Abstract
Sneathia sanguinegens, Sneathia vaginalis, and Mageeibacillus indolicus have been recently described in the female genital tract. We present the first case of a postpartum septic arthritis of the pubic symphysis due to these organisms, identified by next generation sequencing.
Keywords: Septic arthritis of pubic symphysis, Sneathia sanguinegens, Sneathia vaginalis, Mageeibacillus indolicus
1. Introduction
Septic arthritis of the pubic symphysis is a rare infection, representing about 0.8–1.36% of all septic arthritis cases in adults [1]. Risk factors are previous gynecological or urological surgeries, delivery, drug abuse, diabetes, cancer, local macro- and micro-traumatisms, and radiotherapy [2].
We present the case of a patient with septic arthritis of the pubic symphysis during the postpartum period [3,4], where metagenomic next generation sequencing (mNGS) allowed us to detect and identify unusual pathogens.
2. Case report
A 32-year-old woman presented at 40 weeks of gestation in the latent phase of labor with maternal fever and fetal tachycardia. Complaining of upper respiratory tract symptoms, a rapid influenza test (Cobas®Liat®, Roche) turned out positive for influenza A, motivating a treatment with oseltamivir for 5 days. She had an uncomplicated spontaneous vaginal delivery with only a superficial tear on the labia minora.
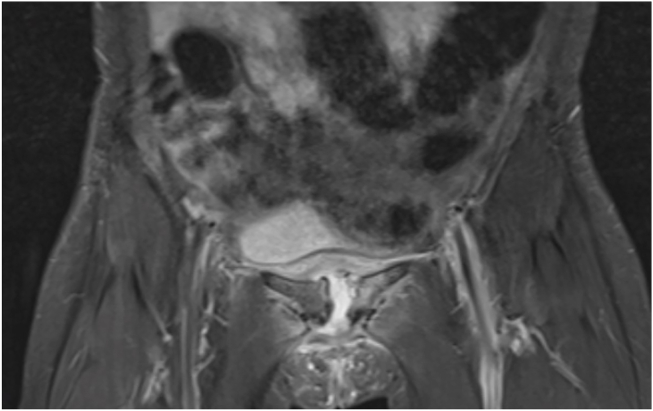
On Day 1 postpartum, she complained of pain in the groin and pubic symphysis, rapidly worsening and resulting in an inability to walk. In parallel, she had persistent fever. A pelvic magnetic resonance imaging (MRI) revealed on postpartum Day 9 a high suspicion of septic arthritis of the pubic symphysis (Fig. 1). An ultrasound-guided aspiration of the joint returned 3 ml of purulent fluid. Surgical drainage of the collection was then performed. During surgery, a pelvic exam was done by a senior gynecologist and no fistula nor previously undiagnosed lesions were found. Immediately following surgery, initial empiric therapy with cefuroxime was switched to broad-spectrum antibiotic (piperacillin-tazobactam). The culture of the ultrasound-guided joint aspiration (without antibiotics) and of multiple perioperative samples did not yield growth under standard conditions. These included blood agar, chocolate agar, MacConkey agar and colistin-nalidixic acid agar plates incubated under aerobic conditions (72 h incubation at 35 °C in 5% CO2 atmosphere), CDC agar plates incubated under anaerobic conditions (48 h incubation at 35 °C) and brain-heart infusion broth (5 days incubation at 35 °C in 5% CO2 atmosphere). Direct staining of the aspirate revealed gram-positive cocci and gram-negative rods. Owing to rapid clinical recovery, the patient was discharged on Day 4 postoperatively with a 3-week course of antibiotics (amoxicillin-clavulanate).
Fig. 1.
Magnetic resonance image of the pelvis (T2 WI tse cor, STIR cor) shows an intra-articular effusion at the pubic symphysis with diastasis and contrast enhancement of the peripheral soft tissues.
2.1. mNGS analysis
Suspecting a polymicrobial infection with fastidious organisms, we performed mNGS of joint fluid after bacterial DNA enrichment using the Ultra-Deep Microbiome Prep (Molzym) with modified sample pretreatment [5,6] protocol. Our mNGS pipeline (HUGe-MAP) consisted in Illumina 2 × 151 iSeq 100 sequencing, ready quality filtering, removal of replicate and human reads, and classification of non-human (paired) reads using CLARK [7] as described previously [6] except for the minimum read length that was set to 90 nt. Of 3,062,842 generated reads (pairs), 2,510,457 passed the quality check and most of them corresponded to human DNA (2,057,545). Among non-human reads (31,992; deposited in the European Nucleotide Archive under study number PRJEB44108), 28,628 were assigned to bacteria, including Sneathia sanguinegens (27,489), Sneathia vaginalis (332), Mageeibacillus indolicus (45) and a common reagent contaminant Cutibacterium acnes (31). Other bacterial species (including Gardnerella vaginalis) were represented by < 24 reads. In the negative control (NEC), C. acnes was the dominant organism (8997 reads). Only one read was assigned to S. sanguinegens, and none to S. vaginalis or M. indolicus.
