Abstract
Skin ageing is characterized by features such as wrinkles, loss of elasticity, laxity, rough-textured appearance, melasma and freckles. Several researches have focused for preventing, and treating skin ageing by many natural ingredients. This study aimed to assess the anti-ageing activities for anti-skin ageing of the ethanolic extracts of Pink rambutan (PR) (Nephelium lappaceum Linn.) from leaves (L), branches (B), seeds (S), and peels from ripe (R) and young (Y) fruits. The extraction yields of all Pink Rambutan (PR) extracted by the Maceration (M) and the Soxhlet extraction (Sox) using 95% ethanol as a solvent, ranged from 10.62% to 30.63%. Flavonoids were found as the main phytochemicals in almost all the PR extracts. The PR-Y-M and PR-Y-Sox extracts gave the highest total phenolic contents by the Folin-Ciocalteu assay of 67.60 ± 4.38 mgGAE/g, and total flavonoid contents by the modified aluminum chloride colorimetric assay of 678.72 ± 23.59 mgQE/g, respectively. The PR-L-M extracts showed the highest three anti-oxidative activities; the free radical scavenging (SC50 of 0.320 ± 0.070 mg/mL), the lipid peroxidation inhibition (LC50 of 0.274 ± 0.029 mg/mL), and the metal chelation activity (MC50 of 0.203 ± 0.021 mg/mL). All the PR extracts at 0.01 and 0.1 mg/mL showed no cytotoxicity on B16F10 cells, and human skin fibroblasts, respectively. Likewise, the PR-R-Sox extract exhibited the highest anti-melanogenesis on B16F10 cells (52.7 ± 0.9%) and, the mushroom tyrosinase inhibition activity (IC50 of 0.04 ± 0.02 mg/mL), which was significantly comparable to kojic acid (p < 0.05). The PR-Y-Sox extract showed the collagen biosynthesis by the Sirius Red method, and the stimulation of anti-ageing genes (Sirt1 and Foxo1) on human skin fibroblasts by the RT-PCR method, which were similar to standards ʟ-ascorbic acid and resveratrol, respectively. This study suggests that the PR-R-Sox and PR-Y-Sox extracts can be further developed as natural anti-ageing agents for whitening and anti-wrinkle in the cosmetics, cosmeceutical, and pharmaceutical industries.
Keywords: Sapindaceae, Antioxidants, Whitening, Anti-wrinkles, Nephelium lappaceum L.
1. Introduction
Globally, the ageing population in the world is growing faster than all the other age groups. The World Health Organization estimated that the world’s population over 60 years and older would be 2.1 billion in 2050, and will be faced with common conditions including osteoarthritis, diabetes, hearing loss, alzheimer’s disease, chronic obstructive pulmonary diseases (COPDs), depression and dementia, cancers, and neurological and cardiovascular diseases (Darawsha et al., 2021, Who, 2021). Skin is one of the most visible organs during the ageing process. Skin aging is related to social communication because good appearances make a favorable impression and improve self-confidence. Skin ageing is affected by environmental or external factors such as air pollutants, xenobiotics, UV radiation, and smoking, and by internal factors such as genetics, cellular metabolism, hormones, and metabolic processes (Darawsha et al., 2021). Many recent researches have focused for maintaining a healthy skin by postponing, preventing, and treating ageing to improve the skin appearance by regenerating and stimulating natural physiological processes to protect the skin from ageing.
The reactive oxygen species (ROS) from the cellular oxidative stress can cause induction of apoptotic cell death by the activation of inflammatory processes and their signaling pathways, increasing of metalloproteinases (MMPs) levels, the degradation of unsaturated lipids, and changing the structures of fibrillar proteins including collagens and elastin that due to the skin ageing (Miastkowska and Sikora, 2018, Darawsha et al., 2021). Following melanogenesis, melanin is a polyphenolic pigment produced in melanosomes of melanocytes that are located in animal’s eyes, hairs and skins, and can protect the skin damaged by ultraviolet radiation (Ali and Naaz, 2018). Tyrosinase is the copper containing enzyme, that can transform L-tyrosine to L-dopa and L-dopa to o-dopaquinone-H + by hydroxylation and oxidation reactions, and then passes through intermediates finally to melanin (Solano, 2018). The ROS also play a significant role in the regulation of cellular oxidative stress in melanocytes which are dendritic cells that localize in the basal layer of the epidermis in the skin. The inflammatory mediators in the skin such as interleukin (IL), prostaglandin E2, tumor necrosis factor, and interferon gamma that are mainly secreted by Th cells, lymphocytes, dendritic cells, and monocytes, regulate to stimulating tyrosinase activity and upregulating TYRP-1 and TYRP-2 expression and promoting melanocyte proliferation which related to melanin production (Fu et al., 2020). However, the overproduction and high accumulation of melanin cause skin ageing such as melasma, freckles, dark spots, lentigo, and hyperpigmentation disorders, which can be aesthetically undesirable (Ali and Naaz, 2018).
Sirtuins are nuclear nicotinamide adenine dinucleotide (NAD + )-dependent class III histone deacetylase that regulate cell senescence and apoptosis, and cell growth by controlling p53, forkhead transcription factors (FOXOs), and peroxisome proliferator-activated receptor gamma (PPAR-γ) involving regulations of metabolism, cell differentiation, survival, and aging. (Son et al., 2021). The SIRT1, SIRT6, and rarely SIRT7 in the sirtuin family have been regulated for dermatologic problems, including skin inflammation, cutaneous infection, cancer, autoimmune diseases, inherited dermatologic diseases, especially skin aging (Son et al., 2021). As well, FOXO1 is a transcription factor that is located in the cytoplasm. SIRT1 deacetylates FOXO1 and activates the FoxO1 transcription in multiple pathways such as MAPK (mitogen-activated protein kinase), protein kinase (AKT), and pancreatic and duodenal homeobox-1 (Pdx1) pathways (Sin et al., 2015). The increase of Sirt1 and Foxo1 mRNA expression regulates glucose metabolism, DNA repairing, neuroprotection, differentiation, vascular protection, and insulin secretion; and decreases ageing and age-related diseases such as oxidative stress, cellular senescence, neurodegeneration, inflammation, insulin resistance, cardiovascular diseases, adiposity, and liver steatosis as well as skin ageing (Son et al., 2021).
The Pharmaceuticals (Drugs) and Cosmetics Act of 1940 defines a drug as “all medicines for internal or external uses by human beings or animals, and all substances intended to be used for, or in the diagnosis, treatment, mitigation, or prevention of any disease or disorder in humans or animals.” Cosmetic is any article intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or applied to any part of the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance, and includes any article intended for use as a component of cosmetic. Cosmeceuticals are cosmetic-pharmaceutical hybrids intended to enhance health and beauty through ingredients that influence the skin's biological textures and functions (Joshi and Pawar, 2015). Presently, anti-ageing cosmetics, cosmeceuticals, and pharmaceuticals contain active compounds which neutralize free radicals such as vitamin E, vitamin C, glutathione, and coenzyme Q10; and delay skin ageing from wrinkles and melasmas such as nucleic acids, protein hydrolysates, algae extracts, plant oils, phytohormones, cytokines, and neuropeptides (Kim et al., 2015, Miastkowska and Sikora, 2018), as well as polyphenols and flavonoids from various natural plant extracts such as the extracts of Vigna subterranean, Stichopus japonicus, Citrus aurantifolia, Ananus comosus and Manihot esculenta (Chutoprapat et al., 2020, Ding et al., 2020 Boonpisuttinant et al., 2022, Jampa et al., 2022).
Pink rambutan or Ngo See Chom Pu (Nephelium lappaceum L.) is a plant from the family of Sapindaceae, which is the indigenous plant of Klung District, Chanthaburi Province, in the eastern part of Thailand. The Pink rambutan (PR) fruits are sweet and have a good taste. They have a thin peel with a yellowish-pink rind and a light-pink spine with a light green tip, which is different from the other species such as Ngo Rongrien and Ngo Nasarn, which have light red rinds and spines. The main phytochemicals found in the rambutan plants are ellagic acid, corilagin, geraniin, quercetin, and rutin (Phuong et al., 2019, Phuong et al., 2020). It has been previously reported that the peels of rambutan from the other species have been found to have a high level of phenolic compounds and have many biological activities such as DPPH radical scavenging, anti-inflammatory, antiviral, anticancer, and antibacterial (Chingsuwanrote et al., 2016, Hernández-Hernández et al., 2019, Rohman, 2017, Sukmandari et al., 2017, Thitilertdecha and Rakariyatham, 2011). However, the Pink rambutan extracts have no scientific report for biological activities, especially anti-ageing activity for skin health and aesthetic purposes. Therefore, the current study aimed to assess and evaluate the potentials of the PR extracts in the skin applications on cosmetics, cosmeceuticals, and pharmaceuticals. The investigations of in vitro anti-ageing biological activities including the tyrosinase inhibition and the anti-melanogenesis, the collagen biosynthesis, and the stimulation of Sirt1 and Foxo1 mRNA expressions, and the three anti-oxidative activities as well as the cytotoxicity on human dermal fibroblasts, indicate the efficiency and safety of the PR extracts which can be further developed to natural skincare products.
