Abstract
Vascular complications from soft tissue fillers can have catastrophic consequences for patients. Adverse events are rare, but they are increasing, and their appearance may be the result of intravascular injection. A comprehensive understanding of the 2-dimensional anatomy (distribution) and 3-dimensional anatomy (depth) of the facial vasculature is fundamental for the safe delivery of nonsurgical cosmetic procedures. The purpose of this review is to provide an illustrated approach to examine surgical anatomy specific to the facial vascular system and the anatomical considerations clinicians need to give in specific danger during injectable cosmetic procedures. A grounding in safety and anatomy will help the new injector to mitigate the risk of vascular complications.
Keywords: Fillers, Hyaluronic acid (HA), Anatomy, Adverse events
Introduction
Nonsurgical injectable procedures to improve facial attractiveness are a challenge complicated by different ethnicity and a demanding and diverse society.1 Internet access and social media platforms are responsible for the rapid globalization of the perception of beauty in all ages. Snapchat and Facetune dysmorphia especially prevalent in Millennials show no geographical boundary and transcend cultural differences.2,3 The societal and cultural pursuit of perfection is increasing and appears to be a key determinant in the rapidly increasing popularity of injectable cosmetic procedures.
Hyaluronic acid (HA) filler products for injection have been globally available since 1996 with the first report of vascular complications described within the literature in 2002.4 The Aesthetic Plastic Surgery National Databank Statistics for 2018 recorded a total of 810,240 HA filler for nonsurgical cosmetic procedures in the United States, with the reported adverse events increased by 130% from 2015 to 2019.5
Adverse events resulting from soft tissue filler treatments are rare but well documented.6,7 Knowledge regarding their proposed prevention and management is a critical factor for patient safety.8 A recent internet survey concluded that 61% of experienced trainers have faced vascular complications in their own practice.9 The most serious adverse event relates to the embolization of soft tissue filler in the arterial circulation, which travels to regions most vulnerable to ischemia because of limited collateral perfusion, e.g., the glabellar region.10 The aim of this narrative review is to provide an illustrated approach to examining facial anatomy specific to the facial vascular system, which is critical to the delivery of lower risk injectable cosmetic procedures and to define the anatomical structures and risk zones critical to avoid complications. By providing a visual and descriptive basis for a grounding in safety and anatomy, this article contributes mitigating the risk of vascular complications for the new injector.
Methods
The methodology for this narrative review included the identification of data from meta- analyses, randomized control studies, and case reports of adverse events following HA filler injections for nonsurgical cosmetic procedures. A review of current publications was performed to determine the relationship between vascular compromise, anatomical danger zones, the use of HA dermal fillers, and protocols for complication. A search strategy was developed and applied to search the Embase (1980-2020) and MEDLINE (1946-2020) databases for relevant literature. A concept table was generated to define the research question according to the patient, intervention, comparator, outcome, and study design (PICOS) principle and also apply inclusion and exclusion criteria (Table 1). The search strategy is shown in Table 2.
Table 1.
Concept table.
| PICOS element | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Problem | Humans Animals |
In vitro experiments |
| Intervention | Hyaluronic acid fillers | Nonhyaluronic acid fillers Other injectables |
| Comparison/control | - | - |
| Outcome | Vascular (arterial and venous) events and complications including thrombus formation, embolization, occlusion, compression, and vasospasm | Plastic surgery intervention Infection Swelling Nodule formation |
| Study design | English language texts Case Studies Randomised controlled trials Review articles Meta-analysis Studies dated 2010-2020 |
NonEnglish language texts Studies dates prior to 2010 |
Table 2.
MeSH (Medical Subject Headings) terms and search strategy.
| Vascular | And | Complications | And | Injectables |
|---|---|---|---|---|
| Vascular* OR Arterial OR Artery OR Vein OR Venous Or Vessel* OR Anatom* |
Complicat* OR Adverse effect OR Thrombosis OR Thrombus OR *embolic OR Embolous OR Necrosis OR Necroti* OR Occlusion Or Occlude* OR Clot OR Blind* OR Danger* OR Compress* Or Spasm |
Injectable* OR Injection* OR Filler OR HA OR Hyaluron* OR Implant OR HA Filler |
Anatomical consideration of vascular risk in the face
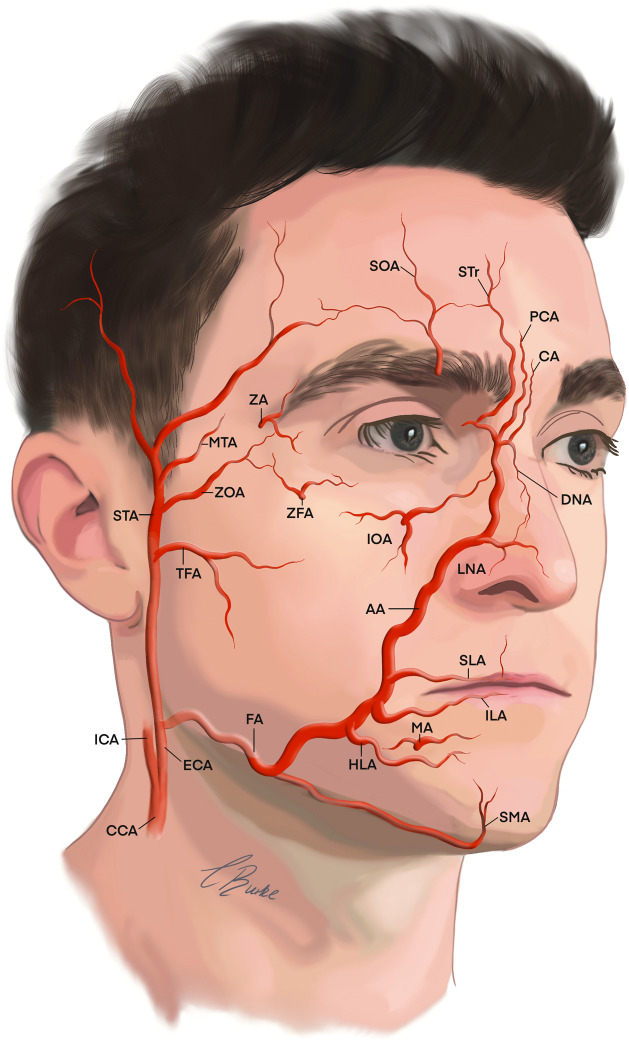
The facial arterial blood supply is highly variable in 2-D pattern (distribution) and 3-D (depth) between faces and contralateral sides of the same face.10 This vascular supply is fundamental for the health of the tissues of the face.11 The arterial supply of the face originates from the two branches of common carotid arteries. The external carotid artery (ECA) generally perfuses the structures within the viscerocranium, while the internal carotid artery (ICA) perfuses the neurocranium.10 Multiple anastomoses exist to ensure a collateral supply with strong ipsilateral contributions between the ECA and ICA through the ophthalmic artery pathway for intra- and extra-cranial perfusion. The ECA and its tributaries are the main arterial supply for the face (Figure 1), while the major contribution of forehead arises from the ophthalmic artery branch of the ICA.11 Deep and superficial fascial vascular plexuses exit connected by perforators over the entire face. The anterior zones of the face have a cutaneous supply related to musculocutaneous perforators, with the lateral arterial supply arising from fasciocutaneous perforators. Predicting 3-D anatomical depth and 2-D anatomical distribution allows practitioners to develop techniques to help mitigate vascular adverse events.10
Figure 1.
The arterial supply to the face is from the ECA and ICA.
