Abstract
Background
Ruboxistaurin (RBX) used to treat retinopathy in diabetic patients which caused by microvascular damage and leakage which contributes to visual loss. There are no published studies on the use of liquid chromatography-tandem mass spectrometry for development and validation of a simple, sensitive, and accurate method for measuring RBX in rat plasma.
Method
Chromatographic separation of RBX was achieved using ultra-performance liquid chromatography. Multiple-reaction monitoring quantification used RBX [M + H] + ion at m/z 469.18 and daughter ions at m/z 84, 58.12, and 98.10. Atorvastatin was used as internal standard (IS), has a single daughter ion, and was identified using m/z 559.6 → 249.9. Validation of the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for RBX in rat plasma for linearity (greater than0.997) was carried out at 25–1000 ng/mL.
Results
In rat plasma, the accuracy was within 3.4%, and the intra- and inter-day precision was within 11.8%. Stability, recovery, and matrix effect were all within acceptable limits. The drug retention time (0.85 ± 0.03 min) was remarkably short.
Conclusion
The method developed in the current study is suitable to quantify RBX in plasma or bulk doses.
Keywords: Ruboxistaurin, Validation, LC-MS/MS, Plasma, Rat
1. Introduction
Millions of people globally suffer from diabetes mellitus, with type 2 accounting for the bulk of diagnosed cases. Microvascular complications, including a progressive condition involving abnormalities in retinal capillaries, affect most patients with type 1 and type 2 diabetes (Beckman et al., 2002, Deissler and Lang, 2016). Diabetic retinopathy is caused by microvascular damage and leakage and leads to macular oedema and progressive vision loss; it is the primary factor contributing to new-onset blindness in Americans between the ages of 20 and 74 (Sheetz et al., 2013). In the presence of the high blood glucose levels typical of diabetes, endothelial dysfunction and decreased retinal blood flow also occur as a result of overactivation of protein kinase C (PKC) and result in ophthalmic vascular injury (Mehta et al., 2009).
Ruboxistaurin (RBX, Arxxant, LY333531, LY338522; hydrochloride structure given in Fig. 1), is a PKC beta inhibitor that is orally bioavailable, selective, and powerful (Burkey et al., 2006), with the mode of enzyme inhibition being competitive and reversible (Burkey et al., 2006, Barbuch et al., 2006, Sheetz et al., 2013, Deissler and Lang, 2016). Phospholipid-dependent intracellular enzymes including PKC control a range of biological processes including cell division, metabolism, and differentiation. RBX has been tested to determine whether it can treat diabetic complications including retinopathy (Ishii et al., 1996, Engel et al., 2000, Burkey et al., 2006, Kunt et al., 2007), has proceeded as far as phase III clinical trials, and is currently awaiting approval for management of diabetic retinopathy in the U.S. and Europe (Ruboxistaurin, 2007, Sheetz et al., 2013, Barbuch et al., 2006). A study testing topical delivery of RBX-containing nanoparticles to the eyes has also shown promise for the treatment of diabetic retinopathy (Alshammari et al., 2022). Other potential applications of RBX are being investigated. Until recently, tamoxifen was the only PKC inhibitor capable of preventing amphetamine or cocaine abuse (Ahmad, 2018, Nestler et al., 2002), but preliminary studies have now shown that injections of RBX have similar effects to tamoxifen administration (Carpenter et al., 2017, Meier et al., 2009, Serova et al., 2006).
Fig. 1.
Structures of (A) ruboxistaurin (RBX) hydrochloride (C₂₈H₂9ClN4O3) and (B) atorvastatin. RBX.HCl has a molecular weight of 505.01 g/mol while the free base (C28H28N4O3) has a molecular weight of 469.18 g/mol.