2. Materials and methods
2.1. Preparation and extraction
The five parts of Pink rambutan (PR) including leaves (L), branches (B), seeds (S), and peels from ripe (R) and young (Y) fruits were collected from Klung District, Chantaburi, Thailand from June to August 2017. The voucher specimens were identified by a botanist at the Forest herbarium of Thailand, and registered at the herbarium (Code: RSPG-RMUTT-0102) of Innovative Natural Products from Thai Wisdom Research Unit, Faculty of Integrative Medicine, RMUTT, Pathum Thani, Thailand. All the parts of the Pink rambutan were cut, washed with tap water, dried at 60 °C in a hot air oven, and ground into powder using a grinder. All parts of Pink rambutans were extracted by Maceration and Soxhlet extraction using 95% ethanol as a solvent, which is the most frequently used for extraction of medicinal plants because it is safe and economical. Briefly, 10 g of each of the PR powders were extracted by (1): the Maceration (M) in 100 mL of 95% (v/v) ethanol (Bangkok Alcohol Industrial, Thailand) with the shaking at 200 rpm at room temperature (25 ± 2 °C) for 48 h; and (2): the Soxhlet extraction (Sox) with 100 mL of 95% (v/v) ethanol at 80 ± 5 °C for 24 h. After that, the extracts were first filtered through cotton wool and then passed through a filter paper connected with a vacuum pump. The filtrates were collected, pooled, and dried by a rotary evaporator. The PR crude extracts were kept in glass bottles and stored at 4 °C in the refrigerator until used. The extraction yields were carried out based on a dry weight basis. The 95% ethanol was used as a solvent for dissolving the PR extracts before the experiments.
2.2. Phytochemicals, total phenolics and flavonoids, and quercetin contents
Phytochemicals such as anthraquinones, alkaloids, carotenoids, glycosides, flavonoids, tannins, and xanthones of the PR extracts were investigated as previously described (Boonpisuttinant et al., 2022). The qualitative results are expressed as (+) for the presence and (-) for the absence of phytochemicals. The total phenolic contents (TPC) were assayed by the Folin-Ciocalteu assay as previously described (Boonpisuttinant et al., 2022). Briefly, 50 µL of the PR extracts, 75 µL of Folin-Ciocalteu reagent (Sigma-Aldrich, USA), and 75 µL of 7.5%(w/v) Na2CO3 (Loba Chemie, India) were added into a 96-well microplate, gently mixed, and then incubated at room temperature in a dark place for 90 min. Then, the absorbances at the wave length of 725 nm were measured by a microplate reader. The phenolic contents were calculated from the standard curve of gallic acid (Sigma-Aldrich, USA). The results were carried out as mg of gallic acid equivalents (GAE)/g of extract.
The total flavonoid content (TFC) by the modified aluminum chloride method was assayed following Shraim et al. (2021). Briefly, 50 µL of the PR extracts, 25 µL of 10%AlCl3 (BDH Prolabo Chemicals, Belgium), and 100 µL of 5% NaNO2 (Loba Chemie, India) were added into a 96-well microplate. After 5 min incubation, the plates were added 25 µL of 1 M NaOH (BDH Prolabo Chemicals, Belgium). The reaction was allowed by incubation at room temperature for 10 min. Then, the absorbances at the wavelength of 450 nm were measured by a microplate reader. The flavonoid contents were calculated from the standard curve of quercetin (Sigma-Aldrich, USA). The results were carried out as mg of Quercetin equivalents (QE)/g of extract.
The quercetin contents in the PR extracts were investigated by the HPLC method (Shaikh and Jain, 2018). Briefly, an accurately weighed amounts of the standard quercetin was dissolved in methanol in a 100 mL of volumetric flask, to obtain a stock solution of 1,000 µg/mL. The standard solutions were subsequently 2-fold serially diluted to 3.125 µg/mL. For sample preparation, 2.5 g of the extracts was dissolved in 25 mL of methanol in a 25 mL of volumetric flask to obtain 1,000 mg/mL. All analysis was performed on an Agilent 1260 infinity HPLC system (Waters, Millford, MA, USA) equipped with a photodiode array detector. The analytical column used was an Agilent Zorbax extended column C18 (5μ × 100 mm × 4.6 mm) with a mobile phase of methanol: 0.1% ortho phosphoric acid (75:25) at a flow rate of 0.6 mL/min. The column temperature was maintained at 25 °C and the detection wavelength was set at 370 nm. All solutions and solvents were filtered through a 0.22 µm filter and degassed. The sample injection volume was 10 µL, and the run time was 8 min.
2.3. Antioxidant activity
2.3.1. Free radical scavenging activity
The free radical scavenging activity of the PR extracts was assayed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method by Boonpisuttinant et al. (2019). An amount of 100 µL of the PR extracts or ʟ-ascorbic acid (Sigma-Aldrich, USA) as the positive control and 100 µL of 0.1 mg/mL of DPPH (Sigma-Aldrich, USA) solution in absolute ethanol were added to a 96 well microplate. After incubation at room temperature in a dark place for 30 min, the absorbances were measured at the wavelength of 515 nm using a microplate reader. The percentages of DPPH radical scavenging activity were calculated according to the following formula:
| (1) |
Where, A0 is the absorbance of the control and A1 is the absorbance of the samples. The concentrations providing 50% scavenging (SC50) were then calculated from the graph plotted between the %free radical scavenging and the sample concentrations.
2.3.2. Lipid peroxidation inhibition
The lipid peroxidation inhibition of the PR extracts was assayed by the Ferric-thiocyanate method Boonpisuttinant et al. (2019). An amount of 50 µL of the PR extracts or α-tocopherol (Sigma-Aldrich, USA) as the positive control and 50 µL of linoleic acid in 50%(v/v) DMSO (Sigma-Aldrich, USA) were added to a 96 well microplate. The reaction was initiated by the addition of 50 µL of 5 mM NH4SCN and 50 µL of 2 mM FeCl2. After incubation at 37 °C in a dark place for 60 min, the absorbances at a wavelength of 490 nm were measured using a microplate reader. The percentages of lipid peroxidation inhibition were calculated according to the following formula:
| (3) |
Where A0 is the absorbance of the control and A1 is the absorbance of the samples. The concentrations providing 50% of lipid peroxidation inhibition (LC50) were calculated from the graph plotted between the % lipid peroxidation inhibition and the sample concentrations.
2.3.3. Metal chelation
The metal chelation of the PR extracts was assayed by the ferrous ion chelating (FIC) method by Boonpisuttinant et al. (2019). An amount of 50 µL of the PR extracts or EDTA (Loba Chemie, India) as the positive control, 1 mg/mL FeCl2, and 50 µL of 1 mg/ml of ferrozine in 1% HCl (TCI, USA) were added to a 96 well microplate. After incubation at room temperature in a dark place for 60 min, the absorbances at the wavelength of 570 nm were measured using a microplate reader. The percentages of metal chelation were calculated according to the following formula:
| (2) |
Where A0 is the absorbance of the control and A1 is the absorbance of the samples. The concentrations providing 50% of metal chelation (MC50) were calculated from the graph plotted between the % metal chelation and the sample concentrations.
2.4. Mushroom tyrosinase inhibition
The mushroom tyrosinase inhibition activity of the PR extracts was assayed by the modified dopachrome method (Boonpisuttinant et al., 2022). An amount of 50 µL of the PR extracts or kojic acid (Sisco Research Laboratories (SRL), India) as the positive control, 50 µL of 0.1 mg/mL L-tyrosine (Sigma-Aldrich, USA), 50 µL of 0.1 mM phosphate buffer, and 50 µL of 0.1 mg/mL mushroom tyrosinase (Sigma-Aldrich, USA) were added to a 96-well microplate. Before and after incubation at 37 °C in a dark place for 60 min, the absorbances at the wavelength of 450 nm were measured using a microplate reader. The percentages of mushroom tyrosinase inhibition were calculated according to the following formula:
| (4) |
Where A is the absorbance of the blank after incubation, B is the absorbance of the blank before incubation, C is the absorbance of the samples after incubation, and D is the absorbance of the samples before incubation. The concentrations providing 50% tyrosinase inhibition (IC50 mg/mL) were calculated from the graph plotted between the % tyrosinase inhibition and the sample concentrations.
2.5. In vitro anti-ageing activities on cell cultures
2.5.1. Cell cultures
Murine melanomas (B16F10) and human skin fibroblasts were used to investigate the anti-melanogenesis, collagen biosynthesis, and anti-ageing of Sirt1 and Foxo1 mRNA expression, respectively. These two cells were obtained from the American Type Culture Collection (ATCC), Virginia, USA. The cells were cultured in the Dulbecco's Modified Eagle Medium (DMEM) (Sigma-Aldrich Biotechnology, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich Corporation, St. Louis, MO, USA), 100 IU/ml of penicillin and 100 mg/mL of streptomycin (Gibco BRL, Gaithersburg, USA) under the standard conditions at 37 °C under the 5% CO2 incubator before experiments.
