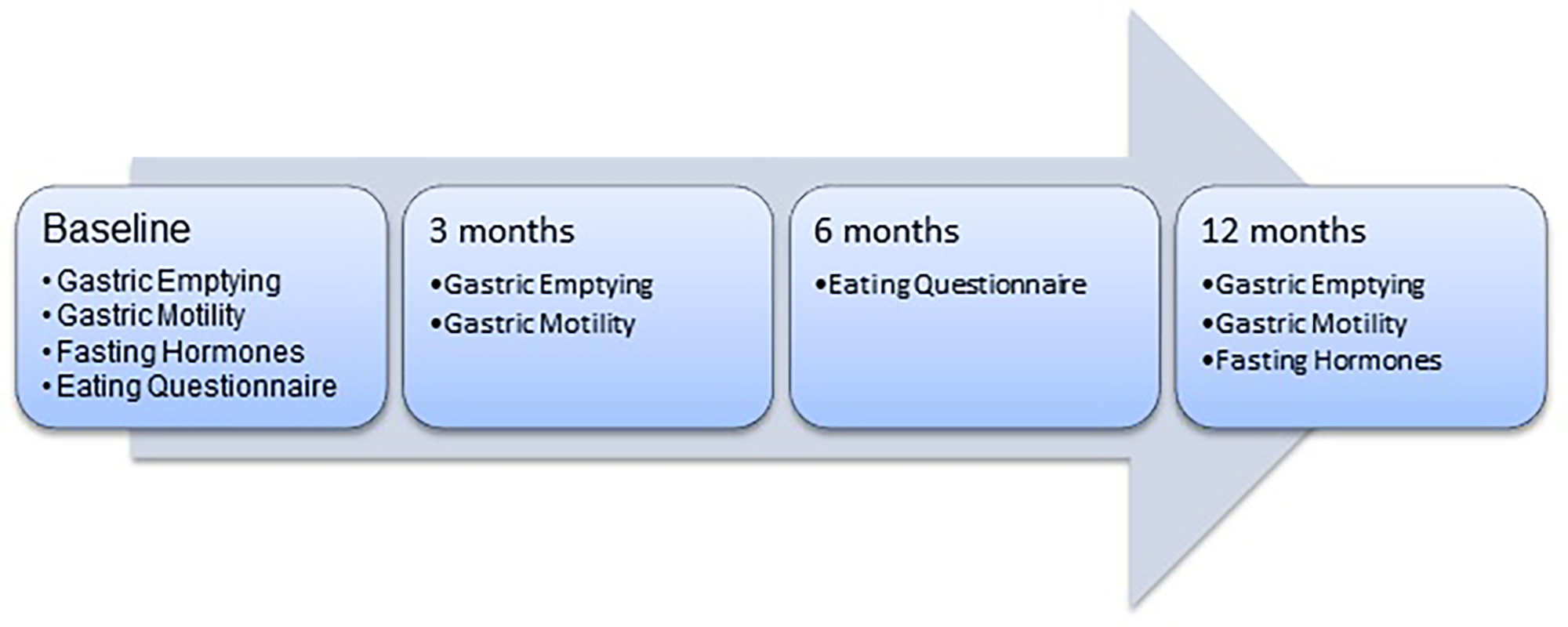
Study Diagram
Participants
Adults aged 21–65 years, with body mass index (BMI) between 30–40 kg/m2 who were enrolled in the main study were invited to participate in this ancillary study. Inclusion criteria included willingness to comply with the lifelong dietary restrictions required by the procedure, history of failure with non-surgical weight loss methods, and willingness to follow protocol requirements including follow-up schedule, laboratory testing, and dietary counseling. Exclusion criteria included prior history of esophageal, bariatric or gastrointestinal surgery (except uncomplicated cholecystectomy or appendectomy), or known abdominal adhesions, inflammatory bowel disease involving the upper gastrointestinal tract, known esophageal, gastric, or severe colonic motility disorders, esophageal strictures, large gastric polyps, ongoing anti-coagulation or anti-thrombotic medication use, insulin-dependent diabetes, A1c > 8.99, uncontrolled hypertension, concomitant anti-obesity medications use, eating disorders, monogenic obesity disorders, endocrine disorders affecting body weight such as Cushing’s or uncontrolled hypothyroidism, pregnancy, breastfeeding and intolerance to Spirulina or dairy. Further details about eligibility are provided on ClinicalTrials.gov: NCT03406975
Interventions
Active Control Group
Study subjects randomized to the active control group received a standardized moderate-intensity lifestyle intervention treatment (12 visits) over 12 months starting on the day of randomization. A reduced-calorie diet recommended by a registered dietician was reviewed and adjusted based on each participant’s needs conducive to weight loss. Physical activity, including 150 minutes of aerobic exercise per week was encouraged.
Treatment Group (ESG)
The ESG procedure was performed by a single endoscopist (BKA) under general anesthesia using a commercially available, FDA-approved, full-thickness endoscopic suturing device (Overstitch; Apollo Endosurgery, Austin, TX, USA) mounted on a double channel therapeutic Olympus endoscope. The procedure was accomplished by placing a series of endolumenally placed full-thickness sutures along the greater curvature of the stomach from the level of the incisura to the level of the gastroesophageal junction, creating a restrictive “sleeve” similar to the surgical sleeve gastrectomy, without removing blood supply or stomach tissue. Procedural details have been previously published. (Supplementary Figure 1)
Post-procedure, the ESG group followed a 6-week transitional diet consisting of 4 weeks of full liquid protein shakes and 2 weeks of a soft pureed diet. The ESG group followed the same moderate intensity lifestyle interventions otherwise as the active control group, with 12 visits over 12 months with the personalized diet and physical activity encouragement.
Gastric Emptying
The gastric emptying rate of solid food was calculated using the FDA-approved breath-based test (GEBT; Cairn Diagnostics ™, Brentwood, TN, USA). The test has been previously validated against the gold standard gastric scintigraphy to measure gastric emptying with a linear concordance of over 95%. 18 The test uses pharmaceutical grade Spirulina, an algae-based nutritional supplement, enriched with a nonradioactive form of carbon, carbon-13 (13C) that can be measured in breath samples. The meal consists of approximately two eggs containing 13C enriched Spirulina, six saltine crackers, and 6 fl oz of water. The meal was prepared in the Clinical Research and Trials Unit (CRTU) in a standard 1100-watt microwave using the standard instructions by trained personnel.
The time for 50% of stomach contents to empty (T1/2), percent emptied at 2 and 4 hours was calculated for each subject. The gastric emptying rate was assessed at baseline, 3 and 12 months into the study.