Arteries contributing to the vascular supply of the face: AA; angular artery, CA; central artery, CCA; common carotid artery, DNA; dorsal nasal artery, ECA; external carotid artery, FA; facial artery, HLA; horizontal labiomental, ICA; internal carotid artery, ILA; inferior labial artery, IOA; infraorbital artery, LNA; lateral nasal artery, MA; mental artery, MTA; middle temporal artery, PCA; paracentral artery, SLA; superior labial artery, SMA; Submental artery, SOA; supraorbital artery, STA; superficial temporal artery, STr; supratrochlear artery, TFA; transverse facial artery, ZA; zygomaticotemporal artery, ZFA; zygomaticofacial artery, ZOA; zygomatico-orbital artery.
Layers of the face: high and low risk zones
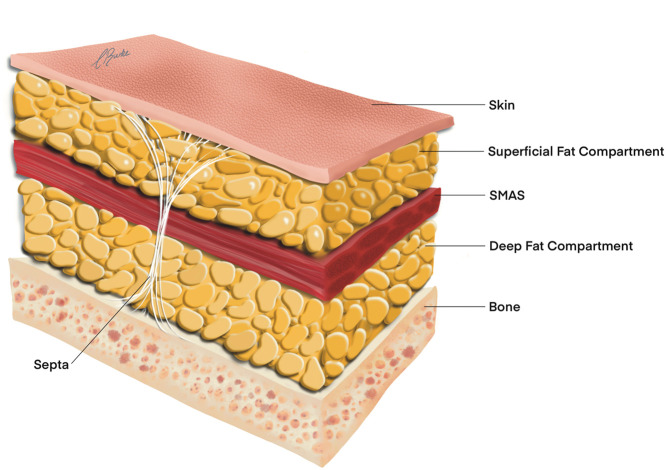
The face generally consists of five concentric anatomical layers from superficial to deep: skin (layer 1), superficial fat (layer 2), muscle (layer three), deep fat (layer four), and bone (layer five) (Figure 2). Layer 4 is also referred to as a layer of spaces/gliding planes and containing retaining ligaments, as described by Mendelson.12,13 There are the only three exceptions to the five layers of the face concept, the temple (10 layers), the tear trough (three layers), and the preauricular region (seven layers). There is a statistical predictable consistency of the 3-D depth of vessels within these layers.10 The authors propose to utilize this anatomical 3-D consistency to formulate lower risk zones for injection.
Figure 2.
Five anatomical layers of the face.
SMAS; superficial musculoaponeurotic system.
Anatomical risk zones
Each anatomical risk zone has different pertinent vascular anastomoses of the ECA and ICA.14 The nose and glabella form are the most reported cases of blindness, though moderate risk sites include the nasolabial folds, forehead, periocular region, temple, and cheek.15 There are no safe zones for injection, and all regions of the face represent a risk.16 Most soft tissue filler injection techniques used are opinion-based, with no evidence-based validation in the cadaveric model for safe practice.1 The assessment of a patient for HA filler injection can be guided by the respective anatomical subunit. Each anatomical subunit differs in risk, injection technique, and product rheology. The main objective remains consistent – to avoid high risk procedures while providing the patient with their ideal aesthetic outcome. We offer consideration of these factors in the following six facial anatomical subunits: (1) forehead and glabella complex; (2) temple; (3) cheeks and nasolabial fold; (4) nose (5) lips and perioral region; and (6) chin and jawline.
Forehead and glabella complex
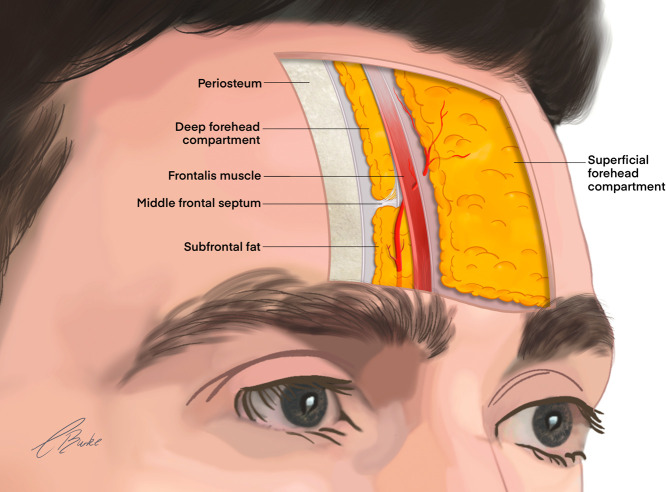
The forehead has distinct anatomical boundaries: superiorly at the hairline, the superior boarder of the orbit inferiorly, and the laterally the superior temporal fusion line.17 The forehead consists of five distinct layers, and the vascular architecture of the region is well documented.
The ophthalmic artery (OA)
The OA is the first intracranial branch of the ICA arising within the middle cranial fossa perfusing the eye and surrounding periorbital region. This artery is the major arterial anastomosis between the ICA and ECA.11 The distribution pattern is complex and unique in individuals.10 The central retinal artery is a significant branch for vascular complications supplying the retina. The superficial branches of OA supply the skin of the forehead: supratrochlear, supraorbital, and dorsal nasal artery.
The supratrochlear artery (STr)
The STr artery is a terminal branch of the OA that exits the orbit 17-22 mm lateral to the midline. The STr artery traverses the margin of the orbit, emerging deep from a supratrochlear notch or foramen before transitioning more superficially as it ascends cranially (Figure 3).18 At the orbital margin, the STr artery perforates or is superficial to corrugator supercilii and deep to both the orbicularis oculi and frontalis muscles. The vessel ascends to reach the middle frontal septum at approximately 13-17 mm in the midline, where it transitions superficially through frontalis and orbicularis oculi to the overlying fascia in the subcutaneous plane.10 An ultrasound-based investigation by Cotofana et al. has shown that the deep branch of the STr artery changes plane from deep to superficial to the frontalis muscle at a mean distance of 14 mm (range, 10.0-19.0 mm in males, 4.0-27.0 mm in females) when measured from the superior orbital rim.19 The topographical landmark for this vessel is the glabellar crease or a zone 3.2 mm lateral to crease.14 The average entire volume of the STr artery from the glabella to the orbital apex is 0.085 ml (range 0.04–0.12 ml), and injection volume should not exceed this volume at critical injection points.20 The STr artery laterally anastomoses with the supraorbital and medially with the angular artery.
Figure 3.
Sagittal course of the supratrochlear artery in the forehead.
The supraorbital artery (SOA)
The SOA is another terminal branch of the OA that exits the orbit via a supraorbital notch or foramina around 32 mm laterally to the midline and corresponds to a line vertical from the medial limbus of the eye.14 The SOA emerges deep and ascends cranially with variable distribution. The medial course perforates the corrugator supercilii, but the lateral course does not contact the muscle.10 The SOA ascends in the inferior frontal septum and at the junction with the middle frontal septum gives perforators to supply the periosteum. In the same ultrasound-based investigation of the STr artery by Cotofana et al., the deep branch of SOA changed plane from deep to superficial to the frontalis muscle at a mean distance of 13 mm (range, 7.0-19.0 mm) in males and at 14 mm (range, 4.0-24.0 mm) in females when measured from the superior orbital rim.19 The SOA has both the deep and superficial branches that anastomose with the superficial temporal artery both medially and laterally.