The reads assigned by the HUGe-MAP pipeline to M. indolicus, S. sanguinegens and S. vaginalis were queried against corresponding reference genome sequences using blastn search application (-word_size 12 -evalue 1e-010 -perc_identity 80 -qcov_hsp_perc 80 -culling_limit 1) from BLAST+ v2.13.0 [8]. This analysis largely confirmed taxonomic assignments to M. indolicus [(100% of reads with blastn hits; median (Q1–Q3) hit sequence identity percentage 99.3 (98–100)] and to S. sanguinegens [(98.7% of reads with blastn hits; median hit sequence identity percentage 99.3 (98.7–100)]. The reads classified as S. vaginalis by HUGe-MAP had a lower similarity to the reference genome sequence of this species [(64.4% of reads with blastn hits; median hit sequence identity percentage 94.9 (91.3–97.3)] and to that of S. sanguinegens [(7.4% of reads with blastn hits; median hit sequence identity percentage 94.1 (89.5–96.7)]. We cannot exclude that (at least some of) these reads originated from a putative yet-to-be-described Sneathia species [9].
3. Discussion
The presence of Sneathia sanguinegens [10] [synonym Leptotrichia sanguinegens [11]], Sneathia vaginalis [12] [synonyms Sneathia amnii [9] and Leptotrichia amnionii [13]] and Mageeibacillus indolicus confirms the diagnosis of septic arthritis of pubic symphysis, the likely source being the female genital tract. In 2015, Austin et al. [14] isolated M. indolicus (family Oscillospiraceae, phylum Firmicutes), a strictly anaerobic gram-positive rod-shaped bacterium from the female genital tract associated with bacterial vaginosis. S. sanguinegens and S. vaginalis are gram-negative rod-shaped and pleomorphic coccobacilli, respectively, which belong to the family Leptotrichiaceae (phylum Fusobacteria) and are part of the vaginal microbiome [9,15]. During vaginal delivery, microtrauma to the pubic symphysis can predispose to local bacterial inoculation. In seven of the nine published cases of septic arthritis of the pubic symphysis during the postpartum period [[2], [3], [4]], Streptococcus, Staphylococcus or Pseudomonas have been identified while Sneathia and Mageeibacillus have not been reported.
Sneathia species have been sporadically reported as causes of infection, including peripartum bacteremia [16], and have recently been considered potential emerging pathogens [15]. The underestimation of their potential pathogenicity may be linked to their complex growth requirements [15]. Therefore, such bacteria could be, for example, etiological agents of culture-negative postpartum septic arthritis reported by Ducrotoy et al. [17]. Our case highlights the advantage of mNGS in the detection of emerging pathogens in particular situations.
CRediT author statement
Aude Nguyen: Writing-original draft preparation, Visualization.
Ludovica Ferrero: Writing-original draft preparation.
Vladimir Lazarevic: Methodology, Investigation, Validation, Writing - Review & Editing.
Nadia Gaia: Methodology, Investigation, Validation.
Begoña Martinez de Tejada: Writing - Review & Editing.
Jacques Schrenzel: Investigation, Methodology, Validation, Resources, Supervision, Writing - Review & Editing.
Nadia Berkane: Supervision, Writing - Review & Editing.
Transparency declaration
The authors have no conflict of interest nor source of funding to declare.
Acknowledgements
Myriam Girard (sample preparation for mNGS).
Handling Editor: Patricia Schlagenhauf
Contributor Information
Aude Nguyen, Email: aude.nguyen@hcuge.ch.
Nadia Berkane, Email: nadia.berkane@hirslanden.ch.