2.5.2. Cytotoxicity test on human skin fibroblasts
The various concentrations of the PR extracts were investigated for the cytotoxicity on the murine melanomas (B16F10) and human skin fibroblasts by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as the previously described Boonpisuttinant et al. (2022). The human skin fibroblasts at the density of 1 × 104 cells per well were plated in 96-well plates, adjusted the volume to 180 µL with the DMEM, and incubated at 37 °C under a 5% CO2 atmosphere for 24 h. The extracts at the various concentrations were then added. The cells were incubated at 37 °C under a 5% CO2 atmosphere for 24 h. After that, the cells were washed with 10 mM PBS at pH 6.8 for 3 times and added 1 mL of 0.5 mg/mL of MTT solution (Sigma-Aldrich, USA). After incubation at room temperature for 3 h, the MTT solution was removed, and 100 µL of dimethyl sulfoxide (DMSO) was added to each well. The plates were gently shaken at 200 rpm for 15 min. The absorbances at the wavelength of 570 nm of the solutions were measured using a microplate reader. The percentages of the cell viability were calculated according to the following formula:
Where Acontrol is the absorbance of the control and Asample is the absorbance of the samples.
2.5.3. Anti-melanogenesis on B16F10 cells
The anti-melanogenesis on B16F10 cells of the PR extracts was examined using the previously described method by Boonpisuttinant et al. (2022). The B16F10 cells at the density of 2.5 × 105 cells per well were seeded in 6-well plates and incubated at 37 °C under a 5% CO2 atmosphere overnight. The extracts at a concentration of 0.01 mg/mL were then added. Kojic acid was used as a positive control. The cells were incubated at 37 °C under 5% CO2 atmosphere for 72 h. After that, the supernatants were collected to the clean microfuge tubes, whereas the cells were washed with 10 mM Phosphate Buffer Saline (PBS) at pH 6.8 for 3 times, dissolved in 200 µL of 10% NaOH, and incubated at 60 °C for 1 h. The absorbances at the wavelength of 450 nm of the supernatants and the cell lysates were measured using a microplate reader. The percentages of the melanin content were calculated according to the following formula:
| (5) |
Where, Msample was the absorbance of the samples, and Mcontrol was the absorbance of the control.
2.5.4. Collagen biosynthesis on human skin fibroblasts
The collagen biosynthesis of the PR extracts was examined as the previously described method by Boonpisuttinant et al. (2022). The human skin fibroblasts at a density of 5 × 105 cells per well were seeded into 6-well plates and incubated at 37 °C under a 5% CO2 atmosphere for overnight. The extracts at a concentration of 0.1 mg/mL were then added. ʟ-ascorbic acid was used as a positive control. The cells were incubated at 37 °C under a 5% CO2 atmosphere for 24 h. After that, the cells were washed with 10 mM PBS at pH 6.8 for 3 times, added 1 mL of 0.1% (w/v) Sirius red solution (Sigma-Aldrich, USA) in the saturated picric acid (Sisco Research Laboratories (SRL), India), and then incubated at room temperature for 1 h. The dye was removed, and the plates were washed with 1 mL of 10 mM HCl (Merck Millipore, Germany) 5 times. Then, the cells were dissolved by adding 1 mL of 0.1 M NaOH. The absorbances at the wavelength of 450 nm of the supernatants and the cell lysates were measured using a microplate reader. The percentages of the collagen amount were calculated according to the following formula:
| (6) |
Where, Csample was the collagen content of the samples, and Ccontrol was the collagen content of the control.
2.5.5. Stimulation of anti-ageing genes on human skin fibroblasts
The stimulation of anti-ageing genes (Foxo1 and Sirt1) of the PR extracts was determined by the RT-PCR as the previously described Polouliakh et al. (2020). The human skin fibroblasts at the density of 5 × 105 cells per well were seeded into 6-well plates incubated at 37 °C under 5% CO2 atmosphere overnight. The extracts at the concentration of 0.01 mg/mL were then added. After incubation for 24 h, the total RNA was extracted with the NucleoSpin RNA Plus (Macherey Nagel, Dueren, Germany) according to the manufacturer’s instructions. The amount of total RNA was quantified by Qubit 2.0 fluorometer (Invitrogen, Massachusetts, USA.). The specific primers for RT-PCR including Foxo1, Sirt1 and β-actin were purchased from Macrogen Oceania, Australia. The sequences of the specific primers were followed; Foxo1: 5′-GAC GCC GTG CTA CTC GTT-3′ (Forward) and 5′-CGG TTC ATA CCC GAG GTG-3′ (Reverse); Sirt1: 5′-TAG CCT TGT CAG ATA AGG AAG GA-3′ (Forward), 5′-ACA GCT TCA CAG TCA ACT TTG T-3′ (Reverse); and β-actin: 5′TCA TGC AGT GTG ACG TTG ACA TCC GT-3′ (Forward), 5′-CCT AGA AGC ATT TGC GGT GCA CGA TG −3′ (Reverse). The PCR mixtures have contained 1x SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA Polymerase Supermix (Invitrogen, Massachusetts, USA.), 250 nM of specific primers, and 2 µg of RNA. All the RT-PCR reactions were performed on TProfessional Basic Gradient 070–601 (Biometra GmbH, Göttingen, Germany) under the amplification conditions: 60 °C 30 min for reverse transcription; then 40 cycles at 94 °C for 15 s of denaturation, 55 °C for 30 s of annealing, and 68 °C for 30 s of extension; and finally at 68 °C for 5 min. After RT-PCR processes, the PCR products were then separated on a 1.5% agarose gel mixed with the Novel Juice 6x DNA Loading Buffer (GeneDireX™, China), viewed with a transilluminator, and then photographed using Quantity One 4.6.8 (basic) (Bio-Rad Laboratories, Inc., California). Subsequently, the band density was quantified using Image J (Version 1.47, National Institutes of Health, Bethesda, MD, USA), and was normalized to the housekeeping gene, β-actin.
2.6. Statistical analysis
All experiments were expressed as the mean ± standard deviation (S.D.) of the independent experiments (n = 3). Statistical differences were analyzed by the ANOVA with the Tukey test (p < 0.05). The relationships between the bioactivities were tested by Pearson's correlation coefficient.
3. Results
3.1. The extraction yields, physical characteristics and phytochemical contents of the PR extracts
The extraction yields, physical characteristics, and phytochemical contents of the PR extracts were exhibited in Table 1. The extraction yields of the PR extracts ranged from 10.62% to 30.63%. The main phytochemical components of the PR extracts were flavonoids. Exceptional, the Pink rambutan extracts from seeds (PR-S-M and PR-S-Sox) did not contain flavonoids but showed only glycosides as their phytochemicals. Only, the PR extracts from the ripe and young peels by the Maceration (M) showed the presence of xanthones, which is a heat-labile polyphenol. In addition, the extraction yields of the PR extracts by the Soxhlet extraction (Sox) seemed to be higher than the Maceration (M).
Table 1.
The extraction yields, physical characteristics, and phytochemical contents of the PR extracts.
| Extracts | % Yields | Physical characteristics |
Phytochemical contents |
||||
|---|---|---|---|---|---|---|---|
| Glycosides | Flavonoids | Carotenoids | Tannins | Xanthones | |||
| PR-L-M | 22.02 | viscous, dark green | – | ++ | ++ | + | – |
| PR-L-Sox | 17.63 | viscous, dark green | – | ++ | + | ++ | – |
| PR-B-M | 10.62 | viscous, brown | – | + | ++ | – | – |
| PR-B-Sox | 22.96 | viscous, brown | – | + | – | – | – |
| PR-S-M | 13.24 | viscous, light yellow | ++ | + | – | – | – |
| PR-S-Sox | 15.30 | viscous, light yellow | + | + | – | – | – |
| PR-R-M | 16.54 | viscous, dark brown | – | ++ | – | + | + |
| PR-R-Sox | 29.90 | viscous, dark brown | – | + | – | ++ | – |
| PR-Y-M | 16.02 | viscous, brown | – | ++ | – | ++ | + |
| PR-Y-Sox | 30.63 | viscous, brown | – | +++ | – | + | – |
Note: The qualitative results are expressed as (+) for the presence and (-) for the absence of phytochemicals. +++, ++, and + is shown the higher, moderate, and low contents, respectively. PR is the Pink rambutan extracts. L, B, S, R, and Y are leaves, branches, seeds, and peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
3.2. Total phenolic (TPC), flavonoid (TFC), and quercetin contents of the PR extracts
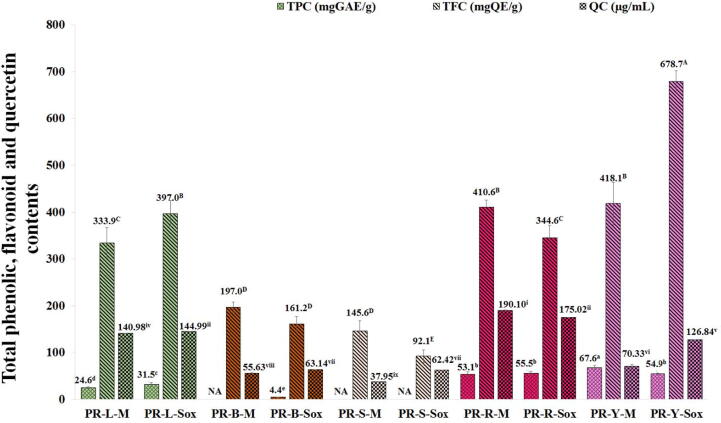
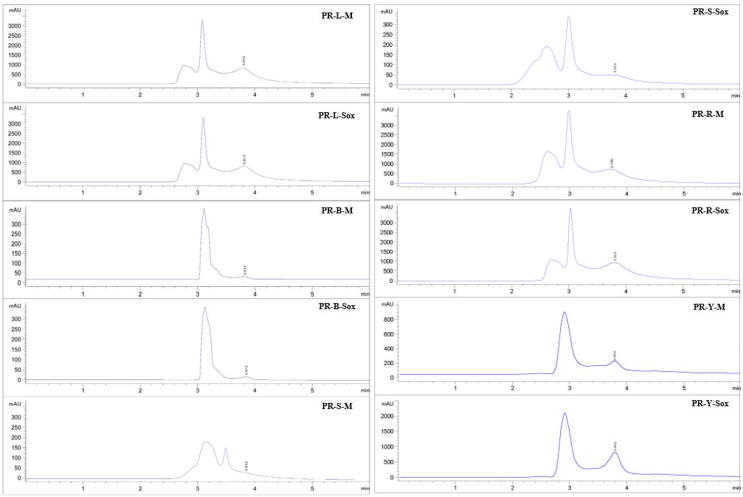
The PR extract from peels of young fruits by the Maceration (PR-Y-M) gave the highest total phenolic contents by the Folin-Ciocalteu assay of 67.60 ± 4.38 mgGAE/g, while the PR extract from peels of young fruits by the Soxhlet extraction (PR-Y-Sox) gave the highest total flavonoid contents by the modified aluminum chloride colorimetric assay of 678.72 ± 23.59 mgQE/g (Fig. 1) (p < 0.05). However, the PR-B-M, PR-S-M, and PR-S-Sox extracts showed no phenolic content. It was revealed that all PR extracts demonstrated the quercetin contents determined by the HPLC analysis. In addition, the PR extract from peels of ripe fruits by the Maceration (PR-R-M) gave the highest quercetin contents of 190.10 ± 0.28 µg/mL. (Fig. 2).