The superficial temporal artery (STA)
The STA is a terminal branch and most cranial tributary of the ECA possessing complex anastomoses within the forehead. The STA connects with the supratrochlear, supraorbital, dorsal nasal (DNA), and angular arteries (AA) generally in the region lateral inferior and middle third of the forehead. The frontal branch of STA is located over the frontalis, approximately 15 mm superior and 14 mm posterior to the peak of the brow, having multiple anastomoses with the contralateral branch.21
Glabella complex
In the glabella, the STr, SOA, and DNA traverse both superficially and deeply with multiple perforators.14 The midline can possess the central artery from the DNA and paracentral arteries branches from the angular artery. The glabella represents a high-risk area for potential anastomoses between branches of ICA and the ECA with direct pathways to the vascular supply of the eye.11
Temple
The temple has distinct boundaries: the superior temporal fusion line forms a curved boundary both superiorly and anteriorly, the frontal process of the zygomatic bone anteriorly and inferiorly on the arch of the zygoma before transitioning to the midface. There are 10, often contested, distinct layers in the temple, and specific vascular distribution can be found in respective layers.10 The temple has potential anastomoses of both the ICA and ECA.11
The zygomatico-orbital artery (ZOA)
The ZOA is a branch of the STA or middle temporal artery and is the largest artery in the temple.22 This vessel can be classified into 3 groups by anatomical relationship and bifurcation from the source vessels. The originate is approximately 11.3 mm in front of the midpoint of the apex of the tragus, with most trunks located less than 20.0 mm above the zygomatic arch.23 The rich plexus of anastomose between the ECA and ICA represents a high risk of vascular complications through the eyelid vessels. The mean diameter of the zygomatico-orbital artery is 1.2 ± 0.2 mm and has an average depth of 5.61 mm and a length of 8.50 cm.22,23
The lacrimal artery (LA)
The LA is the second and largest branch of the OA. It enters the orbit and traverses along the superior edge of the lateral rectus muscle. It supplies the eyelids, lacrimal gland, and conjunctiva. The lacrimal artery is a branch of the OA system and gives 2 branches: zygomaticofacial artery and zygomaticotemporal artery.
The zygomaticotemporal artery (ZA)
The zygomaticotemporal artery (ZA) exits a small foramen in the lateral wall of the orbit and contributes to the blood supply of the temple. The ZA perforates the orbicularis oculi muscle in a variable 2-D pattern, forming a rich plexus in lateral orbital and temple region.
The superficial temporal artery (STA)
At the superior border of the zygomatic arch, the STA gives off the middle temporal artery. Ascending cranially over the zygomatic arch, the vessel bifurcates into frontal and parietal branches on average 3 cm superior to the apex of the tragus, showing a mean deviation of 87 degrees. The vessels perfuse the forehead-temple part of the zygomatic complex and ear.11 The mean diameter of the frontal branch is approximately 2 mm and is always situated 10 mm anterior and 10 mm superior from the apex of the tragus and lies within the superficial temporal fascia.10 The STA traverses the temporal fusion line and changes planes to a subcutaneous plane, anastomosing with the tributaries of the ICA of the forehead. The STA is often visible and palpable following a tortuous course.
The middle temporal arteries (MTA)
The MTA branches from the STA above the zygomatic arch before transition deep to perfuse the deep temporal fascia and gives multiple branches that supply the temporalis muscle. The MTA anastomoses with the anterior and posterior deep temporal arteries.
Deep temporal arteries (DTA)
Deep in the temporal fossa, the anterior deep and posterior deep temporal arteries run cranially at 1.5-2 cm and 2.5-3.0 cm, respectively, from the lateral orbital rim and perfuse the temporalis muscle.10
Cheeks and nasolabial fold
The main blood supply for the cheeks originates from the transverse facial artery and the facial artery. The buccal artery, a branch of the maxillary artery, contributes to the blood supply in the buccal region of the cheek.11
The zygomaticofacial artery (ZFA)
The upper portion of the cheek over the zygoma is supplied by the ZFA, arising deep on the zygoma through a foramen. A branch of the lacrimal artery that represents a potential danger zone back to the OA and vision disturbance via an anastomosis between the ECA and ICA or direct intravascular injection in the region. The ZFA is a small vessel with a diameter of 0.3 mm.11
The transverse facial artery (TFA)
The TFA is the STA's major tributary.11 The TFA has its origin in the parotid gland as a branch from the superficial temporal artery. At the origin, the TFA has a mean diameter of around 1 mm (+/- 0.4 mm) and follows a transverse course parallel approximately 2 cm below the zygomatic arch.24,25 The TFA is divided into superior and inferior trunks in the gland and then crosses the masseter. It has been classified into four types according to branching patterns. The TFA has a significant role in the blood supply to the lateral face.26 This artery can anastomose with the facial and infraorbital arteries and supplies blood to the parotid gland and duct, facial nerve, facial musculature, and skin in the lateral and medial face.
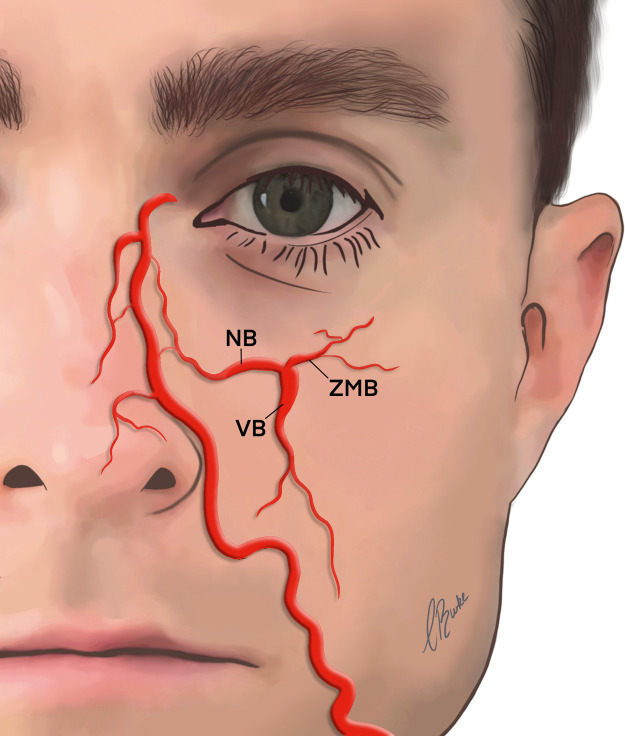
The infraorbital artery (IAO)
The IAO is a terminal branch of the maxillary artery, and on exiting the maxilla via the infraorbital foramen, approximate 9.1 mm below the orbital rim, it divides into multiple branches.11 There are three main branches of the IOA that are involved in the perfusion of the infraorbital, midface, and upper lip regions (Figure 4), with an average diameter for all IOA branches being 0.5 mm.27
-
1.
Nasal artery branch (NB). The NB has an average diameter of 0.6 mm supplying the lateral aspect of the nose.27 This vessel traverses the periosteal plane and has significant anastomoses with DNA, AA, and STr artery.
-
2.
Zygomaticomalar artery branch (ZMB) is also known as the palpebral branch.27,26 The ZMB branch supplies the tissues of the lower eyelids, and the cheekbone region has an average diameter of 0.7 mm.27 The ZMB becomes superficial around 17 mm medial to the edge of the zygomatic arch and travels in the infraorbital fat pad before perfusing skin of the cheek.
-
3.
Vestibular artery branch (VB) is also known as the labial branch.27,28 The VB supplies the vestibule and oral mucosa of the upper jaw. It has a diameter of 0.7 mm and was found to be too narrow to disrupt when cannula technique is performed.27
Figure 4.
Branches of the IOA.
AA; angular artery, DNA; dorsal nasal artery, IOA; infraorbital artery, NB; nasal artery branch, STA; superficial temporal artery, ZMB; zygomaticomalar artery branch.