RBX was used as a (pre-)clinical medication with fewer analytical determination techniques. Only a limited number of quantitative methods using high performance liquid chromatography (HPLC) have been published for analysis of RBX in biological fluids. Chromatographic separation of RBX has been reported for dog plasma after oral dosing (Engel et al., 2000), and human plasma, urine, and faeces have been analysed using radiolabeled RBX (Burkey et al., 2006).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has been used to identify the RBX levels oral dosing in dogs, mice, and rats (Barbuch et al., 2006, Yeo et al., 2006). Where, the RBX disposition and metabolism was discus in details, without mentioned the validation of the method used (Barbuch et al., 2006). On the other hand, study of rifampicin co-administered with RBX (Yeo et al., 2006), showed that RBX and it’s metabolite measurement in human plasma after solid-phase extraction (Yeo et al., 2006), showed acceptable validation intraassay and interassay precision and accuracy, but without mentioned the retention time, and/or the mass chromatograms obtained after RBX administration.
Unfortunately, while previously reported methods provide indications of the pharmacokinetics, disposition, and metabolism of RBX, few fully validated analytical methods have been reported. Existing methods also exhibit drawbacks including having a higher limit of quantification (Yeo et al., 2006), requiring a large volume of plasma (Bansal et al., 2013), and having an extended analytical run time and/or time-consuming sample clean-up (Bansal et al., 2013), which may involve liquid–liquid extraction.
To the best of our knowledge, no fully validated LC-MS/MS method has been reported for RBX in rat plasma. In this study we aimed to develop and validate a novel, straightforward, sensitive, dependable, and accurate method for RBX in rat plasma that can be applied in pharmacokinetic investigations.
2. Materials and methods
2.1. Chemicals and reagent
RBX (hydrochloride, 99.84%) and atorvastatin were obtained from Sigma-Aldrich (St Louis, MO, USA). All other chemicals were of analytical grade. Water was obtained from a Milli-Q water purification system (Millipore, Burlington, MA, USA).
2.2. Chromatographic separation
An Acquity ultra-performance liquid chromatography (UPLC) system (Waters Corp., Milford, MA, USA) was used for separation, using an Acquity UPLC HSS T3 column (1.0 × 100 mm), a quaternary solvent pump, and a refrigerated autosampler (10 °C). To avoid saturating the ionisation source with buffer, solvent A was 10 mM ammonium formate, 0.2% trimethylamine, 1% acetonitrile, pH 7.2, while solvent B was 0.2% formic acid, 0.2% trimethylamine in acetonitrile.
The elution gradient started at 1.0% solvent A, increasing to 80% solvent A over 2.0 min before returning to 1.0% at 2.5 min The flow rate was 0.4 mL/min, and the run time was 3 min. TargetLynx V 4.1 and MassLynx V 4.1 software (Waters Corp) were used to evaluate the results. The UPLC was linked to a triple quadrupole tandem mass detector (Waters Corp) with an electrospray ionisation (ESI) source. With the ESI source in positive ion mode, multiple-reaction monitoring (MRM) was used to measure the most significant mass transitions. The mass spectrometry parameters are given in Table 1. Argon was used as the collision gas with a pressure of 0.25 Pa, and the optimal collision energies for RBX and ATR were 30 and 40 eV, respectively. The most prominent RBX fragments either in positive and negative mode, with a scan time of 0.10 s, are shown in Fig. 2.
Table 1.
RBX liquid chromatography-tandem mass spectrometry detection settings.
| Source (ESI + ) and Analyser | Settings |
|---|---|
| Capillary voltage (kV) | 2.88 |
| Cone voltage (V) | 78 |
| Extractor (V) | 3 |
| Radio frequency lens (V) | 0.2 |
| Source temperature (°C) | 150 |
| Desolvation temperature (°C) | 500 |
| Cone gas flow (L/h) | 150 |
| Desolvation gas flow (L/h) | 650 |
| Collision energy (eV) | 20 |
| Collision gas flow (mL/min) | 0.15 |
Fig. 2.
Fragmentation of RBX in positive (upper panel) and negative (lower panel) electrospray.