Fig. 1.
Total phenolic content (TPC), total flavonoid content (TFC), and quercetin content (QC) of the PR extracts. Superscript asterisks (a-e for TPC, A-E for TFC and i-ix for QC) in the column indicate denote significant differences at p < 0.05 by Tukey test. PR is the Pink rambutan extracts. L, B, S, R and Y are leaves, branches, seeds, peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at the room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
Fig. 2.
HPLC chromatograms of quercetin contents in the PR extracts. PR is the Pink rambutan extracts. L, B, S, R, and Y are leaves, branches, seeds, and peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
3.3. Antioxidant and mushroom tyrosinase inhibition activities of the PR extracts
Table 2 demonstrated the three anti-oxidative activities, including the free radical scavenging activity by the DPPH method, the lipid peroxidation inhibition by the Ferric-thiocyanate method; the metal chelation by the FIC method; and the mushroom tyrosinase inhibition of the PR extracts. Most of the PR extracts except the PR-L-M, PR-B-M, and PR-S-Sox extracts showed superior free radical scavenging activity with the SC50 range of 0.023 – 0.045 mg/mL, which was comparable to standard ʟ-ascorbic acid (SC50 of 0.036 ± 0.003 mg/mL) at p < 0.05. In addition, the PR-L-M extract showed the highest lipid peroxidation inhibition with the LC50 of 0.274 ± 0.029 mg/mL, and the metal chelation activity with the MC50 of 0.203 ± 0.021 mg/mL, which was comparable to standard α-tocopherol (LC50 of 0.122 ± 0.015 mg/mL) and EDTA (MC50 of 0.518 ± 0.034 mg/mL), respectively (p < 0.05). All of the PR extracts demonstrated the mushroom tyrosinase inhibition activity, while the PR-R-Sox extract showed the highest activity with the IC50 of 0.04 ± 0.02 mg/ml, which was comparable to the standard kojic acid (IC50 of 0.02 ± 0.01 mg/ml) (p < 0.05).
Table 2.
Anti-oxidant and mushroom tyrosinase inhibition activities of the PR extracts.
| Extracts |
Free radical scavenging activity (SC50 mg/ml) |
Lipid peroxidation inhibition (LC50 mg/ml) |
Metal chelation (MC50 mg/ml) |
Mushroom tyrosinase inhibition (IC50 mg/ml) |
|---|---|---|---|---|
| PR-L-M | 0.320 ± 0.070b | 0.274 ± 0.029A | 0.203 ± 0.021i | 2.80 ± 0.18VII |
| PR-L-Sox | 0.023 ± 0.004a | 1.852 ± 0.361C | 2.793 ± 0.551ii | 0.66 ± 0.18III |
| PR-B-M | 0.309 ± 0.050b | 1.301 ± 0.120B | 3.999 ± 0.961iii | 1.01 ± 0.30IV |
| PR-B-Sox | 0.045 ± 0.003a | 1.714 ± 0.188C | 4.548 ± 0.521iii | 1.72 ± 0.32VI |
| PR-S-M | NA | NA | 3.494 ± 1.175ii | 0.95 ± 0.07IV |
| PR-S-Sox | 5.106 ± 0.061c | NA | 2.480 ± 0.828ii | 1.03 ± 0.07IV |
| PR-R-M | 0.035 ± 0.001a | 2.317 ± 0.229D | NA | 1.50. ± 0.52 V |
| PR-R-Sox | 0.036 ± 0.003a | 1.582 ± 0.246C | 5.446 ± 1.161iii | 0.04 ± 0.02I |
| PR-Y-M | 0.032 ± 0.005a | 1.606 ± 0.470C | NA | 1.70 ± 0.07VI |
| PR-Y-Sox | 0.034 ± 0.002a | 2.077 ± 0.298D | 4.714 ± 0.454ii | 0.23 ± 0.02II |
| Std. ʟ-ascorbic acid | 0.036 ± 0.003a | – | – | – |
| Std. α-tocopherol | – | 0.122 ± 0.015A | – | – |
| Std. EDTA | – | – | 0.518 ± 0.034i | – |
| Std. Kojic acid | – | – | – | 0.02 ± 0.01I |
Note: The data are expressed as mean ± SD and different superscript asterisks (a-c for SC50, A-D for LC50, i-iii for MC50, I-VII for IC50) in the column indicate denote significant differences at p < 0.05 by Tukey test. PR is the Pink rambutan extracts. L, B, S, R and Y are leaves, branches, seeds, peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at the room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
3.4. Cytotoxicity of the PR extracts
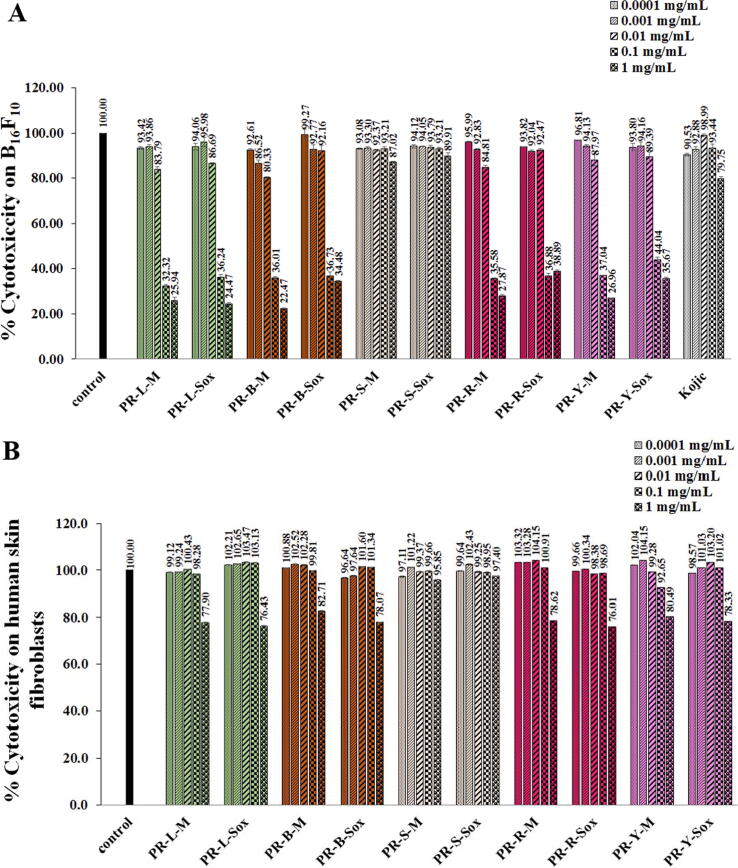
Fig. 3 showed the cytotoxicity on the B16F10 cells (A) human skin fibroblasts by the MTT assay of the PR extracts. All of the PR extracts at the concentrations of 0.01 and 0.1 mg/mL had no cytotoxicity on the B16F10 cells and human skin fibroblasts, respectively since they gave>80% relative viability compared to the untreated group. Despite this, the higher concentrations of all PR extracts were considered cytotoxic on those cells. The higher concentration than that of 1 mg/ml was not tested since it could not completely dissolve in the solvent.
Fig. 3.