Eating Behaviors
Subjects’ eating behaviors were assessed at baseline, before randomization, using the validated Three-Factor Eating Questionnaire (TFEQ-18).19, 20 The self-assessment scale is widely used to assess eating behaviors across excess weight and normal-weight individuals in weight loss studies. It measures eating behaviors across three domains: cognitive restraint (CR), uncontrolled eating (UE), and emotional eating (EE). The questionnaire is comprised of 18 questions, with most answers coded on a four-point scale (1–4), with higher values indicating more of that behavior. Higher CR [range 6–28] means higher restraint, whereas higher UE [range 9–36] or EE [range 3–12] indicate more uncontrolled eating or emotional eating, respectively. The subjects’ eating behaviors were re-assessed 6 months into the study.
Hormone Assays
After an overnight fast, subjects were provided plasma samples as part of the main study for hormone analysis. Total ghrelin, glucagon-like peptide-1 (GLP-1), and PYY were measured at baseline and 12 months into the study. In a post hoc fashion, we also measured hormones at 18 months. We discontinued all the drugs that interfered with hormone measurements 24 h before the analysis. All samples were analyzed at our Immunochemical Core Laboratory (ICL) at Mayo Clinic, Rochester. Information on hormone assay methodology can be found in the supplementary materials.
Gastric motility
Using an established technique21, postprandial gastric motility was visualized at three contiguous 10 mm thick slices oriented in an oblique coronal plane through the antrum with a 2D FISP (FIESTA) sequence (TE 1.8 ms, TR 3.8 ms, a fractional field of view of 40 × 32 cm, and an acquisition matrix of 256 × 192). At each location, 80 images were acquired over 60 s at approximately 15 and 30 minutes after a meal. During imaging, patients were instructed to hold their breath as long as possible and then perform shallow breathing. Images from the slice that encompassed the maximum extent of the antrum and the body were analyzed with ANALYZE software algorithms (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN, USA). After manually demarcating the outer stomach contour, the stomach was segmented. After drawing a line through the gastric longitudinal axis which terminated at the distal boundary of the contrast-filled stomach, gastric cross-sectional diameters were measured at planes perpendicular to this longitudinal axis at one-pixel intervals. Using MATLAB (MathWorks, Natick, MA, USA), these diameters were processed by a semi-automated process to generate a time sequence of gastric diameters (i.e., ‘contractograms’). These contractograms revealed propagating contractions when present. Using Fourier transforms, a spectral analysis of these cross-sectional diameters overtime was performed to identify the frequency of the dominant peak at each position along the longitudinal axis. The phase at this frequency was then plotted against the location along the long axis. In these phase shift plots, a linear change (i.e., R2 ≥ 0.95) in phase vs location was used to document propagated contractions. The velocity of propagation was estimated from the (inverse) slope of this line. The relative amplitude was estimated by calculating the magnitude of the Fourier coefficient at the dominant peak, which was normalized to the largest diameter at that perpendicular plane over the entire 60 s epoch. Data were summarized by averaging relative amplitudes across all planes (locations) spanning the contraction. While motion (e.g., respiratory) artifact at the beginning or end of a contractogram was eliminated before analysis, artifact interspersed within data was retained.
Gastric Accommodation
Gastric accommodation was estimated by calculating the maximum transverse diameter (in mm) of the stomach body and fundus in pre-prandial and post prandial states from cross-sectional imaging. The gastric fundus was measured at the level of the diaphragmatic crus and the proximal gastric body was measured at the level of the entry of the gastric body by the celiac artery takeoff. Postprandial gastric accommodation was computed by the difference (ln [Maximum postprandial diameter] – ln [Maximum fasting diameter]) separately in the gastric fundus and body, at baseline, 3 months, and 12 months after ESG; values were log transformed prior to analysis.
Outcomes
The pre-specified primary outcome of interest was the change in time to empty 50% of stomach contents (T1/2) at 3 months. The pre-specified secondary outcomes were: 1) change in T1/2 at 12 months, 2) change in percent 2 hours emptied and 4 hours emptied at 3 and 12 months, 3) change in total ghrelin, GLP-1, and PYY at 12 months, and 4) change in eating behaviors at 6 months.
Statistical Methods
Using preliminary data, we performed an a priori power calculation for our primary outcome demonstrating that a total sample size of 30 (n=15 ESG; n=15 diet only) provides 90% power to detect a 35-minute change in our primary outcome of T1/2, detecting the change that was seen after a surgical sleeve gastrectomy.26A sample size of 38 (n=15 ESG; n=23 diet only) assuming a 1:1.5 randomization scheme provides 80% power to detect a 29-minute change. These power calculations were performed with a two-sided equal-variances t-test at alpha=0.05 and with a standard deviation of 30 for the change in gastric emptying, a more conservative standard deviation than the 15 found in preliminary studies.14
We expressed continuous variables as the mean (SD) or as median (range). We reported categorical variables as a percentage (%). We compared the 3-month T1/2, 2 hr % emptied and 4% emptied between the two groups using the student’s t-test. We used the paired t-test for comparisons between the baseline and follow-up continuous variables within each group. We additionally performed pre-specified linear regression analyses between the change in T1/2 at 3 months and weight loss (%TBWL), change in 2 hr % emptied and weight loss, and change in 4 hr % emptied and weight loss. We performed a pre-specified analysis of covariance (ANCOVA) examining the change in percent 2-hour retention and weight loss, adjusting for the procedure, sex, and baseline gastric emptying and weight.
The MRI variables (gastric accommodation and motility) were analyzed by analyses of variance, then pairwise comparisons.