2.3. Internal standard and quality control samples
Atorvastatin (Fig. 1) was used as an analogue internal standard (IS), with IS and RBX both present during sample preparation and analysis without evidence of interference. RBX and IS were prepared as stock solutions at 1000 µg/mL in methanol and stored at − 30 °C in 4.0 mL coloured glass vials. To produce working standard solutions of RBX (25–1000 ng/mL), aliquots of stock solutions of RBX were diluted with methanol. Quality control (QC) samples were used at 100 (low), 500 (intermediate), and 1000 ng/mL (high) concentrations. To prepare the QC samples, different concentrations of RBX working solution was spiked into rat plasma containing IS (20 µL). Fresh QC samples were prepared daily.
2.4. Plasma sample preparation
Plasma was collected from six male Wistar rats (250 ± 20 g) after approved by the Institutional Review Board of the King Saud University Institutional Review Board Committee (IRB) (Approved number, SE-22–153). At least six copies of plasma calibration standards were prepared in Eppendorf tubes, with concentrations ranging from 25 to 1000 ng/mL. Aliquots comprised 200 µL of blank rat plasma spiked with RBX solution and 20 µL IS. Protein was precipitated from plasma samples using 1 mL acetonitrile, and samples were vortexed for 1 min before centrifugation at 20,000 rpm for 15 min. The supernatant was then transferred to a clean 5 mL Pyrex glass tube and evaporated with a moderate nitrogen stream until dry. The residue was reconstituted in 900 µL of 90% methanol.
2.5. Matrix effect
Six blank (drug-free) rat plasma samples were collected, and RBX was used to reconstitute extracts at three different concentrations (low, intermediate, and high) of 100, 500, and 1000 ng/mL. The peak regions of samples were contrasted with non-extracted reference standard solutions (n = 6) with the same nominal amount of RBX in the mobile phase.
2.6. Method validation
The following elements were assessed during method validation, in accordance with guidelines of the European Medicines Agency (EMA) (van Amsterdam et al., 2013) and the Food and Drug Administration (FDA) (FDA, 2018, Meesters and Voswinkel, 2018, Food and Industry, 2001): selectivity, accuracy, precision, recovery, matrix effect, and stability.
Calibration standards were prepared using rat plasma. Ultrafiltrate was mixed with a stock solution of RBX (1 mg/mL) to create seven calibrators at concentrations of 25, 50, 100, 250, 500, 750, and 1000 ng/mL. Additionally, one sample was prepared and tested that only included plasma ultrafiltrate and 20 µL of IS. QC samples were prepared at the following concentrations: 100 ng/mL (low), 500 ng/mL (intermediate), and 1000 ng/mL (high) to assess the accuracy and precision (higher QC). Prepared solutions were stored at − 30 °C until use.
To ascertain selectivity, interference was studied in six separate rat plasma samples. When there was no interference from interfering components and/or no interference at the RBX retention time, selectivity was deemed satisfactory. By spiking the lowest standard concentration of blank rat plasma in the calibration curve, three lower limit of quantitation (LLOQ; see below) concentrations for RBX replicates were produced.
At seven different concentration levels, accuracy was evaluated using the coefficient of variation, assessed by computing the percentage difference between the measured and nominal concentrations. By examining standard plots coupled to a seven-point standard calibration curve, linearity was confirmed. The matrix effect was evaluated by comparing the peak area of analyte observed in blank samples to the area observed in methanol, i.e., by comparing the slopes of RBX calibration curves in the rat plasma matrix and mobile phase.
Percentage bias and relative standard deviation (RSD) were used to determine precision and accuracy, whereby all values, with the exception of LLOQ, should be within 15% of one another.