Cytotoxicity on B16F10 cells (A) and human skin fibroblasts (B) of the PR extracts. PR is the Pink rambutan extracts. L, B, S, R, and Y are leaves, branches, seeds, and peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
3.5. Anti-melanogenesis of the PR extracts
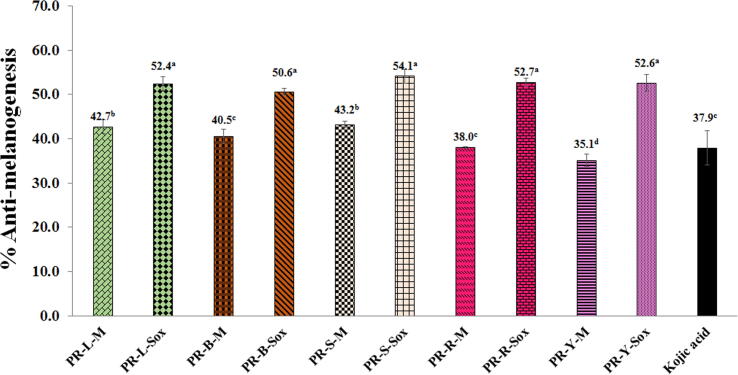
In this study, there is a screening method from many samples, therefore we highlight only the highest concentration that had no cytotoxicity on the both cells, and might have the highest anti-melanogenesis activity. This concentration is the highest concentration that showed no cytotoxicity on the B16F10 cells after being treated with the PR extracts and kojic acid (>80% cell viability) (data not shown). Fig. 4 exhibited the anti-melanogenesis on the murine melanomas (B16F10) cells of the PR extracts at the concentration of 0.01 mg/ml. All PR extracts exhibited anti-melanogenesis on B16F10 cells. The PR extracts from leaves, branches, seeds, and ripe and young fruits by the Soxhlet extraction (PR-L-Sox, PR-B-Sox, PR-S-Sox, PR-R-Sox and PR-Y-Sox) showed the highest anti-melanogenesis of 50.6–54.1%, which was dramatically superior to the standard kojic acid (37.9 ± 3.9%) by about 1.5 folds, whereas the activity of the RB-M and PR-Y-M extracts was comparable to the standard kojic acid (p < 0.05).
Fig. 4.
Anti-melanogenesis on the B16F10 cells of the PR extracts at the concentration of 0.01 mg/ml. Superscript asterisks (a-d) in the column indicate denote significant differences compared to kojic acid at p < 0.05 by the Tukey test. PR is the Pink rambutan extracts. L, B, S, R, and Y are leaves, branches, seeds, and peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
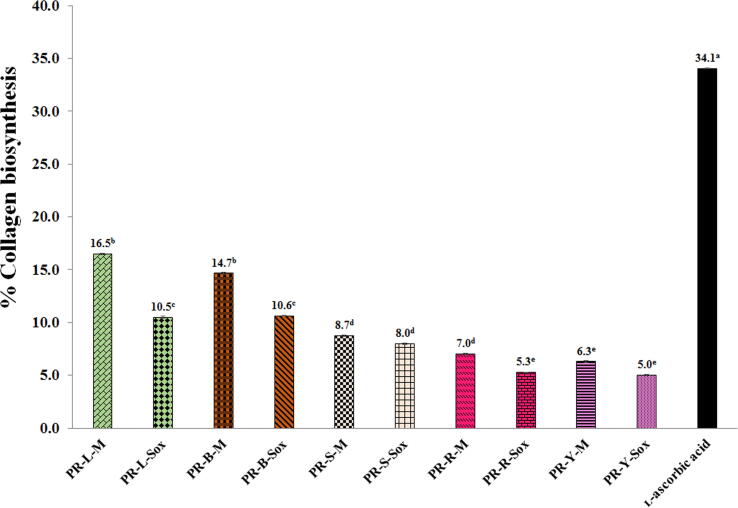
3.6. Stimulation of collagen biosynthesis of the PR extracts
All PR extracts at the concentration of 0.1 mg/ml showed the stimulation of collagen biosynthesis on the human skin fibroblasts determined by the Sirius Red method. The concentration of 0.1 mg/ml of the PR extracts and ʟ-ascorbic acid is the highest concentration that showed no cytotoxicity on the human skin fibroblasts. The PR extracts from leaves and branches by the Soxhlet extraction (the PR-L-M and the PR-B-M extracts) demonstrated the highest collagen biosynthesis (16.46 ± 0.07% and 14.66 ± 0.09%, respectively), which was lower than the standard ʟ-ascorbic acid (34.07 ± 0.03%) by about 2 folds (p < 0.05) (Fig. 5).
Fig. 5.
Collagen biosynthesis on human skin fibroblasts of the PR extracts at the concentration of 0.1 mg/ml. Superscript asterisks (a-e) in the column indicate denote significant differences compared to L-ascorbic acid at p < 0.05 by the Tukey test. PR is the Pink rambutan extracts. L, B, S, R, and Y are leaves, branches, seeds, and peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
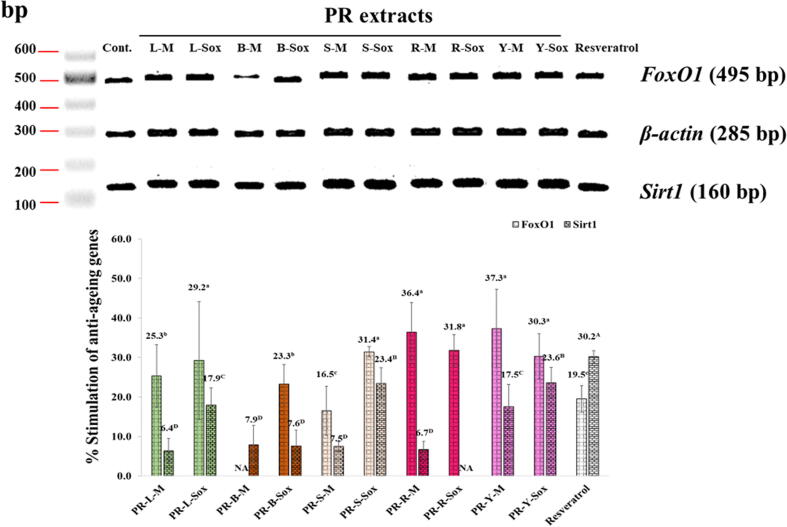
3.7. Stimulation of anti-ageing genes
Fig. 6 showed the stimulation of anti-ageing genes including Sirt1 and Foxo1 genes on the human skin fibroblasts of the PR extracts at 0.01 mg/ml was determined by the RT-PCR technique. The concentration of 0.01 mg/ml of the PR extracts and resveratrol is the highest concentration that showed no cytotoxicity on the human skin fibroblasts. Interestingly, the PR-L-Sox, PR-S-Sox, PR-R-M, PR-R-Sox, PR-Y-M and PR-Y-Sox extracts at 0.01 mg/ml exhibited the highest stimulation of Foxo1 anti-ageing mRNA expression (495 bp), which was dramatically superior to the standard resveratrol by about 2 folds (p < 0.05). On the other hand, the PR-S-Sox and PR-Y-Sox extracts exhibited the highest stimulation of Sirt1 anti-ageing mRNA expression (160 bp), which was lower than that of resveratrol (p < 0.05). Although almost all PR extracts demonstrated the stimulation of anti-ageing gene expression, the PR-R-Sox and PR-B-M extracts did not stimulate Sirt1 and Foxo1 expression, respectively.
Fig. 6.
Stimulation of anti-ageing genes on human skin fibroblasts of the PR extracts at the concentration of 0.1 mg/ml. Superscript asterisks (a-c for Foxo1 and A-D for Sirt1) in the column indicate significant differences compared to kojic acid at p < 0.05 by the Tukey test. PR is the Pink rambutan extracts. L, B, S, R, and Y are leaves, branches, seeds, and peels of ripe and young fruits, respectively. M is the Maceration in 95% (v/v) ethanol by shaking at 200 rpm at room temperature (25 ± 2 °C) for 48 h. Sox is the Soxhlet extraction with 95% (v/v) ethanol at 80 ± 5 °C for 24 h.
4. Discussion
Several natural plants have usually been screened for their phytochemicals, bioactive compounds, and biological properties to find new promising natural ingredients for medicine, food supplements, and cosmetic applications. Pink rambutan is one of the local fruits in Klung District, Chanthaburi Province, Thailand. There are a few scientific reports on its biological and pharmaceutical activities, especially its anti-ageing activity. In this study, the five parts of Pink rambutan, including leaves, branches, seeds, and peels from ripe and young fruits, were extracted by the Maceration and the Soxhlet extraction using 95% ethanol as solvent. Several newly discovered phytochemicals, including quercetin, which are secondary metabolites of plants, have been studied for anti-ageing effects in cells, animals, and humans (Phuong et al., 2020). From the findings of this study, flavonoids were the main phytochemicals of the PR extracts. It has been previously reported that the extracts from the other species of rambutan were presented with phytochemicals including flavonoids, tannins, and carotenoids, which correlated with their antioxidant properties (Rohman, 2017). The absence of xanthones by the Soxhlet extraction might be from high temperatures (Yuvanatemiya et al., 2022, Sankeshwari et al., 2018). Quercetin is one of the most abundant phenolic and flavonoid compounds found in many fruits and vegetables, as well as in rambutan (Phuong et al., 2020). In this study, the higher temperature (80 °C) of Soxhlet process can extract a non-heat labile substance including quercetin more than the cold process. The quercetin amount determined by HPLC analysis can be used as a marker for quality control of the PR extracts on the production scale. The Pearson’s correlation coefficient (R2) between the TFC and the TPC of the PR extracts was 0.825 classifying a very strong relationship (R2 = ± 0.80 to ± 1.00) (p < 0.01) which means if the TFC is increased, the TPC would be dramatically increased as well. In addition, the R2 coefficient between the TPC and the quercetin content of the PR extracts was 0.650 classifying a strong relationship (R2 = ± 0.60 to ± 0.79) (p < 0.05). The difference in extraction yields, phytochemicals, as well as TPC, TFC and quercetin of the PR extracts might be affected by the solvents, extraction processes, and temperatures (Chutoprapat et al., 2020). Several studies have found that flavonoid and phenolic compounds, as well as quercetin, have a wide range of biological and pharmaceutical activities, including anti-microbial, antioxidant, anti-inflammation, and anti-aging activity (Panche et al., 2016, Shahidi and Yeo, 2018, Musika et al., 2021, Wang et al., 2022).