RESULTS
Baseline Demographics and Participant Flow
A total of 38 patients were randomized to ESG (n=20) and LS (n=18) at our institution. From these, 36 (ESG=18; LS=18) agreed to participate in the substudy. Baseline characteristics were similar between the two groups (Table 1). Out of n=36, n=36 subjects have reached 3 and 12 months, respectively. A total of six patients (ESG=2; LS=4) dropped out of the substudy, two at 3 months and four at 12 months, for a total sample size of n=34 at 3 months and n=30 at 12 months. (Supplementary Figure 2)
Table 1:
Baseline characteristics
| Overall N= 36 | ESG N=18 | Control N=18 | P-value | |
|---|---|---|---|---|
| Age, years | 48.3 ± 10.8 | 48.9 ± 8.4 | 47.8 ±12.9 | 0.76 |
| Sex, % female | 75 % | 78% | 72% | 0.7 |
| Weight, kg | 101.3 ± 12.3 | 99.8 ±13 | 102.7±11.7 | 0.48 |
| BMI | 35.1 ± 2.5 | 34.9 ± 2.6 | 35.1 ± 2.5 | 0.8 |
| Type 2 Diabetes | 36% | 33% | 39% | 1.0 |
| T1/2, min | 85.9 ± 18.9 | 83.6 ±17.5 | 88.3 ± 20.2 | 0.46 |
| 2hr % emptied | 67.6 ± 11.3 | 66.7 ±13.2 | 68.4 ±13.2 | 0.65 |
| 4hr % emptied | 87.3 ± 16.5 | 85.2 ± 21.7 | 89.5 ± 8.8 | 0.44 |
| Fasting Total Ghrelin, pg/mL | 896.9 ± 336.7 | 850±302.5 | 943.8±371 | 0.43 |
| Fasting GLP-1, pmol/L | 7.96 ± 3.3 | 7.6 ± 4.2 | 8.3 ± 2.4 | 0.54 |
| Fasting PYY, pg/mL | 84.7 ± 21.8 | 77.5 ± 19.1 | 91.4 ± 22.5 | 0.06 |
Changes in Gastric Emptying T1/2
At 3 months, T1/2 (minutes) was significantly delayed in the ESG group (n=17) compared to the LS group (n=17) (152.3 ± 47.3 vs. 89.1 ±27.9; p<0.001). There was a significant increase in T1/2 in the ESG group at 3 months by 65.9 ±45.4 minutes; p<0.001). There was no significant change in the LS cohort (3.01±19.9 minutes; p=0.54)
At 12 months, the T1/2 (minutes) remained significantly delayed in the ESG group (n=16) compared to the LS group (n=14) (137 ± 37.4 vs. 90.1 ±23.4; p<0.001). There was a significant increase in T1/2 in the ESG group at 12 months by 51.6 ± 37.7 minutes; p<0.001). There was no significant change in the LS cohort (−5.5 ± 12.7 minutes; p=0.17). Notably, there was no significant change in T1/2 in the ESG group between 3 and 12 months (n=16) 155.8± 46.5 vs. 137.1±37.4 minutes; p=0.15) and remained significantly delayed at 12 months compared to baseline (137.1± 37.4 vs. 85.5± 13.5 minutes; p<0.001) Figure 2
Figure 2:
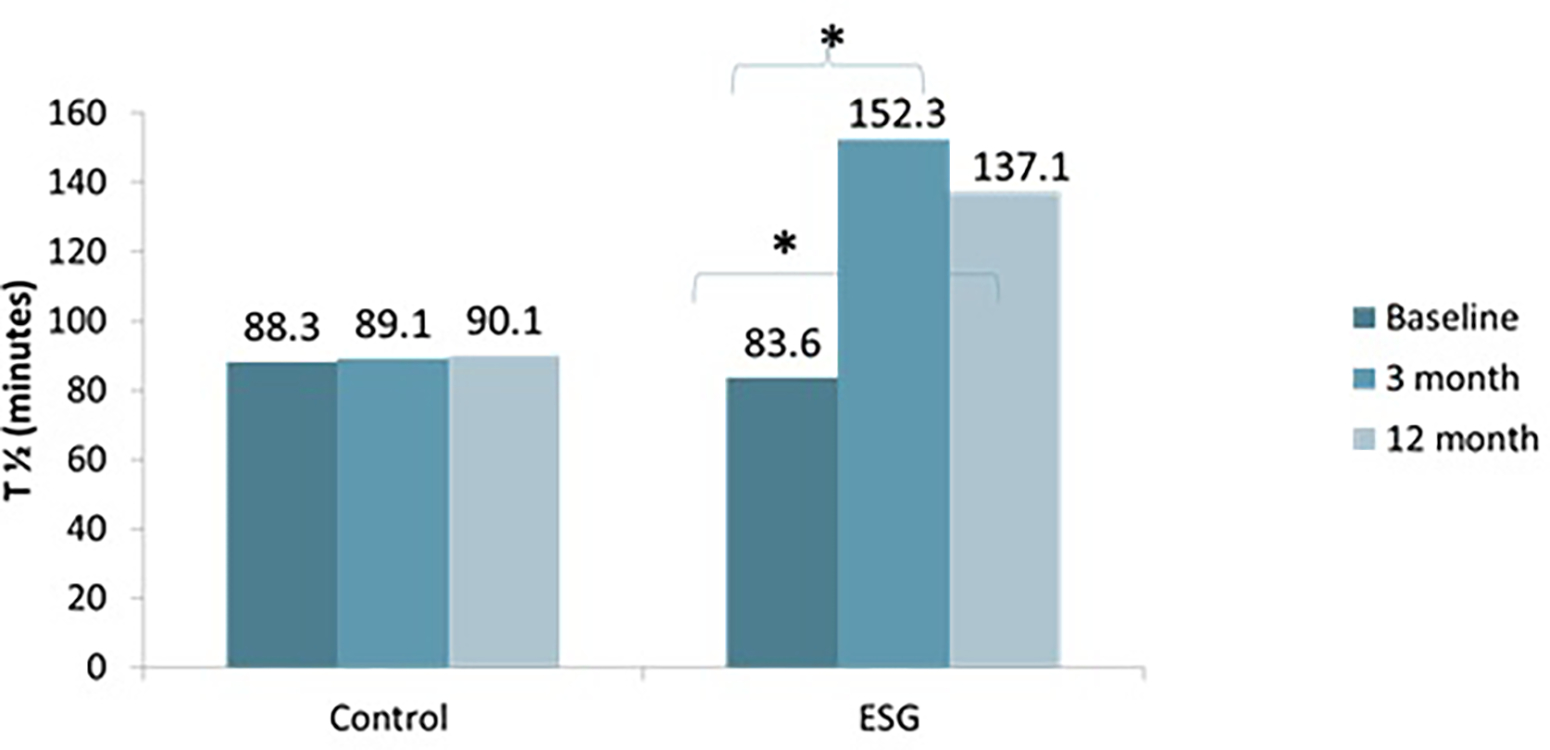
Change in T1/2 over time
2 hour and 4 hour % emptied
At 3 months, the percentage of food emptied in 2 hours from the stomach in the ESG group was significantly decreased compared to the LS group (42.5% vs. 66.6%; p<0.0001). At 12 months, the percent emptied at 2 hours remained significantly decreased in the ESG group compared to the LS group (46.1% vs. 66.8%; p=0.0007). At 4 hours, the percent emptied was also significantly decreased at 3 months in the ESG group compared to the LS group (71.9% vs. 89.2%; p<0.0001). Similarly, at 12 months, the percent emptied at 4 hours remained decreased in the ESG vs. LS group (79.9% vs. 89.5%; p=0.023).
Weight Loss
%TBWL in the ESG group was significantly greater than the %TBWL in the LS group at 3, 6 and 12 months (p<0.0001 all comparisons). The %TBWL in the ESG group was 13.1% (n=17), 15.9% (n=17) and 16% (n=16) at 3, 6 and 12 months, while it was 2.5% (n=17), 2.6% (n=16) and 0.6% (n=14) in the LS group. In an exploratory 18-month weight assessment, the %TBWL in the ESG group (n=10) was 16%.