Using three QC samples for each test, stability was evaluated as freeze–thaw stability, autosampler stability, short-term stability, and long-term stability. Freezing (−30 °C) and thawing (room temperature (RT), e.g., 25 °C) stability were tested for three freeze–thaw cycles. To assess autosampler stability, extracted QC samples were stored in an autosampler at 10 °C for 24 h. Short-term stability was tested at RT for 24 h. After 30 d storage at − 30 °C, the long-term stability of QC samples was assessed; with RSDs of less than 15%, all samples were judged stable.
The limit of detection (LOD) was determined using the lowest analyte concentration with a signal-to-noise (S:N) ratio of greater than 5:1. The lowest analyte concentration giving an S:N ratio greater than 10 that can be determined daily with an RSD of 20% was taken to be the LLOQ.
3. Results
3.1. Method development
Ion mode (ESI), stationary and mobile phases, flow rate, and column temperature were assessed to determine the optimal chromatographic separation and most sensitive analytical conditions for quantifying RBX. Both positive- and negative-ion modes were investigated in the early stages of method development. A LC-MS/MS tandem quadrupole mass spectrometer was used for the parent RBX (m/z 469.18) to track primary (m/z 84, collision energy (CE) = 38 V), secondary (m/z 58.12, CE = 40 V), and third ion transitions in positive ion mode (m/z 98.10, CE = 40 V) (Fig. 2). When the instrument was used in negative-ion mode, only one daughter ion was identified for RBX (m/z 467.18 → 254.03, CE = 72 V).
While RBX displayed responses in both (+) ESI and (−) ESI modes, because of its tendency to lose protons from the carboxyl group, RBX exhibited larger signal intensities in the (+) ESI mode than in the (−) ESI mode. The MRM transition used for the IS was m/z 559.6 → 249.9 (CE 40 eV) with a cone voltage of 10 V.
Best results were obtained using a UPLC HSS T3 column as the stationary phase and 10 mM ammonium formate as the aqueous component of the mobile phase. As RBX compounds have a greater mass spectrometric response, the analysis was found to benefit from addition of 0.2% formic acid, 0.2% trimethylamine, and 1% acetonitrile to the aqueous portion of the mobile phase. Solvent B, comprising acetonitrile with 0.2% formic acid, augmented the MRM response of the drug.
Controlling the column temperature and flow rate also increased selectivity and resolution, in parallel with optimisation of the stationary and mobile phases. The best column temperature was determined to be 40 °C, and a flow rate of 0.4 mL/min was found to be sufficient for separation ionisation modes.
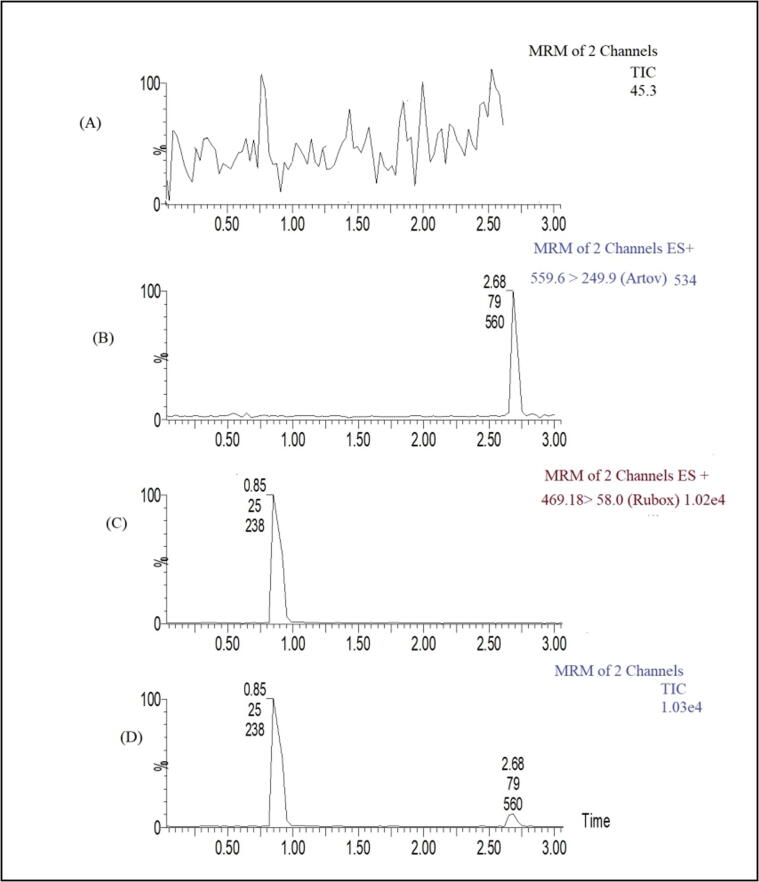
Fig. 3 displays a typical chromatogram of blank rat plasma and plasma spiked with IS and/or RBX (200 ng/mL) with two-channel MRM. RBX was well separated and eluted at 0.85 ± 0.03 min, while IS eluted at 2.68 ± 0.02 min under optimised conditions, representing one of the fastest reported separations for RBX to date. The retention time for RBX (RSD 0.9%) did not change significantly over the course of three months of validation work (P = 0.350).