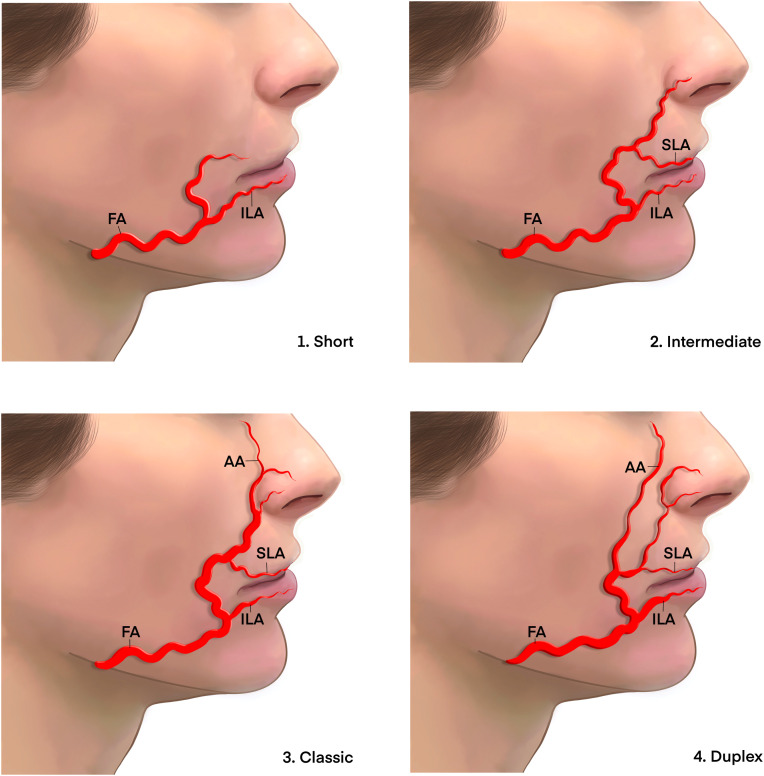
The facial artery (FA)
The principle vascular supply to the mobile anterior face is the FA.29,30 The FA branches from the ECA as a separate branch or rarely as a common branch with the lingual artery.11 The FA has an extremely tortuous course that provides for excess vessel length, allowing for open oral cavity and expression without extension that would constrict the bore and restrict blood flow.31 There is a large heterogeneity in the distribution patterns of the FA generally classified into four main types.32
-
1.
The classic course that extends to the branch of the angular artery terminating at medial canthus (Figure 5.1).
-
2.
An intermediate course that terminates at the superior labial artery (Figure 5.2).
-
3.
The short course that terminates before the superior labial artery (Figure 5.3).
-
4.
The duplex course that shows a dominant lateral angular branch (Figure 5.4).
Figure 5.
Variations in course and branching pattern of the FA.
AA; angular artery, FA; facial artery, DNA; dorsal nasal artery, NB; nasal artery branch, VB; vestibular artery branch, ZMB; zygomaticomalar artery branch.
Adapted from Furukawa M, Mathes DW, Anzai Y. Evaluation of the facial artery on computed tomographic angiography using 64-slice multidetector computed tomography: implications for facial reconstruction in plastic surgery. Plast Reconstr Surg 2013; 131: 526–535.
The FA courses the lower border of the mandible and curves upward to the lateral aspect at the premasseteric notch beneath platysma. Deep in the buccal space, ascending with a tortuous course and a diameter of approximately 2.14 mm.25 The buccal space is a facial space that resists age-related dimensional changes, proving a reliable and predictable zone for aesthetic procedures.33 The symmetry and variation between the 2-D and 3-D distributions ranges from 52% to 68%, with right-sided dominance.32
The FA is fixed by a muscular band from buccinator approximately 15 mm posterior to the commissures close to the modiolus and between the buccinator and platysma and the converging muscles of facial expression.10
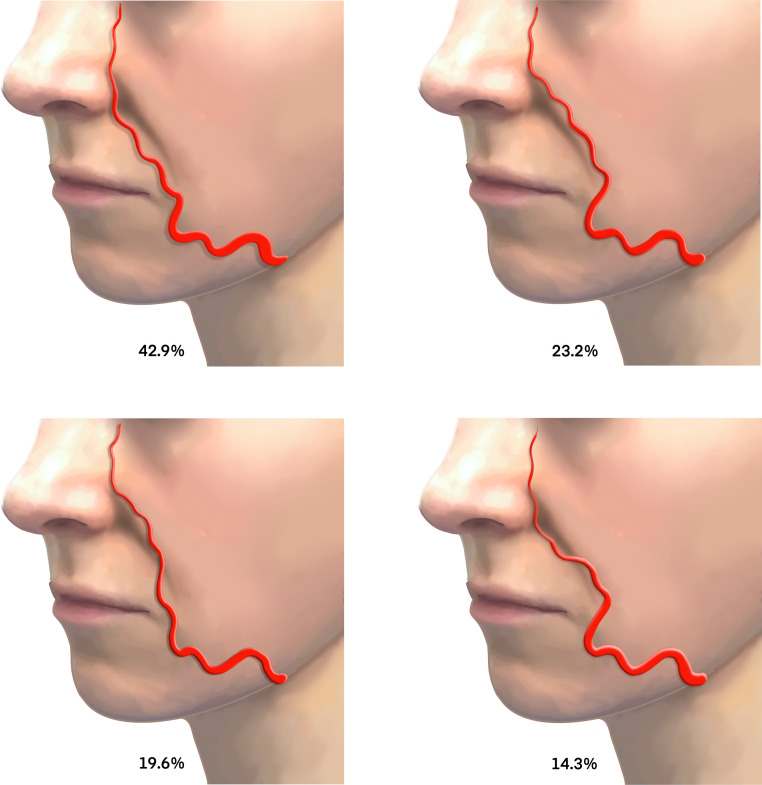
The angular artery (AA)
The FA becomes the AA after it branches from the superior labial artery (SLA).10,30 The AA is a small tributary of the FA traversing a tortuous 2-D path in the dermis of the NLF presenting medial (42.9%), lateral (23.2%), or crossing the NLF (33.9%) (Figure 6).34 The AA may occasionally follow a deeper course along the roof of the deep pyriform space. Injection deep on bone in this region reduces the risk of adverse vascular events.35 As AA ascends, it anastomoses with vascular branches of the lateral nose and medial branches of the infraorbital artery, forming a complex rich plexus of collateral supply.30
Figure 6.
Variations in coursing patterns of the facial artery.
Medial (above left, 42.9%), lateral (above right, 23.2%), and crossing the variations (below left and right, 33.9%).
Adapted from Yang H-M, Lee J-G, Hu K-S, et al. New anatomical insights on the course and branching patterns of the facial artery: clinical implications of injectable treatments to the nasolabial fold and nasojugal groove. Plast Reconstr Surg 2014; 133: 1077–1082.
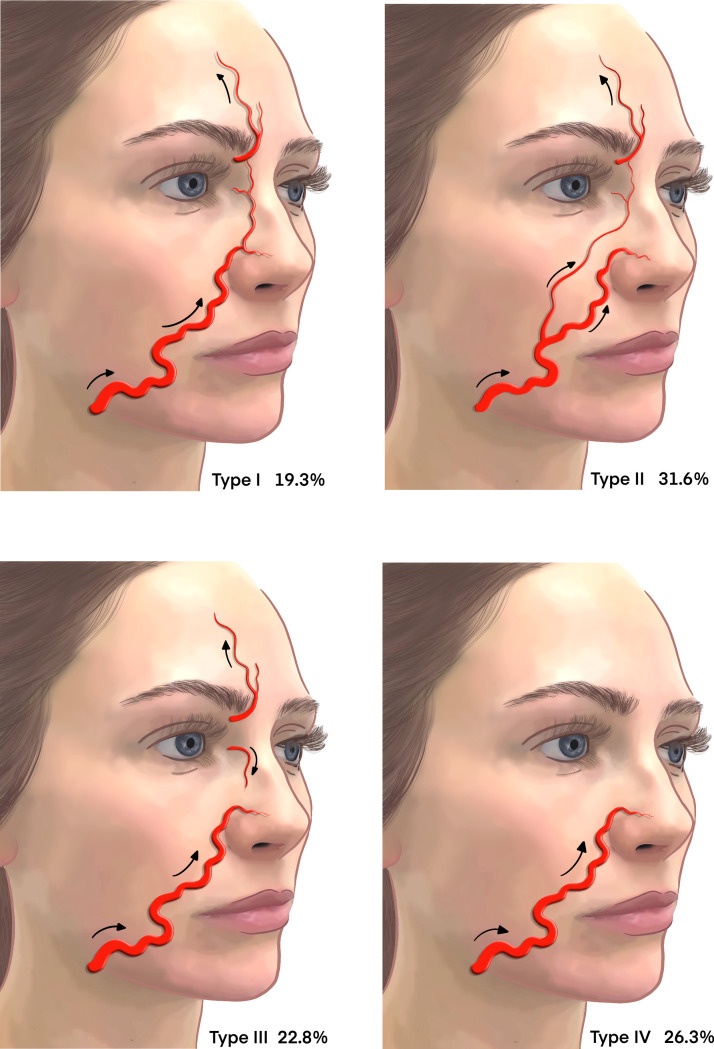
After transitioning the NLF, there are four branching patterns of the AA (Figure 7).