Association between Change in Gastric Emptying and Weight Loss
Overall, greater changes in gastric emptying at 3 months as measured through T1/2 (Figure 3), 2 hr % emptied or 4 hr % emptied significantly correlated with greater weight loss at 3,6 or 12 months. The change in percent emptied at 4 hours demonstrated the highest correlation with weight loss across all three-time points, followed by T1/2. (Supplementary Figure 3)
Figure 3:
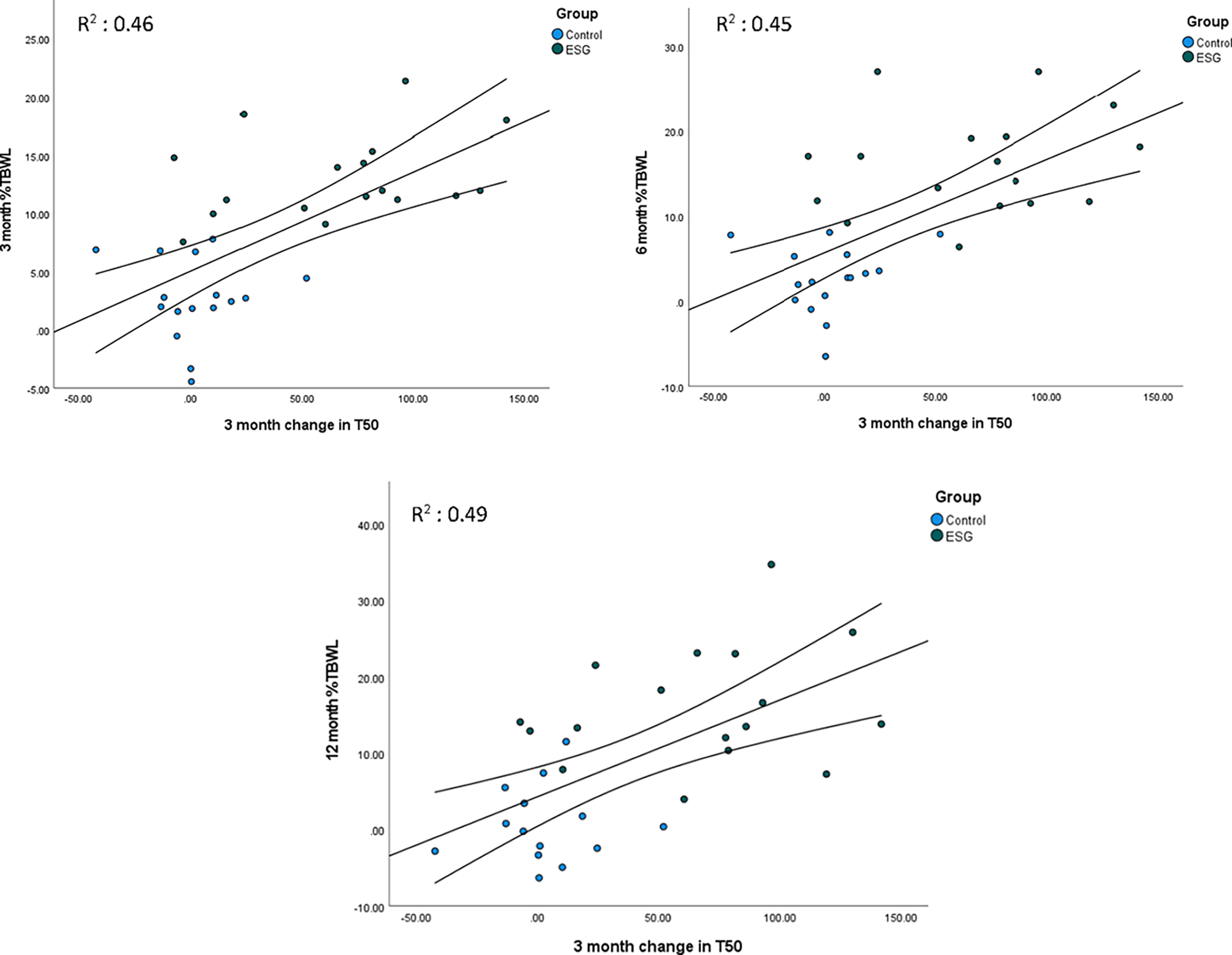
Change in 3-month T1/2 correlated with weight loss at 3, 6, and 12 months
Given the highest correlation with 4 hr % emptied, we divided the cohort into the slowest quartile into one group (≥18% increased retention), and the remainder in another group. We found that those who experienced a greater than 18% increase in gastric retention at 4 hours, experienced more weight loss at 3, 6, and 12 months. Table 2
Table 2:
%TBWL at 3, 6, 12 months by group, >18% retained at 4h
| Slowest | Other | p-value | |
|---|---|---|---|
|
| |||
| 3 month % TBWL | 14.3 ± 3.4 | 5.4 ± 5.5 | <0.0001 |
| 6 month % TBWL | 17.4 ± 5.3 | 6.1 ± 6.2 | <0.0001 |
| 12 month % TBWL | 18.6± 8.8 | 4.6 ± 7.3 | 0.0011 |
On ANCOVA, after adjusting for the ESG procedure, sex, baseline weight and baseline 4 hr % emptied, the change in 3 months 4 hr % emptied was a significant predictor of 3 and 6 months %TBWL (3 months β=0.17 p=0.03; 6-month β=0.28; p=0.03), with higher retained food (delayed emptying) associated with more weight loss, independent of the ESG procedure, which remained the strongest predictor of weight loss (3-month β=8.1 p<0.001; 6-month β=7.7; p=0.01). At 12 months, only the ESG procedure remained a significant predictor of weight loss after adjusting for age, sex, and baseline weight (β=14.7 p<0.0001). In post hoc analysis using the slowest quartile group, we found that at 4 hours, those with an increase in retained food at 3 months by 18% were independently associated with increased weight loss at 3,6 and 12 months, after adjusting for arm, age, sex, baseline weight, and baseline 4 hr % emptied (3-month β= 3.3 p=0.03; 6-month β=5.1; p=0.04; 12-month β=6.5; p=0.09).