Fig. 3.
Elution profiles for (A) blank rat plasma, (B) internal standard (IS) alone, (C) RBX alone, and (D) RBX and IS in rat plasma.
3.2. Method validation
Blank plasma samples from six different animals did not show any endogenous peaks at RBX or IS retention times (Fig. 3), demonstrating the specificity of the test. Low background noise allowed for excellent detection of RBX (Fig. 3). Rat plasma samples had the same chromatographic performance as spike or QC samples, a strong indicator of assay selectivity. A value of S:N of greater than 5 defined the assay LOD of 10 ng/mL with an RSD of 18%, and a value of S:N of greater than 10 defined the LLOQ of 25 ng/mL with an RSD of 15% at an injection volume of 10 µL. The standard curve for RBX in rat plasma was shown to have excellent linearity (y = 0.163 × − 0.026), where × is the concentration of RBX, and y is the peak area for RBX, with a correlation coefficient (R2) > 0.997 ± 0.0055 over the concentration range investigated (25–1000 ng/mL) (n = 6).
The regression parameters for the assay are presented in Table 2. The three calibrations showed no significant differences for three curves obtained on successive days. This approach was shown to be sensitive enough to determine the RBX levels in rats, and within the defined concentration range, the results demonstrated an excellent linear correlation between the peak area and the analyte concentration.
Table 2.
Linearity parameters for RBX (n = 6).
| Linearity parameter | RBX |
|---|---|
| Range | 25–1000 ng/mL |
| Slope | 0.163 ± 0.013 |
| Intercept | −0.026 ± 0.008 |
| Regression coefficient (R2) | 0.997 ± 0.0055 |
3.2.1. Matrix effect
Table 3 shows the matrix effect. At low, intermediate, and high QC levels, the average matrix effect values for RBX were 101.3%, 96.4%, and 103%, respectively. Under these settings, the matrix effect on analyte ionisation was not noticeable.
Table 3.
Matrix effect for RBX in rat plasma (mean ± SD, n = 6).
|
Spiked plasma concentration (ng/mL) |
Measured concentration (ng/mL) |
Matrix effect (%) |
RSD (%) |
|---|---|---|---|
| 100 | 96.5 ± 6.4 | 101.3 ± 4.4 | 9.8 |
| 500 | 490.8 ± 12.4 | 96.4 ± 5.2 | 7.4 |
| 1000 | 1101.3 ± 61.8 | 103.2 ± 6.3 | 5.2 |
3.2.2. Recovery
Recovery was evaluated five times by comparing the peak areas for extracted plasma at 100, 500, and 1000 ng/mL with peak areas obtained from the direct injection of standard solutions at the same concentrations without pretreatment. QC samples at low, intermediate, and high concentrations had extraction recoveries of 98%, 104%, and 101%, respectively. Table 4 summarises the data. The recovery of RBX in rat plasma was consistent, exact, and repeatable.