-
1.
This distribution pattern originates cranially at the branch of LNA adjacent to the ala of the nose (Figure 7, Type I).
-
2.
This is the detouring pattern in which the AA traverses continuously from the detouring branch of the FA and ascends cranially to the nasojugal groove and medial canthal areas (Figure 7, Type II).
-
3.
An alternative pattern in which the AA originates only from the OA and demonstrates a reverse blood flow direction (Figure 7, Type III).36
-
4.
The latent pattern in which the FA terminates around the nasolabial area without giving off an AA branch (Figure 7, Type IV).
Figure 7.
Variations in branching patterns of the AA.
Adapted from Kim YS, Choi DY, Gil YC, Hu KS, Tansatit T, Kim HJ. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatologic Surgery. 2014 Oct 1;40(10):1070-6.
Nose
The nose is a highly vascular structure with complex anastomoses between the ICA and ECA. The literature now indicates that soft tissue filler injections for nonsurgical rhinoplasty are the primary cause of vision loss.15,37
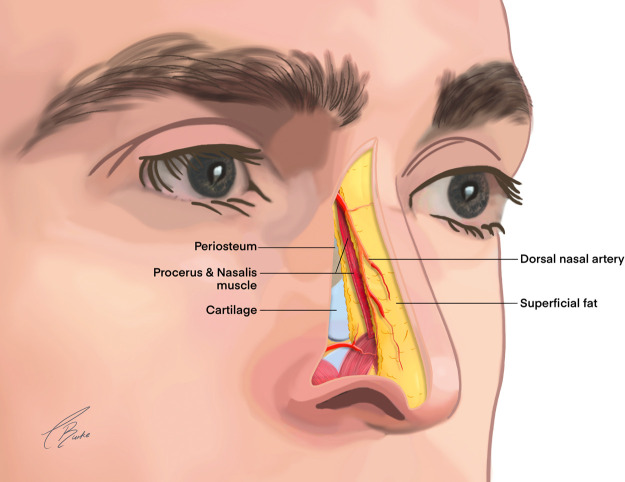
The 5 layers of the nose include skin, superficial fat, muscle and fascia (superficial musculoaponeurotic layer [SMAS]), areolar tissue, and cartilage/bone.14 The superficial blood supply of the nose is located above the nasal SMAS (Figure 8) and is composed of a complex multidirectional anastomotic system of the ECA and ICA.38
Figure 8.
Relationship of the dorsal nasal artery to procerus and nasalis.
The vasculature of the nose has three main arterial distribution patterns in the 2-D plane. The source of the blood supply identifies the patterns according to facial, dorsal nasal, or infraorbital.30 The complex plexus of vessels that supply the highly vascular nose also include the superior labial, columellar, the superior and inferior alar arteries, and a contralateral supply.11
The dorsal nasal artery (DNA)
The AA gives branches to lateral nasal artery that perfuses the nose in a variety of 2-D patterns. The DNA is a bilateral terminal branch of the OA.10 The DNA traverses out of the orbit through the orbital septum and runs approximately 5 mm superiorly to the medial canthal ligament. Deep to orbicularis oculi anastomosis with contralateral DNA on nasal bone at the origin of procerus muscle. The DNA has multiple plexus of anastomoses with angular, palpebral, and supratrochlear arteries.
The lateral nasal artery (LNA)
The LNA is the main blood supply to the tip, with DNA the main blood supply to the upper portion of the nose. The vessel has an internal diameter of approximately 0.5 mm and branches from the AA several times along the lateral border of the nose.11
Lips and perioral region
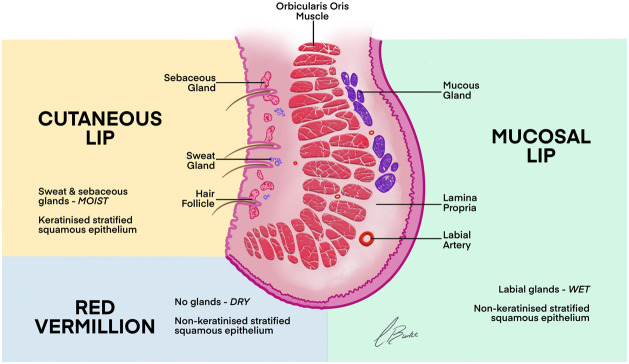
This region is the most common for complications, but the incidence rate is low, and the majority of adverse events are mild.39 The lip can be classified into four zones – cutaneous portion, intramuscular portion containing orbicularis oris, red vermillion (dry oral mucosae), and the mucosal lip (wet oral mucosa) in respect to arterial position (Figure 9). The blood supply includes the following arteries.
Figure 9.
Classification of the lip into four zones.
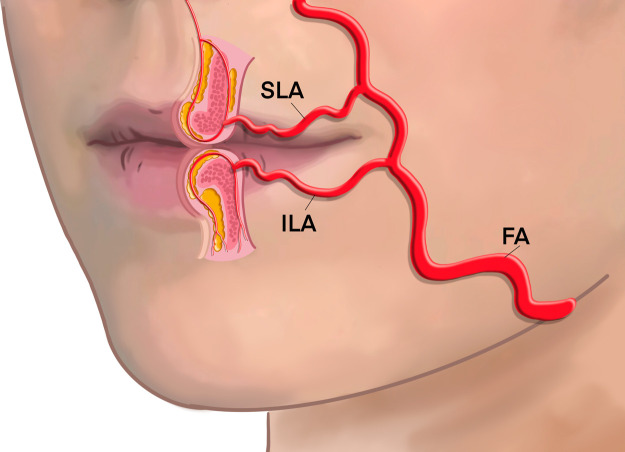
The superior labial artery (SLA)
The SLA branches around the level of the commissure have an average external diameter of 1.6 mm (0.6-2.8 mm) and a lumen diameter of 0.85 mm+/-0.34 mm, diminishing to 0.56+/-0.21 mm at the midline.25,40 The SLA traverses in the subcutaneous layer between orbicularis oris and the skin before transitioning deep to anastomose with the contralateral vessel, forming a rich arterial plexus (Figure 10). In Caucasians, the SLA was found submucosal in 78.1%, intramuscular in 17.6%, and subcutaneous in 2.6%, but it transitioned between planes once 16%, twice 13%, and took a more consistent course than the ILA.41 The SLA provides multiple small caliber superficial and deep perforator septal and columella vessels that ascend to supply the upper cutaneous lip tissue, forming a rich nasal tip plexus. Similar position of this vessel has also been confirmed in Asian lip morphology by ultrasonographic evidence.42
Figure 10.
Positional relationship of SLA and ILA.