As previously stated, radical oxygen species, or ROS, can cause age-related damage at the cell and tissue levels, accelerating chronologic and environmental ageing characterized by wrinkles and atypical pigmentation (Papaccio et al., 2022). Natural antioxidants have the ability to scavenge free radicals, which results in reduced anti-inflammatory effects, the prevention of cancer, diabetes, and brain disorders, the reduction of blood pressure and atherosclerosis, and the slowing of skin aging (Miracle Uwa, 2017). Free radical scavenging, inhibition of lipid peroxidation, and metal chelation have been widely investigated as systems to investigate the antioxidant activity of natural products. For DPPH free radical scavenging, the odd electrons in DPPH molecules are reduced by receiving a hydrogen atom from antioxidants to become hydrazine molecules (Ionita, 2021). The lipid peroxidation method is based on the oxidation of copper (II) to copper (III) ions passed by a linoleic acid emulsion (Bakir et al., 2017), while the metal chelation method is based on the oxidation of copper (II) to form a ferric-ferrozine complex (Wong et al., 2014). The presence of flavonoid and phenolic content in the PR extracts from this study may have contributed to the three antioxidative activities. It was found that the phenolic compounds (e.g., corilagin, ellagic acid, geraniin, and quercetin) from the rambutan extracts exhibit antioxidant activity by DPPH radical scavenging and lipid peroxidation inhibition (Hernández-Hernández et al., 2019, Phuong et al., 2019, Monrroy et al., 2020, Araujo et al., 2021). This is similar to the previous reports that the peel extracts from the other species of rambutan exhibit high TPC and TFC, as well as show stronger antioxidant activities such as ABTS, DPPH, O2-scavenging, ferric reducing assay power (FRAP), chelating agents, and lipid peroxidation (Lourith et al., 2017, Rohman, 2017, Monrroy et al., 2020). Interestingly, the R2 coefficient between the metal chelation and the lipid peroxidation inhibition of the PR extracts showed a very strong positive correlation of 0.953, classifying a strong relationship at a significant level of p < 0.01.
A cytotoxicity study of plant extracts involving normal mammalian or human cells must be conducted before a given plant extract can eventually be investigated for evaluation in animal models and clinical trials. Our study showed that all the PR extracts at the concentrations of 0.01 and 0.1 mg/mL had no cytotoxicity on the B16F10 cells and normal human skin fibroblasts, respectively, indicating non-toxicity for dermal applications such as cosmetics, cosmeceuticals, and pharmaceuticals, and there was the proper concentration for investigation of the anti-melanogenesis on B16F10 cells and collagen biosynthesis on human skin fibroblasts. The cytotoxic effect on the normal cells of the PR extracts at the higher concentrations of > 0.1 mg/mL in this experiment might probably cause cell damage and death. The different cytotoxic effects of the plant extracts depend on the cell types, which have a different response to the nature of plants and their biologically active compounds (Rezk et al., 2015).
Hyperpigmentation can result from melanin overproduction, which is a sign of skin aging and diseases such as melasma and freckles (Ali and Naaz, 2018). Commonly, the inhibition of tyrosinase is a method for reducing skin pigmentation by controlling a rate-limiting enzyme and down-regulating melanogenesis, including tyrosinase-related proteins 1 and 2 (TRP1 and 2) and α-MSH-stimulated microphthalmia-associated transcription factor (MITF) in melanogenesis (Lee et al., 2015, Serre et al., 2018, Panzella and Napolitano, 2019). The amounts of TFC, TPC, and quercetin, as well as tannins in the PR-R-Sox extract, might be responsible for inhibiting the mushroom tyrosinase activity and melanogenesis by forming divalent ions with the Cu (II) ion content of the enzyme to form insoluble complexes (Obaid et al., 2021). The structure of tannin contains a benzene ring with many hydroxyl (—OH) groups, which is very similar to the structure of tyrosine and might inhibit the oxidation process of tyrosinase substrate (Chai et al., 2018). Many enzymes, including trypsin, amylase, and lipase, which contain Fe (II) and Zn (II) ions, can exhibit this effect. Lourith et al. (2017) reported that the extract of rambutan peels suppressed melanin production with a calculated cellular IC50 of 0.040 mg/mL.
Collagen is a fibrillar protein of the extracellular matrix, which is abundant in the body. The breakdown of collagen in organs, including bones, tendons, cartilage, and skin, resulting in a loss of elasticity and durability, can cause ageing process (Reilly et al., 2021). According to collagen biosynthesis, it starts with transcription of collagen genes, translation, and translocation of the nascent polypeptide chain to the rough endoplasmic reticulum (rER). Then, it passes to highly complex processes including co-translational modification and folding, trafficking through the Golgi network, secretion, and maturation (Onursal et al., 2021). It was revealed that the PR extracts by the Maceration seemed to stimulate the collagen biosynthesis more than the Soxhlet extraction. Although, there is no previous report on the collagen biosynthesis of the PR extracts, many reports suggest investigation of this activity in natural plants. Chutoprapat et al. (2020) reported that the ethanolic seed extract of Bambara groundnut, which contains flavonoids and tannins, stimulated collagen biosynthesis. It also suggests that a natural extract high in carotenoids can protect the skin from age-related collagen type I degradation (Meinke et al., 2017). The correlations between collagen biosynthesis and lipid peroxidation; and metal chelation, with the R2 coefficients of 0.677 and 0.689, respectively, were classified as strong positive correlations. As known, collagen can be damaged by ROS, whereas antioxidants inhibit ROS production, which can damage the biosynthesis of connective tissue, including collagen in fibroblasts, resulting in increased collagen biosynthesis (Tu and Quan, 2016). Darawsha et al. (2021) report that carotenoids, polyphenols, and estradiol could protect dermal fibroblasts from oxidative stress-induced damage through a reduction in ROS levels.
Generally, the NAD + -dependent histone deacetylase SIRT1 regulates glucose metabolism, DNA repair, neuroprotection, differentiation, vascular protection, and insulin secretion, whereas the transcription factor FOXO1 plays a role in several biological pathways, including apoptosis, oxidative stress, and cell cycle arrest (Grabowska et al., 2017, Mo et al., 2019). Both SIRT1 and FOXO1 have been studied for aging mechanisms in the oxidative phosphorylation system and restoring mitochondrial dysfunction such as insulin resistance, oxidative stress resistance, and metabolism in mammals, especially the development of ageing (Sin et al., 2015, Son et al., 2021). Natural phytochemicals, both polyphenols (quercetin, resveratrol, fisetin, and curcumin) and non-polyphenols (berberine), can modulate Sirt1 and Foxo1 mRNA expression and activity for the prevention and treatment of stress-related oxidative diseases (Iside et al., 2020). Resveratrol (3,5,4-trihydroxystilbene) derived from grape skin is an effective SIRT1 and FOXO1 activator, extending the lifespan of aged mice, regulating ageing-related factors by decreasing insulin-like growth factor-1, increasing AMP-activated protein kinase and PPAR-coactivator 1, decreasing the level of malondialdehyde and fibronectin in the renal cortex, and increasing superoxide activity (Son et al. Up-regulation of Sirt1 and Foxo1 mRNA expression on various cells in response to natural plant extracts and phytochemicals has been reported in vitro and in vivo. Son et al. (2021) discovered that a Prunus mume seed extract can up-regulate Sirt1 mRNA expression, increase collagen levels, and decrease metalloproteinase 1 mRNA expression in mouse dorsal skin tissue. Verbascoside and lycopene increased SIRT1 activity in rabbit liver and heart tissues while also improving blood lipid and glycemic profiles (Corbi et al., 2018). The extract from Dracocephalum kotschyi significantly enhanced p-FOXO1, p-AKT, SREBP-1, and PPARγ expressions in the lipid metabolism of adipose tissue (Aslian and Yazdanparast, 2018). Tabatabaie and Yazdanparast (2017) study reveals that the Teucrium polium extract could up-regulate Pdx1 and p-FoxO1 proteins and reduce p-JNK expression in diabetic rats. The extracts of Retama monosperma (L.) Boiss seed and flowers, and the isolated flavonoids, including quercetin, genistein, 6-methoxykaempferol, and kaempferol, can enhance Sirt1 and Sirt3 gene expression in HaCaT cells and show anti-oxidative activity (Zefzoufi et al., 2021). The stimulation of Sirt1 and Foxo1 mRNA expression by the extracts from Pink rambutan (Nephelium lappaceum Linn.) is reported for the first time. Punicalagin hydrolysable tannin is found in pomegranates and could increase the total FOXO1 protein and enhance its nuclear translocation (Liu et al., 2019), and enhance SIRT1 nuclear distribution and NRF-2-HO-1 signaling, resulting in a reduction of the inflammatory response, nitrosative stress, and cardiac oxidative stress induced by the myocardial ischemia/reperfusion (MI/R) operation (Yu et al., 2019). It has also been reported that hydrolysable tannins from other plants, including gallic acid, geraniin, ellagic acid, and corilagin, have been found in the peels of N. lappaceum Linn. with anti-hyperglycemic and antioxidant properties (Monrroy et al., 2020), which might have stimulation activity on Sirt1 and Foxo1 genes similar to punicalagin.