Table 4.
Recovery of RBX in rat plasma after solid-phase extraction (SPE) (mean ± SD, n = 5).
|
Spiked plasma concentration (ng/mL) |
Measured concentration (ng/mL) |
Extraction recovery (%) |
RSD (%) |
|---|---|---|---|
| 100 | 97.2 ± 5.2 | 98.3 ± 3.7 | 8.7 |
| 500 | 488.5 ± 10.2 | 104.2 ± 6.1 | 6.7 |
| 1000 | 1090.5 ± 57.3 | 101.8 ± 8.4 | 5.1 |
3.2.3. Accuracy and precision
Table 5 shows accuracy and precision values for intra- and inter-day plasma samples. Assay values were within the permitted variability limits (intra- and inter-day). The inter-day precision was 1.6–11.7%, and the intra-day precision was 4.9–7.6%. The intra-day accuracy was determined to be −1.6–0.33%, and the inter-day accuracy to be −3.4–0.45%. The data demonstrated that the accuracy, precision, and reproducibility of the assay were acceptable, with all results within acceptable limits, according to the U.S. FDA Bioanalytical Method Validation guidelines.
Table 5.
Precision and accuracy for RBX in rat plasma (n = 6; 3 d).
|
Spiked plasma concentration (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Intra-day |
Inter-day |
|||||||
| Measured Concentration (ng/mL) |
Precision (RSD, %) |
Accuracy (RE, %) |
P value | Measured Concentration (ng/mL) |
Precision (RSD, %) |
Accuracy (RE, %) |
P value | |
| 100 | 99.7 ± 12.5 | 7.61 | 0.33 | 0.834 | 98.9 ± 8.8 | 11.7 | −3.4 | 0.927 |
| 500 | 503.8 ± 24.8 | 4.93 | 0.76 | 0.65 | 497.9 ± 34.9 | 9 | 0.45 | 0.78 |
| 1000 | 1016.5 ± 59.6 | 5.86 | −1.62 | 0.47 | 1009.8 ± 69.8 | 1.6 | 0.78 | 0.605 |
3.2.4. Stability
Six replicates of QC samples at three concentrations were assessed for stability during storage and handling. RBX was shown to be stable in rat plasma after 24 h at RT, for three freeze–thaw cycles, and for 30 days at − 30 °C. The processed samples also remained stable in the autosampler for 24 h, and the findings were consistent throughout the procedure. Table 6 presents the results of stability testing.
Table 6.
RBX stability in rat plasma (n = 5).
| Storage conditions |
Concentration (ng/mL) |
RSD (%) | RE (%) | |
|---|---|---|---|---|
| Spiked | Measured (mean ± SD) | |||
| RT for 24 h | 100 | 103.07 ± 6.46 | 6.27 | 2.97 |
| 500 | 507.73 ± 15.99 | 3.15 | 1.52 | |
| 1000 | 1016.17 ± 35.08 | 3.45 | 1.59 | |
| Autosampler (10 °C for 24 h) |
100 | 106.47 ± 8.07 | 7.58 | 6.07 |
| 500 | 508.37 ± 15.87 | 3.12 | 1.64 | |
| 1000 | 1013.87 ± 29.39 | 2.89 | 1.37 | |
| Long-term stability (−30 °C for 30 d) |
100 | 102.77 ± 4.55 | 4.42 | 2.69 |
| 500 | 504.7 ± 12.53 | 2.48 | 0.93 | |
| 1000 | 1022.97 ± 15.17 | 1.52 | 2.24 | |
3.2.5. Robustness
Under changes in the volume of acetonitrile in the aqueous phase, the flow rate, and the column temperature all changed, yet the retention time for RBX remained consistent at the 95% level.