The inferior labial artery (ILA)
At the origin of the ILA, the diameter is 1.6 mm and runs toward the midline, and it forms a rich plexus with the contralateral ILA, labiomental, mental, and submental arteries.25 In Caucasian, the ILA travels inferior to the vermillion border, with a distribution pattern identified to course submucosally 78.1%, intramuscular 17.3%, and subcutaneously 1.7%, but it transitioned between these planes once 9% and twice 23%.41 Similar morphology has been recognized in the Asian population.42
The variable 2-D and 3-D distribution/depth pattern of these labial arteries is determined by embryogenesis. The vessels formation precedes the formation of the precursor cells for orbicularis oris muscle, which forms around the pre-existing perioral vasculature.36
Many previous studies have suggested a guideline for lower risk injection depth of soft tissue fillers for lip augmentation, recommending that intimate knowledge of lip topography is fundamental to prevent complications with superficial injection.41, 42, 43, 44
The horizontal labiomental artery (HLA)
Within the buccal space, the FA give a rise to the HLA as a single branch or as a common trunk with the ILA. The HLA perfuses the labiomental region of the chin anastomoses, with the ILA to create a complex perioral vascular plexus.39
Chin and jawline
The mental area (chin) derives its perfusion from a rich plexus of anastomoses between the mental artery, the submental artery, and the rich plexus of connections between the inferior labial and labiomental arteries.11
The mental artery (MA)
The mental artery emerges from the mental foramen in the body of the mandible and at this point has a diameter of around 1.68 mm.25
The submental artery (SMA)
The submental artery perfuses the lower part of chin and submental region, and perforators extend toward the oral commissure.11 The possibility of an uncommon variation in the perfusion of the midline chin arises from a median artery arising from a midline foramen.45
Discussion and recommendations
The current evidence base suggests that facial vasculature has variable branching patterns, course (2-D) and depth (3-D). This anatomical knowledge is fundamental in the avoidance of complications and safest practice.10 Soft tissue filler complications can be classified according to severity (mild, moderate, or severe); nature (ischemic complications and nonischemic); or time of onset (early or late).39 There is a prerequisite obligation that practitioners can recognize and manage vascular complications.9 Intra-arterial soft tissue filler embolus has been reported in all branches of the facial and ophthalmic artery, which supports the evidence that no region of the face is risk free.46 Vascular complications usually have cutaneous involvement, but they can extend to the subcutaneous fat, muscle, deep fat, tendons, and even bone if unresolved, with potential for life-altering consequences, including tissue ischemia and loss, visual loss, pulmonary embolization, and stroke.15,47 Vascular complications represent different risks associated with variation in arterial distribution patterns. Patients without an anastomosis between the FA and the DNA have a lower risk of visual disturbance than patients with this anastomosis.30
It is important to recognize the vast variation in facial vasculature that exists, with less than 50% of patients possessing the standard description of FA distribution pattern and of those only 25% are bilaterally symmetrical.30 Importantly, statistical consistency exists within the literature as to the vessel depth. Cannula injections rather than needle injections is generally considered to be the safest option, but practitioners still need to be vigilant with good anatomical knowledge as there are case reports of blindness following cannula injections.44
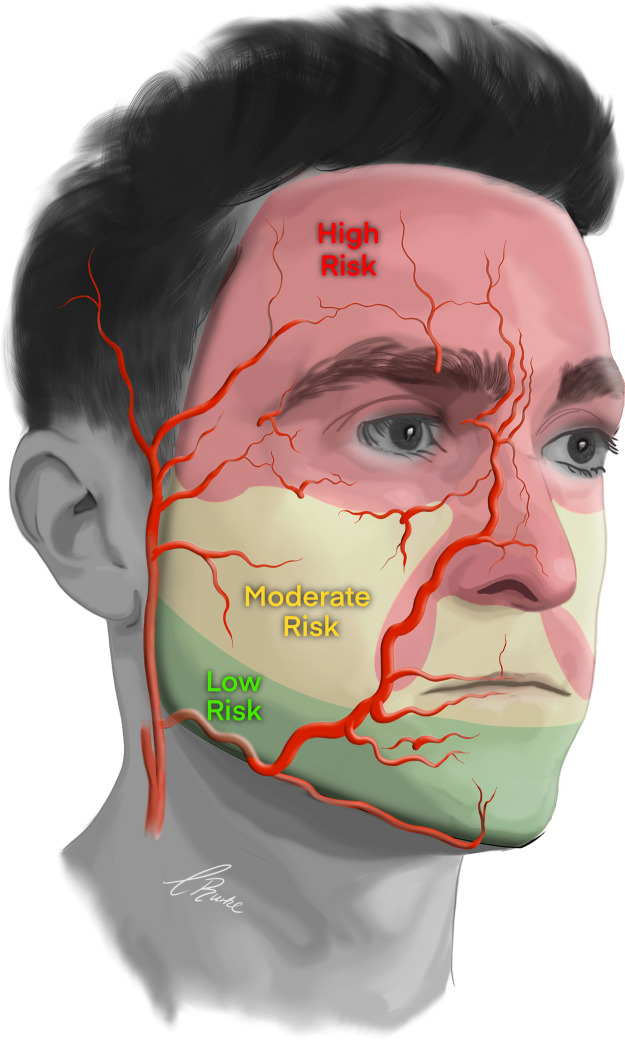
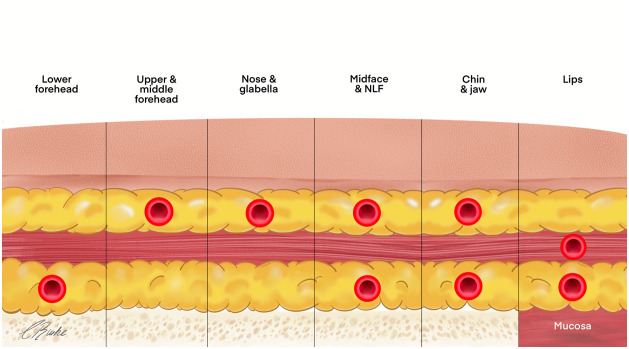
The topographical areas of the face and the corresponding risk of vascular complications are summarized in Figure 11. The main risk of complications is found in layers two, three, and four (Figure 12). The 2-D distribution has highly individual variation, but 3-D anatomy is generally regarded as consistent. Regions of the face can be generally classified by risk, and the risk is statistically related to the layers in which the vessels are located (Table 3). Understanding the 3-D depth of the vessel allows the injection to be in the layers of lower risk. If the vessel has a good 3-D statistical probability to traverse deep in layer 4, then superficial injections in layer 2 reduce the risk, and vice versa.
Figure 11.
Summary of the topographical areas of the face and corresponding risk of vascular complication.
Figure 12.
3-D vasculature areas of the face with vessel signifying the anatomical layer posing the highest risk for intravascular injection.
NLF; nasolabial fold.
Table 3.
Summary of anatomical high- and low-risk zones.
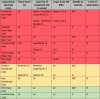 |
A; advanced, B; beginner, HA; hyaluronic acid, I; intermediate, NLF; nasolabial fold, TT; tear trough.
*the superior temple compartment is the target zone. The danger in is the anterior deep temporal arteries in the inferior compartment. The tear trough is a unique area having only three layers, skin, muscle, and bone. The layer of maximum risk is superficial layer two, above the muscle but below the skin. The layer for injection is below the muscle supra-periosteal but still layer two since the technique creates a small channel between bone and muscle.