References
- 1.Hocedez C., Pelissier A., Mosbah R., Raimond E., Gabriel R., Graesslin O. Arthrite septique de la symphyse pubienne au cours de la grossesse [Septic arthritis of the pubic symphysis during pregnancy] Gynecol Obstet Fertil. 2015 Jun;43(6):472–473. doi: 10.1016/j.gyobfe.2015.03.014. [French] [DOI] [PubMed] [Google Scholar]
- 2.Ross J.J., Hu L.T. Septic arthritis of the pubic symphysis: review of 100 cases. Medicine (Baltim) 2003 Sep;82(5):340–345. doi: 10.1097/01.md.0000091180.93122.1c. [DOI] [PubMed] [Google Scholar]
- 3.Dunk R.A., Langhoff-Roos J. Osteomyelitis of the pubic symphysis after spontaneous vaginal delivery. BMJ Case Rep. 2010 Oct 12;2010 doi: 10.1136/bcr.01.2010.2610.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosma S., Borella F., Carosso A., Ingala A., Fassio F., et al. Osteomyelitis of the pubic symphysis caused by methicillin-resistant Staphylococcus aureus after vaginal delivery: a case report and literature review. BMC Infect Dis. 2019 Nov 8;19(1):952. doi: 10.1186/s12879-019-4595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarevic V., Gaïa N., Girard M., Mauffrey F., Ruppé E., Schrenzel J. Effect of bacterial DNA enrichment on detection and quantification of bacteria in an infected tissue model by metagenomic next-generation sequencing. ISME COMMUN. 2022;2:122. doi: 10.1038/s43705-022-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Büchler A.C., Lazarevic V., Gaïa N., Girard M., Eckstein F., Egli A., Sutter S.T., Schrenzel J. Mycobacterium chelonae infection identified by metagenomic next-generation sequencing as the probable cause of acute contained rupture of a biological composite graft-A case report. Int J Mol Sci. 2021 Dec 29;23(1):381. doi: 10.3390/ijms23010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ounit R., Wanamaker S., Close T.J., Lonardi S. CLARK: fast and accurate classification of metagenomic and genomic sequences using discriminative k-mers. BMC Genom. 2015 Mar 25;16(1):236. doi: 10.1186/s12864-015-1419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., et al. BLAST+: architecture and applications. BMC Bioinf. 2009 Dec 15;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harwich M.D., Jr., Serrano M.G., Fettweis J.M., Alves J.M., Reimers M.A., Vaginal Microbiome Consortium (additional members) Buck G.A., Jefferson K.K. Genomic sequence analysis and characterization of Sneathia amnii sp. nov. BMC Genom. 2012;13(Suppl 8):S4. doi: 10.1186/1471-2164-13-S8-S4. Suppl 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins M.D., Hoyles L., Tornqvist E., von Essen R., Falsen E. Characterization of some strains from human clinical sources which resemble "Leptotrichia sanguinegens": description of Sneathia sanguinegens sp. nov., gen. nov. Syst Appl Microbiol. 2001 Nov;24(3):358–361. doi: 10.1078/0723-2020-00047. [DOI] [PubMed] [Google Scholar]
- 11.Hanff P.A., Rosol-Donoghue J.A., Spiegel C.A., Wilson K.H., Moore L.H. Leptotrichia sanguinegens sp. nov., a new agent of postpartum and neonatal bacteremia. Clin Infect Dis. 1995 Jun;20(Suppl 2):S237–S239. doi: 10.1093/clinids/20.supplement_2.s237. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg T., Gronow S., Falgenhauer J., Imirzalioglu C., Mühldorfer K., et al. Sneathia vaginalis sp. nov. (Fusobacteriales, Leptotrichiaceae) as a replacement of the species 'Sneathia amnii' Harwich et al. 2012 and 'Leptotrichia amnionii' Shukla et al. 2002, and emended description of Sneathia Collins et al. Int J Syst Evol Microbiol. 2001;71(3) doi: 10.1099/ijsem.0.004663. 2019 Jun. [DOI] [PubMed] [Google Scholar]
- 13.Shukla S.K., Meier P.R., Mitchell P.D., Frank D.N., Reed K.D. Leptotrichia amnionii sp. nov., a novel bacterium isolated from the amniotic fluid of a woman after intrauterine fetal demise. J Clin Microbiol. 2002 Sep;40(9):3346–3349. doi: 10.1128/JCM.40.9.3346-3349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin M.N., Rabe L.K., Srinivasan S., Fredricks D.N., Wiesenfeld H.C., Hillier S.L. Mageeibacillus indolicus gen. nov., sp. nov.: a novel bacterium isolated from the female genital tract. Anaerobe. 2015 Apr;32:37–42. doi: 10.1016/j.anaerobe.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theis K.R., Florova V., Romero R., Borisov A.B., Winters A.D., et al. Sneathia: an emerging pathogen in female reproductive disease and adverse perinatal outcomes. Crit Rev Microbiol. 2021 Aug;47(4):517–542. doi: 10.1080/1040841X.2021.1905606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Martino S.J., Mahoudeau I., Brettes J.P., Piemont Y., Monteil H., Jaulhac B. Peripartum bacteremias due to Leptotrichia amnionii and Sneathia sanguinegens, rare causes of fever during and after delivery. J Clin Microbiol. 2004 Dec;42(12):5940–5943. doi: 10.1128/JCM.42.12.5940-5943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducrotoy V., Fournet P., Vittecoq O., Daragon A. Arthrites septiques du post-partum. A propos de deux cas [Postpartum septic arthritis. Two case reports] J Gynecol Obstet Biol Reprod. 1998 Jun;27(4):449–454. [French] [PubMed] [Google Scholar]