3.2.6. Carry-over
Following the highest RBX calibration standard, a blank plasma sample was injected to assess carry-over. The average carry-over was 6.2% (or less than 20%), demonstrating that the carry-over had no bearing on the results.
4. Discussion
A highly sensitive LC-MS/MS method for quantifying RBX in rat plasma was developed in this study and fully validated according to FDA guidelines (FDA, 2018, Food and Industry, 2001, Guideline, 2005). It demonstrated appropriate sensitivity and suitability for routine therapeutic drug monitoring.
The selection of an appropriate column for RBX separation was the first challenge in this study. Several elution gradients were tested to achieve baseline separation of RBX. Preliminary results showed that the separation was improved by increasing the aqueous phase ratio.
The (pre-)clinical drug RBX employed and only a few analytical measurement methods were used. Limited publication with a high performance liquid chromatography (HPLC) was reported in biological fluid related to dogs and human (Engel et al., 2000, Burkey et al., 2006, Yeo et al., 2006, Barbuch et al., 2006). None of these articles mentioned the retention time of the RBX and/or the linearity and inter-inra-day validation process. Additionally only one publication applied the liquid mass- tandem mass (LC-MS/MS) analysis for RBX (Barbuch et al., 2006) without mentioned any details for validation, it was determined the pharmacokinetics, disposition, and metabolism of RBX.
There is an urge need to developed a simple method with full validation of the RBX using LC-MS/MS technique have a several advantages, single step protein precipitation (using acetonitrile), high limit of quantification, small volume of plasma (200 µL) and short run time as the retention time of the RBX was recorded as 0.85 ± 0.03.
5. Conclusion
For the first time, a unique, straightforward, sensitive and simple protein precipitation sample preparation based LC-MS/MS method used for assessing RBX in rat plasma was developed. The method has been fully validated and may be used to assess any dosage type containing RBX. Validation studies showed that the assay is linear across the recommended working range, precise, accurate, and specific, and is suitable for use in future bioequivalence studies and pharmacokinetic investigations of RBX.
Author contributions
All authors contributed to data analysis and drafting and revising the article gave final approval of the version to be published, and agreed to be accountable for all aspects of this work.
7. Availability of data and material
All data and material are available upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting project number (RSPD2023R621), King Saud University, Riyadh, Saudi Arabia
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad I. Tamoxifen a pioneering drug: an update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018;143:515–531. doi: 10.1016/j.ejmech.2017.11.056. [DOI] [PubMed] [Google Scholar]
- Alshammari R.A., Aleanizy F.S., Aldarwesh A., Alqahtani F.Y., Mahdi W.A., Alquadeib B., Alqahtani Q.H., Haq N., Shakeel F., Abdelhady H.G. Retinal delivery of the Protein Kinase C-β inhibitor ruboxistaurin using non-invasive nanoparticles of polyamidoamine dendrimers. Pharmaceutics. 2022;14:1444. doi: 10.3390/pharmaceutics14071444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D., Badhan Y., Gudala K., Schifano F. Ruboxistaurin for the treatment of diabetic peripheral neuropathy: a systematic review of randomized clinical trials. Diabetes Metab. J. 2013;37:375. doi: 10.4093/dmj.2013.37.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbuch R.J., Campanale K., Hadden C.E., Zmijewski M., Yi P., O'Bannon D.D., Burkey J.L., Kulanthaivel P. In vivo metabolism of [14C] ruboxistaurin in dogs, mice, and rats following oral administration and the structure determination of its metabolites by liquid chromatography/mass spectrometry and NMR spectroscopy. Drug Metab. Dispos. 2006;34:213–224. doi: 10.1124/dmd.105.007401. [DOI] [PubMed] [Google Scholar]
- Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Burkey J.L., Campanale K.M., Barbuch R., O'Bannon D., Rash J., Benson C., Small D. Disposition of [14C] ruboxistaurin in humans. Drug Metab. Dispos. 2006;34:1909–1917. doi: 10.1124/dmd.106.009894. [DOI] [PubMed] [Google Scholar]
- Carpenter C., Zestos A.G., Altshuler R., Sorenson R.J., Guptaroy B., Showalter H.D., Kennedy R.T., Jutkiewicz E., Gnegy M.E. Direct and systemic administration of a CNS-permeant tamoxifen analog reduces amphetamine-induced dopamine release and reinforcing effects. Neuropsychopharmacology. 2017;42:1940–1949. doi: 10.1038/npp.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deissler H.L., Lang G.E. The protein kinase C inhibitor: ruboxistaurin. Retinal Pharmacotherapeutics. 2016;55:295–301. doi: 10.1159/000431204. [DOI] [PubMed] [Google Scholar]
- Engel G.L., Farid N.A., Faul M.M., Richardson L.A., Winneroski L.L. Salt form selection and characterization of LY333531 mesylate monohydrate. Int. J. Pharm. 2000;198:239–247. doi: 10.1016/s0378-5173(00)00350-1. [DOI] [PubMed] [Google Scholar]
- FDA, F. 2018. Guidance for industry: bioanalytical method validation. http://www.fda.gov/cder/Guidance/4252fnl.pdf.