In a recent consensus, the concept of graded training for nonsurgical cosmetic procedures was introduced, highlighting the anatomical areas of increased risk that require more specific training, greater supervision, and access to medical care.9 The conclusion was that teaching required rethinking and a graded system from low to high risk be employed. The high-risk indications should only be performed as experience and knowledge increases and conducted in correct clinical settings. Grade one procedures are those with a low risk of intravascular injection and embolic events. Excellent areas for beginner training were included jaw line, chin, marionettes, and lateral cheek. Grade two procedures are those with a moderate risk of intravascular injection and embolic events. These include the lips and perioral region, which are considered a high risk of intravascular injection and embolisation but only a moderate risk of visual loss. Grade three procedures are those with a moderate risk with significant risk of serious intravascular injection and visual embolic event. High risk indications require strict technique, good anatomical knowledge, and product placement that include temple nasolabial fold tear trough periorbital and medial cheek. Grade four procedures are those with a very high risk of significant intravascular injection and blindness. These represent the regions perfused directly by the branches of the ICA. The practitioner requires to have progressed the graded framework, acquiring experience, knowledge, and training. These regions represent the highest risk including the nose glabella and forehead.9
Conclusion
An in-depth understanding of the applied surgical anatomy is essential for lower risk soft tissue filler injectable procedures. Regulated pathways of progression with core curriculum focused on education and clinical transferable skills are required. Further consideration for a mandatory qualification with the specific intent to evaluate tangible competence skill judiciously and precisely without variation and subjectivity is paramount in this fast-moving discipline. In the present study, we have focused on the visual and descriptive basis for a safe approach to HA filler injections during cosmetic procedures. Through this illustrated grounding in surgical relevant anatomy, we hope that the new injector will be able to mitigate the risk of vascular complications in their own practice.
Financial Support
SRA is funded by the Welsh Clinical Academic Training (WCAT) Fellowship. ISW is funded via a EURAPS/AAPS Academic Scholarship.
Conflicts of interest: None.
Institutional ethical approval: None.
Reporting standards: Not applicable.
Authorship
All listed authors contributed to; 1) conception and design, acquisition of data, analysis and interpretation of data; 2) drafting the article or revising it critically for important intellectual content; 3) final approval of the version to be published; 4) agreement to be accountable for all aspects of the work.
Acknowledgements
We would like to thank Dr Toni Burke who produced the artwork and illustrations for the article.
References
- 1.Ghannam S, Sattler S, Frank K, et al. Treating the Lips and Its Anatomical Correlate in Respect to Vascular Compromise. Facial Plast Surg. 2019;35:193–203. doi: 10.1055/s-0039-1683856. [DOI] [PubMed] [Google Scholar]
- 2.Cristel RT, Dayan SH, Akinosun M, et al. Evaluation of Selfies and Filtered Selfies and Effects on First Impressions. Aesthet Surg J. 2021;41:122–130. doi: 10.1093/asj/sjz362. [DOI] [PubMed] [Google Scholar]
- 3.Heydenrych I, Kapoor KM, De Boulle K, et al. A 10-point plan for avoiding hyaluronic acid dermal filler-related complications during facial aesthetic procedures and algorithms for management. Clin Cosmet Investig Dermatol. 2018;11:603–611. doi: 10.2147/CCID.S180904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schanz S, Schippert W, Ulmer A, et al. Arterial embolization caused by injection of hyaluronic acid (Restylane) Br J Dermatol. 2002;146:928–929. doi: 10.1046/j.1365-2133.2002.04707.x. [DOI] [PubMed] [Google Scholar]
- 5.Chatrath V, Banerjee PS, Goodman GJ, et al. Soft-tissue Filler–associated Blindness: A Systematic Review of Case Reports and Case Series. Plast Reconstr Surg Glob Open. 2019;7:e2173. doi: 10.1097/GOX.0000000000002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philipp-Dormston WG, Bergfeld D, Sommer BM, et al. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J Eur Acad Dermatol Venereol. 2017;31:1088–1095. doi: 10.1111/jdv.14295. [DOI] [PubMed] [Google Scholar]
- 7.Schelke LW, Velthuis P, Kadouch J, et al. Early ultrasound for diagnosis and treatment of vascular adverse events with hyaluronic acid fillers. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2019.07.032. S0190-9622(19)32392–8. [DOI] [PubMed] [Google Scholar]
- 8.Torbeck RL, Schwarcz R, Hazan E, et al. In Vitro Evaluation of Preinjection Aspiration for Hyaluronic Fillers as a Safety Checkpoint. Dermatol Surg. 2019;45:954–958. doi: 10.1097/DSS.0000000000001767. [DOI] [PubMed] [Google Scholar]
- 9.Goodman GJ, Magnusson MR, Callan P, et al. A Consensus on Minimizing the Risk of Hyaluronic Acid Embolic Visual Loss and Suggestions for Immediate Bedside Management. Aesthet Surg J. 2020;40:1009–1021. doi: 10.1093/asj/sjz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotofana S, Lachman N. Arteries of the Face and Their Relevance for Minimally Invasive Facial Procedures: An Anatomical Review. Plast Reconstr Surg. 2019;143:416–426. doi: 10.1097/PRS.0000000000005201. [DOI] [PubMed] [Google Scholar]
- 11.von Arx T, Tamura K, Yukiya O, et al. The Face – A Vascular Perspective. A literature review. Swiss Dent J. 2018;128:382–392. doi: 10.61872/sdj-2018-05-405. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson BC. Extended sub-SMAS dissection and cheek elevation. Clin Plast Surg. 1995;22:325–339. [PubMed] [Google Scholar]
- 13.Mendelson BC. Correction of the nasolabial fold: extended SMAS dissection with periosteal fixation. Plast Reconstr Surg. 1992;89:822–835. [PubMed] [Google Scholar]
- 14.Scheuer JF, III, Sieber DA, Pezeshk RA, Gassman AA, Campbell CF, Rohrich RJ. Facial danger zones: techniques to maximize safety during soft-tissue filler injections. Plastic and Reconstructive Surgery. 2017 May 1;139(5):1103–1108. doi: 10.1097/PRS.0000000000003309. [DOI] [PubMed] [Google Scholar]
- 15.Sito G, Manzoni V, Sommariva R. Vascular Complications after Facial Filler Injection: A Literature Review and Meta-analysis. J Clin Aesthet Dermatol. 2019;12:E65–E72. [PMC free article] [PubMed] [Google Scholar]
- 16.Beleznay K, Carruthers JDA, Humphrey S, et al. Avoiding and Treating Blindness From Fillers: A Review of the World Literature. Dermatol Surg. 2015;41:1097–1117. doi: 10.1097/DSS.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 17.Cotofana S, Schenck TL, Trevidic P, et al. Midface: Clinical Anatomy and Regional Approaches with Injectable Fillers. Plast Reconstr Surg. 2015;136:219S–234S. doi: 10.1097/PRS.0000000000001837. [DOI] [PubMed] [Google Scholar]
- 18.Berchtold V, Stofferin H, Moriggl B, et al. The supraorbital region revisited: An anatomic exploration of the neuro-vascular bundle with regard to frontal migraine headache. J Plast Reconstr Aesthet Surg. 2017;70:1171–1180. doi: 10.1016/j.bjps.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Cotofana S, Velthuis PJ, Alfertshofer M, Frank K, Bertucci V, Beleznay K, Swift A, Gavril DL, Lachman N, Schelke L. The change of plane of the supratrochlear and supraorbital arteries in the forehead—an ultrasound-based investigation. Aesthet Surg J. 2021 Nov;41(11):NP1589–NP1598. doi: 10.1093/asj/sjaa421. [DOI] [PubMed] [Google Scholar]