Changes in Fasting Total Ghrelin, PYY and GLP-1
At baseline, there were no significant differences in fasting ghrelin, GLP-1, or PYY between the groups (Supplementary Table 1). At 12 months, the ESG group experienced significant increases in total ghrelin (n=16) compared to baseline, but no differences between the two groups were seen. No significant differences between or within groups were seen with GLP-1 or PYY. (Table 3)
Table 3:
Changes in Fasting Hormones at 12 months
| Group | ESG n=16 | LS n=14 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hormone | Baseline | 12 months | p-value | Baseline | 12 months | p-value |
| Total Ghrelin, pg/mL | 772 [556.3–1073.8] | 1116 [680–1562] | <0.001 | 968 [706–1308] | 925 [770–1316] | 0.39 |
| GLP-1, pmol/L | 6.8 [3.9–10.4] | 7.2 [4.9–10.3] | 1 | 8.2 [6.7–10] | 8.5 [6.8–9.9] | 0.77 |
| PYY, pg/mL | 82.5 [59–91.8] | 77 [68.3–100.8] | 0.302 | 97 [77.3–105] | 93 [73–107] | 0.77 |
At 18 months, fasting hormones were assessed in the ESG group alone (n=15). Compared to baseline, fasting total ghrelin (pg/mL) was significantly increased at 18 months (805 [560–1091] vs. 967 [794–1144] p=0.08). Similarly, GLP-1 (pmol/L) was significantly increased compared to baseline 6.1 [3.6–9.2] vs. 8 [4.9–22] p=0.02), and PYY (pg/mL) was also significantly increased at 18 months (82 [58–93] vs. 90 [78–148] p=0.007)
Changes in Eating Behaviors
A total of 32 (ESG=15; LS=17) subjects of the 38 agreed to fill out both questionnaires. At baseline, subjects scored similarly on all three domains of cognitive restraint (CR), uncontrolled eating (UE), and emotional eating (EE). At six months, there were no significant differences in eating behaviors between the two groups despite numerically improved tendencies in all domains. When we compared baseline to final measures using paired t-test analyses, there was a significant change compared to baseline within both groups, showing improvement in all domains except CR, which was only improved in the ESG group (17.7 ± 3.8 vs. 15.5 ± 3.0; p=0.012). (Supplementary Table 2)
Gastric Motility
All 11 participants completed gastric MRI studies at baseline, 3, and 12 months after ESG. Among these participants, the GE T1/2 increased (P < .0001) from 86 ± 17 minutes at baseline to 158 ± 55 minutes at 3 months after ESG but was not different (P < .2776) at 3 vs 12 months (145 ± 36 minutes) after ESG.
The MR images disclosed gastric contractions in all 33 studies except for 1 participant who had no contractions at 12 months. Compared to baseline, the periodicity, amplitude, and propagation velocity of these contractions were not different at 3 or 12 months after ESG f. However, the upper (proximal) extent of these contractions was lower (P = .01) at 3 months after ESG than at baseline; differences between 3 and 12 months after ESG were not significant. Since the lower (distal) boundary of these contractions did not change after vs before ESG, these contractions were propagated for a shorter distance (P = .06) at 3 months after ESG vs baseline; differences between 3 and 12 months after ESG were not significant. The difference (3 months – baseline) in GE T1/2 was correlated with the corresponding change in the average relative amplitude of gastric contractions (r = 0.58, P = .06) but not with the difference (3 months – baseline) in the distance traveled by contractions. Figure 4
Figure 4: Analysis of gastric contractions (‘contractograms’).

Panels A and B are MR images at baseline and 3 months after ESG. Arrows show a contraction (Panel A) and the ESG (Panel B filled arrows) with liquid nutrient in the gastric fundus above and a contraction (open arrow) below the narrow lumen (filled arrows). Panels C, D, and E are time sequences of gastric cross-sectional diameters along the longitudinal axis of the stomach (y-axis) at baseline, 3 and 12 months after ESG. The vertical scale shows the relative diameter. At each timepoint, gastric diameter is expressed relative to the maximum diameter at that location; maximum diameter is shown in dark red and minimum in dark blue. Compared to baseline (Panel C), the contractions were shorter and weaker at 3 months (Panel D) and not visible at 12 months. The phase shift plot (Panel F) shows a contraction that migrated downstream. Panel F shows that this contraction virtually occluded the stomach. Panels H-M compares gastric emptying and characteristics of contractions at baseline, 3, and 12 months after ESG. Compared to baseline, the upper extent of propagated contractions was lower (i.e., more distal) at 3 and 12 months after ESG (Panel L); hence the distance propagated was shorter (Panel K). However, other features (i.e., periodicity, average relative amplitude change, and propagation velocity) were not different after vs before ESG
Gastric Accommodation
The maximum diameter of the gastric fundus and body was measured at all 3 timepoints in 10 of 11 patients who underwent MRI. In the gastric fundus, the postprandial changes varied significantly over the 3 time points of the study (P=.02 for overall model). On average, the difference (ln postprandial – ln fasting volume) between baseline and 3-month visits was positive (P=0.0376), which suggests that postprandial expansion of the gastric fundus increased after ESG (Figure 5). Differences between 3 and 12 months after ESG were not significant. For the gastric body, the postprandial change in gastric diameter also varied significantly over the 3 time points of the study (P=.0458 for overall model) (Figure 5). By contrast to the gastric fundus, the postprandial expansion in the gastric body was lower at 3 months than at baseline (P=.0458 for overall model); hence, the difference (3 months – baseline) was negative in 7 of 10 patients (P=0.023) (Figure 5). By contrast, differences in postprandial expansion of the gastric body between 3 and 12 months were not significantly different (Figure 5).
Figure 5: Change in gastric accommodation of the body and fundus over 12 months.