- FOOD, U. & INDUSTRY, D. A. J. G. F. 2001. Bioanalytical method validation.
- GUIDELINE, I. H. T. 2005. Validation of analytical procedures: text and methodology. Q2 (R1), 1, 05.
- Ishii H., Jirousek M.R., Koya D., Takagi C., Xia P., Clermont A., Bursell S.-E., Kern T.S., Ballas L.M., Heath W.F. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC β inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- Kunt T., Forst T., Kazda C., Harzer O., Engelbach M., Löbig M., Beyer J., Pfützner A. The β-Specific Protein Kinase C Inhibitor Ruboxistaurin (LY333531) suppresses glucose-induced adhesion of human monocytes to endothelial cells in vitro. J. Diabetes Sci. Technol. 2007;1:929. doi: 10.1177/193229680700100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meesters R., Voswinkel S. Bioanalytical method development and validation: from the USFDA 2001 to the USFDA 2018 guidance for industry. J. Appl. Bioanal. 2018;4:67–73. [Google Scholar]
- Mehta N.N., Sheetz M., Price K., Comiskey L., Amrutia S., Iqbal N., Mohler E.R., Reilly M.P. Selective PKC beta inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc. Drugs Ther. 2009;23:17–24. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M., Menne J., Haller H. Targeting the protein kinase C family in the diabetic kidney: lessons from analysis of mutant mice. Diabetologia. 2009;52:765–775. doi: 10.1007/s00125-009-1278-y. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Gould E., Manji H. Preclinical models: status of basic research in depression. Biol. Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Ruboxistaurin A.I. LY333531. Drugs RD. 2007;8:193–199. [Google Scholar]
- Serova M., Ghoul A., Benhadji K.A., Cvitkovic E., Faivre S., Calvo F., Lokiec F., Raymond E. Preclinical and clinical development of novel agents that target the protein kinase C family. Semin. Oncol. 2006:466–478. doi: 10.1053/j.seminoncol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Sheetz M.J., Aiello L.P., Davis M.D., Danis R., Bek T., Cunha-Vaz J., Shahri N., Berg P.H. The effect of the oral PKC β inhibitor ruboxistaurin on vision loss in two phase 3 studies. Invest. Ophthalmol. Vis. Sci. 2013;54:1750–1757. doi: 10.1167/iovs.12-11055. [DOI] [PubMed] [Google Scholar]
- Yeo K.P., Lowe S.L., Lim M.T., Voelker J.R., Burkey J.L., Wise S.D. Pharmacokinetics of ruboxistaurin are significantly altered by rifampicin-mediated CYP3A4 induction. Br. J. Clin. Pharmacol. 2006;61:200–210. doi: 10.1111/j.1365-2125.2005.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available upon reasonable request.