According to the findings, the anti-aging properties of most PR extracts may be attributed to phytochemical constituents such as phenolic and flavonoid content, as well as quercetin. However, there is a weak positive correlation between the quercetin content and all anti-ageing activities in these experiments. As a result, other phytochemicals in the PR extract, such as ellagic acid, corilagin, geraniin, and rutin, may be responsible for the anti-aging properties (Phuong et al., 2019, Phuong et al., 2020).
5. Conclusion
Presently, natural anti-ageing ingredients and products have become popular worldwide, including in Thailand. The rambutan is an indigenous plant of Klung District, Chanthaburi Province, in the eastern part of Thailand. The pharmaceutical activity of the Pink rambutan including its anti-ageing activity would be beneficial to promoting the utilization of valuable natural sources for the development of innovative products. We highlight the Pink rambutan from ripe peels extracted by the Soxhlet extraction (PR-R-Sox) extract as a whitening agent since it exhibits the highest tyrosinase inhibition and anti-melanogenesis activities, and also the Pink rambutan from young peels extracted by the Soxhlet extraction (PR-Y-Sox) extract as an anti-wrinkle agent because it exhibits the highest stimulation of anti-ageing Sirt1 and Foxo1 genes, collagen biosynthesis, and anti-oxidation activity. This study suggested that the PR extracts can be further developed as natural anti-ageing of bioactive substances in cosmetic, cosmeceutical and pharmaceutical industries. Further studies are required to fraction or isolate anti-ageing compounds from the PR extracts as well as the improvement of skin permeation and efficiency by nanotechnology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge the National Research Council of Thailand (NRCT) (Contract No. IRF01126001), Thailand for financial support; and Rajamangala University of Technology Thanyaburi (RMUTT), Thailand for laboratory facility and equipment.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Korawinwich Boonpisuttinant, Email: korawinwich_b@rmutt.ac.th.
Ratakorn Srisuttee, Email: ratakorn.sr@kmitl.ac.th.
Heng Yen Khong, Email: khonghy@uitm.edu.my.
Romchat Chutoprapat, Email: romchat.c@pharm.chula.ac.th.
Waraporn Malilas, Email: waraporn.mas@mahidol.ac.th.
References
- Ali S.A., Naaz I. Biochemical aspects of mammalian melanocytes and the emerging role of melanocyte stem cells in dermatological therapies. Int. J. Health Sci. (Qassim) 2018;12(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- Araujo N.M.P., Arruda H.S., Marques D.R.P., de Oliveira W.Q., Pereira G.A., Pastore G.M. Functional and nutritional properties of selected Amazon fruits: a review. Food Res. Int. 2021;147 doi: 10.1016/j.foodres.2021.110520. [DOI] [PubMed] [Google Scholar]
- Aslian S., Yazdanparast R. Hypolipidemic activity of Dracocephalum kotschyi involves FOXO1 mediated modulation of PPARgamma expression in adipocytes. Lipids Health Dis. 2018;17(1):245. doi: 10.1186/s12944-018-0893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakır T., Sönmezoglu İ., Apak R. Quantification of antioxidant ability against lipid peroxidation with an ‘Area Under Curve’ approach. J. Am. Oil Chem. Soc. 2017;94(1):77–88. doi: 10.1007/s11746-016-2918-2. [DOI] [Google Scholar]
- Boonpisuttinant K., Udompong S., Boonbai R. Tyrosinase inhibition and antioxidant activities of Riceberry (Oryza sativa L.) J. Eng. Appl. Sci. 2019;14(3 SI):6127–6130. [Google Scholar]
- Boonpisuttinant K., Unkeaw S., Chomphoo W., Udompong S., Khong H.Y. In vitro anti-ageing activities of the extracts of low-grade pineapple and lime key from Sob Prab cooperative limited, Lampang, Thailand. J. Smart Sci. Technol. 2022;2(1):46–59. doi: 10.24191/jsst.v2i1.24. [DOI] [Google Scholar]
- Chai W.M., Huang Q., Lin M.Z., Ou-Yang C., Huang W.Y., Wang Y.X., et al. Condensed tannins from Longan Bark as inhibitor of Tyrosinase: structure, activity, and mechanism. J. Agric. Food Chem. 2018;66(4):908–917. doi: 10.1021/acs.jafc.7b05481. [DOI] [PubMed] [Google Scholar]
- Chingsuwanrote P., Muangnoi C., Parengam K., Tuntipopipat S. Antioxidant and anti-inflammatory activities of durian and rambutan pulp extract. Int. Food Res. J. 2016;23:939–947. [Google Scholar]
- Chutoprapat R., Malilas W., Rakkaew R., Udompong S., Boonpisuttinant K. Collagen biosynthesis stimulation and anti-melanogenesis of bambara groundnut (Vigna subterranea) extracts. Pharm. Biol. 2020;58(1):1023–1031. doi: 10.1080/13880209.2020.1822419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbi G., Conti V., Komici K., Manzo V., Filippelli A., Palazzo M., et al. Phenolic plant extracts induce Sirt1 activity and increase antioxidant levels in the rabbit's heart and liver. Oxid. Med. Cell. Longev. 2018;2018:2731289. doi: 10.1155/2018/2731289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darawsha A., Trachtenberg A., Levy J., Sharoni Y. The protective effect of carotenoids, polyphenols, and estradiol on dermal fibroblasts under oxidative stress. Antioxidants (Basel) 2021;10(12) doi: 10.3390/antiox10122023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Jiratchayamaethasakul C., Kim J.S., Kim E.A., Heo S.J., Lee S.H. Antioxidant and anti-melanogenic activities of ultrasonic extract from Stichopus japonicus. Asian Pac. J. Trop. Biomed. 2020;10(1):33–41. doi: 10.4103/2221-1691.273092. [DOI] [Google Scholar]
- Fu C., Chen J., Lu J., Yi L., Tong X., Kang L., et al. Roles of inflammation factors in melanogenesis (Review) Mol. Med. Rep. 2020;21(3):1421–1430. doi: 10.3892/mmr.2020.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska W., Sikora E., Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18(4):447–476. doi: 10.1007/s10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández C., Aguilar C.N., Rodríguez-Herrera R., Flores-Gallegos A.C., Morlett-Chávez J., Govea-Salas M., et al. Rambutan (Nephelium lappaceum L.): Nutritional and functiona l properties. Trends Food Sci. Technol. 2019;85:201–210. doi: 10.1016/j.tifs.2019.01.018. [DOI] [Google Scholar]
- Ionita P. The Chemistry of DPPH. Free Radical and Congeners. Int. J. Mol. Sci. 2021;22(4):1545. doi: 10.3390/ijms22041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iside C., Scafuro M., Nebbioso A., Altucci L. SIRT1 activation by natural phytochemicals: an overview. Front. Pharmacol. 2020;11:1225. doi: 10.3389/fphar.2020.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jampa M., Sutthanut K., Weerapreeyakul N., Tukummee W., Wattanathorn J., Muchimapura S. Multiple bioactivities of Manihot esculenta Leaves: UV filter, anti-oxidation, anti-melanogenesis, collagen synthesis enhancement, and anti-adipogenesis. Molecules. 2022;27(5) doi: 10.3390/molecules27051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi L.S., Pawar H.A. Herbal cosmetics and cosmeceuticals: an overview. Natural Products Chem. Res. 2015;3(2) doi: 10.4172/2329-6836.1000170. [DOI] [Google Scholar]
- Kim M., Shin S., Lee J.A., Park D., Lee J., Jung E. Inhibition of melanogenesis by Gaillardia aristata flower extract. BMC Complement. Altern. Med. 2015;15:1–11. doi: 10.1186/s12906-015-0972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Lee W.J., Chang S.E., Lee G.Y. Hesperidin, a popular antioxidant inhibits melanogenesis via Erk1/2 mediated MITF degradation. Int. J. Mol. Sci. 2015;16(8):18384–18395. doi: 10.3390/ijms160818384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Cao K., Lv W., Feng Z., Liu J., Gao J., et al. Punicalagin attenuates endothelial dysfunction by activating FoxO1, a pivotal regulating switch of mitochondrial biogenesis. Free Radic. Biol. Med. 2019;135:251–260. doi: 10.1016/j.freeradbiomed.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Lourith N., Kanlayavattanakul M., Chaikul P., Chansriniyom C., Bunwatcharaphansakun P. In vitro and cellular activities of the selected fruits residues for skin aging treatment. An. Acad. Bras. Cienc. 2017;89(1 Suppl 0):577–589. doi: 10.1590/0001-3765201720160849. [DOI] [PubMed] [Google Scholar]