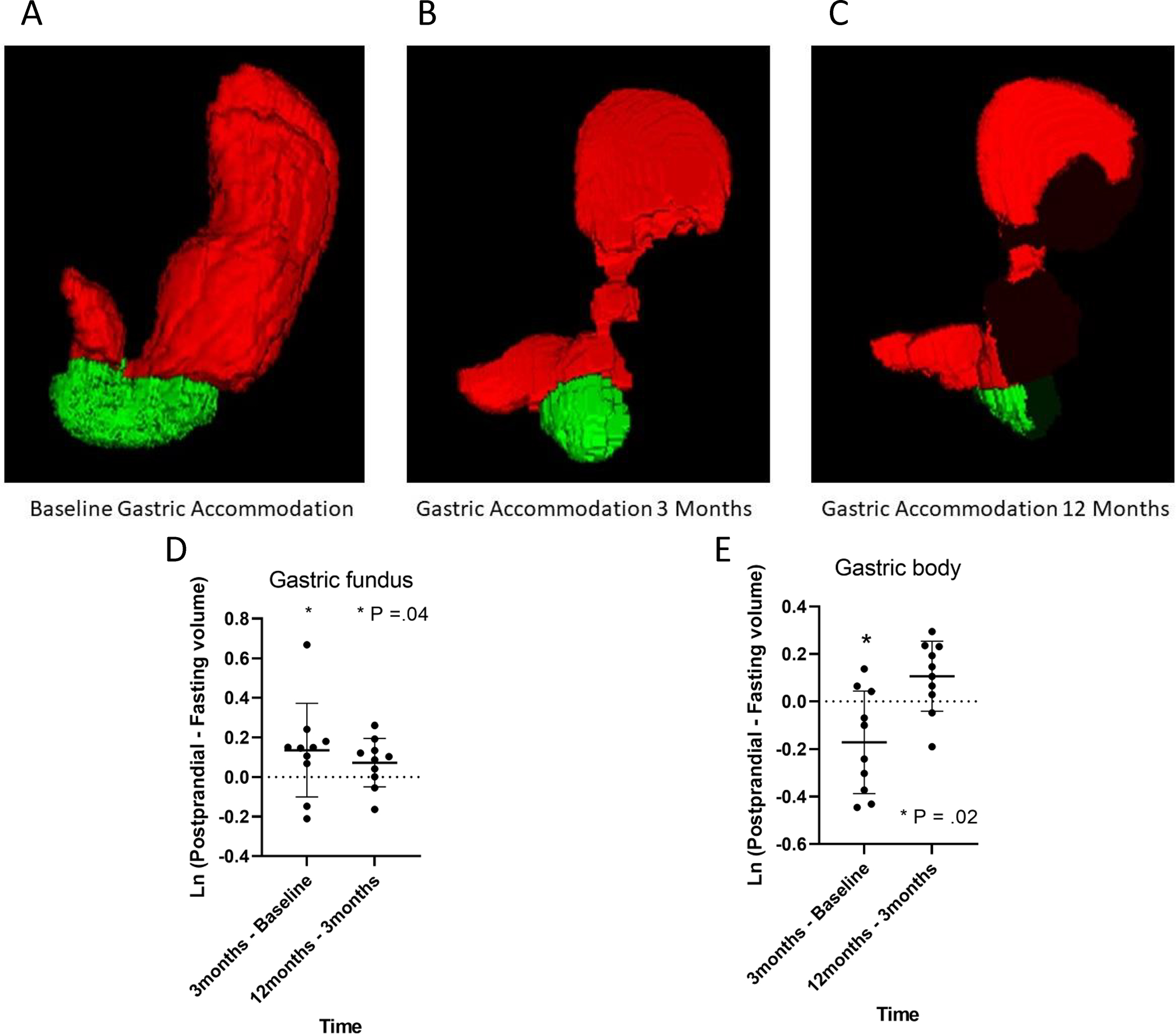
Panels A,B and C MRI images of gastric accommodation at baseline, 3 and 12 months. Panels C and D, significant increase in gastric fundus diameter and significant decrease in body diameter at 3 months compared to baseline
DISCUSSION
In this prospective priori substudy from a randomized clinical trial comparing the ESG vs lifestyle interventions, we established several key findings on gastric emptying, gastric motility, fasting hormones, and eating behaviors. First, we found that the ESG delays gastric emptying by an average of 60 minutes at 3 months, with a sustained delay of at least 50 minutes at 12 months. In post hoc analyses, greater delays in gastric emptying were also associated with weight loss at 3, 6, and 12 months, with higher weight loss seen in those with higher delays at 4 hours. Additionally, we found that at 18 months fasting levels of total ghrelin, PYY, and GLP-1 changed with the ESG procedure. Fasting total ghrelin, an orexigenic hormone, significantly increased following weight loss, yet satiety hormones such as PYY and GLP-1 increased despite the significant delays in gastric emptying and weight loss. Finally, we found that patients in this study experienced positive changes in eating behaviors, but the ESG procedure was the only group to see significant improvements in the cognitive restraint domain. More importantly, we found the procedure likely preserves gastric motility, providing key mechanistic insight into the lack of symptoms experienced by patients who have this procedure.
The relationship between gastric emptying and body weight has been increasingly defined over the past several years. We know that accelerated emptying has been associated with increased adiposity22, but a recent prospective cohort study has also found that early life accelerated emptying is associated with higher odds of weight gain over 4 years compared to normally-emptying counterparts23. Not surprisingly, modifying gastric emptying has been associated with weight loss, with proof-of-concept medication and device trials showing a modest correlation between the change in gastric emptying and weight loss.16, 22, 24–26 The effects, however, were short-lived, highly dependent on device indwelling time or drug tachyphylaxis. Surgical procedures like the laparoscopic sleeve gastrectomy (LSG) accelerated gastric emptying, with changes in GE also associated with weight loss. Altogether, this suggests that the magnitude and persistence of the change may be responsible long term weight loss 27.
Among patients who had a gastric MRI, the GE T1/2 was 72 ± 49 minutes longer at 3 months after ESG, which is similar to the corresponding difference (66 ± 45 minutes) in the entire cohort. The periodicity, amplitude, and velocity of propagation of gastric contractions assessed with our innovative dynamic MRI technique were not significantly different after vs before ESG. This suggests that antral trituration is relatively preserved and argues against a major disruption of antral motility, for example, secondary to a vagal nerve injury during the procedure. However, the gastric contractions originated more distally, distal to the ESG site, after vs before a gastroplasty. Hence, gastric contractions were propagated for a shorter distance, albeit non-significantly, after vs before an ESG. In the closest experimental model of ESG, exclusion of the proximal stomach by a septum in pigs affected the mechanisms responsible for gastric emptying of liquids but not liquid emptying per se.28 While that study did not assess GE of solids, antral motility was reduced after vs before the procedure. This preserved gastric contractility after the ESG may explain the lack of reported symptoms despite delaying gastric emptying, compared to patients for example with gastroparesis with impaired stomach contractility.