- 20.Khan TT, Colon-Acevedo B, Mettu P, et al. An Anatomical Analysis of the Supratrochlear Artery: Considerations in Facial Filler Injections and Preventing Vision Loss. Aesthet Surg J. 2017;37:203–208. doi: 10.1093/asj/sjw132. [DOI] [PubMed] [Google Scholar]
- 21.Lee S-H, Lee H-J, Kim Y-S, et al. What is the difference between the inferior labial artery and the horizontal labiomental artery? Surg Radiol Anat. 2015;37:947–953. doi: 10.1007/s00276-015-1447-2. [DOI] [PubMed] [Google Scholar]
- 22.Choi DH, Eom JR, Lee JW, et al. Zygomatico-orbital artery: The largest artery in the temporal area. J Plast Reconstr Aesthet Surg. 2018;71:484–489. doi: 10.1016/j.bjps.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Liu S., Yan W., Wang G., Zhao R., Qiu H., Cao L., Wang H. Topographic Anatomy of the Zygomatico-Orbital Artery: Implications for Improving the Safety of Temporal Augmentation. Plastic and Reconstructive Surgery. 2021;148(1):19e–27e. doi: 10.1097/PRS.0000000000008100. [DOI] [PubMed] [Google Scholar]
- 24.Koziej M, Polak J, Wnuk J, Trybus M, Walocha J, Chrapusta A, Brzegowy P, Mizia E, Popiela T, Hołda M. The transverse facial artery anatomy: Implications for plastic surgery procedures. PLoS One. 2019 Feb 7;14(2) doi: 10.1371/journal.pone.0211974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tucunduva M-J, Tucunduva-Neto R, Saieg M, et al. Vascular mapping of the face: B-mode and doppler ultrasonography study. Med Oral Patol Oral Cir Bucal. 2016;21:e135–e141. doi: 10.4317/medoral.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierrefeu A, Brosset S, Lahon M, et al. Transverse Facial Artery Perforators: Anatomical, Two- and Three-Dimensional Radiographic Study. Plast Reconstr Surg. 2019;143:820e–828e. doi: 10.1097/PRS.0000000000005421. [DOI] [PubMed] [Google Scholar]
- 27.Hufschmidt K, Bronsard N, Foissac R, et al. The infraorbital artery: Clinical relevance in esthetic medicine and identification of danger zones of the midface. J Plast Reconstr Aesthet Surg. 2019;72:131–136. doi: 10.1016/j.bjps.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Kim H-S, Lee K-L, Gil Y-C, Hu K-S, Tansatit T, Kim H-J. Topographic Anatomy of the Infraorbital Artery and Its Clinical Implications for Nasolabial Fold Augmentation. Plast Reconstr Surg. 2018;142(3):273e–280e. doi: 10.1097/PRS.0000000000004704. [DOI] [PubMed] [Google Scholar]
- 29.Koziej M, Trybus M, Hołda M, et al. Anatomical Map of the Facial Artery for Facial Reconstruction and Aesthetic Procedures. Aesthet Surg J. 2019;39:1151–1162. doi: 10.1093/asj/sjz028. [DOI] [PubMed] [Google Scholar]
- 30.Pilsl U, Anderhuber F, Neugebauer S. The Facial Artery-The Main Blood Vessel for the Anterior Face? Dermatol Surg. 2016;42:203–208. doi: 10.1097/DSS.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 31.DeLorenzi C. Commentary on: Anatomical Variations in the Course of Labial Arteries: A Literature Review. Aesthet Surg J. 2019;39:1236–1240. doi: 10.1093/asj/sjy339. [DOI] [PubMed] [Google Scholar]
- 32.Furukawa M, Mathes DW, Anzai Y. Evaluation of the facial artery on computed tomographic angiography using 64-slice multidetector computed tomography: implications for facial reconstruction in plastic surgery. Plast Reconstr Surg. 2013;131:526–535. doi: 10.1097/PRS.0b013e31827c6f18. [DOI] [PubMed] [Google Scholar]
- 33.Schenck T.L., Koban K.C., Schlattau A., Frank K., Sclafani A.P., Giunta R.E., Roth M.Z., Gaggl A., Gotkin R.H., Cotofana S. Updated anatomy of the buccal space and its implications for plastic, reconstructive and aesthetic procedures. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2018;71(2):162–170. doi: 10.1016/j.bjps.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Yang H-M, Lee J-G, Hu K-S, et al. New anatomical insights on the course and branching patterns of the facial artery: clinical implications of injectable treatments to the nasolabial fold and nasojugal groove. Plast Reconstr Surg. 2014;133:1077–1082. doi: 10.1097/PRS.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 35.Surek CK, Vargo J, Lamb J. Deep Pyriform Space: Anatomical Clarifications and Clinical Implications. Plast Reconstr Surg. 2016;138:59–64. doi: 10.1097/PRS.0000000000002262. [DOI] [PubMed] [Google Scholar]
- 36.Beer G.M., Bitschnau R., Manestar M. Tracing the Blood Flow Direction of the Angular Artery and Vein by Color Doppler Ultrasonography. J Oto Rec Surg. 2016;2(1):113. [Google Scholar]
- 37.Beleznay K, Carruthers JDA, Humphrey S, et al. Update on Avoiding and Treating Blindness From Fillers: A Recent Review of the World Literature. Aesthet Surg J. 2019;39:662–674. doi: 10.1093/asj/sjz053. [DOI] [PubMed] [Google Scholar]
- 38.Saban Y, Andretto Amodeo C, Bouaziz D, et al. Nasal arterial vasculature: medical and surgical applications. Arch Facial Plast Surg. 2012;14:429–436. doi: 10.1001/archfacial.2012.202. [DOI] [PubMed] [Google Scholar]
- 39.Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of Soft Tissue Filler Complications: Expert Consensus Recommendations. Aesthetic Plast Surg. 2018;42:498–510. doi: 10.1007/s00266-017-1063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Money SM, Wall WB, Davis LS, et al. Lumen Diameter and Associated Anatomy of the Superior Labial Artery With a Clinical Application to Dermal Filler Injection. Dermatol Surg. 2020;46:678–684. doi: 10.1097/DSS.0000000000002074. [DOI] [PubMed] [Google Scholar]
- 41.Cotofana S, Pretterklieber B, Lucius R, et al. Distribution Pattern of the Superior and Inferior Labial Arteries: Impact for Safe Upper and Lower Lip Augmentation Procedures. Plast Reconstr Surg. 2017;139:1075–1082. doi: 10.1097/PRS.0000000000003244. [DOI] [PubMed] [Google Scholar]
- 42.Lee K-L, Lee H-J, Youn K-H, et al. Positional relationship of superior and inferior labial artery by ultrasonography image analysis for safe lip augmentation procedures. Clin Anat. 2020;33:158–164. doi: 10.1002/ca.23379. [DOI] [PubMed] [Google Scholar]
- 43.Lee W, Oh W, Oh SM, et al. Comparative Effectiveness of Different Interventions of Perivascular Hyaluronidase. Plast Reconstr Surg. 2020;145:957–964. doi: 10.1097/PRS.0000000000006639. [DOI] [PubMed] [Google Scholar]
- 44.Tansatit T, Apinuntrum P, Phetudom T. A Dark Side of the Cannula Injections: How Arterial Wall Perforations and Emboli Occur. Aesthetic Plast Surg. 2017;41:221–227. doi: 10.1007/s00266-016-0725-7. [DOI] [PubMed] [Google Scholar]
- 45.Iwanaga J, Watanabe K, Saga T, Tabira Y, Yamaki KI. A rare case of an artery passing through the median perforating canal of the mandible. Case Reports in Dentistry. 2016 Jan 1;2016 doi: 10.1155/2016/8183565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashton MW, Taylor GI, Corlett RJ. The Role of Anastomotic Vessels in Controlling Tissue Viability and Defining Tissue Necrosis with Special Reference to Complications following Injection of Hyaluronic Acid Fillers. Plast Reconstr Surg. 2018;141:818e–830e. doi: 10.1097/PRS.0000000000004287. [DOI] [PubMed] [Google Scholar]
- 47.Doerfler L, Hanke CW. Arterial Occlusion and Necrosis Following Hyaluronic Acid Injection and a Review of the Literature. J Drugs Dermatol. 2019;18:587. [PubMed] [Google Scholar]