- Meinke M.C., Nowbary C.K., Schanzer S., Vollert H., Lademann J., Darvin M.E. Influences of orally taken carotenoid-rich curly kale extract on collagen I/Elastin index of the skin. Nutrients. 2017;9(7) doi: 10.3390/nu9070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miastkowska M., Sikora E. Anti-Aging properties of plant stem cell extracts. Cosmetics. 2018;5(4) doi: 10.3390/cosmetics5040055. [DOI] [Google Scholar]
- Miracle Uwa L. The anti-aging efficacy of antioxidants. Curr. Trends Biomed. Eng. Biosci. 2017;7(4) doi: 10.19080/ctbeb.2017.07.555716. [DOI] [Google Scholar]
- Mo X., Wang X., Ge Q., Bian F. The effects of SIRT1/FoxO1 on LPS induced INS-1 cells dysfunction. Stress. 2019;22(1):70–82. doi: 10.1080/10253890.2018.1501022. [DOI] [PubMed] [Google Scholar]
- Monrroy M., Araúz O., García J.R. Active compound identification in extracts of N. lappaceum peel and evaluation of antioxidant capacity. J. Chem. 2020;2020:1–14. doi: 10.1155/2020/4301891. [DOI] [Google Scholar]
- Musika S., Pokratok N., Pliankratoke J., Khongla C., Kupradit C., Ranok A., Mangkalanan S. Antioxidant, antityrosinase and antibacterial activities of fruit peel extracts. Int. J. Agric. Technol. 2021;17(4):1447–1460. [Google Scholar]
- Obaid R.J., Mughal E.U., Naeem N., Sadiq A., Alsantali R.I., Jassas R.S., et al. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: a systematic review. RSC Adv. 2021;11(36):22159–22198. doi: 10.1039/d1ra03196a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onursal C., Dick E., Angelidis I., Schiller H.B., Staab-Weijnitz C.A. Collagen biosynthesis, processing, and maturation in lung ageing. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.593874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:1–15. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzella L., Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: recent advances. Cosmetics. 2019;6(4) doi: 10.3390/cosmetics6040057. [DOI] [Google Scholar]
- Papaccio F., Arino D.A., Caputo S., Bellei B. Focus on the contribution of oxidative stress in skin aging. Antioxidants (Basel) 2022;11(6) doi: 10.3390/antiox11061121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong N.M., Le T., Nguyen M., Camp J., Raes K. Antioxidant activity of Rambutan (Nephelium Lappaceum L.) peel extract in soybean oil during storage and deep frying. Eur. J. Lipid Sci. Technol. 2019;122(2) doi: 10.1002/ejlt.201900214. [DOI] [Google Scholar]
- Phuong N.M., Le T.T., Van Camp J., Raes K. Evaluation of antimicrobial activity of rambutan (Nephelium lappaceum L.) peel extracts. Int. J. Food Microbiol. 2020;321 doi: 10.1016/j.ijfoodmicro.2020.108539. [DOI] [PubMed] [Google Scholar]
- Polouliakh N., Ludwig V., Meguro A., Kawagoe T., Heeb O., Mizuki N. Alpha-Arbutin Promotes Wound Healing by Lowering ROS and Upregulating Insulin/IGF-1 Pathway in Human Dermal Fibroblast. Front. Physiol. 2020;11:1–8. doi: 10.3389/fphys.2020.586843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly D.M., Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plastic and Aesthetic Research. 2021;2021 doi: 10.20517/2347-9264.2020.153. [DOI] [Google Scholar]
- Rezk A., Al-Hashimi A., John W., Schepker H., Ullrich M.S., Brix K. Assessment of cytotoxicity exerted by leaf extracts from plants of the genus Rhododendron towards epidermal keratinocytes and intestine epithelial cells. BMC Complement. Altern. Med. 2015;15:364. doi: 10.1186/s12906-015-0860-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohman A. Physico-chemical properties and biological activities of Rambutan (Nephelium lappaceum L.) Fruit. Res. J. Phytochem. 2017;11(2):66–73. doi: 10.3923/rjphyto.2017.66.73. [DOI] [Google Scholar]
- Sankeshwari R., Ankola A., Bhat K., Hullatti K. Soxhlet versus cold Maceration: Which method gives better antimicrobial activity to licorice extract against Streptococcus mutans? J. Scientific Soc. 2018;45(2) doi: 10.4103/jss.JSS_27_18. [DOI] [Google Scholar]
- Serre C., Busuttil V., Botto J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018;40(4):328–347. doi: 10.1111/ics.12466. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. Int. J. Mol. Sci. 2018;19(6):1–16. doi: 10.3390/ijms19061573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh S., Jain V. Development and validation of a RP-HPLC method for the simultaneous determination of Quercetin, Ellagic Acid and Rutin in hydroalcoholic extract of Triphala Churna. Int. J. Appl. Pharm. 2018;10(3):169–174. [Google Scholar]
- Shraim A.M., Ahmed T.A., Rahman M.M., Hijji Y.M. Determination of total flavonoid content by aluminum chloride assay: a critical evaluation. Lwt. 2021;150 doi: 10.1016/j.lwt.2021.111932. [DOI] [Google Scholar]
- Sin T.K., Yung B.Y., Siu P.M. Modulation of SIRT1-Foxo1 signaling axis by resveratrol: implications in skeletal muscle aging and insulin resistance. Cell. Physiol. Biochem. 2015;35(2):541–552. doi: 10.1159/000369718. [DOI] [PubMed] [Google Scholar]
- Solano F. Feb. On the Metal Cofactor in the Tyrosinase Family. Int. J. Mol. Sci. 2018;19(2):633. doi: 10.3390/ijms19020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H.U., Choi H.J., Alam M.B., Jeong C.G., Lee H.I., Kim S.L., et al. Prunus mume Seed Exhibits Inhibitory Effect on Skin Senescence via SIRT1 and MMP-1 Regulation. Oxid. Med. Cell. Longev. 2021;2021:5528795. doi: 10.1155/2021/5528795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukmandari N.S., Dash G., Jusof W.H.W., Hanafi M. A review on Nephelium lappaceum L. Res. J. Pharm. Technol. 2017;10:2819–2827. doi: 10.5958/0974-360X.2017.00498.X. [DOI] [Google Scholar]
- Tabatabaie P.S., Yazdanparast R. Teucrium polium extract reverses symptoms of streptozotocin-induced diabetes in rats via rebalancing the Pdx1 and FoxO1 expressions. Biomed. Pharmacother. 2017;93:1033–1039. doi: 10.1016/j.biopha.2017.06.082. [DOI] [PubMed] [Google Scholar]
- Thitilertdecha N., Rakariyatham N. Phenolic content and free radical scavenging activities in rambutan during fruit maturation. Sci. Hortic. 2011;129(2):247–252. doi: 10.1016/j.scienta.2011.03.041. [DOI] [Google Scholar]
- Tu Y., Quan T. Oxidative stress and human skin connective tissue aging. Cosmetics. 2016;3(3) doi: 10.3390/cosmetics3030028. [DOI] [Google Scholar]
- Wang G., Wang Y., Yao L., Gu W., Zhao S., Shen Z., Lin Z., Liu W., Yan T. Pharmacological activity of quercetin: an updated review. Evid. Based Complement. Alternat. Med. 2022;2022:3997190. doi: 10.1155/2022/3997190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2021. Ageing and Health. Retrieved (march 2022) from http://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- Wong F.-C., Yong A.-L., Ting E.-P.-S., Khoo S.-C., Ong H.-C., Chai T.-T. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iranian J. Pharm. Res. : IJPR. 2014;13(4):1409–1415. [PMC free article] [PubMed] [Google Scholar]
- Yu L.-M., Dong X., Xue X.-D., Zhang J., Li Z., Wu H.-J., et al. Protection of the myocardium against ischemia/reperfusion injury by punicalagin through an SIRT1-NRF-2-HO-1-dependent mechanism. Chem. Biol. Interact. 2019;306:152–162. doi: 10.1016/j.cbi.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Yuvanatemiya V., Srean P., Klangbud W.K., Venkatachalam K., Wongsa J., Parametthanuwat T., Charoenphun N. A Review of the Influence of Various Extraction Techniques and the Biological Effects of the Xanthones from Mangosteen (Garcinia mangostana L.) Pericarps. Molecules. 2022;27(24) doi: 10.3390/molecules27248775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zefzoufi M., Fdil R., Bouamama H., Gadhi C., Katakura Y., Mouzdahir A., et al. Effect of extracts and isolated compounds derived from Retama monosperma (L.) Boiss. on anti-aging gene expression in human keratinocytes and antioxidant activity. J. Ethnopharmacol. 2021;280 doi: 10.1016/j.jep.2021.114451. [DOI] [PubMed] [Google Scholar]