Overall, the ESG’s effect on gastric emptying persisted over 1 year after the procedure, contrary to intragastric balloons or medications where the effect is short-lived. The change in gastric emptying was also associated with weight loss throughout time, similar to the relationship seen with other interventions 27. Compared to baseline, postprandial expansion of the gastric body had declined at 3 months after ESG, probably because the sleeve restricted expansion of the gastric body. By contrast, postprandial expansion of the gastric fundus was greater after ESG than at baseline. We postulate that the proximal gastric retention of food stretches the fundus to induce satiation and thereby reduced food intake. Arguably, these effects explain the greater cognitive restraint domain scores for eating in the ESG vs sham groups. Taken together, these findings suggest that the ESG not only induces changes in gastric physiology that favor weight loss but allows patients to engage in healthier eating habits throughout time, critical for long-term weight maintenance after a successful weight loss phase. 2, 3, 6, 8, 9, 29
In response to diet-induced weight loss, the human body engages several counter-regulatory measures to return to its previous body weight. First, our body upregulates orexigenic hormones such as ghrelin and downregulates satiety hormones, such as GLP-1, PYY, and CCK, increasing our hunger signals. 30–32 Additionally, the body’s resting energy expenditure becomes more efficient and compounded by behavioral change fatigue, and an overall positive energy state favoring weight regain ensues33 34. These changes explain why the majority of long-term weight maintenance rates following weight loss have been unsuccessful, with bariatric surgery being the exception35–41. In response to bariatric surgery, postprandial PYY and GLP-1 appear to increase, whereas ghrelin levels decrease following sleeve gastrectomy and remain largely unchanged after Roux-en-Y gastric bypass, as the fundus remains intact in the latter.42–48 These changes, along with alterations in bile acid metabolism and intestinal microbiota may lead to more successful weight maintenance.49 Preliminary studies with the ESG in 4 patients showed that active and postprandial ghrelin levels decreased following weight loss, with no significant changes in GLP-1 or PYY being observed.14 The following study with 24 subjects recently confirmed these findings where the ESG did not change postprandial ghrelin, GLP-1, or PYY profiles, despite significant weight loss.50 The laparoscopic sleeve gastrectomy (LSG) however, decreased ghrelin and increased PYY compared to the ESG. These findings suggest that the ESG procedure promotes weight maintenance, but to a lesser extent than the LSG. The changes in gastric emptying, however, were not investigated in this prior study. In our study, the ESG’s effect on hormones slightly differed from prior studies. The fasting total ghrelin, GLP-1, and PYY all increased at varying degrees following weight loss, compared to prior studies where no changes were noted. The difference may be related in part to procedural technique differences, or differences in gastric emptying produced. Ghrelin is known to promote gastric motility and rises in response to weight loss. It’s not surprising that following weight loss and marked delays in gastric emptying, fasting total ghrelin levels rose. In the prior study, gastric emptying was not measured, so whether this was related to changes in motility is unknown. Finally, the hormones in the prior study were assessed at 6 months, where maximal weight loss may have not been reached. In our study, both fasting GLP-1 and PYY which delay gastric emptying were paradoxically increased following weight loss and delays in gastric emptying at 18 months. However, postprandial GLP-1 and PYY were not measured in this study, which limits our understanding of the effects of ESG, weight loss, or counter-regulatory mechanisms on the prandial incretin response. The absence of a sham arm, objective assessment of lifestyle interventions, incomplete blinding, and arguably a small sample size for some assessments (eg, hormones and gastric accommodation) are other limitations of this study. However, those assessing our main outcome of gastric emptying and other outcomes such as hormones were blind to their treatment allocation.
Future Directions
These findings need to be confirmed with larger and more long-term studies. In addition, the effects of ESG, LSG, and Roux-en-Y gastric bypass on postprandial hormone profiles should be compared. The advent of new endoscopic treatment devices provides opportunities to selectively investigate the effects of the pre-procedure hormonal profile, duodenal exclusion, and altered gastric emptying on weight loss, hormones, and the expression of genes. Such studies may refine our understanding of the mechanisms that result in long-term weight loss following bariatric surgery. Finally, these studies will enable a more refined approach to individualized multi-disciplinary therapy of obesity.
Supplementary Material
Supplementary Figure 1: Endoscopic Sleeve Gastroplasty (Left). Sleeve Gastrectomy (Right)
Supplementary Figure 2: Study Flow Diagram
Supplementary Figure 3: Correlation between change in T1/2 ( Left), and 4hr % (Right) emptied with %TBWL at 3, 6 and 12 months
• What is already known on this topic –
The endoscopic sleeve gastroplasty (ESG) results in > 15% TBWL yet its mechanism(s) of action remain largely unknown
• What this study adds –
The procedure preserves stomach contractility while delaying gastric emptying and producing favorable changes in satiation/satiety hormones
• How this study might affect research, practice or policy
The ESG’s effect on gastric and obesity physiology challenges the paradigm set forth by previous obesity research
Grant support:
Dr. Camilleri: Grant R01-DK67071 from National Institutes of Health (NIH) for studies in obesity. Dr. Bharucha: Grant R01-DK78924 from NIH. The study was made possible by CTSA Grant Number UL1-TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Relevant Disclosures
Dr. Vargas, Dr. Jaruvongvanich, Mrs. Rizk, Ms. Gomez Villa, Mr. Edwards, and Mr. Lake: No disclosures; Dr. Storm: Research grant support from Apollo Endosurgery, Boston Scientific, Endogenex, Endo-TAGSS, and Enterasense. Consultant for Apollo Endosurgery, ERBE, GI Dynamics, Intuitive, and Olympus; Dr. Acosta: Stockholder in Gila Therapeutics, Phenomix Sciences; Consultant fees from Rhythm Pharmaceuticals, General Mills; Dr. Abu Dayyeh: Consulting fee from Endogenex, Endo-TAGSS, Metamodix, and BFKW; consulting fee and grant/research support from USGI, Cairn Diagnostics, Aspire Bariatrics, and Boston Scientific; speaker honorarium from Olympus and Johnson and Johnson; speaker honorarium and grant/research support from Medtronic and EndoGastric Solutions; and research support/grant from Apollo Endosurgery and Spatz Medical; Dr. Camilleri: Advisor to Phenomics Sciences, holder of stock options
REFERENCES
- 1.James TW, Reddy S, Vulpis T, et al. Endoscopic Sleeve Gastroplasty Is Feasible, Safe, and Effective in a Non-academic Setting: Short-Term Outcomes from a Community Gastroenterology Practice. Obes Surg 2020;30:1404–1409. [DOI] [PubMed] [Google Scholar]
- 2.Storm AC, Abu Dayyeh BK. Endoscopic sleeve gastroplasty for obesity: defining the risk and reward after more than 1600 procedures. Gastrointest Endosc 2019;89:1139–1140. [DOI] [PubMed] [Google Scholar]
- 3.Cheskin LJ, Hill C, Adam A, et al. Endoscopic sleeve gastroplasty versus high-intensity diet and lifestyle therapy: a case-matched study. Gastrointest Endosc 2020;91:342–349 e1. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, de Moura DTH, Khan A, et al. Intragastric Balloon Versus Endoscopic Sleeve Gastroplasty for the Treatment of Obesity: a Systematic Review and Meta-analysis. Obes Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan Z, Khan MA, Hajifathalian K, et al. Efficacy of Endoscopic Interventions for the Management of Obesity: a Meta-analysis to Compare Endoscopic Sleeve Gastroplasty, AspireAssist, and Primary Obesity Surgery Endolumenal. Obes Surg 2019;29:2287–2298. [DOI] [PubMed] [Google Scholar]
- 6.Fayad L, Cheskin LJ, Adam A, et al. Endoscopic sleeve gastroplasty versus intragastric balloon insertion: efficacy, durability, and safety. Endoscopy 2019;51:532–539. [DOI] [PubMed] [Google Scholar]
- 7.Jalal MA, Cheng Q, Edye MB. Systematic Review and Meta-Analysis of Endoscopic Sleeve Gastroplasty with Comparison to Laparoscopic Sleeve Gastrectomy. Obes Surg 2020. [DOI] [PubMed] [Google Scholar]
- 8.Bazerbachi F, Abu Dayyeh BK. The pressure is on! Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: toward better patient allocation beyond pygmalionism. Gastrointest Endosc 2019;89:789–791. [DOI] [PubMed] [Google Scholar]
- 9.Barrichello S, Hourneaux de Moura DT, Hourneaux de Moura EG, et al. Endoscopic sleeve gastroplasty in the management of overweight and obesity: an international multicenter study. Gastrointest Endosc 2019;90:770–780. [DOI] [PubMed] [Google Scholar]
- 10.Abu Dayyeh BK, Neto MG, Lopez-Nava G, et al. Endoscopic sleeve gastroplasty is safe and effective: pitfalls of a flawed systematic review. Surg Obes Relat Dis 2019;15:1423–1424. [DOI] [PubMed] [Google Scholar]
- 11.Storm AC, Abu Dayyeh BK, Topazian M. Endobariatrics: A Primer. Clin Gastroenterol Hepatol 2018;16:1701–1704. [DOI] [PubMed] [Google Scholar]
- 12.Alqahtani A, Elahmedi M, Alqahtani YA, et al. Endoscopic Sleeve Gastroplasty in 109 Consecutive Children and Adolescents With Obesity: Two-Year Outcomes of a New Modality. Am J Gastroenterol 2019;114:1857–1862. [DOI] [PubMed] [Google Scholar]
- 13.Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients. Clin Gastroenterol Hepatol 2017;15:504–510. [DOI] [PubMed] [Google Scholar]
- 14.Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clinical Gastroenterology & Hepatology 2017;15:37–43.e1. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Nava G, Jaruvongvanich V, Storm AC, et al. Personalization of Endoscopic Bariatric and Metabolic Therapies Based on Physiology: a Prospective Feasibility Study with a Single Fluid-Filled Intragastric Balloon. Obes Surg 2020. [DOI] [PubMed] [Google Scholar]
- 16.Gomez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: Results of a prospective study. Obesity 2016;24:1849–53. [DOI] [PubMed] [Google Scholar]
- 17.Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet 2022;400:441–451. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE, Camilleri M, Veil E, et al. Comprehensive assessment of gastric emptying with a stable isotope breath test. Neurogastroenterology & Motility 2013;25:e60–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson J, Persson LO, Sjostrom L, et al. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord 2000;24:1715–25. [DOI] [PubMed] [Google Scholar]
- 20.de Lauzon B, Romon M, Deschamps V, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr 2004;134:2372–80. [DOI] [PubMed] [Google Scholar]
- 21.Bharucha AE, Manduca A, Lake DS, et al. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil 2011;23:617–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 2015;148:537–546 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pajot G, Camilleri M, Calderon G, et al. Association between gastrointestinal phenotypes and weight gain in younger adults: a prospective 4-year cohort study. Int J Obes (Lond) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2017;2:890–899. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Acosta A. Combination Therapies for Obesity. Metab Syndr Relat Disord 2018;16:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta A, Camilleri M. A working paradigm for the treatment of obesity in gastrointestinal practice. Tech Gastrointest Endosc 2017;19:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas EJ, Bazerbachi F, Calderon G, et al. Changes in Time of Gastric Emptying After Surgical and Endoscopic Bariatrics and Weight Loss: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:57–68 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malbert CH, Mathis C. Antropyloric modulation of transpyloric flow of liquids in pigs. Gastroenterology 1994;107:37–46. [DOI] [PubMed] [Google Scholar]
- 29.de Miranda Neto AA, de Moura DTH, Ribeiro IB, et al. Efficacy and Safety of Endoscopic Sleeve Gastroplasty at Mid Term in the Management of Overweight and Obese Patients: a Systematic Review and Meta-Analysis. Obes Surg 2020;30:1971–1987. [DOI] [PubMed] [Google Scholar]
- 30.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumithran P, Prendergast LA, Delbridge E, et al. Long-Term Persistence of Hormonal Adaptations to Weight Loss. New England Journal of Medicine 2011;365:1597–1604. [DOI] [PubMed] [Google Scholar]
- 32.Scerif M, Goldstone AP, Korbonits M. Ghrelin in obesity and endocrine diseases. Molecular and Cellular Endocrinology 2011;340:15–25. [DOI] [PubMed] [Google Scholar]
- 33.Dulloo AG, Jacquet J, Girardier L. Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. Int J Obes Relat Metab Disord 1996;20:393–405. [PubMed] [Google Scholar]
- 34.Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016;24:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704. [DOI] [PubMed] [Google Scholar]
- 36.Cordero P, Li J, Oben JA. Bariatric surgery as a treatment for metabolic syndrome. Journal of the Royal College of Physicians of Edinburgh 2017;47:364–368. [DOI] [PubMed] [Google Scholar]
- 37.Chantziara K, Laferrere B, Pi-Sunyer X. Bariatric surgery for the treatment of Type 2 diabetes: A step closer? Expert Review of Endocrinology and Metabolism 2014;9:231–237. [DOI] [PubMed] [Google Scholar]
- 38.Van Gaal LF, De Block CEM. Bariatric surgery to treat type 2 diabetes: What is the recent evidence? Current Opinion in Endocrinology, Diabetes and Obesity 2012;19:352–358. [DOI] [PubMed] [Google Scholar]
- 39.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med 2017;376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salminen P, Helmio M, Ovaska J, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018;319:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Look ARG. Eight-Year Weight Losses with an Intensive Lifestyle Intervention: The Look AHEAD Study. Obesity (Silver Spring, Md.) 2014;22:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 2012;22:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. The New England journal of medicine 2002;346:1623–30. [DOI] [PubMed] [Google Scholar]
- 44.Mans E, Serra-Prat M, Palomera E, et al. Sleeve gastrectomy effects on hunger, satiation, and gastrointestinal hormone and motility responses after a liquid meal test. American Journal of Clinical Nutrition 2015;102:540–7. [DOI] [PubMed] [Google Scholar]
- 45.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Annals of surgery 2004;240:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vigneshwaran B, Wahal A, Aggarwal S, et al. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30–35.0 kg/m<sup>2</sup>: a Prospective Study. Obesity Surgery 2016;26:2817–2823. [DOI] [PubMed] [Google Scholar]
- 47.Sista F, Abruzzese V, Clementi M, et al. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surgery for Obesity & Related Diseases 2017;13:7–14. [DOI] [PubMed] [Google Scholar]
- 48.Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3–36 and active GLP-1 levels in non-diabetic humans. Obes Surg 2014;24:241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011;60:1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Nava G, Negi A, Bautista-Castano I, et al. Gut and Metabolic Hormones Changes After Endoscopic Sleeve Gastroplasty (ESG) Vs. Laparoscopic Sleeve Gastrectomy (LSG). Obes Surg 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Endoscopic Sleeve Gastroplasty (Left). Sleeve Gastrectomy (Right)
Supplementary Figure 2: Study Flow Diagram
Supplementary Figure 3: Correlation between change in T1/2 ( Left), and 4hr % (Right) emptied with %TBWL at 3, 6 and 12 months


